Statins reduce cardiovascular morbidity and mortality with dyslipidemia. Although generally well-tolerated, dose-dependent adverse events develop in 10%–15% patients. In addition, up to 3% patients have mildly elevated serum aminotransferase levels within the 1st year of therapy that are rarely associated with symptoms. We report a case of clinically significant hepatotoxicity after a very brief course of rosuvastatin in a statin-naive patient.
A 76-year-old man presented with new-onset right upper quadrant pain. There was no fever, chills, jaundice, hepatosplenomegaly, or indications of chronic liver disease on physical examination. His past medical history included subtotal gastrectomy for advanced gastric cancer 14 years prior to this presentation, hypertension for 10 years, and ischemic heart disease since 2 years. There was no history of liver diseases. Twelve days before this presentation, he was diagnosed with unstable angina pectoris, treated with percutaneous coronary intervention, and administered anti-ischemic medications (clopidogrel, 75 mg/d; rosuvastatin, 10 mg/d) as well as maintenance medications (aspirin, carvedilol, diltiazem, isosorbide mononitrate, barnidipine, and eprosartan) for stable ischemic heart disease, taken since 20 months. Although baseline liver enzyme levels were within normal limits, marked elevation was subsequently observed (serum bilirubin 10.3 mg/L; alkaline phosphatase 174 IU/L; aspartate aminotransferase 1558 IU/L; alanine aminotransferase 613 IU/L). Other pertinent laboratory examinations performed in consideration of the differential diagnoses for abnormal liver biochemistries were negative, including anti-hepatitis A virus antibody IgM, hepatitis B surface antigen, anti-hepatitis C virus antibody, anti-nuclear antibody, anti-smooth muscle antibody, anti-mitochondrial antibody, and anti-liver-kidney microsomal antibody. Computed tomography with contrast showed a normal liver. A liver biopsy was not performed. A re-challenge test with rosuvastatin was not conducted for ethical reasons. Rosuvastatin and the other potentially hepatotoxic medications were discontinued, except for the dual antiplatelet agents. Liver function returned to normal within 4 days. All the medications except rosuvastatin were subsequently resumed without further liver enzyme abnormalities. The patient's biochemical course is summarized in Figure 1.
Figure 1.
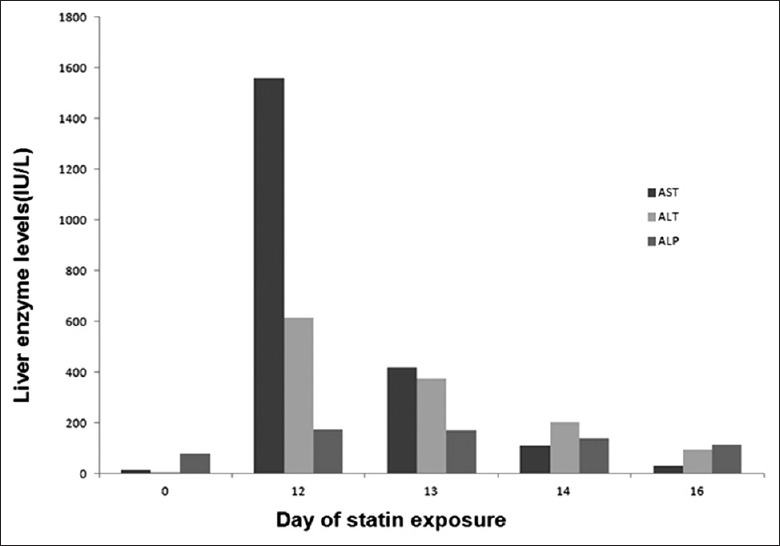
Course of liver enzymes and exposure to statin and other medications. On day 0, percutaneous coronary intervention was done with anti-ischemic medications (clopidogrel, rosuvastatin) as well as maintenance medications (aspirin, carvedilol, diltiazem, isosorbide mononitrate, barnidipine, and eprosartan).
Based on a Roussel Uclaf Causality Assessment Method overall score of 9 point, our patient had drug-induced hepatotoxicity. This method provides a standardized scoring system in which the limits and contents of most criteria were decided by consensus among experts on the basis of organ-oriented characteristics and had its complexity as a major disadvantage. After ruling out other differential diagnoses for the severe hepatitis, we concluded that rosuvastatin-induced hepatotoxicity. Given that rosuvastatin-induced hepatitis usually occurs 3 months after administration,[1] it is extremely rare for severe hepatitis to occur within several days of the initiation of therapy and at commonly prescribed doses.
Dose-dependent transaminase elevations to clinically significant levels are seen in about 0.5%–2.0% of patients taking statins. Most of these statin-induced liver toxicity cases have been reported with lovastatin, simvastatin, and atorvastatin, not rosuvastatin.[2] Significant increased transaminase have been reported in 0.4%, 0, 0 and 0.1% of cases for rosuvastatin 5, 10, 20 and 40 mg, respectively.[3]
Of the eight drugs taken by the patient, isosorbide mononitrate was not considered to cause liver damage. Carvedilol-induced hepatotoxicity is extremely rare. Transient and reversible increases in liver enzymes have likewise been uncommonly reported with barnidipine. Diltiazem-induced hepatotoxicity usually occurs a few days after administration and is usually associated with the characteristic hypersensitivity features that were absent in our patient. Eprosartan may be associated with rare instances of hepatotoxicity that typically occurs within 2–16 weeks of therapy. Although dose-dependent aspirin-induced hepatotoxicity has been reported with anti-inflammatory rather than anti-platelet drugs,[4] there is no evidence that chronic intake of low-dose aspirin leads to hepatotoxicity. Despite being a thienopyridine derivative that inhibits platelet activation via cytochrome P450 (CYP450), clopidogrel-induced hepatotoxicity has been rarely reported.
Rosuvastatin is only minimally metabolized by the CYP450 enzyme system without the significant involvement of the 3A4 enzyme, the isoenzyme implicated in a variety of drug-to-drug interactions. This may be the reason for the fewer drug-to-drug interactions expected with rosuvastatin. Other causes for non-drug-induced hepatotoxicity were effectively ruled out in our case based on the negative results for relevant laboratory tests. The exact mechanism of hepatotoxicity remains unclear. Intracellular accumulation of precursors following statin use may lead to dose-dependent hepatotoxicity. Drug-induced hepatitis can be classified into cholestatic, hepatocellular, or mixed patterns and mostly mimicautoimmune-type hepatitis commonly seen in idiosyncratic or hypersensitivity reactions. Although few case reports have been published describing an autoimmune-type hepatitis induced rosuvastatin use,[5] this was not observed in our patient.
Current guidelines no longer recommend routine monitoring of liver enzymes before and during statin therapy on account of the rarity of serious statin-associated hepatotoxicity. Nevertheless, physicians should consider liver function tests in patients taking statins in the presence of relevant symptoms, including right upper abdominal discomfort, fatigue, decreased appetite, dark urine, or jaundice. As our report suggests, clinically significant hepatitis should be considered as a differential diagnosis after a brief course of common doses of rosuvastatin in the presence of these signs and symptoms of acute liver disease.
Footnotes
Edited by: Ya-Lin Bao
Source of Support: This work was supported by the year 2015 clinical research grant from Pusan National University Hospital, Busan, South Korea.
Conflict of Interest: None declared.
REFERENCES
- 1.Russo MW, Scobey M, Bonkovsky HL. Drug-induced liver injury associated with statins. Semin Liver Dis. 2009;29:412–22. doi: 10.1055/s-0029-1240010. [DOI] [PubMed] [Google Scholar]
- 2.Chalasani N. Statins and hepatotoxicity: Focus on patients with fatty liver. Hepatology. 2005;41:690–5. doi: 10.1002/hep.20671. [DOI] [PubMed] [Google Scholar]
- 3.Robert LT, Pharm D. Safety issues with statin therapy. J Am Pharm Assoc. 2006;46:479–490. doi: 10.1331/154434506778073637. [DOI] [PubMed] [Google Scholar]
- 4.Seaman WE, Ishak KG, Plotz PH. Aspirin-induced hepatotoxicity in patients with systemic lupus erythematosus. Ann Intern Med. 1974;80:1–8. doi: 10.7326/0003-4819-80-1-1. [DOI] [PubMed] [Google Scholar]
- 5.Bhardwaj SS, Chalasani N. Lipid-lowering agents that cause drug-induced hepatotoxicity. Clin Liver Dis. 2007;11:597–613. doi: 10.1016/j.cld.2007.06.010. vii. [DOI] [PMC free article] [PubMed] [Google Scholar]


