INTRODUCTION
Currently, the recommended therapy to control refractory status epilepticus (RSE) is intravenous (IV) anesthetics, such as midazolam, propofol, barbiturates, and so on. However, 15%–26% of RSE cases still cannot be terminated. Three case series studies have demonstrated that the combination of IV anesthetics and moderate (30–31°C) or mild (31–35°C) hypothermia successfully terminated the seizures in RSE patients.[1,2] But the high rebound rate after rewarming, high mortality rate during hospitalization and severe neurological deficits were still unsatisfactory.[1,2] We hypothesized that starting hypothermia earlier, an hour after IV anesthetic, rather than a few days later, may get the best effect on the termination of RSE and has a better prognosis. The purpose of this research was to confirm the effect of the combination of early hypothermia and IV anesthetic on suppressing seizures and improving prognosis.
METHODS
Subjects
Patients were admitted to Neurological Intensive Critical Care Unit at Xuanwu Hospital, Capital Medical University in 2013. This study was approved by the local Ethics Committee in Xuanwu Hospital, Capital Medical University. Informed written consent was obtained from legal representatives of recruited patients.
Inclusion criteria: All patients were 14 years old or older, and were defined as RSE.[3] Exclusion criteria: (1) unstable vital signs; (2) severe systemic diseases; (3) pregnancy; and (4) contraindication of anesthetics.
Treatment protocols
This was a prospective observational case series study. Mechanical ventilation and continuous electroencephalogram (EEG) monitoring were started before IV anesthetic. RSE was initially treated with midazolam infusion. After an IV loading dose of 0.20 mg/kg (2 mg/min), continuous dose ranged from 0.05 to 0.40 mg·kg−1·h−1. If the seizures were not terminated in 10 min, another dose of 0.10 mg/kg was administered. Midazolam began to be tapered after normothermia and weaned off in 24–48 h.
After IV anesthetic for 1 h, if clinical or electrographic seizures were not terminated, an endovascular cooling system (Cool Gard 3000, Alsius Corporation, USA) or an external cooling system (MTRE, Mennen Medical, Israel) was used to induce hypothermia. To avoid system complications, the target temperature was set as 34–35°C (bladder temperature). After 24–48 h at goal temperature with absence of seizures activity, gradual rewarming was initiated at a rate of no more than 0.5°C every 12 h. If the seizures were not terminated at all, rewarming was also begun after 3–5 days considering potential adverse effect.
Electroencephalogram monitoring
Consecutive patients received a 10–20 international system EEG monitoring. As the monitoring target, suppression seizure was maintained for at least 48 h. The monitoring was not stopped until 48 h after weaning off hypothermia and anesthetic.
Data collection
Patients’ basic data including age, gender, past history of epilepsy, etiology, type of status epilepticus (SE), primary antiepileptic drugs, duration of primary therapy, and type of RSE were collected.
Therapeutic effect indexes were as follows: Standard of clinical termination: ≤1 discrete seizure per hour;[4] standard of electrographic termination: Evolving focal or generalized pattern for more than 5 s and <2 min, and ≤1 discrete seizure per hour. In case of periodic epileptiform discharges (PEDs), the average interdischarge interval had to be more than 1 s without clinical twitches.[4] Standard of reduction in seizures: The frequency of clinical or electrographic seizures was reduced by more than 5 times/h on average. Standard of seizure rebound after rewarming: After termination or reduction, limbs twitched again, or seizures reoccurred on EEG, or the frequency of clinical or electrographic seizures increased more than 5 times/h on average. Interictal EEG patterns: Rhythmic fast activities background, interictal epileptiform discharges, PEDs, slow wave background, and burst-suppression.
Complications including sinus bradycardia, hypotension, pneumonia, deep venous thrombosis, gastrointestinal paralysis, acute liver or kidney injury, and so on were collected. Complications prior to treatment should be excluded.
Prognosis including morbidity, Glasgow Outcome Scale, post-SE symptomatic epilepsy at discharge and 3 months after onset, were collected.
RESULTS
Five adult patients were recruited in the study, among which four were viral encephalitis, and one was anti-N-methyl-D-aspartate receptor (anti-NMDAR) encephalitis [Figure 1]. Their primary SE types were all generalized convulsive SE. After the initial therapy, two cases converted to nonconvulsive SE. After early combination of hypothermia and IV anesthetics, RSE were terminated in two patients and markedly reduced in the other three. No clinical or electrographic rebound after rewarming occurred in any case. Complications were not serious and improved after appropriate treatment. All five patients were survival during hospitalization. Three months after onset, two patients had restored from severe disability to normal ability, only one patient had post-SE symptomatic epilepsy, and one patient had died due to cardiac arrest of unknown reason [Table 1].
Figure 1.
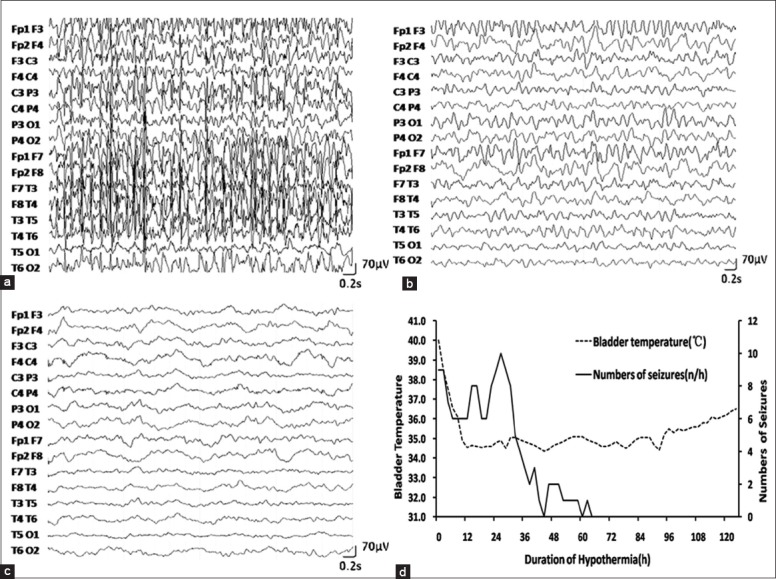
Electroencephalogram (EEG) and correlation between bladder temperature and number of seizures in case 1. (a) EEG after intravenous midazolam. Body temperature was 38.8°C. Midazolam was at 0.2 mg·kg−1·h−1; (b) EEG after reaching goal bladder temperature of 35°C. Midazolam was unchanged; (c) EEG after rewarming to normal bladder temperature of 36.5°C. Midazolam was the same as before; (d) The goal bladder temperature was reached at the 10th h. The patient was rewarmed at the 92nd h.
Table 1.
Clinical characteristics of patients
| Characteristics | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 |
|---|---|---|---|---|---|
| Age (years) | 22 | 45 | 14 | 42 | 42 |
| Gender | Female | Female | Male | Female | Male |
| Past history of epilepsy | No | No | No | No | No |
| Etiology | AntiNMDAR encephalitis | Viral encephalitis | Viral encephalitis | Viral encephalitis | Viral encephalitis |
| The type of SE | GCSE | GCSE | GCSE | GCSE | GCSE |
| Primary therapy | |||||
| Antiepileptic drugs (dosage, mg) | Diazepam (40) Valproate (3540) | Diazepam (10) Phenobarbital (1300) | Diazepam (100) Phenobarbital (1875) | Diazepam (200) Phenobarbital (1800) | Diazepam (60) Phenobarbital (1900) |
| Duration (h) | 23 | 18 | 2 | 30 | 26 |
| Anesthetic therapy | |||||
| The type of RSE | GCSE | GCSE | NCSE | GCSE | NCSE |
| Anesthetic (s) | Midazolam | Midazolam | Midazolam and propofol | Midazolam | Midazolam or propofol |
| Hypothermia therapy | |||||
| Cooling method | External | External | External | Endovascular | External |
| Target temperature (°C) | 35 | 35 | 35 | 34.5 | 35 |
| Time to target temperature (h) | 10 | 3 | 8 | 8 | 11 |
| Time at target temperature (h) | 82 | 48 | 120 | 72 | 216 |
| Rewarming period (h) | 32 | 25 | 32 | 40 | 26 |
| Results | |||||
| Termination of clinical seizures | Yes | Yes | Yes | No | No |
| Termination of electrographic | Yes | Yes | No | Yes | No* |
| seizures | |||||
| Reduction of seizures | Yes | Yes | Yes | Yes | Yes |
| Seizures rebound after rewarming | No | No | No | No | No |
| Interictal EEG pattern before hypothermia | RFAB | PEDs | SWB | IEDs | PEDs |
| Interictal EEG pattern during | IEDs | SWB | SWB | SWB, Burst-suppression | PEDs |
| hypothermia | |||||
| Complication related to treatment | |||||
| Hypotension | Yes | Yes | Yes | Yes | Yes |
| Sinus bradycardia | Yes | No | No | No | No |
| Pneumonia | Yes | No | Yes | No | Yes |
| Gastrointestinal paralysis† | Yes | Yes | Yes | Yes | Yes |
| Increase of amylase and lipase | Yes | Yes | No | No | No |
| Disturbance of coagulation | No | No | No | No | Yes‡ |
| Hypophosphatemia | Yes | No | No | No | No |
| Hyperglycemia | No | No | No | No | Yes |
| Deep venous thrombosis | No | No | No | No | Yes |
| Prognosis | |||||
| Length of NICU stays (d) | 49 | 15 | 95 | 44 | 39 |
| Length of hospital stays (d) | 102 | 15 | 95 | 44 | 39 |
| Discharge from the NICU | |||||
| Outcome | Survival | Survival | Survival | Survival | Survival |
| GOS score | 3 | 2 | 2 | 3 | 3 |
| EEG pattern | SWB | SWB | SWB | RFAB | Normal |
| Three months follow-up | |||||
| Outcome | Survival | Death | Survival | Survival | Survival |
| GOS score | 5 | 1 | 2 | 3 | 5 |
| Post-SE symptomatic epilepsy | No | No | Partial seizures at left corner of mouth | No | No |
*Control of generalized seizures except for periodic seizures; †Gastric retention >200 ml; ‡Prolonged prothrombin time and activated partial thromboplastin time. SE: Status epilepticus; GCSE: Generalized convulsive status epilepticus; NCSE: Nonconvulsive status epilepticus; RFAB: Rhythmic fast activities background; PEDs: Periodic epileptic discharges; SWB: Slow wave background; IEDs: Interictal epileptiform discharges; NMDAR: N-methyl-D-aspartate receptor; RSE: Refractory status epilepticus; EEG: Electroencephalogram; NICU: Neurological Intensive Care Unit; GOS: Glasgow Outcome Scale.
DISCUSSION
This case series first described treatment of RSE using IV anesthetic combined with early hypothermia. Our results showed that the combination of early hypothermia and anesthetic produced better effects in the treatment of RSE. The mechanism to suppress seizures of hypothermia is still unclear, maybe it is mediated by reducing Na+ exchange, decreasing K+ conductance, regulating glutamatergic synaptic transmission, reducing the release of presynaptic vesicle, and disrupting the synchronism of discharges.[1]
The seizure rebound is still an unresolved problem. Using midazolam alone to treat RSE, although most patients initially responded to the anesthetic, over half developed seizures rebound.[5] But in our study, the proportion of seizure rebound was obviously reduced. Besides, the seizure rebound after rewarming was also common. Experts attribute it to the temporarily interrupt of seizures, the reduced threshold of seizures or another time of increased release of excitatory amino acid, especially when rewarm too quickly. In our research, no seizure rebounded after rewarming in any case. Compared with previous study, we thought it was due to the longer time at target temperature (median 82 h vs. 48 h), similar rewarming duration (median 32 h vs. 45 h), and the reasonable consequence of weaning off hypothermia and anesthetic.[1]
Interestingly, all etiologies in our research were encephalitis. Due to the epileptogenicity and the multiple onset zones, this kind of RSE was more intractable. Early hypothermia can not only suppress seizures, but also protect the brain from the pathological injuries of encephalitic. Therefore, early hypothermia combined with an anesthetic may be a better regimen when treating RSE due to encephalitis.
The patients in our research had a lower morbidity and a better prognosis. With earlier hypothermia, earlier control of RSE and earlier brain protection may be the critical factors. The brain protection function was derived from the inhibition of intracellular calcium mobilization and the reduced release of excitatory toxic transmitters. Moreover, many animal experiments have proved the improved prognosis of RSE when using hypothermia, the area of hippocampal lesion was shrunk, seizures were decreased, edema was relieved, and cognitive was improved.
In our study, the adverse reactions were controlled quickly after appropriate treatment. There was no death or poor prognosis related to adverse reactions. To avoid these reactions, the vital signs, biochemical indexes, gastrointestinal movement, and deep venous blood flow should be monitored. In addition, the duration and depth of hypothermia should be limited.
In summary, combination of early hypothermia and anesthetic is a safe and effective therapy for the treatment of RSE and can improve prognosis. This research was a single-center prospective case series study. The limitation of this study included a limited sample size and lack of a control group. A prospective randomized controlled study of a larger study cohort is needed to provide more powerful evidence.
ACKNOWLEDGMENTS
We are thankful for Prof. Xiao-Feng Yang's help on paper writing.
Footnotes
Edited by: Xin Chen
Source of Support: This work is supported by grants from the National Natural Science Foundation of China (No. 81441037), the National Key Department of Neurology funded by the Chinese Health and Family Planning Committee, and National Key Department of Critical-care medicine funded by the Chinese Health and Family Planning Committee.
Conflict of Interest: None declared.
REFERENCES
- 1.Motamedi GK, Lesser RP, Vicini S. Therapeutic brain hypothermia, its mechanisms of action, and its prospects as a treatment for epilepsy. Epilepsia. 2013;54:959–70. doi: 10.1111/epi.12144. [DOI] [PubMed] [Google Scholar]
- 2.Guilliams K, Rosen M, Buttram S, Zempel J, Pineda J, Miller B, et al. Hypothermia for pediatric refractory status epilepticus. Epilepsia. 2013;54:1586–94. doi: 10.1111/epi.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayer SA, Claassen J, Lokin J, Mendelsohn F, Dennis LJ, Fitzsimmons BF. Refractory status epilepticus: frequency, risk factors, and impact on outcome. Arch Neurol. 2002;59:205–10. doi: 10.1001/archneur.59.2.205. [DOI] [PubMed] [Google Scholar]
- 4.Rossetti AO, Milligan TA, Vulliémoz S, Michaelides C, Bertschi M, Lee JW. A randomized trial for the treatment of refractory status epilepticus. Neurocrit Care. 2011;14:4–10. doi: 10.1007/s12028-010-9445-z. [DOI] [PubMed] [Google Scholar]
- 5.Claassen J, Hirsch LJ, Emerson RG, Bates JE, Thompson TB, Mayer SA. Continuous EEG monitoring and midazolam infusion for refractory nonconvulsive status epilepticus. Neurology. 2001;57:1036–42. doi: 10.1212/wnl.57.6.1036. [DOI] [PubMed] [Google Scholar]


