Abstract
Background:
Previous studies have indicated that endoplasmic reticulum stress participates in and mediates liver injury and apoptosis in brain-dead (BD) rats. In this study, we observed the effect of salubrinal (Sal, Sigma, USA) on liver cells in BD rats and explored its relevant mechanisms.
Methods:
Thirty Sprague–Dawley rats were equally randomized into three groups: BD group, Sal group, and DMSO group. The BD models were established by increasing intracranial pressure in a modified, slow, and intermittent way. In the drug groups, Sal was administered 1 h before the induction of BD. After modeling was completed, the blood and liver samples were harvested. CHOP and Caspase-12 mRNA expression was detected using quantitative polymerase chain reaction. PKR-like ER kinase (PERK), P-eukaryotic translation initiation factor 2α (eIF2α), eIF2α, CHOP and caspase-12 expression was detected using western blotting (WB). CHOP and caspase-12 distribution and expression in liver tissues were determined using immunohistochemistry (IHC). Alanine aminotransferase and aspartate aminotransferase level were detected using an automatic biochemical analyzer. Hepatic cell apoptosis was detected using TUNEL. The results were analyzed using Quantity-one v4.62 software (Bio-Rad, USA).
Results:
CHOP and caspase-12 expression and PERK, eIF2α, and P-eIF2α protein expression showed no significant difference between BD group and DMSO group. Compared with BD group, Sal group had a significantly higher P-eIF2C level and a lower P-PERK level 2 h and 6 h after BD (P < 0.05). However, eIF2α expression showed no significant difference (P > 0.05). After the Sal treatment, CHOP and caspase-12 mRNA expression significantly decreased 4 h after BD (P < 0.05). WB and IHC indicated that CHOP and caspase-12 expression also significantly decreased after Sal treatment. Sal was associated with improved liver function and decreased hepatic cell apoptosis.
Conclusions:
Sal can significantly reduce apoptosis in hepatic cells of BD rats. This protective effect may be achieved via the PERK-eIF2α signaling pathway.
Keywords: Apoptosis, Brain Death, Endoplasmic Reticulum Stress, Rat Liver, Salubrinal
INTRODUCTION
Liver transplantation is an effective way to treat end-stage liver disease, with brain death (BD) organ donors being the main source for organ transplantation. However, studies have shown that compared with living donors, organs from BD donors are associated with more postoperative complications and a lower survival rate of the graft.[1,2,3,4] BD can cause a series of pathological changes in the immune, coagulation, fibrinolytic and complement systems.[5,6,7] Therefore, BD has been recognized as an antigen-independent risk factor before organ transplantation.
The endoplasmic reticulum (ER) regulates protein synthesis, and it is where protein folding and accumulation occur. It also regulates cellular stress and intracellular calcium levels.[8] In general, when an external stress occurs, the ER can protect the cells through the unfolded protein response (UPR); however, if such stress persists or is too strong, the UPR will ultimately activate ER stress apoptosis signaling proteins and thereby cause cellular apoptosis.[9] As shown in our previous study, the ER stress response was activated in BD rats, and ER stress-related apoptosis protein expression increased.[10]
PKR-like ER kinase (PERK) is a serine-threonine kinase. After the initiation of the ER stress response, it can mediate cellular apoptosis by phosphorylating the eukaryotic translation initiation factor 2α (eIF2α). By selectively inducing eIF2α phosphorylation and suppressing its dephosphorylation, salubrinal (Sal) can reduce ER stress-induced cellular apoptosis; however, it does not affect apoptosis that is not related to the ER.[11,12,13] Research has demonstrated that Sal can inhibit cellular apoptosis, but it is not known whether Sal can prevent BD-induced injuries, and its potential molecular biological mechanisms remain unclear.
METHODS
Experimental animals
Thirty healthy male Sprague–Dawley rats weighing 200–250 g were purchased from Henan Provincial Experimental Animal Center, Zhengzhou City, China. They were maintained under a controlled light schedule (12 h light: 12 h dark) at room temperature (22 ± 1°C) with constant humidity (50–70%). The rats were randomly divided into three groups of ten rats each: BD group, Sal group (Sal administered before BD), and DMSO group (dimethyl sulfoxide administered before BD). All animals had free access to food and water before the experiment. All animal experiments were conducted after approval and in accordance with the strict guideline of the Institutional Review Board (IRB) at Zhengzhou University, China (FWA00014064, IRB00006861).
Establishment of brain death models
Before anesthesia, 0.25 mg atropine was intraperitoneally injected. Thirty minutes later, the rats were anesthetized with 1% pentobarbital sodium (0.4–0.6 ml/100 g) via intraperitoneal injection, and saphenous artery catheterization, tracheostomy/intubation, cystostomy, and catheterization by cranial drilling were performed. The BD model was established by increasing intracranial pressure in a modified, slow and intermittent way, as described in our previous study.[14,15,16] An airbag was inflated with 20 μl of fluid every 5 min until BD occurred. The criteria for determining BD were as follows: (a) Deep coma; (b) pupillary light reflex disappeared; (c) spontaneous breathing stopped; and (d) cessation of brain electrical activity. A BIOPAC physiological recorder (MP150, BIOPAC system, Santa Barbara, CA, USA) was used to monitor the brain electrical activity, oxygen saturation, and arterial blood pressure throughout the experiment.
Salubrinal treatment
Salubrinal was dissolved in DMSO and then diluted to the final concentration using phosphate buffered saline (PBS). It was intraperitoneally injected (1 mg/kg) 60 min before BD induction.[17]
Protein expression detected using Western blotting
A total of 100 mg of rat liver tissue was harvested, placed into a mortar, and ground to a fine powder in a mortar that was pre-cooled with liquid nitrogen. The powder was transferred to an Eppendorf tube, and 1 ml RIPA (tissue lysis buffer) and 10 μl phenylmethylsulfonyl fluoride were added. Tissue protein was extracted according to the manufacturer's instructions, and the protein concentration was determined using the bicinchoninic acid method. Then, 30 μg of protein was harvested for electrophoresis by SDS-PAGE using a 12% gel. After the protein was wet-transferred onto polyvinylidene difluoride membranes (Millipore Co., Billerica, MA, USA), they were blocked in TBST solution containing 5% skimmed milk powder at room temperature for 1 h. Then, P-PERK (1:1000), eIF2α (1:1000), P-eIF2α (1:1000), CHOP (1:1000), and caspase-12 (1:1000) primary antibodies (all from Cell Signaling Technology, Inc., China) were mixed with ×1 TBST solution. β-actin (1:1000, Beijing Biosynthesis, China) was used as an internal reference. The membranes were incubated at 4°C overnight. After washing 3 times with ×1 TBST, the secondary antibody was diluted with ×1 TBST (1:5000) and incubated at room temperature for 1 h. After washing three times with ×1 TBST, ECL substrate was added in a darkroom for exposure and development processing. The results were analyzed using densitometric analysis software Quantity One v4.62 for Windows (Bio-Rad, CA, USA). Bands standing for CHOP and caspase-12 are scanned the intensities. Actin is used as a loading control.
Detection of mRNA expression using real-time polymerase chain reaction
Rat liver tissue (50 mg) was harvested. Total RNA was extracted from each sample using Trizol reagent (TaKaRa Bio Inc., Dalian, China), according to the manufacturer's instructions. The RNA concentration and A260/A280 ratio were determined using a spectrophotometer (Nanodrop Inc., Washington, USA). The primers were designed according to their gene sequences as follows: CHOP, 5’-CCTGTCCTCAGATGAAATTGG-3’ (forward), 5’-ACCACTCTGTTTCCGTTTCCT-3’ (reverse); and caspase-12, 5’-AAAGGGATAGCCACTGCTGAT-3’ (forward), 5’-GAGAGCCACTCTTGCCTACCT-3’ (reverse). The SYBR® Premix Ex Taq™ II kits (TaKaRa Bio Inc., Dalian, China) was used. The reaction system was 20 μl in size, including ×2 SYBR Green Master Mix (38 μl), cDNA (3 μl), upstream and downstream primers (1.5 μl each) and ddH2O (32 μl). Measurements were repeated three times in each well. Polymerase chain reaction (PCR) conditions were as follows: Initial denaturation at 95°C for 30 s, followed by 40 cycles of denaturation at 95°C for 3 s and annealing at 60°C for 60 s. The reaction was performed using an ABI 7500 Fast System (Applied Biosystems, Foster City, CA, USA). Relative mRNA expression was calculated using the 2−△△CT method.
Liver function
Changes in alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were detected using an automatic biochemical analyzer.
Immunohistochemistry
All specimens were fixed in neutral buffered formalin and dehydrated for embedding in paraffin, and serial slides (4-μm-thick) were prepared. The slides were then dewaxed in xylene and dehydrated in gradient ethanol. Antigen retrieval was performed by heating the slides (immersed in 0.01 mol/L citrate buffer) in a microwave oven. The endogenous peroxidase was quenched by placing the slides in 0.3% hydrogen peroxide. The slides were then blocked with 10% normal goat serum for 1 h at room temperature. CHOP (1:400, Beijing Biosynthesis) and caspase-12 (1:400, Beijing Biosynthesis) were added to the slides and then incubated at 4°C overnight. After rinsing with PBS, the secondary antibody was added to the slides and incubated at room temperature for 1 h. DAB was added for color development and then counterstained with hematoxylin. Positive and negative controls were set for each dye batch.
In situ apoptosis
Hepatic cell apoptosis was detected using the TUNEL method. The tissue specimens were routinely dehydrated for embedding in paraffin, and 4-μm-thick serial slides were prepared before inoculation at 65°C for 30 min. The slides were then dewaxed in xylene and dehydrated in gradient ethanol. Antigen retrieval was performed by heating the slides (immersed in 0.01 mol/l citrate buffer) in a microwave oven. After blocking using 3% BSA and 20% bovine serum at room temperature for 30 min, TUNEL reaction solution was added to the slides and they were incubated at 37°C for 1 h. The slides were counterstained with DAPI and sealed with a coverslip.
Statistical analysis
All data are presented as the mean ± standard deviation (SD) and analyzed using a one-way analysis of variance (ANOVA) or Student's t-test. A value of P < 0.05 was considered statistically significant. All analyses were performed with SPSS (version 13.0; SPSS Inc., Chicago, IL, USA).
RESULTS
Effect of salubrinal on P-eukaryotic translation initiation factor 2α after brain death
To determine whether the PERK/eIF2α signaling pathway mediates ER stress-related apoptosis, P-PERK, eIF2α, and P-eIF2α protein expression was detected using Western blotting (WB). P-PERK, eIF2α, and P-eIF2α expression were not significantly different between the BD group and the DMSO group. In the Sal group, however, P-PERK expression decreased significantly 2 h and 6 h after BD (P < 0.05). P-eIF2α expression significantly increased, although eIF2α expression showed no significant difference [Figure 1].
Figure 1.
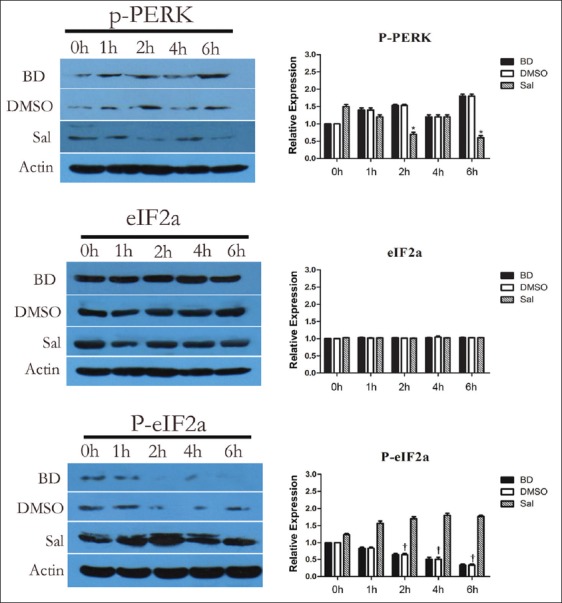
Effects of pretreatment with salubrinal on the expression of P-PKR-like ER kinase (PERK), eukaryotic translation initiation factor 2α (eIF2α) and P-eIF2α. All values shown are mean ± standard deviation. *P < 0.05 versus BD 2 h and 6 h group; †P < 0.05 versus BD 2 h, 4 h, and 6 h group. Actin is used as a loading control.
Salubrinal treatment improved liver function
The ALT and AST levels were not significantly different between the BD group and the DMSO group. After Sal treatment, however, ALT significantly declined 6 h after BD, and AST decreased 0 h, 1 h, 2 h, and 6 h after BD, but AST was not changed 4 h after BD.
Salubrinal treatment reduced the effects on endoplasmic reticulum pathway-related apoptosis proteins
CHOP and caspase-12 mRNA and protein expression before and after Sal treatment were assessed using quantitative PCR (qPCR) and WB, respectively. There were no significant differences for CHOP and caspase-12 mRNA and protein expression, and PERK, eIF2α, and P-eIF2α protein expression between the BD and DMSO groups. After Sal treatment, however, qPCR showed that CHOP mRNA expression decreased 4 h after BD, and caspase-12 mRNA expression was also reduced [Figure 2]. WB and immunohistochemistry (IHC) showed that CHOP and caspase-12 protein expression also decreased significantly [Figures 3–5]. In addition, the TUNEL assay showed that the hepatic cell apoptosis rate dramatically decreased after Sal treatment [Figure 6].
Figure 2.
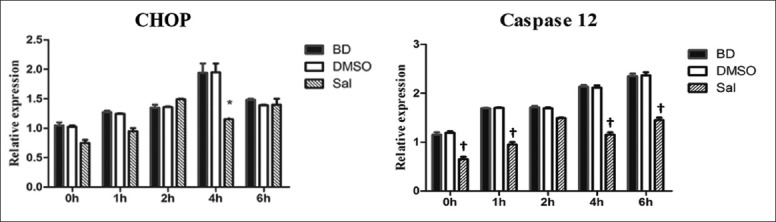
Effects of pretreatment with salubrinal on the CHOP and caspase-12 mRNA expression. All values shown are mean ± standard deviation. *P < 0.05 brain death (BD) 4 h group; †P < 0.05 versus BD 0 h, 1 h, 4 h, and 6 h group.
Figure 3.
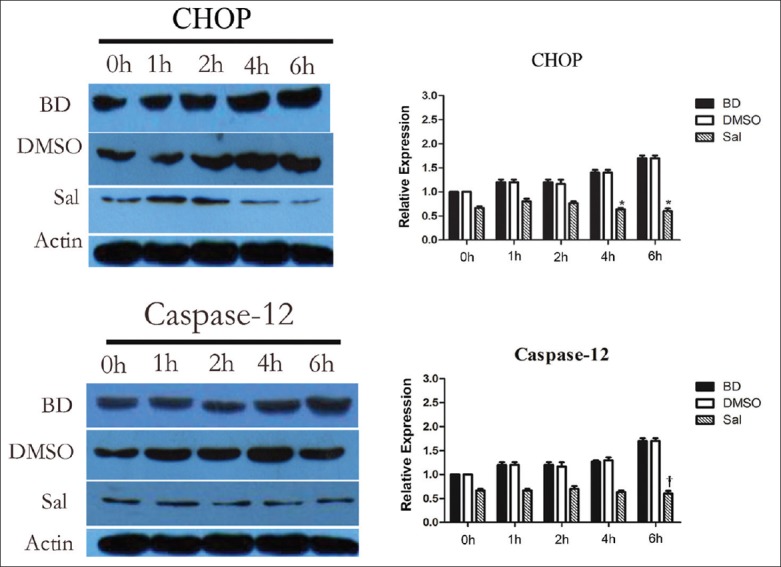
Effects of pretreatment with salubrinal on the expression of CHOP and caspase-12. All values shown are mean ± standard deviation. *P < 0.05 versus BD 4 h and 6 h group; †P < 0.05 versus BD 6 h group. Actin is used as a loading control.
Figure 5.
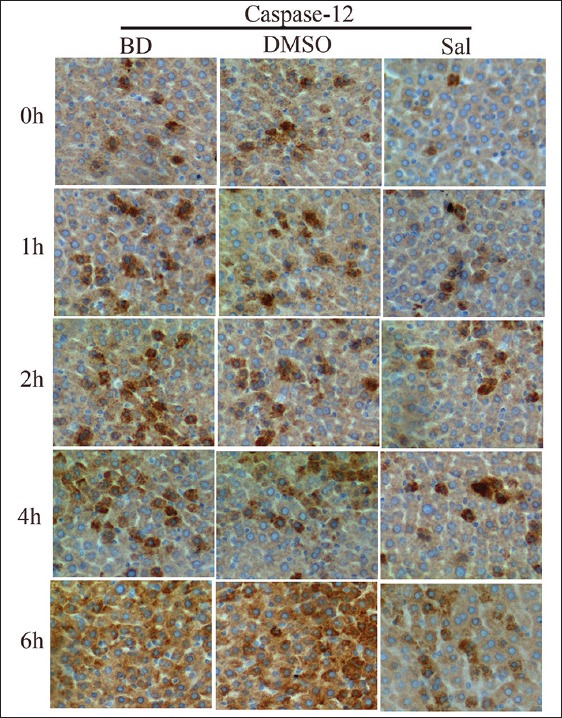
Immunohistochemical staining of caspase-12 in liver sections and the inhibitory effect of salubrinal on caspase-12 expression.
Figure 6.
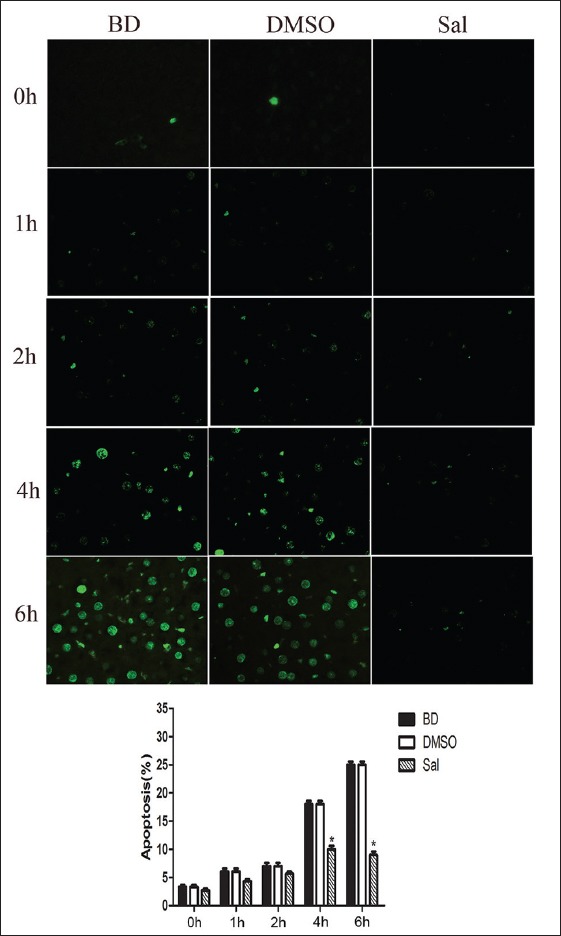
Pretreatment with salubrinal on brain-death-induced apoptosis in liver as demonstrated by TUNEL. All values shown are mean ± standard deviation. *P < 0.05 versus brain death 4 h and 6 h group.
Figure 4.
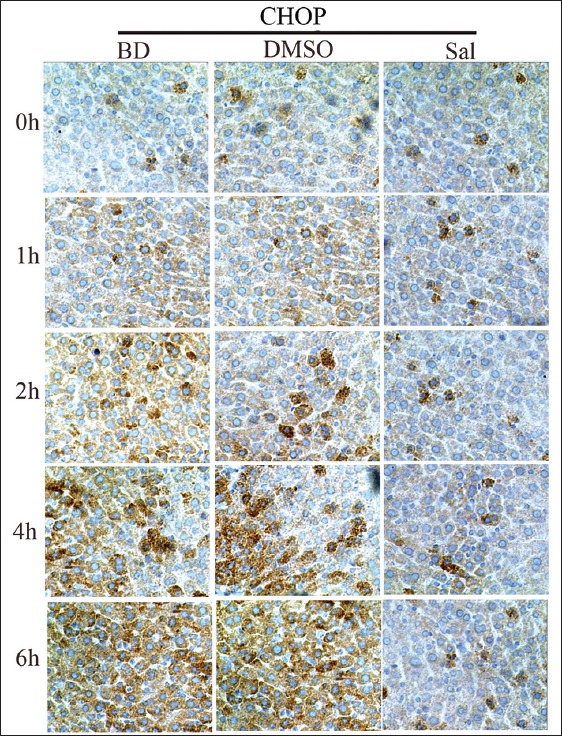
Immunohistochemical staining of CHOP in liver sections and the inhibitory effect of salubrinal on CHOP expression.
DISCUSSION
Brain death is defined as the irreversible cessation of all brain function, including that in the brain stem. Clinical and experimental studies have shown that BD is a complex pathological process that may damage organ morphology and function. Physiological changes after BD can include hemodynamic changes, and blood coagulation, endocrine or electrolyte disorders. Studies have also shown that compared with living donors, BD donors have a lower short- and long-term posttranslational effectiveness and higher incidences of primary graft nonfunction and acute rejection. Therefore, reducing the damage to major organs that results from a series of pathophysiological changes after BD has become a major concern. Studies in this field may help to improve organ donor quality, prolong organ graft survival, and thereby lower the incidence of graft nonfunction or graft failure.
Apoptosis is a programmed cell death regulated by genes and their products. It plays a key role in tissue injury and organ dysfunction. ER stress-mediated apoptosis is the third apoptosis signaling pathway that is independent of the membrane receptors or mitochondrial pathways,[18,19,20] and upregulated Grp78 and XBP-1 expression are regarded as ER stress markers. We showed in our previous study that rat models of BD had upregulated Grp78 and XBP-1 expression,[21] and levels of CHOP and caspase-12, two other ER-stress-related apoptotic proteins, also increased. Thus, BD activates ER stress, and the latter mediates hepatic cell apoptosis in BD rats.
The PERK/eIF2α pathway is one of the key pathways that mediate ER apoptosis. PERK is a serine threonine kinase. After ER stress initiation, PERK can mediate cellular apoptosis by phosphorylating eIF2α. Reducing mRNA transcription can alleviate the protein load on the ER and eventually ER stress mediate apoptosis.[22] By selectively inducing eIF2α phosphorylation and suppressing its dephosphorylation, Sal can reduce ER stress-induced cellular apoptosis; however, it does not protect apoptosis that is not related to the ER. In our current study, the P-eIF2α expression increased after Sal treatment. Both mRNA and protein expression of the ER stress-related pathway proteins CHOP and caspase-12 dramatically decreased. IHC showed that CHOP and caspase-12 proteins were expressed in the cytoplasm, and the expression levels decreased after Sal treatment. Furthermore, the TUNEL assay showed that hepatic cell apoptosis in BD rats was significantly reduced after Sal treatment, and liver function improved.
Our study further confirmed that: (a) Liver cell apoptosis increases in BD rats; (b) phosphorylated eIF2-α expression decreases; and (c) the use of Sal, a selective inhibitor of eIF2α dephosphorylation, can significantly reduce hepatic cell apoptosis and alleviate liver damage in BD rats. These findings indicate that ER stress is involved in hepatic cell apoptosis and that it plays a key role in the PERK/eIF2α signaling pathway. As a core pathway in the ER stress response, the PERK/eIF2α pathway may serve as a potential drug target for improving transplant quality.
ACKNOWLEDGMENTS
The authors thank Open and Key Laboratory of Hepatobiliary and Pancreatic Surgery and Digestive Organ Transplantation at Henan Universities, Zhengzhou Key Laboratory of Hepatobiliary and Pancreatic Diseases and Organ Transplantation for providing equipment and technical support.
Footnotes
Edited by: Yuan-Yuan Ji
Source of Support: This study was supported by a grant of National Natural Science Foundation of China (No. 81171849).
Conflict of Interest: None declared.
REFERENCES
- 1.Marconi L, Moreira P, Parada B, Bastos C, Roseiro A, Mota A. Donor cause of brain death in renal transplantation: A predictive factor for graft function? Transplant Proc. 2011;43:74–6. doi: 10.1016/j.transproceed.2010.12.032. [DOI] [PubMed] [Google Scholar]
- 2.Sam R, Leehey DJ. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med. 2000;342:1837–8. [PubMed] [Google Scholar]
- 3.Weiss S, Kotsch K, Francuski M, Reutzel-Selke A, Mantouvalou L, Klemz R, et al. Brain death activates donor organs and is associated with a worse I/R injury after liver transplantation. Am J Transplant. 2007;7:1584–93. doi: 10.1111/j.1600-6143.2007.01799.x. [DOI] [PubMed] [Google Scholar]
- 4.Zweers N, Petersen AH, van der Hoeven JA, de Haan A, Ploeg RJ, de Leij LF, et al. Donor brain death aggravates chronic rejection after lung transplantation in rats. Transplantation. 2004;78:1251–8. doi: 10.1097/01.tp.0000142679.45418.96. [DOI] [PubMed] [Google Scholar]
- 5.Bugge JF. Brain death and its implications for management of the potential organ donor. Acta Anaesthesiol Scand. 2009;53:1239–50. doi: 10.1111/j.1399-6576.2009.02064.x. [DOI] [PubMed] [Google Scholar]
- 6.Chen EP, Bittner HB, Kendall SW, Van Trigt P. Hormonal and hemodynamic changes in a validated animal model of brain death. Crit Care Med. 1996;24:1352–9. doi: 10.1097/00003246-199608000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Wilhelm MJ, Pratschke J, Laskowski IA, Paz DM, Tilney NL. Brain death and its impact on the donor heart-lessons from animal models. J Heart Lung Transplant. 2000;19:414–8. doi: 10.1016/s1053-2498(00)00073-5. [DOI] [PubMed] [Google Scholar]
- 8.Görlach A, Klappa P, Kietzmann T. The endoplasmic reticulum: Folding, calcium homeostasis, signaling, and redox control. Antioxid Redox Signal. 2006;8:1391–418. doi: 10.1089/ars.2006.8.1391. [DOI] [PubMed] [Google Scholar]
- 9.Jing G, Wang JJ, Zhang SX. ER stress and apoptosis: A new mechanism for retinal cell death. Exp Diabetes Res 2012. 2012 doi: 10.1155/2012/589589. 589589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao S, Wang T, Yan B, Lu Y, Guo W, Zhang S. Protective effects of SP600125 in brain death-induced liver injury. Clin Res Hepatol Gastroenterol. 2014;38:577–82. doi: 10.1016/j.clinre.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, et al. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science. 2005;307:935–9. doi: 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]
- 12.Methippara M, Mitrani B, Schrader FX, Szymusiak R, McGinty D. Salubrinal, an endoplasmic reticulum stress blocker, modulates sleep homeostasis and activation of sleep-and wake-regulatory neurons. Neuroscience. 2012;209:108–18. doi: 10.1016/j.neuroscience.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Liu W, Ma C, Geng J, Li Y, Li S, et al. Endoplasmic reticulum stress contributes to CRH-induced hippocampal neuron apoptosis. Exp Cell Res. 2012;318:732–40. doi: 10.1016/j.yexcr.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Zhang S, Wu Y, Guo W, Zhang Y, Zhai W. Protective effects of N-acetylcysteine on the liver of brain-dead Ba-Ma mini pig. Transplant Proc. 2010;42:195–9. doi: 10.1016/j.transproceed.2009.12.039. [DOI] [PubMed] [Google Scholar]
- 15.Zhang S, Cao S, Wang T, Yan B, Lu Y, Zhao Y. Modified brain death model for rats. Exp Clin Transplant. 2014;12:469–73. doi: 10.6002/ect.2013.0229. [DOI] [PubMed] [Google Scholar]
- 16.Zhu C, Li J, Zhang G, Zhang Y, Zhai W, Shi J, et al. Brain death disrupts structure and function of pig liver. Transplant Proc. 2010;42:733–6. doi: 10.1016/j.transproceed.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 17.Sokka AL, Putkonen N, Mudo G, Pryazhnikov E, Reijonen S, Khiroug L, et al. Endoplasmic reticulum stress inhibition protects against excitotoxic neuronal injury in the rat brain. J Neurosci. 2007;27:901–8. doi: 10.1523/JNEUROSCI.4289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breckenridge DG, Germain M, Mathai JP, Nguyen M, Shore GC. Regulation of apoptosis by endoplasmic reticulum pathways. Oncogene. 2003;22:8608–18. doi: 10.1038/sj.onc.1207108. [DOI] [PubMed] [Google Scholar]
- 19.Kadowaki H, Nishitoh H, Ichijo H. Survival and apoptosis signals in ER stress: The role of protein kinases. J Chem Neuroanat. 2004;28:93–100. doi: 10.1016/j.jchemneu.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7:880–5. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao S, Wang T, Yan B, Lu Y, Zhao Y, Zhang S. Brain death is associated with endoplasmic reticulum stress and apoptosis in rat liver. Transplant Proc. 2014;46:3297–302. doi: 10.1016/j.transproceed.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 22.Hotamisligil GS. Endoplasmic reticulum stress and atherosclerosis. Nat Med. 2010;16:396–9. doi: 10.1038/nm0410-396. [DOI] [PMC free article] [PubMed] [Google Scholar]


