Abstract
Background:
The spectrum of abnormal behaviors in amyotrophic lateral sclerosis/motor neuron disease (ALS/MND) has been described, but its practical meaning, namely its impact on caregiver burden, has not been clearly documented in Chinese population. This study aimed to assess the distribution of abnormal behaviors in Chinese population, and to analyze the relationship between behavior changes and caregiver burden.
Methods:
Sixty-five patients with ALS/MND have been consecutively enrolled into registry platform of Peking Union Medical College Hospital. An investigation was performed to these patients and their caregivers using the revised ALS function rating scale, Frontal Behavioral Inventory-ALS version, the Frontal Assessment Battery, and the Caregiver Burden Inventory.
Results:
Twenty-eight (43.1%) patients displayed abnormal behaviors of varying degrees, with one fulfilling the diagnostic criteria of frontotemporal lobe degeneration. Irritability, logopenia, and inflexibility ranked top 3 of abnormal behavior list. Correlation analysis revealed that the degree of behavioral change and frontal cognitive status were significantly associated with caregiver burden, with more extensive impact from disinhibitive behaviors. Analysis of covariance analysis showed that after associated factors were corrected, caregivers of patients with moderate to severe behavior change reported significantly heavier developmental burden, physical burden, and total burden than those with no behavioral change.
Conclusions:
Neurobehavioral symptoms could present in around 40% of Chinese patients with ALS/MND, and the distribution of these behaviors was also unique. Besides, abnormal behaviors were highly related to caregivers’ burden.
Keywords: Amyotrophic Lateral Sclerosis, Behavioral Symptoms, Caregiver Burden, Motor Neuron Disease
INTRODUCTION
Amyotrophic lateral sclerosis (ALS) is a fatal progressive disorder with median survival time of 3–5 years, clinically characterized by variable combinations of dysarthria, muscle atrophy, limb weakness, and pyramidal signs. Despite predominant manifestation of the physical disability, ALS belongs to a neurodegenerative spectrum beyond motor system. Behavioral changes could be frequently observed in the context of ALS, including perseveration, apathy, disinhibition, and so on.[1] These symptoms apparently exceed the range of depression and anxiety. Frontal lobe involvement serves as a more appropriate explanation: Sharing common pathological basis with frontotemporal lobe degeneration (FTLD), ALS patients could display similar pattern of cognitive and/or behavioral impairment of varying degrees, with 10–15% fulfilling the diagnostic criteria of FTLD.[2,3,4] Besides these frontal behavioral abnormalities, psychotic symptoms such as delusion and hallucination are often of predictive values for the disease complex ALS-FTLD and a specific pathogenic mutation though they are relatively rare in this disease spectrum.[5,6]
Stress and psychological status of ALS caregivers have been shown to be significantly associated with physical disability and negative mood of patients,[7,8] but they are not the only source for caring burden. Due to the fact that the caregivers’ burden of FTLD was highly correlated with the rate of behavioral changes,[9,10] it is reasonable to hypothesize that behavioral symptoms of ALS could impose extra burden to their caregivers, making this phenomenon more than an academic interest.
Genetic background and epidemiological features of ALS patients have been well-depicted in Chinese population.[11,12] Their unique onset age peak and genetic basis give rise to the necessity of performing a neuropsychiatric investigation to them, considering the relationship between clinical phenotype and genotype.[13] Behavioral symptoms have not been illustrated in detail among Chinese patients, and thus their impact on ALS caregivers have not been analyzed either. Under the circumstance of deficiency in specific treatment, it seems to be a practical strategy to help caregivers cope with caring stress and in turn improve the life quality of their patients and themselves. Therefore, the aims of this study were: (1) To assess the distribution of abnormal behaviors in Chinese population; (2) to analyze the relationship between behavior changes and caregiver burden.
METHODS
Subjects
This study was accomplished on the basis of the ongoing registry platform of Peking Union Medical College Hospital for ALS and other motor neuron disease (MND).[11,14] Patients’ medical history was recorded in detail, and all of them underwent neurological, laboratory, neuroimaging, and electrophysiological examinations. The diagnosis was made case-by-case by three ALS/MND specialists after systematically analyzing patients’ medical history and results of the above examinations. Demographic and clinical information including age, gender, level of education, site of symptom onset, disease duration (defined as time lapse between symptom onset and time of evaluation) were collected.
Registered patients were investigated behaviorally, and their caregivers were invited to participate in our research for caregiver burden. Primary caregivers of ALS in the present study referred to those who provided patients with the most care and assistance. Patients were not included into analysis for those with history of other neurological conditions possibly impacting on neuropsychiatric evaluation (major stroke, traumatic brain injury, learning disability, and severe active epilepsy), alcohol-dependence, drug-dependence, severe active mental illness, and use of high-dose psychoactive medication or invasive ventilation support, neither for those accompanied by paid nor underage caregivers. This study was approved by the Research Ethics Committee of Peking Union Medical College Hospital. Patients and their caregivers were included after informed written consent had been obtained, as set forth by the Declaration of Helsinki.
Physical function evaluation
General function status was assessed by the revised ALS functional rating scale (ALSFRS-R), a 12-item scale evaluating bulbar function, fine movement, gross movement, and respiratory function.[15] Each item scored 0 to 4, with a total score of 48 denoting normal function. To further evaluate bulbar function, we then applied swallowing subscale of the ALS severity scale (ALSSS), scoring from 0 to 10, with a lower score indicating worse swallowing function.[16]
Behavioral and cognitive evaluation
The evaluation of personality and behavior change were made through the interview with caregivers about the patients’ daily performance and the administration of the Frontal Behavioral Inventory-ALS version (FBI-ALS). It is a scale originally designed to screen behavioral variant FTLD (bvFTD) and afterward revised for availability to ALS patients by adding scripted questions to distinguish behavioral symptoms from physical disability.[17] It contains 2 subscales, negative and disinhibitive, each including 12 items scoring 0 to 3, making total score range from 0 to 72. Based on previous study conducted with the original version FBI, a total score of zero suggested no behavior change, whereas a total score from 1 to 3, 4 to 14, and ≥15 suggested mild, moderate, and severe behavior change, respectively.[18,19]
The frontal cognitive function was evaluated by the Frontal Assessment Battery (FAB).[20] It contains 6 subsets, namely, similarities, lexical fluency, motor series, conflicting instructions, inhibitory control, and prehension behavior, with each one correlating with medial and dorsolateral frontal lobe activity. Age and education level (years of schooling) have significant effects on performance, original scores were adjusted using the following, previously-reported formula: Adjusted FAB score = raw FAB score − 1.43× [log (100 – age) – 3.65] – 0.98× [square root (years of education) – 3.15].[21]
Demographic characteristics and burden of amyotrophic lateral sclerosis caregivers
Demographic variables of caregivers including age, gender, level of education, and relationship with patients were collected. Caregiver Burden Inventory (CBI) was used to evaluate caring burden quantitatively. It is a 24-item questionnaire of 5 dimensions, time dependence burden, developmental burden, physical burden, social burden, and emotional burden. Its total score ranges from 0 to 96 with each item scoring from 0 to 4, and the total scored increases with caregivers’ burden becoming heavier.[22]
Statistical analysis
Continuous values were shown as mean ± standard deviation (SD) or median and those category ones were in proportion. In the comparison of demographic and clinical variables between different groups, we adopted the one-way analysis of variance (ANOVA) for continuous variables and Chi-square tests for categorical variables. When data failed to meet the criteria for parametric analysis, the nonparametric analysis was applied instead.
Pearson's correlation analysis was adopted to explore the relationship among the adjusted FAB score, FBI-ALS score (total score and its two subsets), and the caregiver burden. Furthermore, multiple one-way analysis of covariances (ANCOVAs) were undertaken to compare the caregiver burden between groups using four covariates: ALSFRS-R, adjusted FAB score, age of patients, and their caregivers.
All tests were two-tailed. Statistical significance was set at P < 0.05. Bonferroni correction was applied to adjust the α value in case of post-hoc analysis and where multiple comparisons were undertaken. Statistical analyses were carried out using SPSS 11.5 software (SPSS Inc., Chicago, IL, USA).
RESULTS
From February 1, 2014 to December 31, 2014, 118 patients were enrolled into registry platform for ALS and other MND, and caregivers of 71 patients were willing to respond to our investigation of caring burden. Then 4 were excluded because they were not primary caregivers, and 2 were excluded due to not finishing the questionnaire, making the remaining 65 into the final analysis. Among them, 56 (86.2%) patients fulfilled the revised El Escorial diagnostic criteria for clinical definite, probable, and lab-supported probable ALS,[23] whereas the other 9 (13.8%) patients without upper motor neuron signs were diagnosed as progressive muscular atrophy.[24] All of them were sporadic cases because none of them had a family history of ALS/MND or dementia. The mean age of included patients was 50.9 ± 9.8 years old, with median disease duration of 12 months (interquartile range 7–20 months). This cohort was mainly composed of male patients and limb-onset type, accounting for 69.2% and 76.9%, respectively. At baseline, median ALSFRS-R score was 42 (interquartile range 36–44), and median ALSSS swallowing score was 10 (interquartile range 9–10). At the time of diagnosis, no one had gastrostomy or noninvasive ventilation. The mean age of patients’ caregivers was 41.5 ± 11.9 years old. They were consisted of 38 (56.9%) spouses, 24 (36.9%) sons or daughters, 2 (3.1%) siblings, and 1 (1.5%) parent.
In the spectrum of abnormal behaviors, irritability ranked the top of the list with 16 patients (24.6%), followed by logopenia (13 patients, 20.0%) and inflexibility (10 patients, 15.4%) [Figure 1]. Categorized behaviorally, 37 patients (56.9%) displayed no behavioral change and scored 0, and those with mild, moderate, and severe behavioral change were 16 (24.6%), 11 (16.9%), and 1 (1.5%), respectively. Besides, the only one patient with severe behavior change fulfilled the diagnostic criteria of bvFTD.[25] The three behavioral groups (patients with no change, patients with mild change, and patients with moderate to severe change) did not differ significantly in any clinical and sociodemographical variables except the swallowing subscale of ALSSS (P = 0.022) [Table 1]. A post-hoc analysis revealed that the difference between the group with moderate to severe change and those with no change remained to be significant (P = 0.014, adjusted α = 0.05/3 = 0.017). Frontal cognitive status (adjusted FAB score) was not significantly different among these three groups [Table 1].
Figure 1.
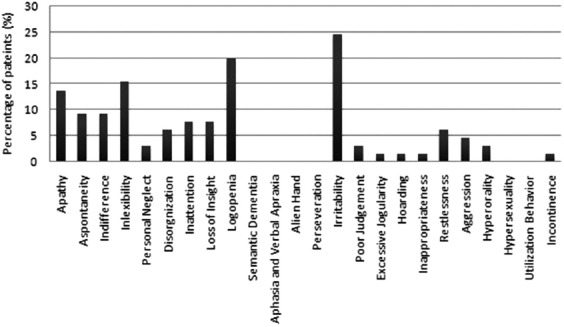
Proportion of ALS/MND patients with changes of each frontal behavior domain of the FBI-ALS. ALS: Amyotrophic lateral sclerosis; MND: Motor neuron disease; FBI-ALS: Frontal Behavioral Inventory-version for amyotrophic lateral sclerosis.
Table 1.
Sociodemographic and clinical characteristics of ALS/MND patients categorized by the degree of behavioral change
| Characteristics | No change (n = 37) | Mild change (n = 16) | Moderate to severe change (n = 12) | P |
|---|---|---|---|---|
| Age, years, mean ± SD | 49.1 ± 9.7 | 50.7 ± 9.8 | 56.8 ± 8.2 | 0.058 |
| Education level, years, mean ± SD | 10.8 ± 3.7 | 11.1 ± 3.6 | 10.3 ± 2.0 | 0.853 |
| Male, % | 64.9 | 68.8 | 83.3 | 0.484 |
| Onset type (bulbar), % | 18.9 | 18.8 | 41.7 | 0.239 |
| Revised El Escorial criteria classification, n | 0.110 | |||
| Clinically definite ALS | 7 | 3 | 2 | |
| Clinically probable ALS | 14 | 2 | 7 | |
| Clinically probable ALSlaboratory supported | 9 | 9 | 3 | |
| Progressive muscular atrophy | 7 | 2 | 0 | |
| Swallowing subscale of ALSSS, mean ± SD | 9.4 ± 1.0 | 9.3 ± 1.7 | 8.3 ± 1.4 | 0.022 |
| ALSFRS-R score, mean ± SD | 39.9 ± 4.5 | 39.5 ± 6.4 | 39.9 ± 5.0 | 0.862 |
| Disease duration, months, mean ± SD | 16.8 ± 15.4 | 14.6 ± 9.0 | 11.0 ± 5.9 | 0.369 |
| Progression rate, mean ± SD | 0.6 ± 0.4 | 0.6 ± 0.3 | 0.9 ± 0.6 | 0.207 |
| Adjusted FAB score, mean ± SD | 14.2 ± 2.0 | 14.7 ± 1.8 | 14.0 ± 2.2 | 0.602 |
| Age of caregivers, years, mean ± SD | 41.2 ± 12.9 | 40.6 ± 11.4 | 43.7 ± 10.1 | 0.728 |
| Caregivers’ relationship with patients, n | 0.875 | |||
| Spouses | 21 | 10 | 7 | |
| Sons or daughters | 13 | 6 | 5 | |
| Siblings | 2 | 0 | 0 | |
| Parent | 1 | 0 | 0 | |
| Education level of caregivers, n | 0.107 | |||
| Less than preliminary school | 0 | 2 | 1 | |
| Preliminary school | 0 | 2 | 1 | |
| Middle school | 22 | 5 | 5 | |
| College | 15 | 7 | 5 |
SD: Standard deviation; ALS: Amyotrophic lateral sclerosis; ALSSS: Amyotrophic lateral sclerosis severity scale; ALSFRS-R: Revised version of amyotrophic lateral sclerosis function rating scale; FAB: Frontal assessment battery; MND: Motor neuron disease; progression rate was defined as (48-ALSFRS-R)/disease duration.
Pearson's correlation analysis showed that scores of FBI-ALS disinhibitive were significantly associated with total CBI score (P = 0.008), developmental burden (P = 0.048), physical burden (P = 0.010), social burden (P = 0.026), and emotional burden (P = 0.003), while scores of FBI-ALS negative were only significantly associated with developmental burden (P = 0.030) and physical burden (P = 0.002). Besides, adjusted FAB score was significantly associated with all FBI-ALS subsets and total score [Table 2].
Table 2.
Pearson's correlation coefficients between behavioral symptoms of ALS/MND and CBI domain
| Items | FBI-ALS negative | FBI-ALS disinhibitive | Total FBI-ALS score | Adjusted FAB score |
|---|---|---|---|---|
| Time dependence burden | −0.035 | 0.238 | 0.106 | −0.344† |
| Developmental burden | 0.270* | 0.246* | 0.297* | −0.366† |
| Physical burden | 0.375† | 0.319* | 0.400† | −0.358† |
| Social burden | 0.219 | 0.276* | 0.282* | −0.295* |
| Emotional burden | 0.161 | 0.359† | 0.291* | −0.278* |
| Total burden | 0.218 | 0.327† | 0.309* | −0.411† |
*P<0.05; †P<0.01. FAB: Frontal assessment battery; FBI-ALS: Frontal behavioral inventoryversion for amyotrophic lateral sclerosis; CBI: Caregiver burden inventory; MND: Motor neuron disease; ALS: Amyotrophic lateral sclerosis.
As revealed by ANCOVA analysis, intergroup differences of developmental burden, physical burden, and total CBI score were shown to be significant [Table 3]. And post-hoc analysis showed that patients with moderate to severe behavior change imposed significantly higher burden than those without any behavior change in total CBI score (P = 0.005), developmental burden (P = 0.003), and physical burden (P = 0.001) (adjusted α = 0.05/3 = 0.017).
Table 3.
ANCOVA analysis for the intergroup differences of CBI among ALS/MND categorized by the degree of behavioral change (mean ± SD)
| Items | No change (n = 37) | Mild change (n = 16) | Moderate to severe change (n = 12) | P |
|---|---|---|---|---|
| Time dependence burden | 7.2 ± 5.6 | 5.9 ± 4.6 | 8.8 ± 5.7 | 0.304 |
| Developmental burden | 4.8 ± 4.7 | 5.3 ± 5.5 | 9.5 ± 3.8 | 0.005 |
| Physical burden | 2.4 ± 3.0 | 3.3 ± 3.9 | 6.0 ± 2.6 | 0.001 |
| Social burden | 1.4 ± 2.2 | 1.9 ± 2.9 | 2.9 ± 1.5 | 0.140 |
| Emotional burden | 0.6 ± 1.4 | 1.1 ± 1.7 | 1.8 ± 1.6 | 0.081 |
| Total CBI score | 16.4 ± 14.1 | 17.6 ± 16.2 | 29.1 ± 10.8 | 0.007 |
SD: Standard deviation; ALS: Amyotrophic lateral sclerosis; ANCOVA: Analysis of covariance; CBI: Caregiver burden inventory; MND: Motor neuron disease
DISCUSSION
This is the first study to investigate the factors associated with ALS/MND caregiver burden from the perspective of behaviors in a Chinese population. The demographic and clinical variables of this cohort were similar to those published before by us and others,[11,14] adding its credibility of truly reflecting characteristic of Chinese patients, although it was a single-center observation of small sample size.
It has reached a consensus that cognitive function occurs as a continuous changing in ALS/MND, from cognitive intactness to mild impairment or even overt dementia. Concerning behavioral status, our study suggested a similar result: Behavioral disturbance was presented in varying degree in this cohort, also in favor of the relationship between ALS/MND and FTLD. Based on FBI-ALS, the rate of mild to moderate behavioral changes was 41.5% in patients with ALS/MND in this cohort. Previous reports showed that this prevalence varied greatly from 17% to 88% in patients with MND, partly due to diverging definitions and diagnostic tools adopted among different studies.[26]
The impact of investigation instrument selection was more than this: The distribution of these abnormal behaviors could also differ from one to another. Investigations by the Frontal Systems Behavior Scale (FrSBe) and Cambridge Behavioral Inventory Revised (CBI-R) displayed behavioral changes weighing more on the subscale of apathy.[27,28] In this study, it was irritability of disinhibitive subscale that ranked top of the list, followed by logopenia and inflexibility subject to the negative subscale. Coincidentally, the research using the original version of FBI conducted in Southwest China reached a similar conclusion.[19] This consistency might result not only from using a same behavioral questionnaire but could also indicate a possibility that Chinese patients might have a distinct type of behavior disorders. Genetic basis, especially C9ORF72, has been blamed for bonding ALS/MND with FTLD. Lacking carriers of this mutation, Chinese patients, and even Eastern Asian population seemed to be protected from comorbid dementia.[12,29] However, the differences of behavioral spectrum waited to be substantiated in future between Chinese and Caucasian cohort, and its underlying genetic basis still needed to be explored. Deciphering the abnormal behaviors served to be of practical significance. Perseveration was remarkably frequent in the context of ALS-bvFTD,[26] but it did not exist in our cohort mainly composed of nondemented patients. This might be potentially regarded as a sign of the transmission from behavioral impairment to frank dementia.
Clinically, bulbar onset type and low ALSFRS-R score were correlated with behavioral impairment or dementia, not conclusively though.[4,29] None of these results was replicated in our study except for the degree of bulbar palsy. Patients with moderate to severe behavioral change scored significantly lower in this variable, also suggesting the connection of bulb dysfunction to frontal lobe involvement. Furthermore, the progression rate was not associated with severity of behavioral change in this cohort. It was in line with previous findings that behavioral impairment was not a predictor of fast progression,[4,29] while comorbid FTLD would worsen the prognosis for patients with ALS.[4,5,29] This controversy might imply that the state of behavioral impairment tends to be a relatively stable status, or ALS is a nonlinear course, inflexion point of which would emerge when frontal-mediated behavioral dysfunction deteriorates to the threshold of bvFTD. Our results seemed to be in favor of the former one, given the insignificant differences of disease duration and El Escorial classification among the three behavioral subgroups.
Behavioral disturbance of ALS detected by FrSBe and CBI-R have been proved to be relevant to caregiver burden.[30,31] Our results verified this relationship again, regardless of the diagnostic instruments. In the perspective of behavioral changes, frequently appearing as an abnormal behavior, though apathy was once shown to contribute little to caregivers’ burden.[30] We did not analyze apathy separately due to the design of FBI-ALS, but it seemed that behaviors of disinhibitive part influenced caregiver burden more than the negative part did, which apathy belonged to. Concerning the component of caregiver burden, caregivers of ALS once reported development burden and emotional burden were the most affected areas by neurobehavioral symptoms.[31] Correlation between developmental burden and abnormal behaviors remained to be significant in the present study, not as much as physical burden, however, possibly reflecting different attitudes and demands of different cultures in caring ALS. Frontal cognitive status was shown to be negatively associated with caregiver burden, but the significance of different caregiver burden among three behavioral groups remained to exist on ANCOVA analysis after associated factors were corrected. As the strongest contributor to the total CBI score, the time dependence burden was surprisingly unrelated to abnormal behaviors, no matter whether it was compared between groups or assessed by correlation. In accordance with previous research,[31] this finding suggested that other factors, such as physical disability, should take more responsibility to this burden category instead. A better understanding of caregiver burden and its related factors might help to design tailored interventions for families with ALS/MND patients.
Our findings should be viewed with consideration of several limitations. Questionnaires of life quality, depression, and anxiety were not included into the analysis, neither for patients nor caregivers. These assessments might be crucial in that they could help tell endogenous frontal dysfunction from negative moods of progressive physical disability. And it was also these measures that could provide evidence that ALS caring burden would actually add more risk of mood disorders to their caregivers and affect their life quality, although it was almost a common knowledge.
In conclusion, patients with ALS could exhibit behavioral disturbance to some extent, but the frequency and spectrum of these behaviors in Chinese patients were different from previous reports. Moreover, ALS caregivers’ burden could be influenced by patients’ abnormal behaviors, with more extensive impact from disinhibitive behaviors. Developmental burden and physical burden are the most affected components in caregiver burden.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Xin Chen
REFERENCES
- 1.Phukan J, Pender NP, Hardiman O. Cognitive impairment in amyotrophic lateral sclerosis. Lancet Neurol. 2007;6:994–1003. doi: 10.1016/S1474-4422(07)70265-X. [DOI] [PubMed] [Google Scholar]
- 2.Phukan J, Elamin M, Bede P, Jordan N, Gallagher L, Byrne S, et al. The syndrome of cognitive impairment in amyotrophic lateral sclerosis: A population-based study. J Neurol Neurosurg Psychiatry. 2012;83:102–8. doi: 10.1136/jnnp-2011-300188. [DOI] [PubMed] [Google Scholar]
- 3.Ringholz GM, Appel SH, Bradshaw M, Cooke NA, Mosnik DM, Schulz PE. Prevalence and patterns of cognitive impairment in sporadic ALS. Neurology. 2005;65:586–90. doi: 10.1212/01.wnl.0000172911.39167.b6. [DOI] [PubMed] [Google Scholar]
- 4.Montuschi A, Iazzolino B, Calvo A, Moglia C, Lopiano L, Restagno G, et al. Cognitive correlates in amyotrophic lateral sclerosis: A population-based study in Italy. J Neurol Neurosurg Psychiatry. 2015;86:168–73. doi: 10.1136/jnnp-2013-307223. [DOI] [PubMed] [Google Scholar]
- 5.Lillo P, Garcin B, Hornberger M, Bak TH, Hodges JR. Neurobehavioral features in frontotemporal dementia with amyotrophic lateral sclerosis. Arch Neurol. 2010;67:826–30. doi: 10.1001/archneurol.2010.146. [DOI] [PubMed] [Google Scholar]
- 6.Cui B, Cui L, Liu M, Ma J, Fang J. Amyotrophic lateral sclerosis with frontotemporal dementia presented with prominent psychosis. Chin Med J. 2014;127:3996–8. [PubMed] [Google Scholar]
- 7.Gauthier A, Vignola A, Calvo A, Cavallo E, Moglia C, Sellitti L, et al. A longitudinal study on quality of life and depression in ALS patient-caregiver couples. Neurology. 2007;68:923–6. doi: 10.1212/01.wnl.0000257093.53430.a8. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein LH, Atkins L, Landau S, Brown R, Leigh PN. Predictors of psychological distress in carers of people with amyotrophic lateral sclerosis: A longitudinal study. Psychol Med. 2006;36:865–75. doi: 10.1017/S0033291706007124. [DOI] [PubMed] [Google Scholar]
- 9.Mourik JC, Rosso SM, Niermeijer MF, Duivenvoorden HJ, Van Swieten JC, Tibben A. Frontotemporal dementia: Behavioral symptoms and caregiver distress. Dement Geriatr Cogn Disord. 2004;18:299–306. doi: 10.1159/000080123. [DOI] [PubMed] [Google Scholar]
- 10.Knutson KM, Zamboni G, Tierney MC, Grafman J. Neural correlates of caregiver burden in cortical basal syndrome and frontotemporal dementia. Dement Geriatr Cogn Disord. 2008;26:467–74. doi: 10.1159/000167268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu MS, Cui LY, Fan DS. Chinese ALS Association. Age at onset of amyotrophic lateral sclerosis in China. Acta Neurol Scand. 2014;129:163–7. doi: 10.1111/ane.12157. [DOI] [PubMed] [Google Scholar]
- 12.Zou ZY, Li XG, Liu MS, Cui LY. Screening for C9orf72 repeat expansions in Chinese amyotrophic lateral sclerosis patients. Neurobiol Aging. 2013;34:1710.e5–6. doi: 10.1016/j.neurobiolaging.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 13.Woollacott IO, Mead S. The C9ORF72 expansion mutation: Gene structure, phenotypic and diagnostic issues. Acta Neuropathol. 2014;127:319–32. doi: 10.1007/s00401-014-1253-7. [DOI] [PubMed] [Google Scholar]
- 14.Cui F, Liu M, Chen Y, Huang X, Cui L, Fan D, et al. Epidemiological characteristics of motor neuron disease in Chinese patients. Acta Neurol Scand. 2014;130:111–7. doi: 10.1111/ane.12240. [DOI] [PubMed] [Google Scholar]
- 15.Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, et al. The ALSFRS-R: A revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III) J Neurol Sci. 1999;169:13–21. doi: 10.1016/s0022-510x(99)00210-5. [DOI] [PubMed] [Google Scholar]
- 16.Hillel AD, Miller RM, Yorkston K, McDonald E, Norris FH, Konikow N. Amyotrophic lateral sclerosis severity scale. Neuroepidemiology. 1989;8:142–50. doi: 10.1159/000110176. [DOI] [PubMed] [Google Scholar]
- 17.Murphy J, Ahmed F, Lomen-Hoerth C. The UCSF screening exam effectively screens cognitive and behavioral impairment in patients with ALS. Amyotroph Lateral Scler Frontotemporal Degener. 2014;10:1–7. doi: 10.3109/21678421.2014.960873. [DOI] [PubMed] [Google Scholar]
- 18.Josephs KA, Whitwell JL, Eggers SD, Senjem ML, Jack CR., Jr Gray matter correlates of behavioral severity in progressive supranuclear palsy. Mov Disord. 2011;26:493–8. doi: 10.1002/mds.23471. [DOI] [PubMed] [Google Scholar]
- 19.Wei Q, Chen X, Zheng Z, Huang R, Guo X, Cao B, et al. Frontal lobe function and behavioral changes in amyotrophic lateral sclerosis: A study from Southwest China. J Neurol. 2014;261:2393–400. doi: 10.1007/s00415-014-7508-3. [DOI] [PubMed] [Google Scholar]
- 20.Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: A Frontal Assessment Battery at bedside. Neurology. 2000;55:1621–6. doi: 10.1212/wnl.55.11.1621. [DOI] [PubMed] [Google Scholar]
- 21.Appollonio I, Leone M, Isella V, Piamarta F, Consoli T, Villa M, et al. The frontal assessment battery (FAB): Normative values in an Italian population sample. Ital J Neurol Sci. 2005;26:108–16. doi: 10.1007/s10072-005-0443-4. [DOI] [PubMed] [Google Scholar]
- 22.Chou KR, Jiann-Chyun L, Chu H. The reliability and validity of the Chinese version of the caregiver burden inventory. Nurs Res. 2002;51:324–31. doi: 10.1097/00006199-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Brooks BR, Miller RG, Swash M, Munsat TL. World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–9. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 24.Visser J, van den Berg-Vos RM, Franssen H, van den Berg LH, Wokke JH, de Jong JM, et al. Disease course and prognostic factors of progressive muscular atrophy. Arch Neurol. 2007;64:522–8. doi: 10.1001/archneur.64.4.522. [DOI] [PubMed] [Google Scholar]
- 25.Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–54. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 26.Raaphorst J, Beeldman E, De Visser M, De Haan RJ, Schmand B. A systematic review of behavioural changes in motor neuron disease. Amyotroph Lateral Scler. 2012;13:493–501. doi: 10.3109/17482968.2012.656652. [DOI] [PubMed] [Google Scholar]
- 27.Terada T, Obi T, Yoshizumi M, Murai T, Miyajima H, Mizoguchi K. Frontal lobe-mediated behavioral changes in amyotrophic lateral sclerosis: Are they independent of physical disabilities? J Neurol Sci. 2011;309:136–40. doi: 10.1016/j.jns.2011.06.049. [DOI] [PubMed] [Google Scholar]
- 28.Lillo P, Mioshi E, Zoing MC, Kiernan MC, Hodges JR. How common are behavioural changes in amyotrophic lateral sclerosis? Amyotroph Lateral Scler. 2011;12:45–51. doi: 10.3109/17482968.2010.520718. [DOI] [PubMed] [Google Scholar]
- 29.Oh SI, Park A, Kim HJ, Oh KW, Choi H, Kwon MJ, et al. Spectrum of cognitive impairment in Korean ALS patients without known genetic mutations. PLoS One. 2014;9:e87163. doi: 10.1371/journal.pone.0087163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lillo P, Mioshi E, Hodges JR. Caregiver burden in amyotrophic lateral sclerosis is more dependent on patients’ behavioral changes than physical disability: A comparative study. BMC Neurol. 2012;12:156. doi: 10.1186/1471-2377-12-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiò A, Vignola A, Mastro E, Giudici AD, Iazzolino B, Calvo A, et al. Neurobehavioral symptoms in ALS are negatively related to caregivers’ burden and quality of life. Eur J Neurol. 2010;17:1298–303. doi: 10.1111/j.1468-1331.2010.03016.x. [DOI] [PubMed] [Google Scholar]


