Abstract
Background:
Gastric stromal tumors arising from the muscularis propria are located in deeper layers. Endoscopic resection may be contraindicated due to the possibility of perforation. These tumors are therefore usually removed by surgical or laparoscopic procedures. This study evaluated the curative effects, safety and feasibility of endoscopic full-thickness resection (EFR) of gastric stromal tumors originating from the muscularis propria.
Methods:
This study enrolled 92 patients with gastric stromal tumors >2.5 cm originating from the muscularis propria. Fifty patients underwent EFR, and 42 underwent laparoscopic intragastric surgery. Operation time, complete resection rate, length of hospital stay, incidence of complications, and recurrence rates were compared in these two groups.
Results:
EFR resulted in complete resection of all 50 gastric stromal tumors, with a mean procedure time of 85 ± 20 min, a mean hospitalization time of 7.0 ± 1.5 days and no complications. Laparoscopic intragastric surgery also resulted in a 100% complete resection rate, with a mean operation time of 88 ± 12 min and a mean hospitalization period of 7.5 ± 1.6 days. The two groups did not differ significantly in operation time, complete resection rates, hospital stay or incidence of complications (P > 0.05). No patient in either group experienced tumor recurrence.
Conclusions:
EFR technique is effective and safe for the resection of gastric stromal tumors arising from the muscularis propria.
Keywords: Endoscopy, Full-thickness Excision, Gastric Stromal Tumor, Muscularis Propria, Treatment
INTRODUCTION
Since gastric stromal tumors arising from the muscularis propria are located in deeper layers, endoscopic resection may easily lead to perforation, and tumor excision is often incomplete. The source and location of these tumors are therefore considered contraindications to endoscopic resection, requiring these tumors to be removed by surgical or laparoscopic procedures.[1,2,3] More recently, however, endoscopic full-thickness resection (EFR) has achieved satisfactory results in the removal gastric stromal tumors arising from the muscularis propria.[4,5,6]
METHODS
Patients
This retrospective study analyzed 92 patients with gastric stromal tumors arising from the muscularis propria, who were diagnosed by gastroscopy and endoscopic ultrasound and treated at Yantai Yuhuangding Hospital affiliated to Medical College of Qingdao University, China, between January 2009 and October 2014. The 92 patients included 61 males and 31 females, ranging in age between 23 and 70 years, with a mean age of 43.5 years. Twenty-eight tumors were present in the gastric antrum, 42 in the gastric body, and 22 in the gastric fundus. Tumor sizes ranged from 2.5 to 5.0 cm. Each patient had a single tumor, with none having metastasis on computed tomography (CT) examination. Before EFR or laparoscopic surgery, all patients underwent routine blood tests for coagulation enzymes and liver and kidney function, as well as undergoing electrocardiography, abdominal CT scan, and other tests. All patients and their families were informed of the benefits and risks of EFR and laparoscopic surgery. The patients were randomly divided into two groups, one undergoing EFR and the other undergoing laparoscopic surgery, according to a random digits table produced by a computer. This clinical control study was approved by the ethic committees of Yantai Yuhuangding Hospital (clinical trial registration number 237).
The EFR group consisted of 50 patients, with tumor sizes 2.5–5.0 cm (mean, 3.4 cm), and the laparoscopy group consisted of 42 patients, with tumor sizes 3.0–5.0 cm (mean, 3.8 cm). The demographic and clinical characteristics of the two groups are shown in Table 1. Patients were included if they had single tumors <5.5 cm in diameter, with ultrasonography and CT showing no evidence of metastasis preoperatively. Patients were excluded if they had multiple tumors, if any tumor was larger than 5.5 cm in diameter, or if ultrasonography or CT showed evidence of preoperative metastasis.
Table 1.
Demographic and clinical characteristics of the EFR and laparoscopy groups
| Item | EFR group (n = 50) | Laparoscopy group (n = 42) | P |
|---|---|---|---|
| Mean age, year | 44.3 | 47.6 | 0.083 (t = 1.231) |
| Sex (male:female) | 28:22 | 23:19 | 0.536 (χ2 = 0.905) |
| Tumor location | 13:23:14 | 15:19:8 | 0.478 (χ2 = 1.476) |
| (gastric antrum:gastric body:gastric fundus) | |||
| Mean tumor size, cm | 3.4 | 3.8 | 0.092 (t = 1.322) |
EFR: Endoscopic full-thickness resection.
Endoscopic full-thickness resection method
A transparent cap was mounted on the end of the gastroscope before EFR. Following intravenous anesthesia with propofol, the edge of the stromal tumor was marked by argon plasma coagulation. Each marked submucosal position was injected with 2–3 ml of a solution consisting of 2–3 ml indigo carmine, 1 ml epinephrine, and 100 ml saline. The mucosa and submucosa surrounding the tumor were precut along the marked points and the tumor was exposed using a hook knife (Olympus, Japan). The body of the tumor was isolated along the capsule from the muscularis propria to the serosal layer using a hook knife or IT knife (Olympus). The serosa was cut along the edge of the tumor; in most cases, the serosa was tightly adherent to the tumor body, making it impossible to remove the tumor directly using an IT knife. Therefore, the serosa was penetrated using a needle knife or hook knife, resulting in an artificial perforation. The liquid in the gastric cavity was fully absorbed, and an IT or hook knife was used to cut the serosa along the edge of the tumor and to remove the tumor completely. Under endoscopic guidance, the incisions on the gastric body from the two ends to the middle were fully closed with titanium clips, and the gastric wound was sealed. If the wound was too large for direct sealing, negative pressure was applied to suck the omentum into the gastric cavity, and the titanium clips were used to seal the wound by clipping the omentum to the gastric mucosa [Figure 1].
Figure 1.
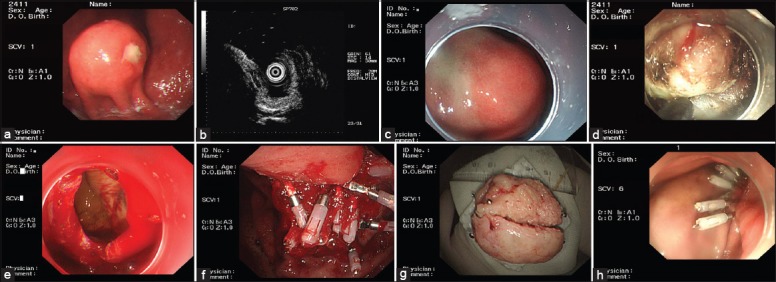
Endoscopic full-thickness resection treatment of gastric stromal tumors arising from the muscularis propria. (a) A protruding submucosal lesion in the gastric fundus; (b) Endoscopic ultrasound showing that the lesion arose from the muscularis propria; (c) Submucosal injection of saline containing adrenaline and indigo carmine; (d) Application of the IT knife to isolate the stromal tumor along its periphery; (e) An artificial perforation observed after stromal tumor resection; (f) Sealing of a perforation with multiple titanium clips; (g) Resected tumor with the mucosa removed (5 cm in diameter); (h) View 9 days after the operation, showing that the perforation healed well.
Method for laparoscopic surgery
Gastric stromal tumors were laparoscopically resected under tracheal intubation and general anesthesia. Patients were placed in a supine, trendelenburg position with their legs apart. The umbilicus was punctured and pneumoperitoneum was established with intra-abdominal pressures maintained at 12–15 mmHg. Usually, a 4–5 hole method was used. A 30° lens was placed into an incision approximately 10 mm above the umbilicus, and a 12-mm trocar was placed horizontally at the main operating site 2 cm above the umbilicus along the left clavicular midline. Finally, 5-mm trocars were placed at auxiliary operating sites located 2 cm above the umbilicus along the right clavicular midline, below the costal margin along the left clavicular midline, and below the costal margin along the right clavicular midline. Based on the location and growth of each gastric stromal tumor, they were removed by wedge resection, transgastric tumor-everting resection, sleeve gastrectomy, or partial gastrectomy [Figure 2].
Figure 2.
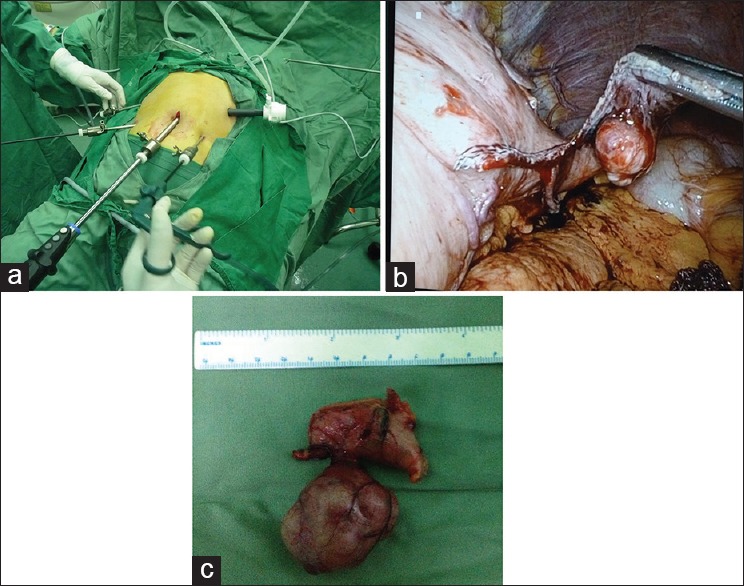
Laparoscopic resection of gastric stromal tumors. (a) Layout of instruments for laparoscopic surgery; (b) Laparoscopic resection of a gastric stromal tumor; (c) Removed tumor (4 cm in diameter).
Postoperative treatment
Patients in the EFR group with an artificial perforation or significant pneumoperitoneum underwent puncture of the right upper quadrant of the abdomen with an abdominal puncture needle to reduce bloating during or after EFR. After EFR, the patients were placed in a semi-supine position and subjected to fasting and gastrointestinal decompression for 3 days, during which they were closely monitored for abdominal pain, bloating, or peritoneal irritation. Three days after EFR, patients were administered oral diatrizoate to determine whether there was contrast agent extravasation and gastric motility. In addition, patients underwent ultrasound examinations to determine whether there were effusions in the abdominal and pelvic cavities. Patients underwent endoscopic examination 1 month after EFR or laparoscopic resection to monitor wound healing and to determine whether there was residual or recurrent tumor.
Statistical analysis
SPSS for Windows version 17.0 software (SPSS Inc., USA) was used for all statistical analyses, with the level of significance set at P < 0.05. Continuous data were compared using independent sample t-tests and categorical data using χ2 tests.
Follow-up
Gastroscopy 1 month after EFR or laparoscopic surgery was performed to detect if there were residual or recurrent tumors.
RESULTS
Complete resection rate and operation time
In the EFR group, each of the 50 stromal tumors arising from the muscularis propria was removed successfully and completely in one operation. All perforation wounds were sealed with titanium clips. Operation times ranged from 55 to 155 min, with mean of 85 ± 20 min. In the laparoscopic surgery group, 39 of the 42 stromal tumors arising from the muscularis propria were removed laparoscopically; operation times ranged from 45 to 215 min, with a mean of 88 ± 12 min. The other three patients had tumors located near the cardia in the posterior wall of the gastric fundus; because of operational difficulties, these patients were converted from laparoscopy to open laparotomy half-way through the operation. Complete resection rate and operation times were similar in the EFR and laparoscopy groups (P > 0.05).
Hospital stay
The mean length of hospital stay was similar in the EFR (7.0 ± 1.5 days; range 4–11 days) and laparoscopy (7.5 ± 1.6 days; range, 4–13 days) groups (P > 0.05).
Complications
In the EFR group, angiography of the upper gastrointestinal tract using diatrizoate 3 days after the operation showed no leakage of contrast agent in any patient. Postoperative reexamination showed good wound healing. There were no complications, such as bleeding, signs of peritonitis, and abdominal abscesses, in any patient. In the laparoscopy group, two patients experienced gastroparesis, which was alleviated after conservative treatment. The complication rate was 4.8%, similar to the rate in the EFR group (P > 0.05).
Recurrence rates
Gastroscopy 1 month after EFR or laparoscopic surgery showed good wound healing in both groups, with no residual or recurrent tumors, making the recurrence rates 0% in both groups.
DISCUSSION
Because of the tendency of gastric stromal tumors to be malignant, surgical resection has been recommended.[7,8,9] The traditional method for resection is surgical or laparoscopic removal,[10,11,12] especially for tumors larger than 2 cm in diameter.[13,14] More recently, however, based on endoscopic submucosal dissection and endoscopic submucosal excavation, and because of improvements in the application of titanium clips under endoscopic guidance, EFR treatment of gastrointestinal tumors arising from the muscularis propria has become possible.
Laparoscopic resection of gastric stromal tumors may reduce surgical trauma and hospital stay. Of our 42 patients, however, three were difficult to resect laparoscopically, requiring conversion to open laparotomy. The ability to resect these tumors laparoscopically is associated with tumor localization. Most subserosal tumors can be removed laparoscopically without using an endoscope. However, gastric stromal tumors in the submucosa and inside the gastric wall and those <1 cm in diameter are difficult to detect laparoscopically, requiring the help of endoscopy. Since the tumors in this study were large, laparoscopic resection did not require endoscopic assistance.
Successful EFR depends on sealing the wound under endoscopic guidance, thus avoiding surgery and postoperative peritonitis.[15] These wounds were most frequently sealed using titanium clips under endoscopy,[16,17] with small perforations requiring one or a few clips. Perforation occurred in all 50 patients in the EFR group, with all wounds sealed with titanium clips. Intra-abdominal pressure was relieved by abdominal puncture, with conservative methods utilized during postoperative care. Nineteen patients required 1–6 clips, and 25 with large perforations underwent suturing from both ends toward the middle. Five patients with larger perforations and some normal mucosa at the periphery required suturing to reduce wound size, and one patient with the largest perforation required the wallet method with nylon rope to seal the perforation.
During EFR, perforations cause pneumoperitoneum, blocking the operative field in the stomach and making endoscopic operations difficult. Thus, during EFR, abdominal palpation should be performed repeatedly. If abdominal pressure increases, timely exhaustion should be applied. The puncture site should be located in the right lower quadrant of the abdomen, with the puncture made using a 20 ml injection needle. After puncture, the abdomen should be manipulated to exhaust air, and the puncture needle should be positioned at the puncture site until the wounds are completely sealed and the pneumoperitoneum is significantly improved. The puncture needle should be withdrawn after confirming that no air continues to flow through it. Other key elements for successful EFR treatment include avoiding the entrance of excessive gastric acid into the abdominal cavity, thus preventing postoperative infection; hemostasis to avoid repeated rinsing during the excision; complete removal of gas and fluid from the gastric lumen prior to incision of the serosa; continuous effective postoperative gastrointestinal decompression; and administration of proton pump inhibitors and antibiotics postoperatively to prevent abdominal infection.[18,19,20,21] None of the patients in our EFR group experienced peritonitis or intra-abdominal abscess.
The complete resection rate in our EFR group was 100%, with a recurrence rate of 0. Operation time and length of hospital stay were similar in the EFR and laparoscopic surgery groups. Three patients in the laparoscopy group required conversion to an open laparotomy, because the stromal tumors were located near the cardia in the posterior wall of the gastric fundus, making a laparoscopic approach difficult. Unlike EFR, which is not affected by the location of the tumor, laparoscopic surgery is difficult and limited to stromal tumors located in the posterior gastric wall and cardia. Thus, tumor size, location, and relationship with the cardia should be clearly determined prior to laparoscopy to avoid conversion to open laparotomy during surgery, causing more trauma and prolonging the patient's hospital stay.
Two patients in the laparoscopy group experienced postoperative gastroparesis, compared with none in the EFR group, further suggesting the advantage of the minimally invasive EFR method. Another advantage of EFR is its accurate localization of the tumor. Without assistance of the gastroscope, it is sometimes difficult during laparoscopic surgery to determine the extent of the excision; thus, excess normal gastric tissue may be removed. In patients with giant stromal tumors protruding into the gastric lumen, it is difficult for the laparoscope to distinguish the tumor from the serosal layer or to pull and remove the tumor body. Therefore, gastroscope-assisted laparoscopic operation, or EFR without laparoscopic assistance may be optimal for treating patients with gastric stromal tumors arising from the muscularis propria.
Endoscopic full-thickness resection can completely remove some stromal tumors arising from the muscularis propria, thus replacing several surgical and laparoscopic procedures. Its application should be recommended in future.
Footnotes
Edited by: Li-Shao Guo
Source of Support: This study was supported by a grant from the National Natural Science Foundation of China (No. 81470909).
Conflict of Interest: None declared.
REFERENCES
- 1.Yu QX, He ZK, Wang J, Sun C, Zhao W, Wang BM. Clinical presentations of gastric small gastrointestinal stromal tumors mimics functional dyspepsia symptoms. World J Gastroenterol. 2014;20:11800–7. doi: 10.3748/wjg.v20.i33.11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Thani H, El-Menyar A, Rasul KI, Al-Sulaiti M, El-Mabrok J, Hajaji K, et al. Clinical presentation, management and outcomes of gastrointestinal stromal tumors. Int J Surg. 2014;12:1127–33. doi: 10.1016/j.ijsu.2014.08.351. [DOI] [PubMed] [Google Scholar]
- 3.Sandvik OM, Søreide K, Gudlaugsson E, Søreide JA. Surgery for gastrointestinal stromal tumors (GISTs) of the stomach and small bowel: Short-and long-term outcomes over three decades. World J Surg. 2015;39:446–52. doi: 10.1007/s00268-014-2824-4. [DOI] [PubMed] [Google Scholar]
- 4.Choi CI, Lee SH, Hwang SH, Kim DH, Jeon TY, Kim DH, et al. Single-incision intragastric resection for upper and mid gastric submucosal tumors: A case-series study. Ann Surg Treat Res. 2014;87:304–10. doi: 10.4174/astr.2014.87.6.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim IH, Kim IH, Kwak SG, Kim SW, Chae HD. Gastrointestinal stromal tumors (GISTs) of the stomach: A multicenter, retrospective study of curatively resected gastric GISTs. Ann Surg Treat Res. 2014;87:298–303. doi: 10.4174/astr.2014.87.6.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmidt A, Bauder M, Riecken B, von Renteln D, Muehleisen H, Caca K. Endoscopic full-thickness resection of gastric subepithelial tumors: A single-center series. Endoscopy. 2015;47:154–8. doi: 10.1055/s-0034-1390786. [DOI] [PubMed] [Google Scholar]
- 7.Kong J, Wu SD, Su Y, Fan Y. Single incision versus conventional laparoscopic resection in gastrointestinal stromal tumors: A retrospective cohort analysis at a single tertiary care center. Onco Targets Ther. 2014;7:995–9. doi: 10.2147/OTT.S62687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agaimy A, Bihl MP. Microscopic gastrointestinal stromal tumors in the gastric antrum. Hum Pathol. 2014;45:1792–3. doi: 10.1016/j.humpath.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 9.Nan G, Siyu S, Sheng W, Xiang L, Jintao G. The role of hemoclips reinforcement in the ligation-assisted endoscopic enucleation for small GISTs in gastric fundus. Biomed Res Int 2014. 2014 doi: 10.1155/2014/247602. 247602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lv M, Wu C, Zheng Y, Zhao N. Incidence and survival analysis of gastrointestinal stromal tumors in shanghai: A population-based study from 2001 to 2010. Gastroenterol Res Pract 2014. 2014 doi: 10.1155/2014/834136. 834136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin J, Huang C, Zheng C, Li P, Xie J, Wang J, et al. Laparoscopic versus open gastric resection for larger than 5 cm primary gastric gastrointestinal stromal tumors (GIST): A size-matched comparison. Surg Endosc. 2014;28:2577–83. doi: 10.1007/s00464-014-3506-x. [DOI] [PubMed] [Google Scholar]
- 12.Kim DJ, Lee JH, Kim W. Laparoscopic resection for 125 gastroduodenal submucosal tumors. Ann Surg Treat Res. 2014;86:199–205. doi: 10.4174/astr.2014.86.4.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du CY, Zhou Y, Song C, Wang YP, Jie ZG, He YL, et al. Is there a role of surgery in patients with recurrent or metastatic gastrointestinal stromal tumours responding to imatinib: A prospective randomised trial in China. Eur J Cancer. 2014;50:1772–8. doi: 10.1016/j.ejca.2014.03.280. [DOI] [PubMed] [Google Scholar]
- 14.Bischof DA, Kim Y, Dodson R, Carolina Jimenez M, Behman R, Cocieru A, et al. Open versus minimally invasive resection of gastric GIST: A multi-institutional analysis of short-and long-term outcomes. Ann Surg Oncol. 2014;21:2941–8. doi: 10.1245/s10434-014-3733-3. [DOI] [PubMed] [Google Scholar]
- 15.Iorio N, Sawaya RA, Friedenberg FK. Review article: The biology, diagnosis and management of gastrointestinal stromal tumours. Aliment Pharmacol Ther. 2014;39:1376–86. doi: 10.1111/apt.12761. [DOI] [PubMed] [Google Scholar]
- 16.Antonopoulos P, Leonardou P, Barbagiannis N, Alexiou K, Demonakou M, Economou N. Gastrointestinal and extragastrointestinal stromal tumors: Report of two cases and review of the literature. Case Rep Gastroenterol. 2014;8:61–6. doi: 10.1159/000354724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong HY, Wang YL, Jia XY, Li J, Li GD, Li YQ. Modified laparoscopic intragastric surgery and endoscopic full-thickness resection for gastric stromal tumor originating from the muscularis propria. Surg Endosc. 2014;28:1447–53. doi: 10.1007/s00464-013-3375-8. [DOI] [PubMed] [Google Scholar]
- 18.Ye LP, Yu Z, Mao XL, Zhu LH, Zhou XB. Endoscopic full-thickness resection with defect closure using clips and an endoloop for gastric subepithelial tumors arising from the muscularis propria. Surg Endosc. 2014;28:1978–83. doi: 10.1007/s00464-014-3421-1. [DOI] [PubMed] [Google Scholar]
- 19.Honda M, Hiki N, Nunobe S, Ohashi M, Kiyokawa T, Sano T, et al. Long-term and surgical outcomes of laparoscopic surgery for gastric gastrointestinal stromal tumors. Surg Endosc. 2014;28:2317–22. doi: 10.1007/s00464-014-3459-0. [DOI] [PubMed] [Google Scholar]
- 20.Feng Y, Yu L, Yang S, Li X, Ding J, Chen L, et al. Endolumenal endoscopic full-thickness resection of muscularis propria-originating gastric submucosal tumors. J Laparoendosc Adv Surg Tech A. 2014;24:171–6. doi: 10.1089/lap.2013.0370. [DOI] [PubMed] [Google Scholar]
- 21.Chan SM, Chiu PW, Teoh AY, Lau JY. Use of the Over-The-Scope Clip for treatment of refractory upper gastrointestinal bleeding: A case series. Endoscopy. 2014;46:428–31. doi: 10.1055/s-0034-1364932. [DOI] [PubMed] [Google Scholar]


