Abstract
Background:
Morbidity and mortality after resuscitation largely depend on the recovery of brain function. Ventricular fibrillation cardiac arrest (VFCA) and asphyxial cardiac arrest (ACA) are the two most prevalent causes of sudden cardiac death. Up to now, most studies have focused on VFCA. However, results from the two models have been largely variable. So, it is necessary to characterize the features of postresuscitation cerebral metabolism of both models.
Methods:
Forty-four Wuzhishan miniature inbred pigs were randomly divided into three groups: 18 for VFCA group, ACA group, respectively, and other 8 for sham-operated group (SHAM). VFCA was induced by programmed electric stimulation, and ACA was induced by endotracheal tube clamping. After 8 min without treatment, standard cardiopulmonary resuscitation (CPR) was initiated. Following neurological deficit scores (NDS) were evaluated at 24 h after achievement of spontaneous circulation, cerebral metabolism showed as the maximum standardized uptake value (SUVmax) was measured by 18F-fluorodeoxyglucose positron emission tomography/computed tomography. Levels of serum markers of brain injury, neuron specific enolase (NSE), and S100β were quantified with an enzyme-linked immunosorbent assay.
Results:
Compared with VFCA group, fewer ACA animals achieved restoration of spontaneous circulation (61.1% vs. 94.4%, P < 0.01) and survived 24-h after resuscitation (38.9% vs. 77.8%, P < 0.01) with worse neurological outcome (NDS: 244.3 ± 15.3 vs. 168.8 ± 9.71, P < 0.01). The CPR duration of ACA group was longer than that of VFCA group (8.1 ± 1.2 min vs. 4.5 ± 1.1 min, P < 0.01). Cerebral energy metabolism showed as SUVmax in ACA was lower than in VFCA (P < 0.05 or P < 0.01). Higher serum biomarkers of brain damage (NSE, S100β) were found in ACA than VFCA after resuscitation (P < 0.01).
Conclusions:
Compared with VFCA, ACA causes more severe cerebral metabolism injuries with less successful resuscitation and worse neurological outcome.
Keywords: Asphyxia, Cardiac Arrest, Positron Emission Tomography/Computed Tomography, Ventricular Fibrillation
INTRODUCTION
Cardiac arrest (CA) is the cessation of cardiac mechanical activity, which is confirmed by the absence of signs of circulation.[1,2] Brain injury after CA is triggered by hypoxic-ischemia and reperfusion.[3] Morbidity and mortality after resuscitation largely depend on the recovery of brain function.[4] Based on a study of CA patients who survived to the intensive care unit admission, 61.5% of patients died in hospital, and 46.0% of the death cause was neurological injury.[5]
Cardiac and respiratory etiologies account for vast majority of CAs in clinical. Ventricular fibrillation CA (VFCA) and asphyxia CA (ACA) are the two most used animal models, representing cardiac and respiratory etiologies respectively.[6] Up to now, most studies have focused on VFCA, studies on ACA are relatively few.[7,8] Results from using VFCA models have been largely different with ACA models.[6,9,10] So, there is clearly a need to characterize the features of postresuscitation cerebral metabolism of both models under identical experimental conditions. For this purpose, we designed this study to focus on the cerebral metabolism in both models, neurologic outcome and biochemical markers of brain injury were also assessed.
METHODS
This study was performed with the approval of the Animal Care and Use Committee of the Chao-Yang Hospital of Capital Medical University. Anesthesia was used in all surgical interventions. All CA data were collected implementing the updated Utstein reporting guidelines.[11]
Animals preparation
Forty-four Wuzhishan inbred miniature pigs (provided by Chinese Academy of Agricultural Sciences, 6–8 months of age, 20.5 ± 1.5 kg) were randomly divided into three groups with the use of sealed envelopes: VFCA group (n = 18, weighing 20.4 ± 1.4 kg), ACA group (n = 18, weighing 20.8 ± 1.6 kg) and the sham-operated group (SHAM group, n = 8, weighing 20.2 ± 1.1 kg).
Before experiments, all animals were under standard condition of temperature (25°C ± 2°C), controlled humidity (relative humidity 60% ± 5%) and 12 h-light/dark cycle. Prior to procedures, pigs were fasted overnight except for free access to water.
After premedication with intramuscular injection of ketamine (10 mg/kg), anesthesia was induced by intravenous (IV) administration of propofol (1.0 mg/kg) and maintained by injection of propofol (9 mg·kg-1·h-1) and fentanyl (1 μg/kg/h). Following endotracheal intubation, animals were mechanically ventilated using a Servo 900C ventilator (Siemens, Berlin, Germany) with a tidal volume of 8 ml/kg, inspired oxygen fraction of 0.21 and respiratory frequency of 12 breaths/min. The end-tidal carbon dioxide pressure was monitored by a CO2SMO Plus monitor (Respironics Inc., Murrysville, PA, USA) and maintained between 35 and 40 mmHg by adjusting the parameters of the ventilator. A 7F Swan-Ganz catheter (Edwards Life Sciences, Irvine, USA) was advanced from the left femoral vein to the pulmonary artery to obtain mixed venous blood samples and measure right atria pressure. A 5F pacing catheter was placed in the right internal jugular vein into the right ventricle to induce ventricular fibrillation (VF). A fluid-filled catheter was advanced from the left femoral artery into the thoracic aorta to measure aortic pressure.
Experimental protocol
To achieve a stable resting level, the animals were allowed to stabilize for 30 min after surgery and the baseline values were obtained. To avoid gasping, animals were paralyzed with 0.2 mg/kg cisatracurium.
Establishment of cardiac arrest models
In the VFCA group, VF was induced by programmed electric stimulation as previously described elsewhere by Wu et al.,[12] using an electrical stimulator (GY- 600A; Kaifeng Huanan Equipment Co., Ltd., Henan, China) programmed in the S1S2 protocol (300/200 ms), 40 V, 8:1 proportion, and −10 ms step length, and was verified by electrocardiogram and blood pressure. Mechanical ventilation was ceased while inducing VF.
In the ACA group, asphyxia was induced by clamping of the endotracheal tube after mechanical ventilation was removed. ACA was defined as an aortic systolic pressure of <30 mmHg.[13] The endotracheal tube was reopened in the ACA group after asphyxia had been successfully induced.
Anesthetic administration in both groups was ceased after CA had been induced.
Cardiopulmonary resuscitation and advanced life support
For both VFCA and ACA animals, mechanical ventilation was resumed with 100% oxygen after 8 min of untreated CA, and cardiopulmonary resuscitation (CPR) was initiated manually at a frequency of 100 compressions/min. In all animals, chest compressions were performed by the same investigators. A HeartStart MRx Monitor/Defibrillator with Q-CPR (Philips Medical Systems, Best, Holland) was adopted to control the quality of chest compressions.
After 2 min of CPR, epinephrine (0.02 mg/kg) was administered by IV injection. After another 2 min of CPR, if VF occurred, defibrillation (Smart Biphasic, Philips Medical Systems, Best, Holland; 3 J/kg) was attempted. CPR was continued for a further 2 min if spontaneous circulation was still not achieved, and then defibrillation was attempted once more (energy was increased in 1 J/kg increments). In 30 min, the above sequence continued until restoration of spontaneous circulation (ROSC) was achieved. ROSC was defined as aortic systolic pressure greater than 50 mmHg that was continuously sustained for at least 10 min. If ROSC was not achieved within 30 min, CPR was ceased and the animals were regarded as dead.
Pigs in the SHAM group underwent anesthesia, surgery, mechanical respiration and 8 min ventilation withheld, without CA initiation and CPR.
After ROSC, advanced life support was followed. 10 ml·kg−1·h−1 normal saline was given intraoperatively to all animals for replenishing fluid losses. The animals underwent intensive care for 6 h. At the end of 6 h postresuscitation monitoring, all catheters were removed. After adequate inspiration depth was ascertained, the animals were extubated. The animals were allowed to recover from anesthesia, and were then placed in observation areas and monitored for a further 18 h.
Measurements
Biochemical markers of brain injury
Venous blood samples were taken at baseline, 1, 2, 4, 6, and 24 h after ROSC. Blood was centrifuged at 2000 × g for 20 min. The isolated serum was immediately frozen at − 80°C and stored until the time of assay. Enzyme-linked immunosorbent assay kits (Rapid Bio, USA) were used for determination of serum neuron specific enolase (NSE) and S100β.
Neurological deficit scores
Neurological function in pigs was evaluated according to the method of neurological deficit scores (NDS) at 24 h after ROSC.[14] The NDS was scored from 0 (no neurological impairment) to 400 (death or brain death). Investigators who examined and confirmed NDS were blinded to the groups.
Cerebral metabolism imaging
On the end of NDS evaluation at 24 h after ROSC, the animals were positioned prone on the scanning table following intramuscular injection of ketamine (8 mg/kg) and ear vein injection of propofol (1.0 mg/kg). Cerebral metabolism was assessed by 18F-fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (18F-FDG PET/CT, GE Discovery STE, GE Healthcare, Waukesha, WI, USA). The imaging tracer (18F-FDG, 5.55 mBq/kg, >95% purity coefficient generated, HTA, Co., Ltd., Beijing, China) was injected into the vein 40 min before imaging. CT images were reconstructed with a 3.75-mm section thickness at 3-mm intervals. PET scan was performed following CT scan. 3D-surface rendering technology was used to visualize the region of interest (ROI; diameter 0.3 cm) and the maximum standardized uptake value (SUVmax) of the parietal lobe, frontal lobe, brain stem and cerebellum were measured by two nuclear medicine physicians blinded to the groups. SUV = (peak kBq/ml in ROI)/(injected activity/gram body weight).
Brain ultramicrostructure
Upon completion of the PET/CT scan, surviving animals were euthanized with potassium chloride (20 ml of 10 mol/L) intravenously. The brain was immediately removed by craniotomy and divided midsagittally. For morphologic examination, the frontal cortex was collected on ice and preserved in 10% formaldehyde and 4% paraformaldehyde. The ultramicrostructure of brain tissue was observed using transmission electron microscopy (H-7650; Hitachi, Ibaraki, Japan).
Statistical analysis
Statistical analysis was performed with SPSS 17.0 software (SPSS, Chicago, IL, USA). Continuous data were presented as mean ± standard deviation. One-way repeated-measures analysis of variance (ANOVA) was used to determine differences over time within groups. One-way ANOVA was used to determine differences between groups and the Bonferroni's test for multiple comparisons. The Fisher's exact test was used to compare discrete variables (ROSC and 24-h survival rates). P < 0.05 was considered statistically significant.
RESULTS
Characters of resuscitation
The CPR duration in the VFCA group was shorter than that in the ACA group (4.5 ± 1.1 min vs. 8.1 ± 1.2 min, P < 0.01). ROSC rate was higher in the VFCA group than in ACA animals (94.4% vs. 61.1%, P < 0.01). Fewer animals survived at 24 h post-ROSC in the ACA group than in VFCA group (38.9% vs. 77.8%, P < 0.01), while 100% (8/8) pigs in the SHAM group survived.
Neurological outcome
All animals enrolled in the evaluation of NDS (n = 18 in the VFCA group, ACA group, respectively, n = 8 in the SHAM group) The NDS was significantly higher in the ACA group when compared with the VFCA group at 24 h after ROSC [P < 0.01; Figure 1], verifying that animals in VFCA group had better neurological outcome.
Figure 1.
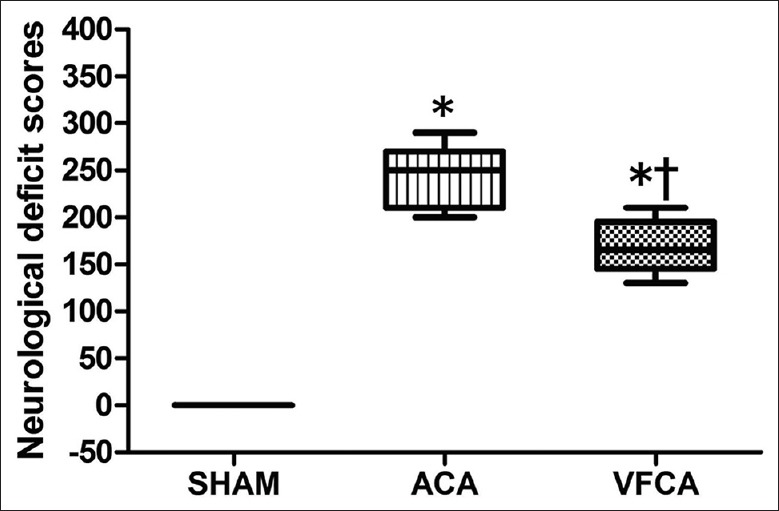
Comparisons of neurological outcome at 24 h after restoration of spontaneous circulation. SHAM: Sham-operated; VFCA: Ventricular fibrillation cardiac arrest group; ACA: Asphyxiation cardiac arrest group. *P < 0.01 versus SHAM group; †P < 0.01 versus ACA group (n = 18 in the VFCA group, ACA group, respectively, n = 8 in the SHAM group).
Biochemical markers of brain injury
There was no significant difference in serum NSE and S100β at baseline among three groups [P > 0.05; Figure 2]. Serum NSE and S100β significantly increased after ROSC in VFCA and ACA groups compared with SHAM [P < 0.05 or P < 0.01; Figure 2]. However, serum NSE and S100β were higher in the ACA group than in the VFCA group at various time points after ROSC [P < 0.01; Figure 2] (n = 14 in the VFCA group, n = 7 in the ACA group, n = 8 in the SHAM group).
Figure 2.

Comparison of biochemical markers within 24 h after restoration of spontaneous circulation (mean ± standard deviation) SHAM: Sham-operated; VFCA: Ventricular fibrillation cardiac arrest group; ACA: Asphyxiation cardiac arrest group. *P < 0.05 and †P < 0.01 vs. baseline; ‡P < 0.05 and §P < 0.01 vs. SHAM group; ||P < 0.01 vs. ACA group (n = 14 in the VFCA group, n = 7 in the ACA group, n = 8 in the SHAM group).
Glucose metabolism of brain tissue
The dosages of propofol and levels of blood glucose among three groups did not differ before PET/CT scans [P > 0.05; Table 1].
Table 1.
Comparisons of propofol dosage and blood glucose before PET/CT scan (mean ± SD)
| Groups | n | Propofol dosage (mg) | Blood glucose (mmol/L) |
|---|---|---|---|
| SHAM | 8 | 24.9 ± 2.7 | 7.7 ± 0.6 |
| VFCA | 14 | 23.8 ± 2.4 | 7.9 ± 0.8 |
| ACA | 7 | 22.9 ± 2.2 | 8.2 ± 0.8 |
SHAM: Sham-operated; VFCA: Ventricular fibrillation cardiac arrest; ACA: Asphyxiation cardiac arrest; SD: Standard deviation; PET: Positron emission tomography; CT: Computed tomography.
Figure 3 shows the images of PET/CT scan after 24 h of ROCS. Compared with SHAM, the SUVmax of the parietal lobe, frontal lobe, brain stem and cerebellum decreased in both VFCA and ACA groups at 24 h post-ROSC [P < 0.01; Figure 4]. In the ACA group, SUVmax of different regions were lower than those in the VFCA group [P < 0.05 or P < 0.01; Figure 4] (n = 14 in the VFCA group, n = 7 in the ACA group, n = 8 in the SHAM group).
Figure 3.
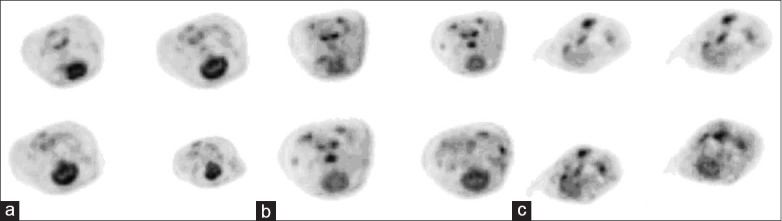
Image of an individual positron emission tomography/computed tomography scan at 24 h after restoration of spontaneous circulation. (a) sham-operated group; (b) ventricular fibrillation cardiac arrest group; (c) asphyxiation cardiac arrest group.
Figure 4.
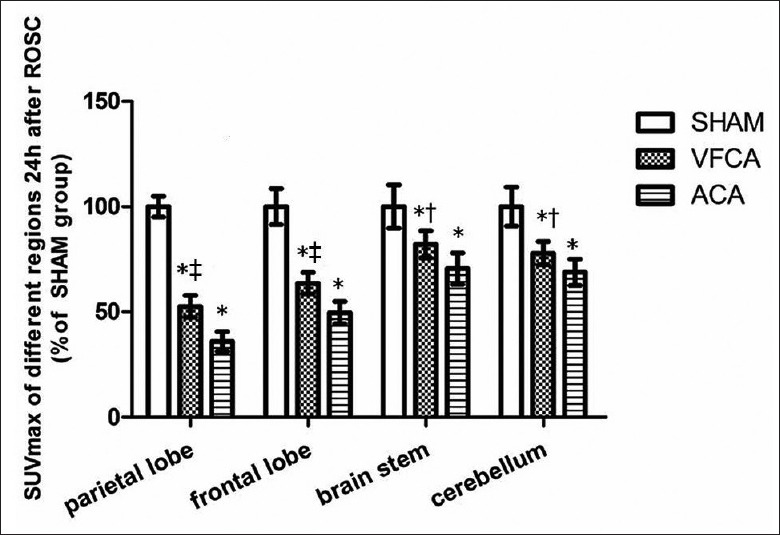
Comparison of cerebral metabolism 24 h after restoration of spontaneous circulation (mean ± standard deviation). SHAM: Sham-operated; VFCA: Ventricular fibrillation cardiac arrest group; ACA: Asphyxiation cardiac arrest group. *P < 0.01 versus SHAM group; †P < 0.05, ‡P < 0.01 versus ACA group (n = 14 in the VFCA group, n = 7 in the ACA group, n = 8 in the SHAM group).
Brain ultramicrostructure
The ACA group showed obvious nerve cell damage, including loss of normal nerve cell form, presence of nuclear deformation and solid shrinkage. Animals in VFCA exhibited little intracellular damage at 24 h after ROSC [Figure 5].
Figure 5.
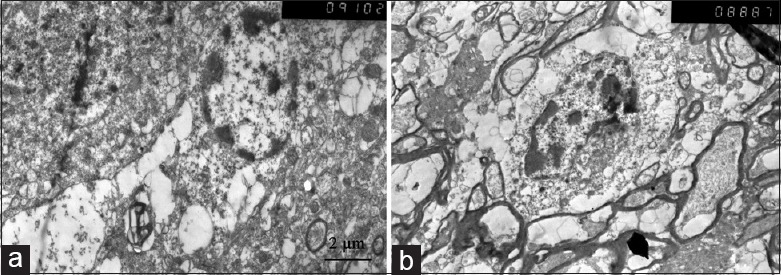
Brain ultramicrostructure of the frontal cortex tissue by electron microscopy (×5000). (a) ventricular fibrillation cardiac arrest group; (b) asphyxiation cardiac arrest group.
DISCUSSION
Here we present this study to characterize the features of postresuscitation cerebral metabolism of the both VFCA and ACA models. The main findings of the present study include: Fewer ACA animals achieved ROSC and survived 24-h post-ROSC compared with the same duration of VF arrest; the CPR duration was longer in ACA; brain tissue suffered more severe cerebral metabolism disorders in ACA, with higher levels of serum biochemical markers and worse neurological outcome.
Vaagenes et al.[10] reported that ROSC was difficult to achieve in VFCA dogs (84%) than in ACA dogs (100%) and the functional brain damage caused by VFCA is similar to that caused by ACA, which is in contrast to the result of this study. Two possible explanations may account for this disparity. The first possibility is the different duration of CA before CPR in the Vaagenes study (10 min for VFCA, while only 7 min for ACA). The second was the different animal species used in previous study. In this study, the same period of 8 min without treatment after CA induction was adopted because resuscitation could not be performed only by compression and mechanical ventilation in the 7–9 min ACA model.[15]
Biochemical markers of brain injury (NSE, S100β) were adopted in this study to evaluate the degree of postresuscitation brain injury. Serum NSE is significantly positively related with neuronal cell death, while serum S100β is a sign of blood brain barrier damage, related with the severity of glial cell damage.[16] High serum levels of NSE and S100β can predict severe neuronal injury in resuscitation.[17,18] In this study, serum levels of NSE and S100β in two resuscitation groups increased within 24 h after ROSC compared with baseline and the SHAM group at the same time, and both NSE and S100β in the ACA group were much higher than in the VFCA group [Figure 2]. These results indicate that cerebral damage was more severe in ACA than in VFCA.
18F-fluorodeoxyglucose PET/CT was adopted to investigate the cerebral metabolic activity in three groups. Glycometabolism has a close relationship with neuronal activity because glucose is the only energy source of brain cells.[19] 18F-FDG PET/CT is used as an in vivo quantitative method to noninvasively measure glucose use in different brain regions, and has been suggested as a useful index of residual brain tissue function after anoxia.[20,21] Schaafsma et al.[22] has verified decreased cerebral glucose metabolism in VFCA patients by PET at 24 h after ROSC, while no obvious hypoxia changes were found by CT and MRI. However, the cerebral glucose metabolism of ACA patients is still unknown.
In this study, because propofol is known to influence PET/CT results,[23] we measured dosage of propofol in all groups before the PET/CT scan and no differences were found among groups. 18F-FDG competes with glucose for transportation into the cells phosphorylated.[24] Thus, levels of blood glucose were also measured before PET/CT scan to avoid influence of blood glucose on SUVs, and no significant differences among groups were found [Table 1].
At 24 h after ROSC, consistent with the Schaafsma study,[22] we found cerebral metabolism in VFCA animals decreased compared with SHAM animals. The abnormal glycometabolism after CA involved both the anterior (parietal lobe and frontal lobe) and posterior circulation (cerebellum and brain stem) regions. These results support the view that CA is a model of global hopoxic-iscimia brain injury. We further found that cerebral glycometabolism in the ACA group decreased more obviously than in the VFCA group, indicating that neuronal activity decreased more seriously in the ACA model. This finding is consistent with our results concerning the NDS and levels of serum biochemical markers of brain injury.
The differences between ACA and VFCA models may partly explain the more severe brain injure in ACA. During VF, ischemia is sudden and complete while the development of ACA is gradual. So, global hypoxia and hypercarbia can fully develop during ACA before resuscitation, and the cellular energy stores were critically depleting.[15] In this study, CPR duration was much longer in ACA than in VFCA. It is recognized that long duration of the CPR is associated with decreased rate of ROSC and worse neurological outcome.[25] Therefore, the longer CPR duration in ACA may partly explain the more severe brain damage.
Some limitations of this study should be noted. This study was performed on healthy animals, whereas most VFCA patients have underlying diseases. Furthermore, we only evaluated outcomes at 24 h post-ROSC, a further study looking at 72 h or longer might help refine the evidence.
In conclusion, Compared with the same duration of VF arrest, ACA animals experienced more severe cerebral metabolism injuries with less successful resuscitation and worse neurological outcome.
Footnotes
Edited by: Xiu-Yuan Hao
Source of Support: Beijing Natural Science Foundation (No.7132092).
Conflict of Interest: None declared.
REFERENCES
- 1.McNally B, Robb R, Mehta M, Vellano K, Valderrama AL, Yoon PW, et al. Out-of-hospital cardiac arrest surveillance-Cardiac Arrest Registry to Enhance Survival (CARES), United States, October 1, 2005 - December 31, 2010. MMWR Surveill Summ. 2011;60:1–19. [PubMed] [Google Scholar]
- 2.Iwami T, Nichol G, Hiraide A, Hayashi Y, Nishiuchi T, Kajino K, et al. Continuous improvements in “chain of survival” increased survival after out-of-hospital cardiac arrests: A large-scale population-based study. Circulation. 2009;119:728–34. doi: 10.1161/CIRCULATIONAHA.108.802058. [DOI] [PubMed] [Google Scholar]
- 3.Neumar RW, Nolan JP, Adrie C, Aibiki M, Berg RA, Böttiger BW, et al. Post-cardiac arrest syndrome: Epidemiology, pathophysiology, treatment, and prognostication.A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008;118:2452–83. doi: 10.1161/CIRCULATIONAHA.108.190652. [DOI] [PubMed] [Google Scholar]
- 4.Binks A, Nolan JP. Post-cardiac arrest syndrome. Minerva Anestesiol. 2010;76:362–8. [PubMed] [Google Scholar]
- 5.Laver S, Farrow C, Turner D, Nolan J. Mode of death after admission to an intensive care unit following cardiac arrest. Intensive Care Med. 2004;30:2126–8. doi: 10.1007/s00134-004-2425-z. [DOI] [PubMed] [Google Scholar]
- 6.Tsai MS, Huang CH, Tsai SH, Tsai CY, Chen HW, Cheng HJ, et al. The difference in myocardial injuries and mitochondrial damages between asphyxial and ventricular fibrillation cardiac arrests. Am J Emerg Med. 2012;30:1540–8. doi: 10.1016/j.ajem.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Varvarousi G, Xanthos T, Lappas T, Lekka N, Goulas S, Dontas I, et al. Asphyxial cardiac arrest, resuscitation and neurological outcome in a Landrace/Large-White swine model. Lab Anim. 2011;45:184–90. doi: 10.1258/la.2011.010176. [DOI] [PubMed] [Google Scholar]
- 8.McCaul CL, McNamara P, Engelberts D, Slorach C, Hornberger LK, Kavanagh BP. The effect of global hypoxia on myocardial function after successful cardiopulmonary resuscitation in a laboratory model. Resuscitation. 2006;68:267–75. doi: 10.1016/j.resuscitation.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 9.Lah K, Križmaric M, Grmec S. The dynamic pattern of end-tidal carbon dioxide during cardiopulmonary resuscitation: Difference between asphyxial cardiac arrest and ventricular fibrillation/pulseless ventricular tachycardia cardiac arrest. Crit Care. 2011;15:R13. doi: 10.1186/cc9417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaagenes P, Safar P, Moossy J, Rao G, Diven W, Ravi C, et al. Asphyxiation versus ventricular fibrillation cardiac arrest in dogs.Differences in cerebral resuscitation effects – A preliminary study. Resuscitation. 1997;35:41–52. doi: 10.1016/s0300-9572(97)01108-8. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs I, Nadkarni V, Bahr J, Berg RA, Billi JE, Bossaert L, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: Update and simplification of the Utstein templates for resuscitation registries: A statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Councils of Southern Africa) Resuscitation. 2004;63:233–49. doi: 10.1016/j.resuscitation.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Wu JY, Li CS, Liu ZX, Wu CJ, Zhang GC. A comparison of 2 types of chest compressions in a porcine model of cardiac arrest. Am J Emerg Med. 2009;27:823–9. doi: 10.1016/j.ajem.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Pantazopoulos IN, Xanthos TT, Vlachos I, Troupis G, Kotsiomitis E, Johnson E, et al. Use of the impedance threshold device improves survival rate and neurological outcome in a swine model of asphyxial cardiac arrest. Crit Care Med. 2012;40:861–8. doi: 10.1097/CCM.0b013e318232d8de. [DOI] [PubMed] [Google Scholar]
- 14.Enna B, Wenzel V, Schocke M, Krismer AC, Voelckel WG, Klima G, et al. Excellent coronary perfusion pressure during cardiopulmonary resuscitation is not good enough to ensure long-term survival with good neurologic outcome: A porcine case report. Resuscitation. 2000;47:41–9. doi: 10.1016/s0300-9572(00)00200-8. [DOI] [PubMed] [Google Scholar]
- 15.Mayr VD, Wenzel V, Voelckel WG, Krismer AC, Mueller T, Lurie KG, et al. Developing a vasopressor combination in a pig model of adult asphyxial cardiac arrest. Circulation. 2001;104:1651–6. doi: 10.1161/hc3901.095896. [DOI] [PubMed] [Google Scholar]
- 16.Loy DN, Sroufe AE, Pelt JL, Burke DA, Cao QL, Talbott JF, et al. Serum biomarkers for experimental acute spinal cord injury: Rapid elevation of neuron-specific enolase and S-100beta. Neurosurgery. 2005;56:391–7. doi: 10.1227/01.neu.0000148906.83616.d2. [DOI] [PubMed] [Google Scholar]
- 17.Floerchinger B, Philipp A, Foltan M, Keyser A, Camboni D, Lubnow M, et al. Neuron-specific enolase serum levels predict severe neuronal injury after extracorporeal life support in resuscitation. Eur J Cardiothorac Surg. 2014;45:496–501. doi: 10.1093/ejcts/ezt370. [DOI] [PubMed] [Google Scholar]
- 18.Böttiger BW, Möbes S, Glätzer R, Bauer H, Gries A, Bärtsch P, et al. Astroglial protein S-100 is an early and sensitive marker of hypoxic brain damage and outcome after cardiac arrest in humans. Circulation. 2001;103:2694–8. doi: 10.1161/01.cir.103.22.2694. [DOI] [PubMed] [Google Scholar]
- 19.Lin X, Tang Y, Sun B, Hou Z, Meng H, Li Z, et al. Cerebral glucose metabolism: Influence on perihematomal edema formation after intracerebral hemorrhage in cat models. Acta Radiol. 2010;51:549–54. doi: 10.3109/02841851003660065. [DOI] [PubMed] [Google Scholar]
- 20.Nakajo M, Nakajo M, Kajiya Y, Jinguji M, Mori S, Aridome K, et al. High FDG and low FLT uptake in a thyroid papillary carcinoma incidentally discovered by FDG PET/CT. Clin Nucl Med. 2012;37:607–8. doi: 10.1097/RLU.0b013e318252d80f. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Gu L, Feng DF, Ding F, Zhu G, Rong J. Exploring temporospatial changes in glucose metabolic disorder, learning, and memory dysfunction in a rat model of diffuse axonal injury. J Neurotrauma. 2012;29:2635–46. doi: 10.1089/neu.2012.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schaafsma A, de Jong BM, Bams JL, Haaxma-Reiche H, Pruim J, Zijlstra JG. Cerebral perfusion and metabolism in resuscitated patients with severe post-hypoxic encephalopathy. J Neurol Sci. 2003;210:23–30. doi: 10.1016/s0022-510x(03)00063-7. [DOI] [PubMed] [Google Scholar]
- 23.Sun X, Zhang H, Gao C, Zhang G, Xu L, Lv M, et al. Imaging the effects of propofol on human cerebral glucose metabolism using positron emission tomography. J Int Med Res. 2008;36:1305–10. doi: 10.1177/147323000803600618. [DOI] [PubMed] [Google Scholar]
- 24.Landau BR, Spring-Robinson CL, Muzic RF, Jr, Rachdaoui N, Rubin D, Berridge MS, et al. 6-Fluoro-6-deoxy-D-glucose as a tracer of glucose transport. Am J Physiol Endocrinol Metab. 2007;293:E237–45. doi: 10.1152/ajpendo.00022.2007. [DOI] [PubMed] [Google Scholar]
- 25.Matos RI, Watson RS, Nadkarni VM, Huang HH, Berg RA, Meaney PA, et al. Duration of cardiopulmonary resuscitation and illness category impact survival and neurologic outcomes for in-hospital pediatric cardiac arrests. Circulation. 2013;127:442–51. doi: 10.1161/CIRCULATIONAHA.112.125625. [DOI] [PubMed] [Google Scholar]


