Abstract
Background:
Hemorrhagic shock (HS) results in myocardial contractile dysfunction. Studies showed that 17β-estradiol protects the myocardium against contractile dysfunction. The study investigated the cardioprotective effects of treatment with 17β-estradiol before resuscitation following 1 h of HS and resuscitation.
Methods:
Male Sprague-Dawley rats were assigned to 2 sets of experimental protocols: Ex vivo and in vivo treatment and resuscitation. Each set had three experimental groups (n = 6 per group): Normotensive (N), HS and resuscitation (HS-R) and HS rats treated with 17β-estradiol (E) and resuscitated (HS-E-R). Rats were hemorrhaged over 60-min to reach a mean arterial blood pressure of 40 mmHg. In the ex vivo group, hearts were resuscitated by perfusion in the Langendorff system. In the 17β-estradiol treated group, 17β-estradiol 280 µg/kg was added for the first 5 min. Cardiac function was measured. Left ventricular generated pressure (LVGP) and +dP/dt were calculated. In the in vivo group, rats were treated with 17β-estradiol 280 µg/kg s.c. after 60-min HS. Resuscitation was performed in vivo by the reinfusion of the shed blood for 30-min to restore normotension.
Results:
Treatment with 17β-estradiol before resuscitation in ex vivo treated and resuscitated isolated hearts and in the in vivo treated and resuscitated rats following HS improved myocardial contractile function. In the in vivo treated group, LVGP and +dP/dt max were significantly higher in 17β-estradiol treated rats compared to the untreated group (LVGP 136.40 ± 6.61 compared to 47.58 ± 17.55, and +dP/dt 661.85 ± 49.88 compared to 88.18 ± 0.85). Treatment with 17β-estradiol improved LVGP following HS.
Conclusions:
The results indicate that treatment with 17β-estradiol before resuscitation following HS protects the myocardium against dysfunction.
Keywords: Contractility, Heart, Hemorrhage, Rats, Resuscitation
INTRODUCTION
Hemorrhagic shock (HS) and trauma are one of the major causes of death.[1] Despite the research that has been done to improve the resuscitation strategies, myocardial dysfunction and multiple organ injury remain one of the major causes of mortality and morbidity following HS and resuscitation.[1,2,3] Resuscitation strategies are concerned to reestablish tissue perfusion, but they do not prevent multiple organ injury. The precise mechanism of multiple organ injury and failure is not well-established. Previous studies suggested the involvement of inflammatory pathways and the production of nitric oxide.[4]
It is well-known that sex is a major determinant of the outcome of patients after trauma and sepsis.[5,6] Estrogens, progesterone, and testosterone mediate a number of the differences in the response to trauma based on different sex.[6] Studies have shown that estradiol decreases lung injury after HS down-regulating the inflammatory mediator production.[7] Estradiol exerts protective effects after ischemia reperfusion.[8,9] Experimental studies have shown that female rats are resistant to organ injury after HS.[10] In addition, intestinal blood flow is protected in female than in male rats after HS.[11]
The present study examined the protective effects of treatment with estradiol before resuscitation on myocardial contractile function following HS in a rat model.
METHODS
The Ethical Committee at the National Plan for Sciences and Technologies, King Saud University approved the study.
Preparation of animals
Male Sprague-Dawley rats were anesthetized by injecting urethane 125 mg/kg intraperitonealy (i.p.). Rats were injected i.p. with heparin sodium 2000 I.U. The left carotid artery was cauterized using polyethylene tubing size 60. For measurement of blood pressure, the left carotid artery was connected to the pressure transducer. Animals were left to stabilize for 30 min. The animals were assigned randomly to two experimental protocols: (a) HS followed by ex vivo treatment and resuscitation and (b) HS followed by in vivo treatment and resuscitation.
Hemorrhagic shock model
Hemorrhage was done by utilizing a 10-ml syringe that is attached to the three-way stopcock connected to the carotid artery. Hemorrhage was achieved by opening the stopcock and aspirating gradually with the syringe. Hemorrhage was done at a rate of 1 ml/min. Mean arterial blood pressure (MABP) was maintained at approximately 40 mmHg. Blood was withdrawn or re-infused to the animal to maintain the blood pressure. For the normotensive group, the same methods were done but the rats were be hemorrhaged.
Resuscitation was done in vivo by reinfusion of the shed blood after 60 min hemorrhage to restore blood pressure. Blood pressure was recorded for 30 min.
Perfusion of hearts outside the body
Hearts were excised and removed. Hearts were perfused outside the animal body (ex vivo). The Langendorff system was used for hemodynamic measurements. Hearts were perfused with normal physiological buffer Krebs-Henseleit-Bicarbonate (KHB) solution for 60 min.
Hearts were hanged by the aorta to the cannula in the Langendorff system. The hearts were perfused with KHB buffer consisting of the following (in mmol/L): Sodium chloride, 118; calcium chloride, 1.25; potassium chloride, 4.7; sodium bicarbonate, 21; magnesium sulfate, 1.2; glucose, 11; potassium biphosphate, 1.2; and ethylenediaminetetraacetic acid (EDTA), 0.5. An incision was made in the apex of the left ventricle using a No. 15 scalpel blade. A cellophane balloon filled with saline and attached to a catheter was inserted into the left ventricle. The catheter was placed through the mitral valve. The catheter was used to measure the left ventricular pressures by attaching the catheter to a transducer that is placed at the levels of the heart and aorta. Stimulation of the hearts was performed electrically at a rate of 300 beats/min using the electrical stimulator (6020 stimulator from Harvard Apparatus). The perfusion pressure (PP) was adjusted to be constant at 50 mmHg. The left end-diastolic pressure was adjusted and maintained at a pressure of 5 mmHg by injecting saline in the left ventricular balloon. The temperature of the perfusate fluid was adjusted and stays constant at 37°C. The perfusion fluid was oxygenated using a mixture of 95% O2+ 5% CO2. The pH of the perfusion fluid was maintained at 7.4.
Study design
Ex vivo treatment and resuscitation
After 60-min HS, hearts were harvested and resuscitated ex vivo by perfusion using the Langendorff apparatus for 60-min [Figure 1]. In the treated group, hearts were perfused with 17β-estradiol for 5 min followed by 55 min of ex vivo resuscitation in the Langendorff system.
Figure 1.
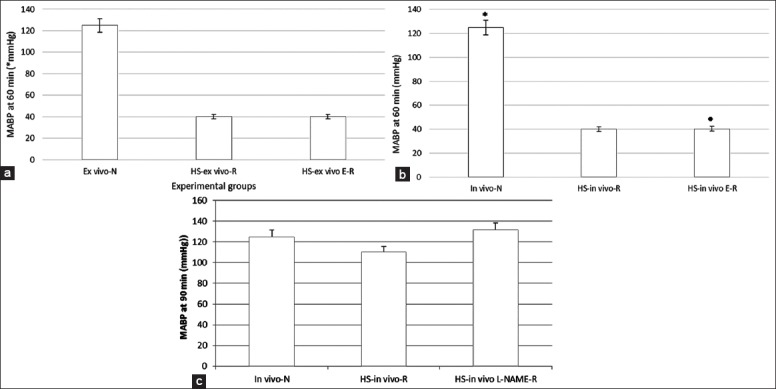
Recording of mean arterial blood pressure (MABP). (a) The ex vivo resuscitated group (MABP) after 60-min. (1) Normotensive rats (ex vivo-N), (2) Hemorrhagic shock (HS) rats (HS-ex vivo R), and (3) ex vivo treatment with 17β-estradiol and resuscitation (HS-ex vivo E-R). (b) In vivo resuscitated group (MABP). (a) Recording of MABP for 60-min. (c) Recording of MABP for 60-min hemorrhage and 30-min resuscitation. (1) Normotensive rats (in vivo-N), (2) HS rats (HS-in vivo R), and (3) in vivo treatment with 17β-estradiol and resuscitation (HS-in vivo E-R). All values are means ± standard deviation. *P < 0.05 versus hemorrhagic shock group, •P < 0.05 versus HS group (n = 6 per group).
Hearts were excised quickly and rapidly placed onto a Langendorff apparatus, and perfused at a flow rate of 10 ml/min with KHB buffer (KHB, in mmol/L: NaCl 118, CaCl21.25, KCl 4.7, NaHCO321, MgSO41.2, glucose, 11, KH2 PO41.2, and EDTA 0.5). Perfusate temperature was maintained at 37°C and was gassed with a mixture of 95% O2 and 5% CO2 at a pH of 7.4 as described previously.[12]
Ex vivo experimental groups
Three experimental groups (n = 6) were assigned:
Normotensive rats (ex vivo-N): The rats underwent the same surgical preparation, and continuous blood pressure measurements were obtained for the 60-min experimental period. Hearts were harvested and perfused ex vivo in the Langendorff system for 60 min with the physiological buffer.
Hemorrhagic shock rats (HS-ex vivo R): After a 30-min stabilization period, the rats were hemorrhaged to 40 mmHg for 60 min. Hearts were then harvested and resuscitated ex vivo in the Langendorff system for 60 min.
Ex vivo treatment with 17β-estradiol and resuscitation (HS-ex vivo E-R): After a 30-min stabilization period, the rats were hemorrhaged to 40 mmHg for 60 min. Hearts were then harvested and treated with 17β-estradiol ex vivo by perfusion for 5 min in the Langendorff system followed by ex vivo resuscitation for 55 min with KHB.
In vivo treatment and resuscitation
In the in vivo resuscitated groups, rats underwent the same surgical procedure and protocol for hemorrhage as described earlier. The rats were then resuscitated in vivo by reinfusion of the shed blood to restore normotension, and the MABP was monitored for 30 min. In the treated group, 17β-estradiol, 280 µg/kg s.c. injected after 60 min of HS. The rats were then resuscitated and monitored for 30 min. Hearts were then excised and perfused in the Langendorff system for measurement of myocardial contractile function, described later.
In vivo experimental groups
Experimental protocols
Three experimental groups (n = 6) were assigned:
Normotensive rats (in vivo-N): The rats underwent the same surgical preparation, and continuous blood pressure measurements were obtained for 60-min experimental HS period and 30 min of resuscitation, total of 90 min. Hearts were then excised and perfused in the Langendorf system for 60 min for measurement of myocardial function.
Hemorrhagic shock and in vivo resuscitation (HS-in vivo R): After a 30-min stabilization period, the rats were hemorrhaged to 40 mmHg for 60 min. The rats were then resuscitated by reinfusion of the shed blood to restore normotension and monitored for 30 min. Hearts were then excised and perfused in the Langendorf system for 60 min for measurement of myocardial function.
Treatment with 17β-estradiol before in vivo resuscitation following HS (HS-in vivo E-R): After a 30-min stabilization period, the rats were hemorrhaged to 40 mmHg for 60 min. 17β-estradiol 280 µg/kg s.c. was injected after 60 min of HS. The rats were then resuscitated and monitored for 30 min. Same procedure was followed for measurement of myocardial function.
Mean arterial blood pressure
Mean arterial blood pressure was monitored for the 60-min duration of hemorrhagic shock and for 30 min after resuscitation in the in vivo treated group.
Hemodynamic measurements
Using the Langendorff apparatus, hemodynamic parameters were measured in the isolated hearts for 60 min. Left ventricular end-diastolic pressure, left ventricular peak systolic pressure, coronary PP was recorded. The left ventricular ±dP/dt, which is the left ventricular index of contractility, was calculated.
Statistical analysis
Data were presented as mean ± standard deviation (SD). Data were analyzed with one-way analysis of variance (ANOVA). The values of P < 0.05 were considered significant. The Student's t-test was used to compare mean values between the two experimental groups.
RESULTS
Mean arterial blood pressure
In the first experimental group, rats were hemorrhaged to 40 mmHg and MABP was monitored for 60 min. As shown in Figure 1a, there was no difference between the hemorrhage and the hemorrhage ex vivo treated groups. In the in vivo resuscitated group, rats were hemorrhaged to 40 mmHg and MABP was monitored for 60 min. The rats were either treated or not in vivo and resuscitated by reinfusion of the shed blood to restore normotension. MABP was monitored for additional 30 min. As shown in Figure 1b, there was no difference between the two hemorrhage groups treated or not. In Figure 1c, there was no difference in MABP between the treated and untreated resuscitated HS groups.
Myocardial contractile function
In this group, rats underwent the same surgical procedure but the rats were not hemorrhaged or resuscitated. There was no contractile dysfunction.
Ex vivo treatment with 17β-estradiol and ex vivo resuscitation
Figure 2a shows the changes in the left ventricular generated pressure (LVGP) the end of the 1 h ex vivo resuscitation following HS. LVGP was significantly lower in the HS group compared to the normotensive group, while the hemorrhage treated group showed a significant increase in the LVGP compared to the hemorrhage group (P < 0.05). Left ventricular maximum +dP/dt [Figure 2b] was significantly lower in the hemorrhage group as compared to the normotensive group and significantly higher in the hemorrhage treated compared to the hemorrhage group (P < 0.05).
Figure 2.
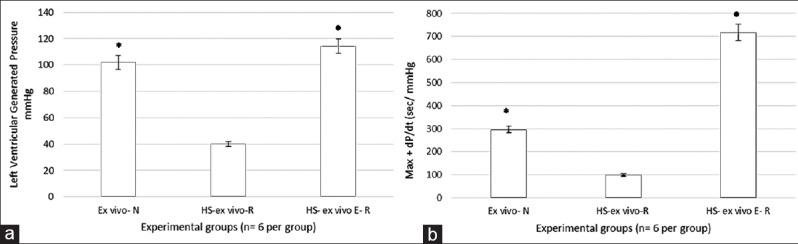
Myocardial contractile function in the ex vivo resuscitated group. (a) Left ventricular generated pressure. (b) Left ventricular +dP/dt. All values are means ± standard deviation. *P < 0.05 versus hemorrhagic shock (HS) group, •P < 0.05 versus HS group (n = 6 per group).
In vivo treatment with 17β-estradiol and in vivo resuscitation
Figure 3 shows the changes in the LVGP and +dP/dt after 1 h of HS followed by in vivo resuscitation. LVGP was significantly lower in the HS group compared to the normotensive group (P < 0.05). The hemorrhage treated in vivo with 17β-estradiol showed a significant increase in the LVGP compared to the hemorrhage group (P < 0.05). Left ventricular maximum +dP/dt [Figure 3b] was significantly lower in the hemorrhage group as compared to the normotensive group and significantly higher in the hemorrhage treated compared to the hemorrhage group (P < 0.05).
Figure 3.
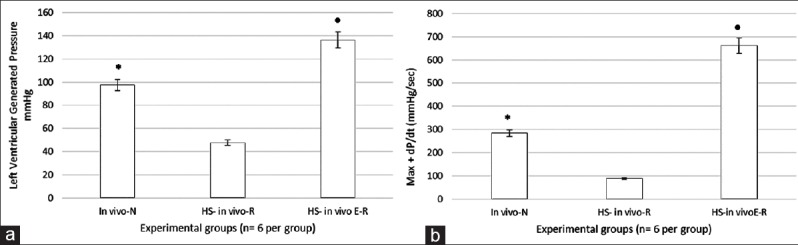
Myocardial contractile function in the in vivo resuscitated group. (a) Left ventricular generated pressure (LVGP). (b) Left ventricular +dP/dt. All values are means ± standard deviation (SD). In (a and b), LVGP and +dP/dtmax was significantly improved in estradiol-treated rats compared to untreated. All values are means ± SD. *P < 0.05 versus hemorrhagic shock (HS) group, •P < 0.05 versus HS group (n = 6 per group).
DISCUSSION
Although previous studies have demonstrated that 17β- estradiol has cardioprotective properties, the present data are the first data demonstrating that treatment with 17β-estradiol before resuscitation following HS protect against myocardial contractile dysfunction. Treatment with 17β-estradiol before resuscitation following HS improved myocardial contractile function and protect the myocardium against failure. The present results are in agreement with the study of Hsu et al.[13] who demonstrated the cardioprotective effects of estradiol following hemorrhage. However, the present study is the first to demonstrate that treatment with 17β- estradiol before resuscitation following HS protect the myocardium against myocardial contractile dysfunction and failure in the ex vivo as well as the in vivo resuscitated rats.
The ex vivo resuscitated group, gives direct information about the cardioprotective effects of the treatment with estradiol outside the body, away from any endocrine, neural and other inflammatory pathways interference. While the in vivo treatment and resuscitation is important to prove the cardioprotective effects inside the body to confirm the protective effects with the interaction with other regulatory mechanisms that acts in response to hemorrhage and resuscitation.
Hemorrhagic shock is one of the major causes of death in trauma.[14] Despite the improvement in resuscitation strategies, myocardial and multiple organ dysfunction and failure remain the major causes of mortality and morbidity after resuscitation following HS.[15] Resuscitation fluids are concerned with the reestablishment of tissue perfusion; however, they do not protect against multiple organ injury and failure. The precise mechanism of multiple organ injury after resuscitation is not well-established. There are multiple mechanisms that contribute in the pathogenesis of cardiac dysfunction, including energy depletion, intracellular acidosis, and activation of inflammatory pathways and excessive production of nitric oxide. HS and resuscitation can produce inflammatory responses with the production of inflammatory mediators, which might be more harmful than the original hemorrhage[16,17,18]
It is well-known that sex is a major determinant in the outcome of trauma patients.[5,19] Clinical and experimental studies have shown that females are more tolerant to injuries than males.[5,19,20] Evidence indicates that men and women have a different response to injury. Recent studies have shown that there are differences in immune responses to diseases depending on the gender difference.[6] Hormones are one of the causes of these different responses in male and female. Experimental studies have shown that sex hormones modulate acute lung injury caused by ischemia-reperfusion.[6] Estradiol decreases acute lung injury caused by HS in male rats and downregulates the inflammatory mediators.[7] Furthermore, intestinal blood flow is protected in female than male rats following HS and that the gut of female is more resistant than that of the male to damaging effects of the ischemic injury.[10] Evidence showed that estradiol may play a protective role against multiple organ injury and dysfunction following HS. The present study demonstrated the cardioprotective effects of administration of estradiol before resuscitation in HS model.
Sex-hormones actions are mediated by their receptors. The mechanism by which estradiol protects against organ dysfunction following ischemia reperfusion is not known. One suggested mechanism is by decreasing the inflammatory response. Multiple organ dysfunction and failure as a complication to systemic inflammatory responses are the major cause of mortality and morbidity following trauma.[21] The mechanism underlying these actions on the myocardial injury after HS and resuscitation are not totally understood. Future studies are needed to investigate the involvement of inflammatory pathways in the mechanism of cardioprotection effects of estradiol. The results of the present study might be a new therapeutic tool for the management of trauma patients that will help to improve the outcomes after HS and resuscitation.
Financial support and sponsorship
This work was supported by a grant from the Research Groups at Deanship of Scientific Research at King Saud University, Riyadh, Saudi Arabia.
Conflicts of interest
There are no conflicts of interest.
ACKNOWLEDGMENT
Technical help from Mr. Sabirine Sahiba is acknowledged.
Footnotes
Edited by: Xiu-Yuan Hao
REFERENCES
- 1.Rossaint R, Cerny V, Coats TJ, Duranteau J, Fernández-Mondéjar E, Gordini G, et al. Key issues in advanced bleeding care in trauma. Shock. 2006;26:322–31. doi: 10.1097/01.shk.0000225403.15722.e9. [DOI] [PubMed] [Google Scholar]
- 2.Moore EE, Moore FA, Harken AH, Johnson JL, Ciesla D, Banerjee A. The two-event construct of postinjury multiple organ failure. Shock. 2005;24(Suppl 1):71–4. doi: 10.1097/01.shk.0000191336.01036.fe. [DOI] [PubMed] [Google Scholar]
- 3.Cotton BA, Guy JS, Morris JA, Jr, Abumrad NN. The cellular, metabolic, and systemic consequences of aggressive fluid resuscitation strategies. Shock. 2006;26:115–21. doi: 10.1097/01.shk.0000209564.84822.f2. [DOI] [PubMed] [Google Scholar]
- 4.Cai B, Dong W, Sharpe S, Deitch EA, Ulloa L. Survival and inflammatory responses in experimental models of hemorrhage. J Surg Res. 2011;169:257–66. doi: 10.1016/j.jss.2009.11.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choudhry MA, Schwacha MG, Hubbard WJ, Kerby JD, Rue LW, Bland KI, et al. Gender differences in acute response to trauma-hemorrhage. Shock. 2005;24(Suppl 1):101–6. doi: 10.1097/01.shk.0000191341.31530.5e. [DOI] [PubMed] [Google Scholar]
- 6.Fish EN. The X-files in immunity: Sex-based differences predispose immune responses. Nat Rev Immunol. 2008;8:737–44. doi: 10.1038/nri2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu JT, Kan WH, Hsieh CH, Choudhry MA, Bland KI, Chaudry IH. Role of extracellular signal-regulated protein kinase (ERK) in 17beta-estradiol-mediated attenuation of lung injury after trauma-hemorrhage. Surgery. 2009;145:226–34. doi: 10.1016/j.surg.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Dubal DB, Shughrue PJ, Wilson ME, Merchenthaler I, Wise PM. Estradiol modulates bcl-2 in cerebral ischemia: A potential role for estrogen receptors. J Neurosci. 1999;19:6385–93. doi: 10.1523/JNEUROSCI.19-15-06385.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fraser H, Davidge ST, Clanachan AS. Enhancement of post-ischemic myocardial function by chronic 17 beta -estradiol treatment: Role of alterations in glucose metabolism. J Mol Cell Cardiol. 1999;31:1539–49. doi: 10.1006/jmcc.1999.0986. [DOI] [PubMed] [Google Scholar]
- 10.Ba ZF, Wang P, Kuebler JF, Rue LW 3rd, Bland KI, Chaudry IH. Flutamide induces relaxation in large and small blood vessels. Arch Surg. 2002;137:1180–6. doi: 10.1001/archsurg.137.10.1180. [DOI] [PubMed] [Google Scholar]
- 11.Deitch EA, Feketeova E, Lu Q, Zaets S, Berezina TL, Machiedo GW, et al. Resistance of the female, as opposed to the male, intestine to I/R-mediated injury is associated with increased resistance to gut-induced distant organ injury. Shock. 2008;29:78–83. doi: 10.1097/shk.0b013e318063e98a. [DOI] [PubMed] [Google Scholar]
- 12.Soliman M. Dimethyl amiloride, a Na+–H+ exchange inhibitor, and its cardioprotective effects in hemorrhagic shock in in vivo resuscitated rats. J Physiol Sci. 2009;59:175–80. doi: 10.1007/s12576-009-0024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsu JT, Hsieh YC, Kan WH, Chen JG, Choudhry MA, Schwacha MG, et al. Role of p38 mitogen-activated protein kinase pathway in estrogen-mediated cardioprotection following trauma-hemorrhage. Am J Physiol Heart Circ Physiol. 2007;292:H2982–7. doi: 10.1152/ajpheart.01303.2006. [DOI] [PubMed] [Google Scholar]
- 14.Mannucci PM, Levi M. Prevention and treatment of major blood loss. N Engl J Med. 2007;356:2301–11. doi: 10.1056/NEJMra067742. [DOI] [PubMed] [Google Scholar]
- 15.Rushing GD, Britt LD. Reperfusion injury after hemorrhage: A collective review. Ann Surg. 2008;247:929–37. doi: 10.1097/SLA.0b013e31816757f7. [DOI] [PubMed] [Google Scholar]
- 16.Cai B, Deitch EA, Grande D, Ulloa L. Anti-inflammatory resuscitation improves survival in hemorrhage with trauma. J Trauma. 2009;66:1632–9. doi: 10.1097/TA.0b013e3181a5b179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deitch EA. Multiple organ failure. Pathophysiology and potential future therapy. Ann Surg. 1992;216:117–34. doi: 10.1097/00000658-199208000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ulloa L. The vagus nerve and the nicotinic anti-inflammatory pathway. Nat Rev Drug Discov. 2005;4:673–84. doi: 10.1038/nrd1797. [DOI] [PubMed] [Google Scholar]
- 19.Kher A, Wang M, Tsai BM, Pitcher JM, Greenbaum ES, Nagy RD, et al. Sex differences in the myocardial inflammatory response to acute injury. Shock. 2005;23:1–10. doi: 10.1097/01.shk.0000148055.12387.15. [DOI] [PubMed] [Google Scholar]
- 20.Hsieh YC, Yang S, Choudhry MA, Yu HP, Bland KI, Schwacha MG, et al. Flutamide restores cardiac function after trauma-hemorrhage via an estrogen-dependent pathway through upregulation of PGC-1. Am J Physiol Heart Circ Physiol. 2006;290:H416–23. doi: 10.1152/ajpheart.00865.2005. [DOI] [PubMed] [Google Scholar]
- 21.Yu HP, Chaudry IH. The role of estrogen and receptor agonists in maintaining organ function after trauma-hemorrhage. Shock. 2009;31:227–37. doi: 10.1097/SHK.0b013e31818347e7. [DOI] [PMC free article] [PubMed] [Google Scholar]


