Abstract
Background:
Current views on continuous positive airway pressure (CPAP) treatment to improve the cognitive deficits of patients with obstructive sleep apnea syndrome (OSAS) are controversial, so we performed a meta-analysis.
Methods:
A comprehensive literature search was undertaken in PubMed, CINAHL, Medline, PsycInfo, EMBASE, Cochrane Library, CNKI, WanFang, VIP, and CBMdisc for studies published from June 1971 to July 2014. The outcome measures included neuropsychological tests of the 7 cognitive domains detailed below.
Results:
After screening the titles and abstracts and thoroughly reading the full text, we obtained 13 studies with little risk of bias that incorporated 1744 middle-aged obese participants with mild to severe OSAS. The studies were published from 1994 to 2012. Treatment durations varied from 1 to 24 weeks. The effect sizes of attention, vigilance, processing speed, working memory, memory, verbal fluency, and visuoconstructive skills domains were −0.10 (P = 0.24), −0.12 (P = 0.04), −0.08 (P = 0.16), 0.00 (P = 0.95), −0.04 (P = 0.30), −0.06 (P = 0.34), and −0.01 (P = 0.92), respectively.
Conclusions:
Cognition partially improved in patients with OSAS after CPAP treatment. The only domain with significant improvement was vigilance. Rigorous randomized controlled trials need to be performed to obtain clear results.
Keywords: Cognition, Continuous Positive Airway Pressure, Meta-analysis, Obstructive Sleep Apnea Syndrome
INTRODUCTION
Obstructive sleep apnea syndrome (OSAS), which is characterized by repeated episodes of apnea and hypopnea during sleep, occurs in 1 of 20 adults,[1] but it is usually unrecognized and undiagnosed. The long-term consequences of untreated OSAS include neurocognitive impairments; increased accident risk; and cerebrovascular, cardiovascular, and metabolic morbidities[2] which reduce social functioning and quality of life. Well-established risk factors for OSAS include obesity, male sex, age, adenotonsillar hypertrophy, and smoking and alcohol consumption.[2]
Previous studies have shown that patients with OSAS exhibit cognitive and psychological impairments in attention, vigilance, executive functioning, constructive abilities, psychomotor functioning, and memory.[3,4,5,6,7,8]
The standard therapy for OSAS is continuous positive airway pressure (CPAP), which alleviates apnea and hypopnea during sleep and improves sleep fragmentation and hypoxemia. Many studies have examined whether pretreatment cognitive deficits are permanent or remit with CPAP treatment. Some studies showed improvement in certain areas, such as global functioning, executive functioning, memory, and attention while others did not.[3,9] Previous meta-analyses have shown CPAP significantly improves attention, symptoms of sleepiness, quality of life, and health-related quality of life,[10,11,12] but the data were relatively unclear. More recent studies with a large number of participants have examined neurocognitive dysfunction improvements. Many reviews have documented that CPAP partially remits cognitive impairments.[3,13,14] However, some studies showed slight or no improvement in neurocognitive function, and the qualitative reviews did not weigh them properly. In addition, differences in the OSAS inclusion criterion; OSAS baseline severity; methodology, duration and compliance of CPAP treatment; and nonstandardized cognitive tests might account for the different findings and conclusions of the reviews. In addition, reviews are qualitative and less convincing than the quantitative results of meta-analyses.
To determine whether CPAP improved cognitive function with thorough quantitative evidence, we conducted a rigorous meta-analysis of randomized controlled trials (RCTs) of CPAP treatment in patients with OSAS with cognitive deficits.
METHODS
Literature search
The current literature was systematically searched in PubMed, CINAHL, Medline, PsycInfo, EMBASE, Cochrane Library, CNKI, WanFang, VIP, and CBMdisc for the period from June 1971 to July 2014. The search words for OSAS were apnea (MeSH), apn(o)ea, sleep apn(o)ea, obstructive sleep apne(o)a, OSA, OSAS, sleep apnea/hypopnea syndrome, obstructive sleep apnea hypopnea syndrome, and upper airway resistance sleep apnea syndrome. The search items for CPAP were CPAP (MeSH), CPAP, bilevel positive airway pressure, BiPAP, nasal CPAP, nCPAP, positive pressure therapy, and nocturnal ventilation. The search items for cognitive measures were mental processes (MeSH), mental status, neuropsychology, cognition, cognitive ability, memory, attention, vigilance, executive, and psychomotor. Two independent investigators assessed whether the literature was relevant, according to title and abstract. The investigators thoroughly read the full text of the relevant studies and determined whether they should be included according to the inclusion criteria set in advance. If the investigators held different opinions, they made a final decision together.
Inclusion criteria
The studies included in the meta-analysis had to meet the following criteria:
The OSAS diagnosis was based on the apnea/hypopnea index (AHI) or respiratory disturbance index (RDI) results from overnight polysomnography, which was performed within 12 months, for patients with or without symptoms, such as snoring or excessive daytime sleepiness.
The study design was a RCT.
The baseline demographic characteristics of the patients with OSAS, including age, gender, and body mass index (BMI), were required.
Cognitive function was tested by at least one standardized neuropsychological test.
Neuropsychological test scores included pre- and post-treatment assessments (mean ± standard deviation [SD] or other statistics) by which to calculate effect size in both the CPAP treatment and control groups.
Exclusion criteria
Studies that met any one of the following criteria were excluded:
Studies conducted in young (<18 years) or older (>65 years) patients because pediatric patients with OSAS often have different etiologies than adults, and older people commonly have comorbidities, such as hypertension, coronary heart disease, and diabetes mellitus.
Studies performed in a special patient population, such as those with Alzheimer's disease, dementia, stroke, Down syndrome, insomnia, or traumatic brain injury.
Studies examining if central sleep apnea or mixed sleep apnea syndrome had different mechanisms than obstructive sleep apnea and CPAP was not the standard treatment.
Studies of medications, such as modafinil or armodafinil, which might alter cognition.
Book chapters, commentaries, review articles, case reports, or conference abstracts.
Outcome measures
Multiple neuropsychological tests were used to assess cognitive functioning. We divided cognition into the following 7 domains and incorporated different neuropsychological tests in each domain following a standard textbook of neuropsychological assessment:[15] attention, vigilance, processing speed, working memory, memory, verbal fluency, and visuoconstructive skills.
Participants and study variables
The study and patient characteristics from each trial included the number of patients, placebo type, trial design, treatment duration, country, gender, mean age, mean BMI (kg/m2), mean AHI or RDI, and mean CPAP use per night. BMI was classified according to the World Health Organization criteria as normal (20.0–24.9 kg/m2), overweight (25.0–29.9 kg/m2), or obese (>30 kg/m2). OSAS severity was classified according to the AHI or RDI: Mild (AHI 5–15 events/h), moderate (15–30 events/h), or severe (>30 events/h). Good compliance meant that usage was >5 days/week and >4 h/night.
Calculation of effect sizes and statistical analysis
For parallel studies, we calculated the effect size with Cohen's d according to the following formula:

where,
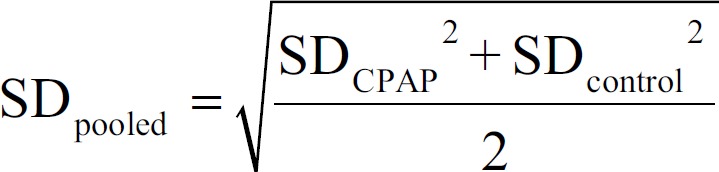
An effect size of 0.20 was considered small, 0.50 was medium, 0.80 was large, and 1.00 was very large.[16] A negative d indicated better performance with CPAP treatment than control. When the studies used more than one test in a single cognitive domain, we computed an averaged effect size to avoid the study over-influencing the results for any given domain.
For cross-over trials, we conducted paired t-tests with the mean difference in P values. When we could not get the above data required to include in a paired test, the available data were analyzed as if the trial had a parallel-group design with treatment versus control intervention. The advantage of the reduced influence of confounding covariates in cross-over designs was disregarded in the latter condition.
In order to pool the results across studies, we calculated a pooled d-value for all seven cognitive domains, which were weighed according to the sample sizes of the individual studies.
Heterogeneity
Considering the diversity in study design, participants, neuropsychological tests, and risks of bias in the included studies, we employed Cochrane's I2 statistics to assess the percentage of variability in the effect estimates that was due to heterogeneity rather than sampling error. I2 was calculated with the equation 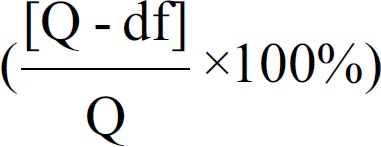 where df signifies the degrees of freedom (number of studies-1).[16] A rough guide of I2 interpretation is that 0–40% might not be important, 30–60% might represent moderate heterogeneity, 50–90% might represent substantial heterogeneity, and 75–100% might represent considerable heterogeneity.[16]
where df signifies the degrees of freedom (number of studies-1).[16] A rough guide of I2 interpretation is that 0–40% might not be important, 30–60% might represent moderate heterogeneity, 50–90% might represent substantial heterogeneity, and 75–100% might represent considerable heterogeneity.[16]
Publication bias
Statistically significant positive results that indicate an intervention works are more likely to be published and cited by others, resulting in publication bias. Publication bias is usually evaluated visually with a funnel plot, which is a simple scatter plot of the intervention effect estimates of individual studies against some measure of each study's size. In the absence of bias, the plot should approximately resemble a symmetrical funnel. Bias, such as when studies without significant effects remain unpublished, results in an asymmetrical funnel plot.[16]
Statistical analysis
The most common meta-analysis approach is the fixed-effects model, which is based on the assumption that the true effect of interventions (in both magnitude and direction) is the same in every study. This model is questionable considering the diverse criteria, OSA severity, and clinical design of the included studies. Here, we employed a random-effects model as an alternative. This model was based on the assumption that the estimated effects in different studies are not identical, and the variance in true effect sizes results from numerous unknown or unmeasured differences across studies.
This analysis was conducted with Review Manager 5.0 (Cochrane center, Nordic Europe). To pool samples of different sizes, generic inverse variance weights within each sample were computed. Inverse variance weights placed greater emphasis on large sample studies than on small sample studies.
RESULTS
Identification of studies
The final search yielded 12,895 articles from June 1971 to July 2014. Duplicate checking and title and abstract screening resulted in 347 publications. The inclusion and exclusion criteria resulted in 148 articles. Of these, 98 were excluded because of an absence of standardized neuropsychological tests, 21 were excluded for no control group, 8 were excluded because healthy volunteers served as controls, 5 were excluded for nonrandomized designs, and 3[17,18,19] were excluded because effect sizes could not be calculated from the available data. Finally, 13 studies were included [Figure 1].
Figure 1.
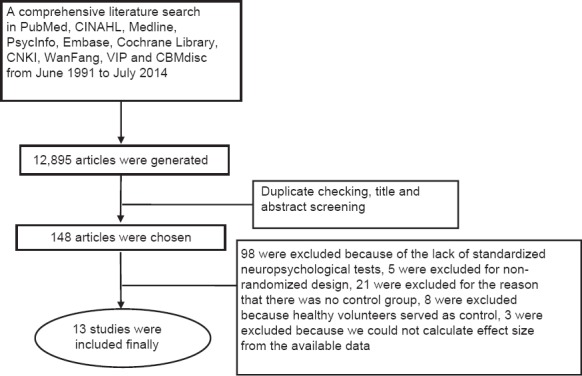
Flow diagram for selection of studies.
The characteristics of the 13 studies are shown in Table 1.
Table 1.
Characteristics of included studies
| Studies | Year | RCT design | Control treatment | Country | Jadad |
|---|---|---|---|---|---|
| Barbé et al.[27] | 2001 | Parallel | Sham CPAP | Spain | 5 |
| Bardwell et al.[28] | 2001 | Parallel | Sham CPAP | USA | 4 |
| Barnes et al.[23] | 2004 | Crossover | Oral placebo | Australia | 3 |
| Engleman et al.[22] | 1994 | Crossover | Oral placebo | UK | 3 |
| Engleman et al.[20] | 1997 | Crossover | Oral placebo | UK | 3 |
| Engleman et al.[21] | 1999 | Crossover | Oral placebo | UK | 3 |
| Gast et al.[30] | 2006 | Parallel | Untreated | USA | 3 |
| Engleman et al.[26] | 1998 | Crossover | Oral placebo | UK | 3 |
| Kushida et al.[31] | 2012 | Parallel | Sham CPAP | USA | 5 |
| Marshall et al.[24] | 2005 | Crossover | Sham CPAP | New Zealand | 5 |
| Monasterio et al.[25] | 2001 | Parallel | Conservative treatment | Spain | 4 |
| Pelletier–Fleury et al.[29] | 2004 | Parallel | Untreated | France | 4 |
| Prilipko et al.[32] | 2012 | Parallel | Sham CPAP | USA | 5 |
RCT: Randomized controlled trial; CPAP: Continuous positive airway pressure; USA: United States of American; UK: United Kingdom; Jadad: The Jadad rating score.
The study dates ranged from 1994 to 2012. Seven were parallel RCTs, while the rest were cross-over studies. Five studies adopted sham CPAP (at 1–2 cmH2O) as a control. Five adopted oral placebos, and the patients were told the placebo might improve upper airway function. Two studies had untreated control patients. One adopted conservative measures, including a home weight-loss program, avoidance of sedatives and alcohol consumption, avoidance of a supine sleep position, and adequate sleep every night. The countries of the studies varied largely, but most participants were Caucasian.
To evaluate the quality of the 13 studies, we calculated the Jadad rating score, which assesses randomization, blinding, and dropouts. Four[24,27,31,32] had maximum scores of 5, 3[25,28,29] had scores of 4, and the others scored 3. Jadad scores of 3 or more indicate high quality of the study. Thus, we concluded that the 13 RCTs were of high quality.
Participants and study characteristics
The characteristics of the participants of the studies are shown in Table 2.
Table 2.
Characteristics of participants of the included studies
| Studies | n (E/C) | Mean age (years) | Male (%) | Mean BMI (kg/m2) | Mean AHI (events/h) | Duration of treatment (weeks) | CPAP usage (h/night) |
|---|---|---|---|---|---|---|---|
| Barbé et al., 2001[27] | 54 (29/25) | 53.1 | 0.91 | 29 | 55.4 | 6 | 4.5 |
| Bardwell et al., 2001[28] | 36 (20/16) | 47.4 | 0.81 | 31.4 | 50.9 | 1 | 5.2 |
| Barnes et al., 2004[23] | 80 | 46.4 | 0.79 | 31.0 | 21.5 | 12 | 3.6 |
| Engleman et al., 1994[22] | 32 | 49.0 | 0.81 | 33.0 | 28.0 | 4 | 3.7 |
| Engleman et al., 1997[20] | 16 | 52.0 | 1.00 | 29.8 | 11.0 | 4 | 2.8 |
| Engleman et al., 1999[21] | 23 | 47.0 | 0.91 | 30.0 | 43.0 | 4 | 2.8 |
| Gast et al., 2006[30] | 29 (17/12) | 52.3 | 1.00 | 37.1 | 43.1 | 1 | – |
| Engleman et al., 1998[26] | 34 | 44.0 | 0.62 | 30.0 | 10.0 | 4 | 2.8 |
| Kushida et al., 2012[31] | 1098 (556/542) | 51.5 | 0.65 | 32.3 | 40.1 | 24 | 3.8 |
| Marshall et al., 2005[24] | 29 | 50.5 | 0.76 | 31.5 | 22.0 | 2 | 4.9 |
| Monasterio et al., 2001[25] | 125 (66/59) | 53.5 | 0.54 | 29.4 | 20.5 | 24 | 4.8 |
| Pelletier–Fleury et al., 2004[29] | 171 (82/89) | 52.9 | 0.70 | 30.1 | 53.2 | 24 | 5.4 |
| Prilipko et al., 2012[32] | 17 (9/8) | 43.2 | 1.00 | 27.8 | 39.7 | 8 | – |
| Total | 1744 | 51.2 | 0.68 | 31.6 | 38.5 | 20.3 | – |
n: Number of participants; E: CPAP treatment group; C: Control group; Male (%): Ratio of male in all the participants; AHI: Apnea/hypopnea index; CPAP: Continuous positive airway pressure.
The total number was 1744, and males accounted for 68%. The participants were middle-aged (mean age, 51.2 years), obese (mean BMI, 31.6 kg/m2) and had severe OSAS (mean AHI, 38.5). The average OSAS severity was mild in 2 studies,[20,26] moderate in 4,[22,23,24,25] and severe in 7.[21,27,28,29,30,31,32] The mean CPAP duration was 20.3 weeks (range: 1–24 weeks). CPAP use was considered as compliant when usage >2.9 h/night (>4 h/night for >5 days/week equaled 2.9 h/night). Therefore, 8 studies were compliant,[22,23,24,25,27,28,29,31] and 3 were almost complained.[20,21,26] As for the other 2 studies,[30,32] 1[30] showed a mean CPAP usage of >5 h/night in 47% of the participants. The another study[32] showed a mean CPAP of more than 4 h/night in 59% of the days.
Table 3 shows the baseline demographics of the CPAP and control groups. Six studies[20,21,22,23,24,26] had a cross-over design, in which all of the participants received CPAP and control treatments in random order. Thus, the CPAP and control group demographics were the same. No significant difference was found except for BMI in one study.[32] The another study[28] contained 29 men and 7 women in total but did not show the sex ratio in each group.
Table 3.
Baseline demographics of CPAP treatment and control groups of the included studies
| Studies | CPAP demographics | Control demographics | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Age (years) | Male (%) | BMI (kg/m2) | AHI (events/h) | n | Age (years) | Male (%) | BMI (kg/m2) | AHI (events/h) | |
| Barbé et al., 2001[27] | 29 | 54 | 89.7 | 29.0 | 30.0 | 25 | 52 | 92.0 | 29.0 | 34.0 |
| Bardwell et al., 2001[28] | 20 | 47 | – | 32.8 | 56.8 | 16 | 48 | – | 29.6 | 43.6 |
| Barnes et al., 2004[23] | 80 | 47 | 79.8 | 31.1 | 21.3 | 80 | 47 | 79.8 | 31.1 | 21.3 |
| Engleman et al., 1994[22] | 32 | 49 | 81.2 | 33.0 | 28.0 | 32 | 49 | 81.2 | 33.0 | 28.0 |
| Engleman et al., 1997[20] | 16 | 52 | 75.0 | 29.8 | 11.0 | 16 | 52 | 75.0 | 29.8 | 11.0 |
| Engleman et al., 1999[21] | 23 | 47 | 91.3 | 30.0 | 43.0 | 23 | 47 | 91.3 | 30.0 | 43.0 |
| Gast et al., 2006[30] | 17 | 52 | 100 | 40.0 | 45.5 | 12 | 52 | 100 | 33.0 | 39.7 |
| Engleman et al., 1998[26] | 34 | 44 | 61.8 | 30.0 | 10.0 | 34 | 44 | 61.8 | 30.0 | 10.0 |
| Kushida et al., 2012[31] | 556 | 52.2 | 65.3 | 32.4 | 39.7 | 542 | 50.8 | 65.7 | 32.1 | 40.6 |
| Marshall et al., 2005[24] | 29 | 50.5 | 76.9 | 31.5 | 21.6 | 29 | 50.5 | 76.9 | 31.5 | 21.6 |
| Monasterio et al., 2001[25] | 66 | 53 | 81.0 | 29.4 | 220.0 | 59 | 54 | 91 | 29.5 | 21.0 |
| Pelletier–Fleury et al., 2004[29] | 82 | 53.8 | 84.3 | 30.5 | 55.6 | 89 | 52.1 | 80.5 | 29.7 | 49.0 |
| Prilipko et al., 2012[32] | 9 | 44.7 | 100 | 29.9* | 45.8 | 8 | 41.6 | 100 | 25.5 | 32.8 |
*Meant P<0.05 between CPAP and control groups; n: Number of the participants in the group; Male (%): Ratio of male in all the participants. BMI: Body mass index; AHI: Apnea/hypopnea index; CPAP: Continuous positive airway pressure.
A risk of bias graph assessed the bias risk in each study [Figure 2]. Three studies[24,27,31] had negligible risk of bias, 7 studies[20,21,22,23,25,28,32] had small risk, and 3 studies[26,29,30] had considerable risk. Thus, most of the included studies had a satisfactory RCT design.
Figure 2.
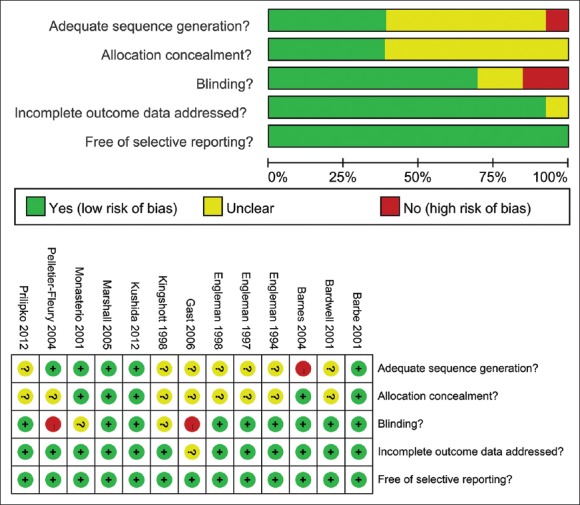
Risk of bias graph-review authors’ judgments about each “risk of bias” item presented as percentages across all included studies.
Effect size
The effect size was calculated for each cognitive domain with the above formulas [Table 4]. As mentioned above, a negative d indicated better performance with CPAP treatment. The effect sizes of each domain were the following: Attention, −0.10 (P = 0.24); vigilance, −0.12 (P = 0.04); processing speed, −0.08 (P = 0.16); working memory, 0.00 (P = 0.95); memory, −0.04 (P = 0.30); verbal fluency, −0.06 (P = 0.34), and visuoconstructive skills, −0.01 (P = 0.92). Thus, CPAP had beneficial and statistically significant effects on vigilance impairment. CPAP improved attention, processing speed, memory, verbal fluency, and visuoconstructive skills, but the data were inadequate because the differences between the CPAP and control groups were not significant in these domains. The forest plot for attention, vigilance, processing speed, working memory, memory, verbal fluency, and visuoconstructive skill domains are shown in Figure 3.
Table 4.
Mean weighted effect sizes, CI and heterogeneity for each cognitive domain
| Cognitive domain | K | n | d | 95% CI | P (d) | χ2 | P (χ2) | I2 (%) |
|---|---|---|---|---|---|---|---|---|
| Attention | 11 | 1598 | −0.10 | −0.27–0.07 | 0.24 | 66.46 | <0.001 | 85 |
| Vigilance | 8 | 375 | −0.12 | −0.23–−0.01 | 0.04* | 7.86 | 0.34 | 11 |
| Processing speed | 8 | 400 | −0.08 | −0.20–0.03 | 0.16 | 8.80 | 0.27 | 20 |
| Working memory | 7 | 1439 | 0.00 | −0.14–0.15 | 0.95 | 19.76 | 0.003 | 70 |
| Memory | 6 | 1345 | −0.04 | −0.11–0.04 | 0.30 | 5.57 | 0.35 | 10 |
| Verbal fluency | 5 | 280 | −0.06 | −0.19–0.07 | 0.34 | 4.57 | 0.33 | 12 |
| Visuoconstructive skills | 3 | 182 | −0.01 | −0.15–0.14 | 0.92 | 1.20 | 0.55 | 0 |
*Statistical significance between CPAP and control. K: Number of studies including this cognitive domain; n: Number of participants included in the assessed cognitive domain; d: Effect size calculated for the cognitive domain; 95% CI: 95% confidence interval of d; P (d): P value of d for each domain; χ2: Within domain heterogeneity; P (χ2): P value of χ2; I2: Percentage of heterogeneity; CPAP: Continuous positive airway pressure.
Figure 3.
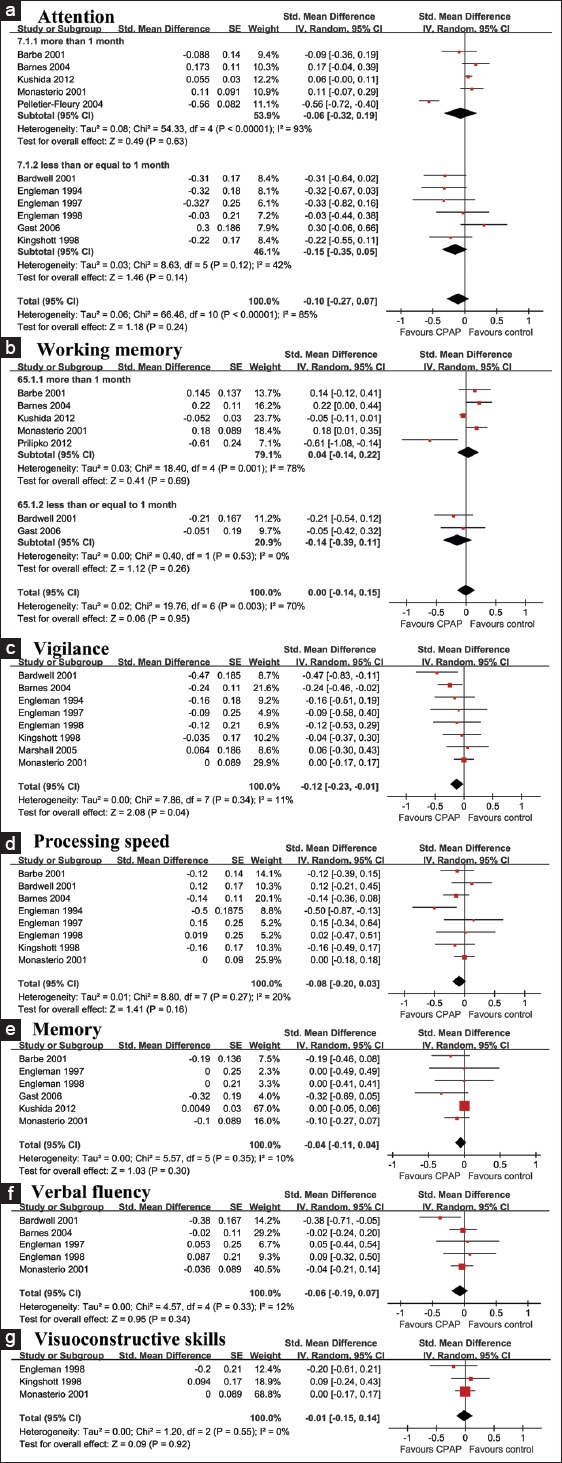
Forest plots of attention (a), working memory (b), vigilance (c), processing speed (d), memory (e), verbal fluency (f), and visuoconstructive skills (g).
Publication bias
We conducted funnel analyses in all of the cognitive domains and did not find obvious asymmetry in any of the funnel plots. Thus, publication bias was not indicated in the selected studies. The funnel plot for vigilance is shown in Figure 4.
Figure 4.
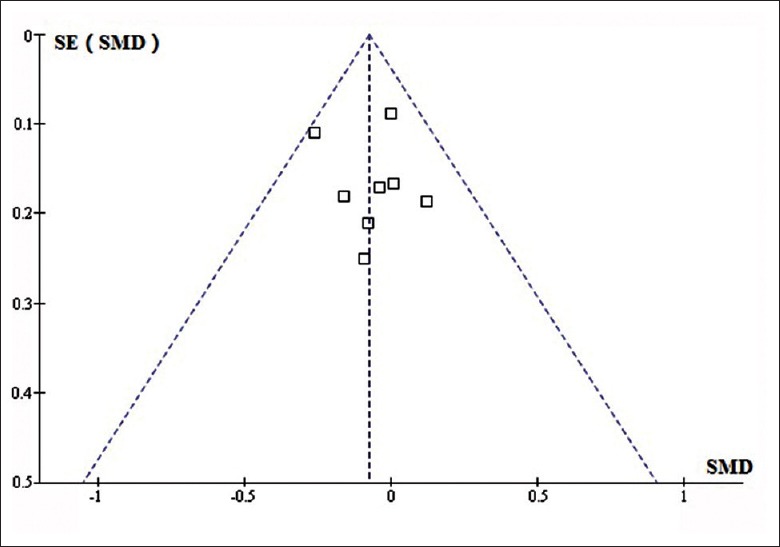
Funnel plot for vigilance domain. The effect estimates on the horizontal scale, and the measure of study size on the vertical axis.
DISCUSSION
CPAP treatment, which is the standard treatment of OSAS, is thought to substantially improve cognitive impairments in patients with OSAS. However, the small treatment effect observed in the current quantitative meta-analysis did not support this positive conclusion drawn in previous reviews.
The reliability of the meta-analysis depends on the quality of the included studies. All of the studies were RCTs. A Jadad rating score was used to assess the quality and a risk of bias graph was used to assess the risk of bias in each study. We observed that 3 studies[24,27,31] had negligible risk of bias, 7 studies[20,22,23,25,26,28,32] had small risk, and 3 studies[21,29,30] had considerable risk. All of the studies had a relatively high Jadad score, which indicated high quality of the studies. Thus, most of the included studies had a satisfactory RCT design.
A dose-response relationship between the length of CPAP treatment and the level of cognitive improvement has been suggested.[33,34] We divided the studies into two subgroups according to treatment duration (subgroup 1: >1-month; subgroup 2: <1-month). We did not find a significant change in the effect size, P (d), or I2 in any cognitive domain, and considerable heterogeneity was observed in the effect size in working memory and attention before and after the subgroup analysis. Considering the diversity of OSAS severity, we performed another subgroup analysis for mean AHI (mild to moderate: <30 events/h, severe: >30 events/h), but no notable change in heterogeneity or effect size was found in any cognitive domain (data not shown). The unavoidable heterogeneity supported the use of random-effects statistical models and suggested the presence of other unanticipated determinants of effect size.
We performed a sensitivity analysis to evaluate the analysis stability. After removing Gast et al.,[30] I2(attention, subgroup 2) decreased from 42% to 0%, and d changed from −0.15 (P = 0.14) to −0.25 (P = 0.004). After deleting any one of the remaining studies,[20,21,22,23,24,25,26,27,28,29,30,31,32] no results, including heterogeneity and effect size, changed significantly. Thus, this analysis was relatively stable.
Our analysis had some limitations. First, we found unavoidable substantial heterogeneity in working memory and attention, even after a subgroup analysis for treatment duration and OSAS severity. Our belief that AHI was the best index of OSAS severity was empirical and lacked solid evidence. Other factors, such as obstructive event duration, oxygen desaturation degree, or arousal frequency, might be more suitable for representing OSAS severity. Thus, our subgroup analysis that was based on AHI might be slightly inaccurate. More studies are necessary to explore the disease features. The included studies covered a long time range (from 1994 to 2012), and the scoring and diagnosing of OSAS changed after the introduction of the nasal cannula pressure transducer, which might have introduced heterogeneity to the results. In addition, the selected studies were placebo-controlled, but the placebo included oral placebo, sham CPAP, and no treatment, which might have resulted in unpredictable effects on the results.
Second, we calculated the effect size from the original study data, which were not adjusted for age, gender, race, occupation, treatment compliance, premorbid intelligence, or education. These possible confounders were not recorded in detail, except for age, race, and compliance. All of the participants were middle-aged, and most participants were Caucasian. Most of the studies were considered as compliant, such that the recorded possible confounders were consistent in most studies while many other important confounders were absent and could not be evaluated properly. Further studies should record this data for a thorough understanding of cognitive dysfunction in OSAS and the resulting improvements of CPAP treatment.
Third, more than one neuropsychological test often assessed each cognitive domain. We divided cognition into 7 domains and incorporated different neuropsychological tests in each, but the lack of specificity of the tests might have influenced the reliability of the conclusions. More specific neuropsychological tests should be explored to produce more credible results.
Fourth, we included 6 cross-over studies,[20,21,22,23,24,26] and only 2[23,24] had a washout period (both were 2 weeks). Yet, in all of the cross-over studies, statistical analysis was conducted to check whether a significant interaction of treatment had occurred. All of the cross-over studies in our analysis either employed a design to minimize possible carry-over effects or statistically adjusted for such effects. Thus, this design issue seemed to not affect our results.
Fifth, the current meta-analysis had rigorous inclusion and exclusion criteria, which ensured the quality of the included studies to the greatest extent. However, strict criteria might miss valuable information, such as studies comparing CPAP with surgery (tonsillectomy, uvulopalatopharyngoplasty, laser-assisted uvulopalatopharyngoplasty, etc.), oral appliances, postural therapy, and other positive airway therapies, and studies aimed at improving CPAP compliance with behavioral approaches compared with regular CPAP therapy.
Finally, the present findings were based on group averages. Any patient might show neuropsychological characteristics that differ from the average because of his or her unique genetic, medical, environmental, and historical circumstances. Thus, we should consider every single patient separately in clinical practice.
Despite the limitations, this meta-analysis provides valuable evidence for clinicians about the use of CPAP in middle-aged patients with OSAS and cognitive deficits. The reliability of the meta-analysis results from: (1) The comprehensive literature search, including Chinese databases, over a long time range; (2) Large number of participants; (3) Rigorous inclusion and exclusion criteria based on guidelines and standard neuropsychological tests; (4) Subdivision of cognition into seven domains; (5) Balanced baseline demographics in the CPAP and control groups; (6) Subgroup analysis based on AHI and CPAP duration; (7) Sensitivity analysis of the stability of the analysis; (8) High quality of the included studies with high Jadad scores and negligible risk of bias; and (9) Little publication bias with symmetric funnel plots.
According to the quantitative analysis, we concluded that CPAP partially improved cognitive dysfunction, especially vigilance, in middle-aged patients with OSAS. And CPAP might improve attention, processing speed, working memory, memory, verbal fluency, and visuoconstructive skills despite the absence of statistical significance. Further rigorous RCT studies with a focus on possible confounders are needed.
Financial support and sponsorship
This work was supported by a grant from the National Natural Science Foundation of China (No. 81370185).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yuan-Yuan Ji
REFERENCES
- 1.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: A population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 2.Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet. 2014;383:736–47. doi: 10.1016/S0140-6736(13)60734-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aloia MS, Arnedt JT, Davis JD, Riggs RL, Byrd D. Neuropsychological sequelae of obstructive sleep apnea-hypopnea syndrome: A critical review. J Int Neuropsychol Soc. 2004;10:772–85. doi: 10.1017/S1355617704105134. [DOI] [PubMed] [Google Scholar]
- 4.Shpirer I, Elizur A, Shorer R, Peretz RB, Rabey JM, Khaigrekht M. Hypoxemia correlates with attentional dysfunction in patients with obstructive sleep apnea. Sleep Breath. 2012;16:821–7. doi: 10.1007/s11325-011-0582-1. [DOI] [PubMed] [Google Scholar]
- 5.Bawden FC, Oliveira CA, Caramelli P. Impact of obstructive sleep apnea on cognitive performance. Arq Neuropsiquiatr. 2011;69:585–9. doi: 10.1590/s0004-282x2011000500003. [DOI] [PubMed] [Google Scholar]
- 6.Tippin J, Sparks J, Rizzo M. Visual vigilance in drivers with obstructive sleep apnea. J Psychosom Res. 2009;67:143–51. doi: 10.1016/j.jpsychores.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salorio CF, White DA, Piccirillo J, Duntley SP, Uhles ML. Learning, memory, and executive control in individuals with obstructive sleep apnea syndrome. J Clin Exp Neuropsychol. 2002;24:93–100. doi: 10.1076/jcen.24.1.93.973. [DOI] [PubMed] [Google Scholar]
- 8.Lis S, Krieger S, Hennig D, Röder C, Kirsch P, Seeger W, et al. Executive functions and cognitive subprocesses in patients with obstructive sleep apnoea. J Sleep Res. 2008;17:271–80. doi: 10.1111/j.1365-2869.2008.00660.x. [DOI] [PubMed] [Google Scholar]
- 9.Saunamäki T, Himanen SL, Polo O, Jehkonen M. Executive dysfunction and learning effect after continuous positive airway pressure treatment in patients with obstructive sleep apnea syndrome. Eur Neurol. 2010;63:215–20. doi: 10.1159/000278301. [DOI] [PubMed] [Google Scholar]
- 10.Marshall NS, Barnes M, Travier N, Campbell AJ, Pierce RJ, McEvoy RD, et al. Continuous positive airway pressure reduces daytime sleepiness in mild to moderate obstructive sleep apnoea: A meta-analysis. Thorax. 2006;61:430–4. doi: 10.1136/thx.2005.050583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDaid C, Griffin S, Weatherly H, Durée K, van der Burgt M, van Hout S, et al. Continuous positive airway pressure devices for the treatment of obstructive sleep apnoea-hypopnoea syndrome: A systematic review and economic analysis. Health Technol Assess. 2009;13:iii–iv. doi: 10.3310/hta13040. xi-xiv, 1-119, 143-274. [DOI] [PubMed] [Google Scholar]
- 12.Kylstra WA, Aaronson JA, Hofman WF, Schmand BA. Neuropsychological functioning after CPAP treatment in obstructive sleep apnea: A meta-analysis. Sleep Med Rev. 2013;17:341–7. doi: 10.1016/j.smrv.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Weaver TE, Chasens ER. Continuous positive airway pressure treatment for sleep apnea in older adults. Sleep Med Rev. 2007;11:99–111. doi: 10.1016/j.smrv.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sánchez AI, Martínez P, Miró E, Bardwell WA, Buela-Casal G. CPAP and behavioral therapies in patients with obstructive sleep apnea: Effects on daytime sleepiness, mood, and cognitive function. Sleep Med Rev. 2009;13:223–33. doi: 10.1016/j.smrv.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Lezak MD, Howieson DB, Loring DW. 4th ed. New York: Oxford University Press; 2006. Neuropsychological Assessment. [Google Scholar]
- 16.Julian PT, Higgins SG, Acquadro C, Acquadro C, Alderson P, Altman DG, et al. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration. (5th ed) 2011 [Google Scholar]
- 17.Lim W, Bardwell WA, Loredo JS, Kim EJ, Ancoli-Israel S, Morgan EE, et al. Neuropsychological effects of 2-week continuous positive airway pressure treatment and supplemental oxygen in patients with obstructive sleep apnea: A randomized placebo-controlled study. J Clin Sleep Med. 2007;3:380–6. [PMC free article] [PubMed] [Google Scholar]
- 18.Lee IS, Bardwell WA, Kamat R, Tomfohr L, Heaton RK, Ancoli-Israel S, et al. A model for studying neuropsychological effects of sleep intervention: The effect of 3-week continuous positive airway pressure treatment. Drug Discov Today Dis Models. 2011;8:147–54. doi: 10.1016/j.ddmod.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barnes M, Houston D, Worsnop CJ, Neill AM, Mykytyn IJ, Kay A, et al. A randomized controlled trial of continuous positive airway pressure in mild obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:773–80. doi: 10.1164/ajrccm.165.6.2003166. [DOI] [PubMed] [Google Scholar]
- 20.Engleman HM, Martin SE, Deary IJ, Douglas NJ. Effect of CPAP therapy on daytime function in patients with mild sleep apnoea/hypopnoea syndrome. Thorax. 1997;52:114–9. doi: 10.1136/thx.52.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engleman HM, Kingshott RN, Wraith PK, Mackay TW, Deary IJ, Douglas NJ. Randomized placebo-controlled crossover trial of continuous positive airway pressure for mild sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159:461–7. doi: 10.1164/ajrccm.159.2.9803121. [DOI] [PubMed] [Google Scholar]
- 22.Engleman HM, Martin SE, Deary IJ, Douglas NJ. Effect of continuous positive airway pressure treatment on daytime function in sleep apnoea/hypopnoea syndrome. Lancet. 1994;343:572–5. doi: 10.1016/s0140-6736(94)91522-9. [DOI] [PubMed] [Google Scholar]
- 23.Barnes M, McEvoy RD, Banks S, Tarquinio N, Murray CG, Vowles N, et al. Efficacy of positive airway pressure and oral appliance in mild to moderate obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:656–64. doi: 10.1164/rccm.200311-1571OC. [DOI] [PubMed] [Google Scholar]
- 24.Marshall NS, Neill AM, Campbell AJ, Sheppard DS. Randomised controlled crossover trial of humidified continuous positive airway pressure in mild obstructive sleep apnoea. Thorax. 2005;60:427–32. doi: 10.1136/thx.2004.032078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monasterio C, Vidal S, Duran J, Ferrer M, Carmona C, Barbé F, et al. Effectiveness of continuous positive airway pressure in mild sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med. 2001;164:939–43. doi: 10.1164/ajrccm.164.6.2008010. [DOI] [PubMed] [Google Scholar]
- 26.Engleman HM, Martin SE, Kingshott RN, Mackay TW, Deary IJ, Douglas NJ. Randomised placebo controlled trial of daytime function after continuous positive airway pressure (CPAP) therapy for the sleep apnoea/hypopnoea syndrome. Thorax. 1998;53:341–5. doi: 10.1136/thx.53.5.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barbé F, Mayoralas LR, Duran J, Masa JF, Maimó A, Montserrat JM, et al. Treatment with continuous positive airway pressure is not effective in patients with sleep apnea but no daytime sleepiness. A randomized, controlled trial. Ann Intern Med. 2001;134:1015–23. doi: 10.7326/0003-4819-134-11-200106050-00007. [DOI] [PubMed] [Google Scholar]
- 28.Bardwell WA, Ancoli-Israel S, Berry CC, Dimsdale JE. Neuropsychological effects of one-week continuous positive airway pressure treatment in patients with obstructive sleep apnea: A placebo-controlled study. Psychosom Med. 2001;63:579–84. doi: 10.1097/00006842-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Pelletier-Fleury N, Meslier N, Gagnadoux F, Person C, Rakotonanahary D, Ouksel H, et al. Economic arguments for the immediate management of moderate-to-severe obstructive sleep apnoea syndrome. Eur Respir J. 2004;23:53–60. doi: 10.1183/09031936.03.00066903. [DOI] [PubMed] [Google Scholar]
- 30.Gast H, Schwalen S, Ringendahl H, Jörg J, Hirshkowitz M. Sleep-related breathing disorders and continuous positive airway pressure-related changes in cognition. Sleep Med Clin. 2006;1:499–511. [Google Scholar]
- 31.Kushida CA, Nichols DA, Holmes TH, Quan SF, Walsh JK, Gottlieb DJ, et al. Effects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: The Apnea Positive Pressure Long-term Efficacy Study (APPLES) Sleep. 2012;35:1593–602. doi: 10.5665/sleep.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prilipko O, Huynh N, Schwartz S, Tantrakul V, Kushida C, Paiva T, et al. The effects of CPAP treatment on task positive and default mode networks in obstructive sleep apnea patients: An fMRI study. PLoS One. 2012;7:e47433. doi: 10.1371/journal.pone.0047433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferini-Strambi L, Baietto C, Di Gioia MR, Castaldi P, Castronovo C, Zucconi M, et al. Cognitive dysfunction in patients with obstructive sleep apnea (OSA): Partial reversibility after continuous positive airway pressure (CPAP) Brain Res Bull. 2003;61:87–92. doi: 10.1016/s0361-9230(03)00068-6. [DOI] [PubMed] [Google Scholar]
- 34.Muñoz A, Mayoralas LR, Barbé F, Pericás J, Agusti AG. Long-term effects of CPAP on daytime functioning in patients with sleep apnoea syndrome. Eur Respir J. 2000;15:676–81. doi: 10.1034/j.1399-3003.2000.15d09.x. [DOI] [PubMed] [Google Scholar]


