Abstract
Background:
Regulatory T-cells (Treg) play key roles in suppressing cell-mediated immunity in cancer patients. Little is known about perioperative Treg fluctuations in nonsmall cell lung cancer (NSCLC). Video-assisted thoracoscopic (VATS) lobectomy, as a minimal invasive procedure for treating NSCLC, may have relatively less impact on the patient's immune system. This study aimed to observe perioperative dynamics of circulating Treg and natural killer (NK) cell levels in NSCLC patients who underwent major lobectomy by VATS or thoracotomy.
Methods:
Totally, 98 consecutive patients with stage I NSCLC were recruited and assigned into VATS or thoracotomy groups. Peripheral blood samples were taken on 1-day prior to operation, postoperative days (PODs) 1, 3, 7, 30, and 90. Circulating Treg and NK cell counts were assayed by flow cytometry, defined as CD4+CD25+CD127low cells in CD4+ lymphocytes and CD56+16+CD3− cells within CD45+ leukocytes respectively. With SPSS software version 21.0 (SPSS Inc., USA), differences between VATS and thoracotomy groups were determined by one-way analysis of variance (ANOVA), and differences between preoperative baseline and PODs in each group were evaluated by one-way ANOVA Dunnett t-test.
Results:
In both groups, postoperative Treg percentages were lower than preoperative status. No statistical difference was found between VATS and thoracotomy groups on PODs 1, 3, 7, and 30. On POD 90, Treg percentage in VATS group was significantly lower than in thoracotomy group (5.26 ± 2.75 vs. 6.99 ± 3.60, P = 0.012). However, a higher level of NK was found on all PODs except on POD 90 in VATS group, comparing to thoracotomy group.
Conclusions:
Lower Treg level on POD 90 and higher NK levels on PODs 1, 3, 7, 30 in VATS group might imply better preserved cell-mediated immune function in NSCLC patients, than those in thoracotomy group.
Keywords: Natural Killer Cell, Regulatory T-cell, Surgical Invasiveness, Tumor Immunology, Video-assisted Thoracoscopic Lobectomy
INTRODUCTION
Video-assisted thoracoscopic (VATS) lobectomy has gradually gained popularity,[1,2,3] and been accepted as an alternative procedure to thoracotomy for resectable lung cancer by National Comprehensive Cancer Network (NCCN) since 2013.[4,5,6] Interest has developed on whether VATS preserves better immune status and benefits long-term survival.[7,8] Regulatory T-cell (Treg), known as a negative regulator of the cellular immunity, has been considered as a negative prognostic factor for cancer progression. Little is known[9] on the influence of lobectomy on Treg population in lung cancer patients. Natural killer (NK) cells are known to play a significant role in tumor immunosurveillance through their ability to recognize, target, and directly destroy tumor cells without prior sensitization.
In this study, we examined peripheral levels of Treg and NK cells in stage I nonsmall cell lung cancer (NSCLC) patients undergoing lobectomy by VATS or thoracotomy, to disclose perioperative fluctuation of Treg and NK, as well as the correlation between surgical invasiveness and postoperative cell-mediated immune functions.
METHODS
Study population
Totally, 110 consecutive patients with clinical stage I NSCLC in our team were recruited from October 2012 to November 2013. Preoperative evaluations and TNM stage were performed according to 2014 NCCN clinical guideline for NSCLC. Exclusion criteria were as follows: History of malignancies; receiving surgical or intervene operation in recent 1-year; receiving immune therapy including traditional Chinese medicine within 6 months; concomitant immune-enhancing or immune-suppressing diseases including lupus erythematosus, Crohn's disease, and myasthenia gravis; allergic constitution; acquired human immune deficiency syndrome; and any known infection by microorganisms. Ethical approval was given by the clinical research ethics committee of the Second Affiliated Hospital of Zhejiang University, School of Medicine. Informed consent for the study was obtained from all patients.
Surgical techniques
The choice of VATS or open procedure was made by the patient himself/herself and was independent to enrollment. All patients received identical anesthesia with selective one lung ventilation and major lung resection, followed by lymphadenectomy (4, 5, 6, 7, 8, 9, 10 stations for left side cases and 2, 3, 4, 7, 8, 9, 10 for right side ones. Levels 11-13 nodes were dissected during anatomic lobectomy and proceeding in vitro dissection). All resected nodes were individually labeled and submitted entirely for pathological examination. Compared with conventional posterolateral thoracotomy, our three-ports VATS technique used one 3–4 cm utility thoracotomy in the fourth or fifth intercostal space near anterior axillary line without rib spreading. Exploring port was designed in the seventh or eighth intercostal space on the middle axillary line, and an 1–2 cm auxiliary operation hole was made below the inferior angle of the scapula. No artificial pneumothorax was used in VATS. Severe pleural adhesions and completely fused fissures were no longer contraindications of VATS on anatomic grounds. In conditions of lymph nodes (LNs) firmly adhering to pulmonary vessels which imply a high risk of life-threatening hemorrhage, VATS procedures were converted to thoracotomy for safety reasons. Those whose dissected LNs were positive for metastasis were excluded from the study because subsequent adjuvant therapy could impact immune function. Postoperative pain control was achieved by patient-controlled intravenous or paravertebral analgesia within first 48 h followed by oral analgesics if indicated.
Blood sample collection, cell isolation, and antibody reagents
Two milliliters of heparinized peripheral venous blood samples were collected on 10 AM 1-day before surgery as a baseline, and at the same time on postoperative day (POD) 1, 3, 7, 30 and 90. Samples were immediately processed. Mononuclear cells were isolated from the peripheral blood by centrifugation over Histopaque (Hettich UNIVERSAL 320R, Germany). A flow cytometry of Treg and NK subpopulations was performed using appropriate isotype controls and the following markers:
CD4− FITC (Cat No. 340133, BD Biosciences, USA)
CD25− PE (Cat No. 341009, BD Biosciences)
CD45APC (Cat No. 340943, BD Biosciences)
CD127− PerCP− Cy5.5 (lot: 90916, BD Pharmingen, USA)
CD3− FITC/CD16+ 56− PE (Cat No. 340042, BD Biosciences).
All these antibodies were mouse anti-human. Cell membrane staining of CD127 was performed according to the product application.
Flow cytometry
Flow cytometry was performed on a FACSCanoII instrument (BD Biosciences). Data were obtained and analyzed using the FACSDiva6.0 software (BD Biosciences). In order to identify the Treg subsets, mononuclear cells were gated on side scatter versus CD4. CD4+ cells were then analyzed for CD25 high expression and low expression of CD127. Treg percentage was defined as CD4+ CD25+ CD127low cells in CD4+ lymphocytes.[10,11,12,13] NK percentage was defined as CD56+ 16+ CD3− cells within CD45+ leukocytes.
Statistical analysis
SPSS version 21.0 (SPSS Inc., USA) was used for statistical analysis. Differences of age (years), tumor diameter (cm), number of dissected LN, operation duration (min) and blood loss (ml) between VATS and thoracotomy groups were determined by independent t-test. Chi-square and nonparametric test (Kruskal–Wallis test) were used to compare the distribution of sex and tumor histopathology in these two groups. Time trend and variances in Treg/NK level with the change in postoperative time in either VATS or thoracotomy group were calculated by repeated measures test of general linear model (GLM) according to Mauchly's test of sphericity results. Differences of Treg/NK level between VATS and thoracotomy groups were determined by one-way analysis of variance (ANOVA). Comparison of Treg/NK level in various times after the operation with preoperative level as a control in each group was carried out by one-way ANOVA Dunnett t-test. Equality of variances was confirmed by Levene's test. Differences were considered statistically significant when P < 0.05.
RESULTS
Clinical outcomes
In total, 2284 dissected regional LNs, 37 LNs from 12 patients were pathologically diagnosed as positive for metastasis. Those 12 patients were then confirmed as advanced stage NSCLC and were excluded from the study. Two VATS procedures were converted to open thoracotomy after encountering LNs firmly adhere to pulmonary vessels, imposing the high risk of massive hemorrhage. Eventually 98 stage I patients were enrolled, with 70 patients in VATS group and 28 in thoracotomy group. Clinical information is summarized in Table 1. No demographic differences were found between the two groups. No blood transfusion or blood product was required for any patient. There was no perioperative mortality or major complications in each group. No patient withdrew, and no death or tumor recurrence was observed during the 3-month follow-up.
Table 1.
Clinical demographics of patients in VATS and thoracotomy groups
| Variables | VATS group (n=70) | Thoracotomy group (n=28) | P |
|---|---|---|---|
| Sex (male/female) | 39/31 | 17/11 | 0.651 |
| Age (years)* | 63.1 ± 11.1 | 61.0 ± 7.8 | 0.294 |
| Smoker/nonsmoker | 44/26 | 18/10 | 0.895 |
| Adeno/SCC/other† | 34/32/4 | 15/12/1 | 0.613 |
| Lobectomy/biolobectomy‡ | 68/2 | 27/1 | 1.000 |
| Tumor diameter (cm)* | 2.3 ± 1.2 | 2.6 ± 1.2 | 0.375 |
| Number of dissected LN* | 24.5 ± 6.7 | 22.9 ± 6.3 | 0.267 |
| Operation duration (min)* | 142.4 ± 22.3 | 134.1 ± 25.0 | 0.121 |
| Blood loss (ml)* | 74.7 ± 42.7 | 94.3 ± 49.0 | 0.055 |
*Mean ± SD; †Kruskal–Wallis test; ‡Chi-square tests-Fisher’s exact test. Adeno: Adenocarcinoma; SCC: Squamous cell carcinoma; VATS: Video-assisted thoracoscopic; SD: Standard deviation; LN: Lymph node.
Dynamics of circulating regulatory T-cells and natural killer levels after major lung resection
Flow cytometric analysis of Treg cells and NK cells in the peripheral blood of VATS and thoracotomy groups was demonstrated in Figures 1 and 2. Repeated measures test of GLM results demonstrated that there were significant differences of Treg/NK level in various times in each group (F = 35.34, P = 0.000 of NK, F = 6.284, P = 0.000 of Treg). Time trend was obvious in NK (F = 72.972, P = 0.000) but not significant in Treg (F = 16.74, P = 0.185). Level of NK was found increasing with the extension of postoperative recovery time in both VATS and thoracotomy groups.
Figure 1.
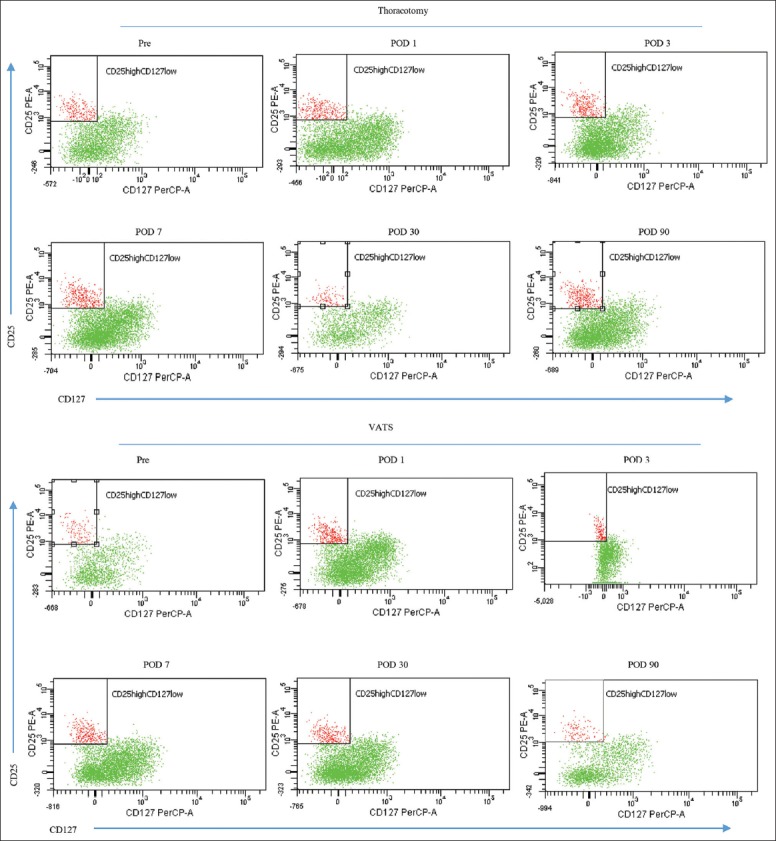
Representative dot plot of CD4+CD25+CD127low expression in the peripheral blood from patients undergoing thoracotomy (upper) or video-assisted thoracoscopic (lower). CD4+ cells were analyzed for CD25−PE and CD127−PerCP-Cy5.5 staining, CD25 high expression, and low expression of CD127 were then gated.
Figure 2.
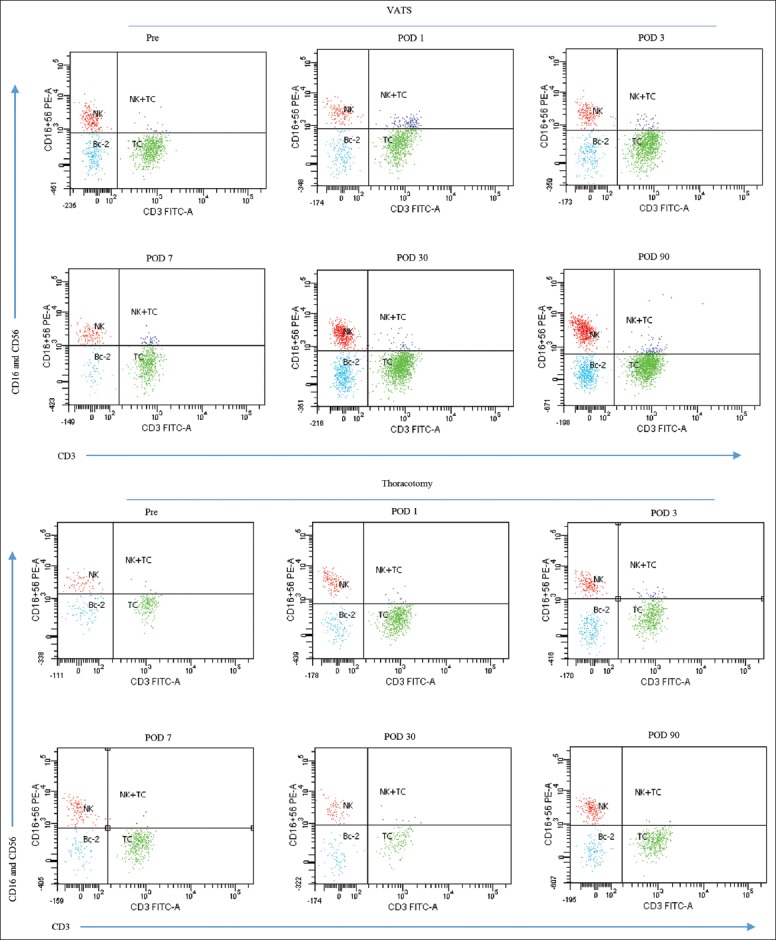
Representative dot plot of CD56+16+CD3− cells in the peripheral blood CD45+ leukocytes from patients undergoing video-assisted thoracoscopic lobectomy (upper) or thoracotomy (lower). CD45+ leukocytes were analyzed for CD3−FITC/CD16+56−PE staining, subsequently CD16+56 high expression, and negative expression of CD3 were gated.
Comparison of Treg/NK level in various times after operation with preoperative level as a control in each group was demonstrated in Table 2. In VATS group, all postoperative Treg levels were found statistically lower than preoperative status. In thoracotomy group, differently, significant lower Treg percentages was only found on POD 30 (5.76 ± 2.71, P = 0.042). In both groups, on POD 1, NK percentages were significantly lower than preoperative status, and there was a clear rising time trend. In thoracotomy group, NK population did not recover to preoperative level until POD 7. Nevertheless, in VATS group, NK population recovered to preoperative status on POD 3 and kept rising, and peripheral NK levels on PODs 7, 30, and 90 were statistically higher than preoperative level [Table 2].
Table 2.
Comparison of postoperative Treg/NK subpopulation with preoperative levels in VATS and thoracotomy groups by one-way ANOVA Dunnett t-test
| Treg/NK | Sampling day | Value (%) | P |
|---|---|---|---|
| VATS | |||
| Treg | Preoperative | 7.61 ± 3.08 | |
| POD 1 | 5.88 ± 2.81 | 0.004 | |
| POD 3 | 5.83 ± 3.39 | 0.003 | |
| POD 7 | 5.32 ± 3.15 | 0.000 | |
| POD 30 | 5.05 ± 3.13 | 0.000 | |
| POD 90 | 5.26 ± 2.75 | 0.000 | |
| NK | Preoperative | 18.44 ± 6.19 | |
| POD 1 | 15.38 ± 6.39 | 0.038 | |
| POD 3 | 19.15 ± 6.23 | 0.961 | |
| POD 7 | 20.78 ± 6.62 | 0.169 | |
| POD 30 | 24.86 ± 8.03 | 0.000 | |
| POD 90 | 25.90 ± 8.52 | 0.000 | |
| Thoracotomy | |||
| Treg | Preoperative | 8.01 ± 4.20 | |
| POD 1 | 5.98 ± 2.59 | 0.079 | |
| POD 3 | 6.43 ± 3.30 | 0.240 | |
| POD 7 | 6.39 ± 2.70 | 0.219 | |
| POD 30 | 5.76 ± 2.71 | 0.042 | |
| POD 9 | 6.99 ± 3.60 | 0.645 | |
| NK | Preoperative | 19.28 ± 7.63 | |
| POD 1 | 12.60 ± 5.68 | 0.001 | |
| POD 3 | 12.90 ± 4.54 | 0.002 | |
| POD 7 | 16.16 ± 7.40 | 0.282 | |
| POD 30 | 20.86 ± 7.02 | 0.848 | |
| POD 90 | 22.72 ± 7.34 | 0.201 |
POD: Postoperative day; NK: Natural killer; VATS: Video-assisted thoracoscopic; Treg: Regulatory T-cells.
There was no significant difference on preoperative Treg or NK levels between VATS and thoracotomy groups (Treg: 7.61 ± 3.08 vs. 8.01 ± 4.20, P = 0.603; NK: 18.44 ± 6.19 vs. 19.28 ± 7.63, P = 0.572). Furthermore, in comparison between Treg levels in VATS and thoracotomy groups, no significant difference was confirmed on PODs 1, 3, 7 and 30 (POD 1: 5.88 ± 2.81 vs. 5.98 ± 2.59, P = 0.874; POD 3: 5.83 ± 3.39 vs. 6.43 ± 3.30, P = 0.426; POD 7: 5.32 ± 3.15 vs. 6.39 ± 2.70, P = 0.117; POD 30: 5.05 ± 3.13 vs. 5.76 ± 2.71, P = 0.295), unless that on POD 90, Treg percentages in VATS group were significantly lower than those in thoracotomy group (5.26 ± 2.75 vs. 6.99 ± 3.60, P = 0.012) [Figure 3a]. Meanwhile, compared with thoracotomy group, in VATS group, higher levels of NK were found on all PODs except on POD 90 (15.38 ± 6.39 vs. 12.60 ± 5.68 on POD 1, P = 0.024; 19.15 ± 6.23 vs. 12.90 ± 4.54 on POD 3, P = 0.000; 20.78 ± 6.62 vs. 16.16 ± 7.40 on POD 7, P = 0.003; 24.86 ± 8.03 vs. 20.86 ± 7.02 on POD 30, P = 0.023; 25.90 ± 8.52 vs. 22.72 ± 7.34 on POD 90, P = 0.087) [Figure 3b]. The results show that in stage I NSCLC patients, VATS approach indicates less inhibition and faster recovery of circulating NK cells, compared to thoracotomy. In our observation, the benefit to NK counts from less invasive VATS approach diminished on POD 90.
Figure 3.
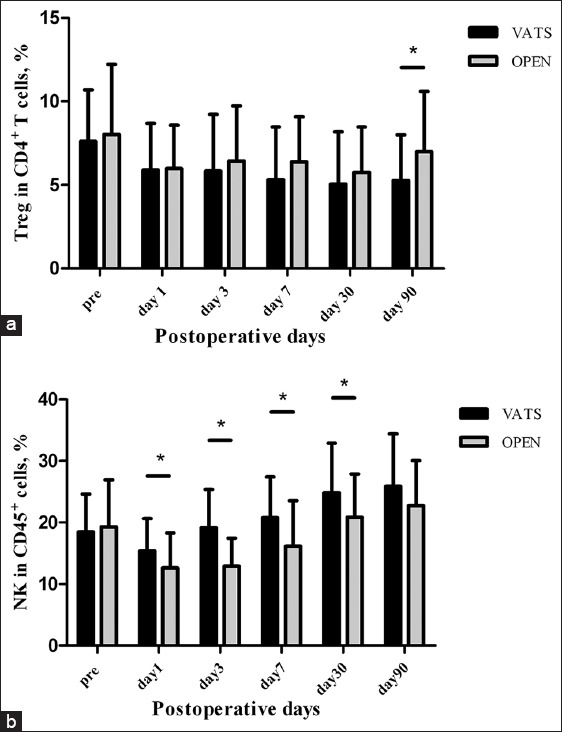
Comparison of peripheral regulatory T-cells (a) and natural killer cells (b) levels in patients undergoing video-assisted thoracoscopic lobectomy or thoracotomy by one-way ANOVA, *P < 0.05. Pre: preoperative.
DISCUSSION
In spite of improvements on surgical skills, new chemical agents, evolution of radiation therapy, prognosis of lung cancer remains poor, the curing rate for NSCLC is reported lower than 20%.[14] Treg is a subset of T-cells with immune-suppressive function,[15,16] defined[10,17,18,19,20] by CD4+CD25+FoxP3+/CD127low. Treg is considered to play key roles in suppressing cell-mediated immunity in lung cancer patients. In NSCLC patients, circulating Treg levels were elevated from an early stage,[9] and a higher level of Treg in tumor tissue, regional LNs, and peripheral blood is regarded as a negative prognostic factor.[16,21,22] Interestingly, peripheral blood Treg levels in advanced cancer patients dropped significantly after effective chemotherapy or chemo-radiation therapy, parallel to clinical remission.[23,24,25]
As tumors may promote expansion, recruitment, and activation of Treg, surgical tumor removal may directly lead to a reduction of Treg population in cancer patients. After monitoring peripheral levels of Treg in gastric and esophageous cancer patients, Kono et al.[26] reported that elevated number of CD4+CD25+ Treg was significantly reduced after curative resection but increased again in the cases of cancer relapse. There are very few reports on perioperative Treg levels in lung cancer patients. Recently, Chen et al.[9] reported a direct assessment of the perioperative circulating Treg population in 36 NSCLC patients, demonstrating decreased CD4+ CD25+ FoxP3+ Treg percentages on POD 30 (preoperative vs. POD 30: 3.16% ± 1.32% vs. 2.46% ± 1.43%, P = 0.007). Similarly in our observation, in both VATS and thoracotomy groups, CD4+ CD25+ CD127low Treg levels dropped significantly and remained below preoperative status within 30 days after surgery. Furthermore, it was suggested by Hanbo et al. that postoperative Treg values in early stage patients were not statistically different from Treg frequencies of control patients,[9] which implies that tumor mediated immune suppression by Treg may be completely eliminated after tumor removal in early NSCLC.
Meanwhile, although with controversy,[27] Saito et al.[28] suggested that Treg could also be an efficient indicative biomarker for surgical invasiveness. In their study, markedly lower levels of peripheral Treg were found after laparoscopic surgeries when compared to open ones. In this study, we examined circulating Treg in NSCLC patients undergoing major lung resection by VATS or thoracotomy. To minimize the influence of tumor progression on Treg level,[9,21,22,29] only stage I patients were enrolled. To our knowledge, it is a relatively early continuous observation on postoperative Treg population in stage I NSCLC cases, focusing on variances of surgical invasiveness by VATS or conventional thoracotomy.
Mechanisms of expansion, recruitment, and activation of Treg cells in postoperative patients are undoubtedly very complex, but tumor removal and surgical stress are believed to play important roles. Our study showed that peripheral CD4+ CD25+ CD127low Treg proportions significantly dropped promptly after operations in both VATS and thoracotomy groups, but there was no significant difference between the two groups on PODs 1, 3, 7, and 30. This result suggests that within the 1st month postoperative, direct tumor removal ceases the tumor mediated Treg proliferation and release, and may be the most important factor on Treg reduction in early postoperative period. However, in VATS group, Treg proportion on POD 90 was found statistically lower than that in thoracotomy group. It may be explained that the diminished surgical trauma by VATS approach does not change the Treg level directly, but gradually induces less immune disturbance in multiple ways[7,8,30,31] and hence inhibits Treg for a longer time.
Less immune disturbance after VATS lobectomy was previously shown in several researches on plasma immunomodulatory mediators[7,8] and NK cells.[30,31] Notably, differences in nearly all of the postoperative cytokines and plasma immunomodulatory mediators measured thus far between VATS and thoracotomy groups are short-lived, in the order of hours to a couple of days.[7,8] In our study, on PODs 1, 3, 7, 30, NK cell numbers were found to be significantly higher following VATS lobectomy compared to the open approach, suggesting that VATS is associated with less inhibition and quicker recovery of NK cell than conventional thoracotomy. The rationale behind remains to be fully elucidated and actually encompasses numerous factors, including reduced acute-phase cytokine responses and postoperative pain, differences in patient expectations, and possible psychological effects.[30] On the contrary, the difference on Treg levels between VATS and thoracotomy group was not significant except on POD 90. This phenomenon suggests that, unlike plasma immune molecules and NK counts, difference of Treg levels between VATS and thoracotomy is a “delayed” and perhaps longer-lived marker for lesser immune disturbance. The mechanism is undoubtedly very complex and remains to be explored.[32]
Thus, VATS major lung resection for NSCLC is associated with less postoperative immune-suppression when compared with the thoracotomy approach. Nevertheless, whether a better-preserved postoperative immune status could be linked to survival benefit remains uncertain. Study including large-scale randomized trial and longer time follow-up recording multiple immune function markers after VATS and thoracotomy is needed for further evidence.[33] However, for the reasons of patients’ intention[32] and 2015 NCCN recommendation for NSCLC, it's difficult to randomize patients into thoracotomy group. In addition, diversities in VATS technique may have additional effects on the postoperative immune markers.
The correlation between Treg proportions and histological subtypes in NSCLC was not taken into consideration in our study. Several reports claimed higher Treg proportions in adenocarcinoma than squamous carcinoma, but this difference seems existed in tumor and invaded LNs,[34] but not in peripheral blood.[9]
It is also noteworthy that identification of pure or naive immune-suppressive Treg population is still a challenge, explaining the varying results by different groups in clinical studies. Treg cells in cancer patients were initially determined by CD4 and CD25 co-expression.[35,36] Since the discovery of transcription factor FoxP3 in 2003, it became the most common marker for Treg cells. However, even the inclusion of FoxP3 assessment has been interpreted differentially when assessing frequencies of Treg cells in healthy individuals and cancer patients.[37,38] Furthermore, given that FoxP3 is an intracellular protein, detection of FoxP3 requires permeabilization of cells; hence this technique cannot be used to isolate viable Treg population.[39] Therefore, a unique or additional surface marker of Treg cells is needed. On basis of several reports,[10,12,13] the low cell-surface expression of CD127 allows for an accurate estimation of Treg numbers and the isolation of pure populations for in vitro studies, providing a flexible alternative to the transcription factor FoxP3. On the other hand, In contrast to CD4+ CD25+ FoxP3+ cells, gating of a clear CD4+ CD25+ CD127low/− population was difficult.[39] Moreover, Klein et al.[39] demonstrated these two markers did not represent the same population of Treg cells. Recently, Beyer et al.[40] supported the superiority of combining CD127 and FoxP3 for further quantification of Treg cells in malignant diseases. In our study, CD4+ CD25+ CD127low were used to define Treg cells. The conclusion that VATS better preserves postoperative cellular immune function from our observation is not going to be confirmed unless the CD4+ CD25+ CD127low cells assessed in our study displayed immune depressive activity in future in vitro or even in vivo studies.
Footnotes
Edited by: Yuan-Yuan Ji
Source of Support: This research was supported by a grant of Zhejiang Science and Technology Agency Research Program (No. 2012R10031).
Conflict of Interest: None declared.
REFERENCES
- 1.Nicastri DG, Wisnivesky JP, Litle VR, Yun J, Chin C, Dembitzer FR, et al. Thoracoscopic lobectomy: Report on safety, discharge independence, pain, and chemotherapy tolerance. J Thorac Cardiovasc Surg. 2008;135:642–7. doi: 10.1016/j.jtcvs.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Higuchi M, Yaginuma H, Yonechi A, Kanno R, Ohishi A, Suzuki H, et al. Long-term outcomes after video-assisted thoracic surgery (VATS) lobectomy versus lobectomy via open thoracotomy for clinical stage IA non-small cell lung cancer. J Cardiothorac Surg. 2014;9:88. doi: 10.1186/1749-8090-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berry MF, D’Amico TA, Onaitis MW, Kelsey CR. Thoracoscopic approach to lobectomy for lung cancer does not compromise oncologic efficacy. Ann Thorac Surg. 2014;98:197–202. doi: 10.1016/j.athoracsur.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao C, Zhu ZH, Yan TD, Wang Q, Jiang G, Liu L, et al. Video-assisted thoracic surgery versus open thoracotomy for non-small-cell lung cancer: A propensity score analysis based on a multi-institutional registry. Eur J Cardiothorac Surg. 2013;44:849–54. doi: 10.1093/ejcts/ezt406. [DOI] [PubMed] [Google Scholar]
- 5.Lee PC, Nasar A, Port JL, Paul S, Stiles B, Chiu YL, et al. Long-term survival after lobectomy for non-small cell lung cancer by video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg. 2013;96:951–60. doi: 10.1016/j.athoracsur.2013.04.104. [DOI] [PubMed] [Google Scholar]
- 6.Taioli E, Lee DS, Lesser M, Flores R. Long-term survival in video-assisted thoracoscopic lobectomy vs open lobectomy in lung-cancer patients: A meta-analysis. Eur J Cardiothorac Surg. 2013;44:591–7. doi: 10.1093/ejcts/ezt051. [DOI] [PubMed] [Google Scholar]
- 7.Yim AP, Wan S, Lee TW, Arifi AA. VATS lobectomy reduces cytokine responses compared with conventional surgery. Ann Thorac Surg. 2000;70:243–7. doi: 10.1016/s0003-4975(00)01258-3. [DOI] [PubMed] [Google Scholar]
- 8.Jones RO, Anderson NH, Murchison JT, Brittan M, Simon EJ, Casali G, et al. Innate immune responses after resection for lung cancer via video-assisted thoracoscopic surgery and thoracotomy. Innovations (Phila) 2014;9:93–103. doi: 10.1097/IMI.0000000000000061. [DOI] [PubMed] [Google Scholar]
- 9.Chen C, Chen D, Zhang Y, Chen Z, Zhu W, Zhang B, et al. Changes of CD4+CD25+FOXP3+ and CD8+CD28- regulatory T cells in non-small cell lung cancer patients undergoing surgery. Int Immunopharmacol. 2014;18:255–61. doi: 10.1016/j.intimp.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Hartigan-O’Connor DJ, Poon C, Sinclair E, McCune JM. Human CD4+regulatory T cells express lower levels of the IL-7 receptor alpha chain (CD127), allowing consistent identification and sorting of live cells. J Immunol Methods. 2007;319:41–52. doi: 10.1016/j.jim.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+T reg cells. J Exp Med. 2006;203:1701–11. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ziegler SF. FOXP3: Of mice and men. Annu Rev Immunol. 2006;24:209–26. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 14.Sandler A, Yi J, Dahlberg S, Kolb MM, Wang L, Hambleton J, et al. Treatment outcomes by tumor histology in Eastern Cooperative Group Study E4599 of bevacizumab with paclitaxel/carboplatin for advanced non-small cell lung cancer. J Thorac Oncol. 2010;5:1416–23. doi: 10.1097/JTO.0b013e3181da36f4. [DOI] [PubMed] [Google Scholar]
- 15.Terabe M, Berzofsky JA. Immunoregulatory T cells in tumor immunity. Curr Opin Immunol. 2004;16:157–62. doi: 10.1016/j.coi.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 16.Schneider T, Kimpfler S, Warth A, Schnabel PA, Dienemann H, Schadendorf D, et al. Foxp3(+) regulatory T cells and natural killer cells distinctly infiltrate primary tumors and draining lymph nodes in pulmonary adenocarcinoma. J Thorac Oncol. 2011;6:432–8. doi: 10.1097/JTO.0b013e31820b80ca. [DOI] [PubMed] [Google Scholar]
- 17.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 18.Gambineri E, Torgerson TR, Ochs HD. Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance (IPEX), a syndrome of systemic autoimmunity caused by mutations of FOXP3, a critical regulator of T-cell homeostasis. Curr Opin Rheumatol. 2003;15:430–5. doi: 10.1097/00002281-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 20.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+T regulatory cells. Nat Immunol. 2003;4:337–42. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 21.Beyer M, Schultze JL. Regulatory T cells in cancer. Blood. 2006;108:804–11. doi: 10.1182/blood-2006-02-002774. [DOI] [PubMed] [Google Scholar]
- 22.Esendagli G, Bruderek K, Goldmann T, Busche A, Branscheid D, Vollmer E, et al. Malignant and non-malignant lung tissue areas are differentially populated by natural killer cells and regulatory T cells in non-small cell lung cancer. Lung Cancer. 2008;59:32–40. doi: 10.1016/j.lungcan.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 23.Hu M, Li K, Maskey N, Xu Z, Peng C, Wang B, et al. Decreased intratumoral Foxp3 Tregs and increased dendritic cell density by neoadjuvant chemotherapy associated with favorable prognosis in advanced gastric cancer. Int J Clin Exp Pathol. 2014;7:4685–94. [PMC free article] [PubMed] [Google Scholar]
- 24.Ladoire S, Arnould L, Apetoh L, Coudert B, Martin F, Chauffert B, et al. Pathologic complete response to neoadjuvant chemotherapy of breast carcinoma is associated with the disappearance of tumor-infiltrating foxp3+regulatory T cells. Clin Cancer Res. 2008;14:2413–20. doi: 10.1158/1078-0432.CCR-07-4491. [DOI] [PubMed] [Google Scholar]
- 25.Tsuchikawa T, Hirano S, Tanaka E, Matsumoto J, Kato K, Nakamura T, et al. Novel aspects of preoperative chemoradiation therapy improving anti-tumor immunity in pancreatic cancer. Cancer Sci. 2013;104:531–5. doi: 10.1111/cas.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kono K, Kawaida H, Takahashi A, Sugai H, Mimura K, Miyagawa N, et al. CD4(+) CD25 high regulatory T cells increase with tumor stage in patients with gastric and esophageal cancers. Cancer Immunol Immunother. 2006;55:1064–71. doi: 10.1007/s00262-005-0092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao HJ, Zhang YJ, Liang HH, Li P, Peng ZW, Pang XH, et al. Radiofrequency ablation does not induce the significant increase of CD4(+) CD25(+) Foxp3(+) regulatory T cells compared with surgical resection in Hepal-6 tumor model. Arch Immunol Ther Exp (Warsz) 2013;61:333–40. doi: 10.1007/s00005-013-0226-1. [DOI] [PubMed] [Google Scholar]
- 28.Saito Y, Shimada M, Utsunomiya T, Morine Y, Imura S, Ikemoto T, et al. Regulatory T cells in the blood: A new marker of surgical stress. Surg Today. 2013;43:608–12. doi: 10.1007/s00595-013-0517-5. [DOI] [PubMed] [Google Scholar]
- 29.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 30.Ng CS, Lee TW, Wan S, Wan IY, Sihoe AD, Arifi AA, et al. Thoracotomy is associated with significantly more profound suppression in lymphocytes and natural killer cells than video-assisted thoracic surgery following major lung resections for cancer. J Invest Surg. 2005;18:81–8. doi: 10.1080/08941930590926320. [DOI] [PubMed] [Google Scholar]
- 31.Leaver HA, Craig SR, Yap PL, Walker WS. Lymphocyte responses following open and minimally invasive thoracic surgery. Eur J Clin Invest. 2000;30:230–8. doi: 10.1046/j.1365-2362.2000.00622.x. [DOI] [PubMed] [Google Scholar]
- 32.Ng CS, Wan IY, Yim AP. Impact of video-assisted thoracoscopic major lung resection on immune function. Asian Cardiovasc Thorac Ann. 2009;17:426–32. doi: 10.1177/0218492309338100. [DOI] [PubMed] [Google Scholar]
- 33.Ng CS, Whelan RL, Lacy AM, Yim AP. Is minimal access surgery for cancer associated with immunologic benefits? World J Surg. 2005;29:975–81. doi: 10.1007/s00268-005-0029-6. [DOI] [PubMed] [Google Scholar]
- 34.Black CC, Turk MJ, Dragnev K, Rigas JR. Adenocarcinoma contains more immune tolerance regulatory t-cell lymphocytes (versus squamous carcinoma) in non-small-cell lung cancer. Lung. 2013;191:265–70. doi: 10.1007/s00408-013-9455-7. [DOI] [PubMed] [Google Scholar]
- 35.Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, et al. Regulatory CD4(+) CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766–72. [PubMed] [Google Scholar]
- 36.Woo EY, Yeh H, Chu CS, Schlienger K, Carroll RG, Riley JL, et al. Cutting edge: Regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol. 2002;168:4272–6. doi: 10.4049/jimmunol.168.9.4272. [DOI] [PubMed] [Google Scholar]
- 37.Beyer M, Kochanek M, Giese T, Endl E, Weihrauch MR, Knolle PA, et al. In vivo peripheral expansion of naive CD4+CD25high FoxP3+regulatory T cells in patients with multiple myeloma. Blood. 2006;107:3940–9. doi: 10.1182/blood-2005-09-3671. [DOI] [PubMed] [Google Scholar]
- 38.Prabhala RH, Neri P, Bae JE, Tassone P, Shammas MA, Allam CK, et al. Dysfunctional T regulatory cells in multiple myeloma. Blood. 2006;107:301–4. doi: 10.1182/blood-2005-08-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klein S, Kretz CC, Krammer PH, Kuhn A. CD127(low/-) and FoxP3(+) expression levels characterize different regulatory T-cell populations in human peripheral blood. J Invest Dermatol. 2010;130:492–9. doi: 10.1038/jid.2009.313. [DOI] [PubMed] [Google Scholar]
- 40.Beyer M, Classen S, Endl E, Kochanek M, Weihrauch MR, Debey-Pascher S, et al. Comparative approach to define increased regulatory T cells in different cancer subtypes by combined assessment of CD127 and FOXP3. Clin Dev Immunol 2011. 2011 doi: 10.1155/2011/734036. 734036. [DOI] [PMC free article] [PubMed] [Google Scholar]


