Abstract
Background:
There are few studies for evaluating plaque characteristics of nonstenotic basilar arteries (BA). Our aim was to determine entire BA plaques with a three-dimensional volumetric isotropic turbo spin-echo acquisition (VISTA) and investigate the differences between the patients with and without isolated pontine infarction (IPI).
Methods:
Twenty-four consecutive symptomatic patients with nonstenotic BA on time of flight magnetic resonance angiography (TOF MRA) were enrolled from China-Japan Friendship Hospital between January 2014 and December 2014. BA was classified as “normal” or “irregular” based on TOF MRA, and “normal wall”, “slight wall-thickening”, and “plaque” based on three-dimensional VISTA images. Outcomes from MRA and VISTA were compared. Patients were categorized as IPI and non-IPI groups based on the diffusion-weighted imaging. Clinical and plaque characteristics were compared between the two groups.
Results:
A total of 1024 image slices including 311 (30.37%) plaque slices, 427 (41.70%) slight wall-thickening slices, and 286 (27.93%) normal wall slices for the entire BA from 23 patients were finally included for analysis. VISTA images detected plaques in all the 9 (100%) irregular MRA patients and 7 of 14 (50%) normal MRA patients. IPI was found in 11 (47.83%) patients. Compared to non-IPI group, the IPI group had a higher percentage of plaque slices (P = 0.001) and lower percentage of normal wall slices (P = 0.014) than non-IPI group.
Conclusions:
Three-dimensional VISTA images enable detection of BA plaques not visualized by MRA. BA plaques could be found in both the IPI and non-IPI group. However, IPI group showed plaques more extensively in BA than the non-IPI group.
Keywords: Atherosclerosis, Basilar Artery, High Resolution, Isolated Pontine Infarction, Magnetic Resonance Imaging
INTRODUCTION
Globally, stroke is the second leading cause of death in those ≥60 years,[1,2] and has become the first leading cause of death and adult disability in China.[3,4] However, stroke of unknown cause still represents 30–40% of all ischemic strokes.[5,6] A few pathological studies of intracranial arteries have shown that the nonstenotic plaque can occlude small penetrating arteries, causing acute stroke.[7] Furthermore, a recent multicenter prospective study in China showed 3.25% of symptomatic patients with no stenosis would face recurrent stroke after 12 months, only slightly lower than the patients with 50% to 69% stenosis (3.82%).[8] However, the prevalence of these nonstenotic arterial plaques is underestimated by conventional imaging techniques including magnetic resonance angiography (MRA), computed tomography angiogram, and digital subtract angiography.
High-resolution magnetic resonance imaging (MRI) has emerged as an effective tool for identifying plaque of intracranial arteries,[9,10,11,12,13] and has been used for assessment of the artery wall of normal or irregular basilar arteries (BA) in recent years,[14,15,16] which may be the reason of isolated pontine infarction (IPI). However, only little slices were obtained in a limited time because the acquainted time was too long to cover entire BA with two-dimensional MRI technique. In addition, those studies generally assessed the narrowest lumen locations, but the narrowest lumen location may not be representative of the entire BA.
Recently, a three-dimensional MRI sequence with good black blood effect called the volumetric isotropic turbo spin-echo acquisition (VISTA) was reported with high resolution and large coverage in a clinically accepted time.[17,18] In this study, we used three-dimensional VISTA to assess the plaques in the nonstenotic BA of symptomatic patients, and explore the association between the plaques and the IPI.
METHODS
Patients
This study was approved by Institutional Ethics Committees of China-Japan Friendship Hospital, and written informed consent was obtained from all patients before each MRI examination. Between January 2014 and December 2014, patients were enrolled into this study from China-Japan Friendship Hospital according to the following criteria: (1) Ischemic stroke or transient ischemic attack (TIA) in the target BA territory; (2) normal or irregular BA (stenosis <30%) on MRA; and (3) two or more atherosclerotic risk factors including hypertension, hyperlipidemia, diabetes mellitus, cigarette smoking, and obesity.[9] Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or the patients was currently on an antihypertensive drug. Hyperlipidemia was defined as a total cholesterol level ≥2.40 g/L or low-density lipoprotein cholesterol level ≥1.60 g/L or current medication use for lowering blood cholesterol level. Patients who used antidiabetic medications (insulin or oral hypoglycemics) were considered to have diabetes mellitus. Patients who smoked in the past or who currently smoke were considered to have cigarette smoking history. Obesity was defined as >30 kg/m2 of body mass index. According to the traditional clinical definition, we defined ischemic stroke as a new focal neurologic deficit of sudden onset lasting ≥24 h and not caused by hemorrhage and TIA as acute onset of a focal neurologic deficit lasting <24 h.[9] Patients with the following conditions were excluded: (1) Contraindications to MRI; (2) nonatherosclerotic vasculopathy, such as dissection, arteritis, or moyamoya disease; (3) atrial fibrillation, rheumatic heart, and metallic heart valve.
Magnetic resonance imaging protocol
A 3T MR scanner (Ingenia; Philips Healthcare, The Nederland) with a 15-channel phased-array head coil was used in this study. A three-dimensional time of flight (TOF) MRA was first acquired to localize the intracranial artery with the following parameters: Repetition time/echo time = 21 ms/3.2 ms, field of view (FOV) =200 mm × 200 mm × 344 mm, matrix = 400 × 287 × 287, and number of signal averages (NSA) = 1. Acquisition (ACQ) voxel volume was 0.5 mm × 0.7 mm × 1.2 mm. Reconstruction (REC) voxel volume was 0.5 mm × 0.5 mm × 0.6 mm. Three-dimensional VISTA images were then acquired in a traversal plane to cover the major intracranial arteries as identified on the TOF MRA. Imaging parameters were as follows: Repetition time/echo time = 1300 ms/36 ms, FOV = 140 mm × 200 mm × 135 mm, matrix = 280 × 332 × 270, NSA = 2. ACQ voxel volume was 0.5 mm × 0.6 mm × 0.5 mm. REC voxel volume was 0.5 mm × 0.5 mm × 0.5 mm. The short axial cross-sections were constructed automatically with 0.5 mm slice thickness. Diffusion-weighted imaging (DWI) of the whole brain was performed in the axial plane with b values of 0 and 1000 s/mm2 using a single-shot spin-echo planar imaging sequence (repetition time/echo time of 2279/60 ms, 136 × 133 matrix, 230 mm × 230 mm FOV, one excitation, and 5 mm thickness). ACQ voxel size was 1.69 mm × 1.73 mm. REC voxel size was 0.9 mm × 0.9 mm. Diffusion gradients were applied along three orthogonal directions (x -, y -, and z-axes).
Basilar arteries plaque assessment
Magnetic resonance imaging was transferred to a digital picture archiving and communication workstation. Two experienced neuroradiologists who were blinded to clinical data assessed TOF MRA and VISTA images by visual inspection independently. Analytical data were used to calculate the observer reproducibility. Then differences between two observers were solved by consensus. Image quality was assessed using a previously developed four-point scale (1 = poor quality, 2 = adequate quality, 3 = good quality, and 4 = excellent).[19]
Based on three-dimensional TOF MRA constructed views (both coronal and sagittal views), stenosis was classified as: (1) “Normal” when the lumen was clear; (2) “irregular” when luminal boundaries of BA were not strictly parallel but without significant stenosis (<30%).
Basilar arteries plaques were assessed on all cross-sectional image slices with a score of 2 or greater, and were classified as: (1) “Plaque” when marked wall-thickening. (2) “Slight wall-thickening” when the BA wall was slight wall-thickening but did not reach the criteria of plaque; and (3) “Normal wall” when the BA wall was clear [Figure 1]. Because obliquity artifacts for the tortuous BA in the cross-sections would lead to overestimation of the true wall thickness, and mimic plaque, we angled reconstructed planes of three-dimensional VISTA to ensure that all the cross-sectional images were perpendicular the long axial of BA. For each patient, the percentage of the individual plaque, slight wall-thickening, and normal wall slices were calculated.
Figure 1.
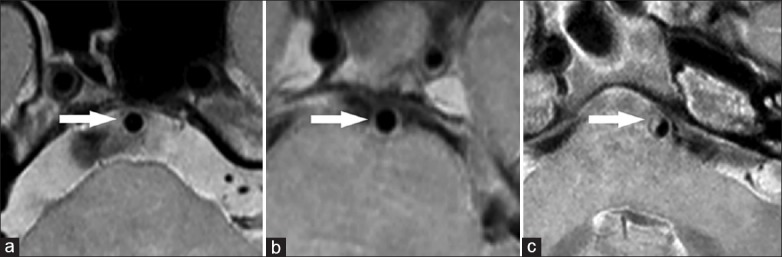
High isotropic resolution magnetic resonance imaging of the basilar artery showing normal wall (a, arrow), slight wall-thickening (b, arrow), and plaque (c, arrow).
Isolated pontine infarction was assessed based on DWI. Patients were classified into two groups (IPI group and non-IPI group).
Statistical analysis
Cohen's k-statistic was computed to assess the observer reproducibility. A value of k > 0.75 was used to indicate a high level of reproducibility, and 0.40 ≤ k ≤ 0.75 denoted moderate reproducibility. Continuous variables were assessed for normality by the Kolmogorov-Smirnov test. The normally distributed continuous variables were summarized as mean ± standard deviation (SD), and were analyzed by independent sample t-test. Continuous variables not normally distributed were summarized as median (interquartile range [IQR]), and were analyzed by Mann-Whitney U-test. Categorical variables such as male sex, risk factors, and qualifying event were presented as percentage. Fisher exact test (for percentage) was used to compare categorical variables. SPSS 11.5 (SPSS Inc., Chicago, IL, USA) was used as the statistical analysis software. All reported P values were two-sided, and P < 0.05 were considered statistically significant.
RESULTS
Patient characteristics
Between January 2014 and December 2014, a total of 24 patients were enrolled. One patient was excluded from this study due to poor image quality. Therefore, 23 (95.83%) suitable patients (19 men and 4 women) were left for investigating those variables. Concomitant ≥50% vertebral artery stenoses were found in 4 patients. The mean age of 23 patients was 56.65 ± 13.31 years. The patients had 2 (n = 10, 43.48%) or 3 or more (n = 13, 56.52%) atherosclerosis risk factors, including hypertension (n = 21, 91.30%), hyperlipidemia (n = 16, 69.57%), diabetes mellitus (n = 12, 52.17%), smoking (n = 12, 52.17%), and obesity (n = 0). The median time from the qualifying events to three-dimensional VISTA was 13 days (IQR, 8–90 days).
Observer variability
The observer reproducibility was excellent for assessment of the BA irregularity on the TOF MRA with k = 0.810 and BA plaques on VISTA images with k = 0.912, respectively.
Morphological characteristics of basilar arteries lesions
Normal and irregular BA on three-dimensional TOF MRA were found in 14 and 9 of 23 patients, respectively [Table 1]. However, 7 and 4 of 14 normal BA patients on MRA showed plaques and slight wall-thickening on VISTA images, respectively [Figure 2]. On the VISTA images, a total of 1024 cross-sectional image slices were obtained from entire BA of 23 patients including 311 (30.37%) plaque slices, 427 (41.70%) slight wall-thickening slices, and 286 (27.93%) normal wall slices.
Table 1.
Comparison of the outcomes from MRA and VISTA images
| MRA | VISTA images | Total | ||
|---|---|---|---|---|
| Normal wall | Slight wall-thickening | Plaque | ||
| Normal | 3 | 4 | 7 | 14 |
| Irregular | 0 | 0 | 9 | 9 |
| Total | 3 | 4 | 16 | 23 |
VISTA: Volumetric isotropic turbo spin echo acquisition; MRA: Magnetic resonance angiography.
Figure 2.
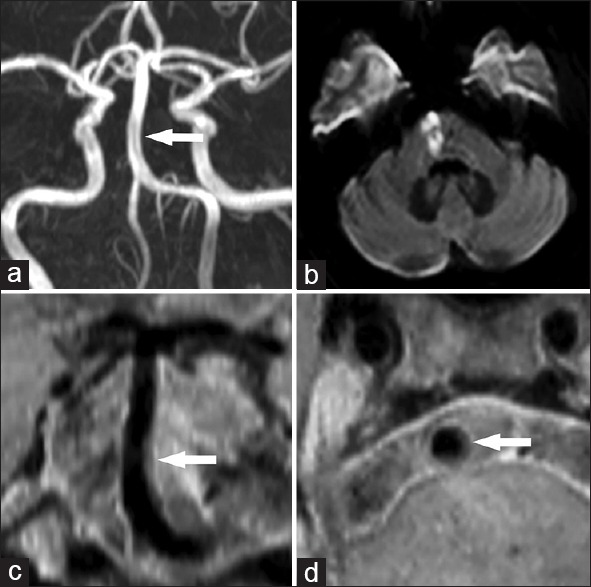
Normal basilar artery on magnetic resonance angiography (a, arrow) with isolated pontine infarction (b). High isotropic resolution magnetic resonance imaging showed an eccentric plaque on the coronal reconstructed image (c, arrow) and axial reconstructed image (d, arrow).
Comparison between patients with and without new infarction
Isolated pontine infarction was found in 11 patients (46.25%) on DWI including 6 paramedian pontine infarction (PPI) patients and 5 lacunar pontine infarction (LPI) patients. No IPI was found in the 4 patients with concomitant ≥50% vertebral artery stenoses. On the contrast to non-IPI group, the IPI group had a shorter time from qualifying event with a borderline significance (P = 0.053). No other significant difference was found in the patient characteristics between the two groups [Table 2].
Table 2.
Comparison of clinical characteristics between IPI and non-IPI groups
| Characteristics | IPI group (n = 11) | Non-IPI group (n = 12) | t | P |
|---|---|---|---|---|
| Age, years, mean ± SD | 59.91 ± 14.36 | 53.67 ± 12.09 | 1.131 | 0.271 |
| Men, n (%) | 11 (100) | 8 (66.67) | NA | 0.093 |
| Hypertension, n (%) | 11 (100) | 10 (83.33) | NA | 0.478 |
| Hyperlipidemia, n (%) | 9 (81.82) | 7 (58.33) | NA | 0.371 |
| Diabetes mellitus, n (%) | 6 (54.55) | 6 (50.00) | NA | 1.000 |
| Smoking, n (%) | 7 (63.64) | 5 (41.67) | NA | 0.414 |
| Qualifying stroke event | 11 (100) | 10 (83.33) | NA | 0.478 |
| Days from the qualifying event to VISTA, days, median (IQR) | 10.00 (6–17) | 26.50 (9–201.25) | –1.910 | 0.056 |
SD: Standard deviation; IQR: Interquartile range; VISTA: Volumetric isotropic turbo spin echo acquisition; IPI: Isolated pontine infarction; NA: The variables between two groups were compared by Fisher exact test
Patients with irregular BA on TOF MRA or plaques on VISTA images were higher in the IPI group than in the non-IPI group, however, there was no statistical significance (P > 0.05). Based on the total image slices of entire BA on VISTA, the IPI group had a higher percentage of plaque slices (P = 0.001) and lower percentage of normal wall slices (P = 0.014) than non-IPI group. But, there was no significant difference in the percentage of slight wall-thickening slices between the two groups [Table 3].
Table 3.
Comparison of wall features between the IPI and non-IPI groups
| Variables | IPI group (n = 11) | Non-IPI group (n = 12) | t | P |
|---|---|---|---|---|
| Patients on MRA, n (%) | NA | 0.680 | ||
| Normal | 6 (54.55) | 8 (66.67) | ||
| Irregular | 5 (45.45) | 4 (33.33) | ||
| Patients on VISTA, n (%) | NA | 0.231 | ||
| Plaque | 10 (90.91) | 6 (50.00) | ||
| Slight wall-thickening | 1 (9.09) | 3 (25.00) | ||
| Normal wall | 0 (0) | 3 (25.00) | ||
| Slices on VISTA, %, mean ± SD | ||||
| Apparent plaque slices | 50.49 ± 23.14 | 15.16 ± 22.08 | 3.746 | 0.001 |
| Slight wall-thickening | 41.97 ± 16.86 | 40.59 ± 35.55 | 0.117 | 0.908 |
| slices | ||||
| Normal wall slices | 7.54 ± 18.33 | 44.25 ± 42.04 | –2.669 | 0.014 |
VISTA: Volumetric isotropic turbo spin echo acquisition; SD: Standard deviation; IPI: Isolated pontine infarction; MRA: Magnetic resonance angiography; NA: The variables between two groups were compared by Fisher exact test.
DISCUSSION
The three-dimensional VISTA is a new method introduced recently with high-isotropic resolution and large volume coverage for intracranial arteries.[17,18] The three-dimensional volume acquisition enables imaging reconstruction in any plane for the tortuous intracranial arteries. This feature was very important for the accurate assessment of plaques by reducing mimic plaques due to obliquity artifacts, which was relatively common in the two-dimensional image acquisition.[20] In our study, most of the images had good or adequate quality enough for analyzing the nonstenotic BA wall with a high observer reproducibility. These results suggested that this noninvasive VISTA images might have great potential to evaluate early atherosclerosis, and help to find possible mechanism of new infarction in the pontine.
In our study, all the patients with irregular BA and half of patients with normal BA on MRA showed plaques on VISTA images suggesting arterial remodeling at the early stage of atherosclerosis. This phenomenon was found by Glagov et al.[21] in coronary arteries at first and also reported in studies of middle cerebral arteries[9] and BA[10] recently. This finding suggested this high-resolution technique could assess arteries with no or mild stenosis more sensitively for detecting the early stage of intracranial atherosclerotic disease (ICAD).
Basilar arteries provided blood supply to the pons so that the BA plaques may lead to IPI by obstructing or extending into BA branches.[7] IPI are usually classified as PPI and LPI according to the lesion shapes and location.[22] Recent studies[6,14,15] showed both the PPI and LPI might associated with BA plaques with or without lumen stenosis. Our study showed plaques or slight wall-thickening in all the patients with IPI including PPI and LPI, suggesting BA plaques were possible causes of both the PPI and LPI, similar with the results of previous studies.[6,14,15] In our study, patients with irregular BA or plaques were more frequently found in IPI group, although no statistical significance was obtained. Furthermore, based on image slices of entire BA on VISTA, the IPI group had a higher percentage of apparent plaque slices and lower percentage of no plaque slices than non-IPI group with statistical significance. This result further strengthens the evidence linking atherosclerosis with IPI in the no-stenotic BA.
Our study has several limitations. First, patients with PPI and LPI were grouped together due to a small sample size. However, an increasing number of studies suggested BA plaques might be the cause for both PPI and LPI. In addition, further study with large sample will be preformed in the future, and the PPI, LPI, and control groups will be compared. Second, we could not exclude other concomitant etiology. In our study, 4 cases with concomitant ≥50% vertebral artery stenoses were included. However, no IPI was found in those 4 patients, and we believe this will not change the direction of our conclusion. Finally, the high-resolution magnetic resonance imaging findings had no histopathologic confirmation, as biopsy was impracticable in surviving patients.
In conclusion, three-dimensional VISTA images with postprocessing reconstruction in any plane enable detection of BA plaques not visualized by MRA. BA plaques could be found in both the IPI and non-IPI group. However, IPI group showed plaques more extensively in BA than the non-IPI group.
Footnotes
Edited by: Jian Gao
Source of Support: This study was supported by grants from China- Japan Friendship Hospital Youth Science and Technology Excellence Project (No. 2014-QNYC-A-04), National Natural Science Foundation of China (No. 81173595, No. 30670731, No. 81070925 and No. 81471767), and National Basic Research Program (973 Program) of China (No. 2013CB733805).
Conflict of Interest: None declared.
REFERENCES
- 1.Johnston SC, Mendis S, Mathers CD. Global variation in stroke burden and mortality: Estimates from monitoring, surveillance, and modelling. Lancet Neurol. 2009;8:345–54. doi: 10.1016/S1474-4422(09)70023-7. [DOI] [PubMed] [Google Scholar]
- 2.Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, et al. Global and regional burden of stroke during 1990-2010: Findings from the Global Burden of Disease Study 2010. Lancet. 2014;383:245–54. doi: 10.1016/s0140-6736(13)61953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu L, Wang D, Wong KS, Wang Y. Stroke and stroke care in China: Huge burden, significant workload, and a national priority. Stroke. 2011;42:3651–4. doi: 10.1161/STROKEAHA.111.635755. [DOI] [PubMed] [Google Scholar]
- 4.Wang PL, Zhao XQ, DU WL, Wang AX, Ji RJ, Yang ZH, et al. In-hospital medical complications associated with patient dependency after acute ischemic stroke: Data from the China National Stroke Registry. Chin Med J. 2013;126:1236–41. [PubMed] [Google Scholar]
- 5.Klein IF, Labreuche J, Lavallée PC, Mazighi M, Duyckaerts C, Hauw JJ, et al. Is moderate atherosclerotic stenosis in the middle cerebral artery a cause of or a coincidental finding in ischemic stroke? Cerebrovasc Dis. 2010;29:140–5. doi: 10.1159/000262310. [DOI] [PubMed] [Google Scholar]
- 6.Feng C, Xu Y, Bai X, Hua T, Li Q, Tang GY, et al. Basilar artery atherosclerosis and hypertensive small vessel disease in isolated pontine infarctions: A study based on high-resolution MRI. Eur Neurol. 2013;70:16–21. doi: 10.1159/000346577. [DOI] [PubMed] [Google Scholar]
- 7.Caplan LR. Intracranial branch atheromatous disease: A neglected, understudied, and underused concept. Neurology. 1989;39:1246–50. doi: 10.1212/wnl.39.9.1246. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Zhao X, Liu L, Soo YO, Pu Y, Pan Y, et al. Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: The Chinese Intracranial Atherosclerosis (CICAS) Study. Stroke. 2014;45:663–9. doi: 10.1161/STROKEAHA.113.003508. [DOI] [PubMed] [Google Scholar]
- 9.Zhu XJ, Du B, Lou X, Hui FK, Ma L, Zheng BW, et al. Morphologic characteristics of atherosclerotic middle cerebral arteries on 3T high-resolution MRI. AJNR Am J Neuroradiol. 2013;34:1717–22. doi: 10.3174/ajnr.A3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma N, Jiang WJ, Lou X, Ma L, Du B, Cai JF, et al. Arterial remodeling of advanced basilar atherosclerosis: A 3-tesla MRI study. Neurology. 2010;75:253–8. doi: 10.1212/WNL.0b013e3181e8e714. [DOI] [PubMed] [Google Scholar]
- 11.Ma N, Lou X, Zhao TQ, Wong EH, Jiang WJ. Intraobserver and interobserver variability for measuring the wall area of the basilar artery at the level of the trigeminal ganglion on high-resolution MR images. AJNR Am J Neuroradiol. 2011;32:E29–32. doi: 10.3174/ajnr.A2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang WJ, Yu W, Ma N, Du B, Lou X, Rasmussen PA. High resolution MRI guided endovascular intervention of basilar artery disease. J Neurointerv Surg. 2011;3:375–8. doi: 10.1136/jnis.2010.004291. [DOI] [PubMed] [Google Scholar]
- 13.Lou X, Ma N, Ma L, Jiang WJ. Contrast-enhanced 3T high-resolution MR imaging in symptomatic atherosclerotic basilar artery stenosis. AJNR Am J Neuroradiol. 2013;34:513–7. doi: 10.3174/ajnr.A3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein IF, Lavallée PC, Schouman-Claeys E, Amarenco P. High-resolution MRI identifies basilar artery plaques in paramedian pontine infarct. Neurology. 2005;64:551–2. doi: 10.1212/01.WNL.0000150543.61244.06. [DOI] [PubMed] [Google Scholar]
- 15.Klein IF, Lavallée PC, Mazighi M, Schouman-Claeys E, Labreuche J, Amarenco P. Basilar artery atherosclerotic plaques in paramedian and lacunar pontine infarctions: A high-resolution MRI study. Stroke. 2010;41:1405–9. doi: 10.1161/STROKEAHA.110.583534. [DOI] [PubMed] [Google Scholar]
- 16.Kim YS, Lim SH, Oh KW, Kim JY, Koh SH, Kim J, et al. The advantage of high-resolution MRI in evaluating basilar plaques: A comparison study with MRA. Atherosclerosis. 2012;224:411–6. doi: 10.1016/j.atherosclerosis.2012.07.037. [DOI] [PubMed] [Google Scholar]
- 17.Qiao Y, Steinman DA, Qin Q, Etesami M, Schär M, Astor BC, et al. Intracranial arterial wall imaging using three-dimensional high isotropic resolution black blood MRI at 3.0 Tesla. J Magn Reson Imaging. 2011;34:22–30. doi: 10.1002/jmri.22592. [DOI] [PubMed] [Google Scholar]
- 18.Qiao Y, Zeiler SR, Mirbagheri S, Leigh R, Urrutia V, Wityk R, et al. Intracranial plaque enhancement in patients with cerebrovascular events on high-spatial-resolution MR images. Radiology. 2014;271:534–42. doi: 10.1148/radiol.13122812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Underhill HR, Yarnykh VL, Hatsukami TS, Wang J, Balu N, Hayes CE, et al. Carotid plaque morphology and composition: Initial comparison between 1.5- and 3.0. T magnetic field strengths. Radiology. 2008;248:550–60. doi: 10.1148/radiol.2482071114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antiga L, Wasserman BA, Steinman DA. On the overestimation of early wall thickening at the carotid bulb by black blood MRI, with implications for coronary and vulnerable plaque imaging. Magn Reson Med. 2008;60:1020–8. doi: 10.1002/mrm.21758. [DOI] [PubMed] [Google Scholar]
- 21.Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987;316:1371–5. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- 22.Erro ME, Gállego J, Herrera M, Bermejo B. Isolated pontine infarcts: Etiopathogenic mechanisms. Eur J Neurol. 2005;12:984–8. doi: 10.1111/j.1468-1331.2005.01119.x. [DOI] [PubMed] [Google Scholar]


