Abstract
Background:
Intravascular ultrasound (IVUS) examination can provide useful information during endovascular stent graft repair. However, its actual clinical utility in thoracic endovascular aortic repair (TEVAR) for type B aortic dissection (type B-AD) remains unclear, especially in complicated aortic dissection. We evaluated the effect of IVUS as a complementary tool during TEVAR.
Methods:
From September 2011 to April 2012, we conducted a prospective cohort study of 47 consecutive patients with “complicated” type B-AD diagnosed. We divided the patients into two groups: IVUS-assisted TEVAR group and TEVAR using angiography alone group. The general procedure of TEVAR was performed. We evaluated the perioperative and follow-up events. Patient demographics, comorbidities, preoperative images, dissection morphology, details of operative strategy, intraoperative events, and postoperative course were recorded.
Results:
A total of 47 patients receiving TEVAR were enrolled. Among them (females, 8.51%; mean age, 57.38 ± 13.02 years), 13 cases (27.66%) were selected in the IVUS-assisted TEVAR group, and 34 were selected in the TEVAR group. All patients were symptomatic. The average diameter values of IVUS measurements in the landing zone were greater than those estimated by computed tomography angiography (31.82 ± 4.21 mm vs. 30.64 ± 4.13 mm, P < 0.001). The technique success rate was 100%. Among the postoperative outcomes, statistical differences were only observed between the IVUS-assisted TEVAR group and TEVAR group for total operative time and the amount of contrast used (P = 0.013 and P < 0.001, respectively). The follow-up ranged from 15 to 36 months for the IVUS-assisted TEVAR group and from 10 to 35 months for the TEVAR group (P = 0.646). The primary endpoints were no statistical difference in the two groups.
Conclusions:
Intraoperative IVUS-assisted TEVAR is clinically feasible and safe. For the endovascular repair of “complicated” type B-AD, IVUS may be helpful for understanding dissection morphology and decrease the operative time and the amount of contrast used.
Keywords: Aortic Dissection, Endovascular, Intravascular Ultrasound, Stent Repair
INTRODUCTION
Ever since the first introduction of thoracic endovascular aortic repair (TEVAR), acute and chronic type B aortic dissection (type B-AD) has been increasingly treated by TEVAR.[1,2] Although several controversies continue to exist regarding the optimal treatment strategy for type B-AD, the recent interdisciplinary expert consensus document suggests that TEVAR should be used for complicated cases with suitable anatomy to decrease the mortality and morbidity of open surgery, whereas medical management with close follow-up is the best strategy for uncomplicated type B-AD.[3]
As to TEVAR for type B-AD, the discrimination of true and false lumen and successful sealing of the primary intimal tear are the prerequisite, However, predictors of aortic remodeling of the aorta after TEVAR would affect the survival rate and the re-intervention rate. A comprehensive evaluation of the preoperative and intraoperative aortic dissection (AD) morphology using computed tomography angiography (CTA), magnetic resonance angiography (MRA), transesophageal echocardiography (TEE), or intravascular ultrasound (IVUS) is important. IVUS is a unique image modality that visualizes vessels from within. Recently, IVUS examinations of the coronary arteries[4] and peripheral arteries or venous diseases[5,6,7,8,9] have enabled to assess the morphological characteristics of the lesions and have enabled treatment to be optimized. The use of IVUS in the management of AD, however, is not a routine practice. Literature[10,11,12] regarding TEVAR for type B-AD suggested that it had the capability of delineating false and true lumens and visualizing whether a major side branch is emanating from the true or false lumen. The origins of the dissection and re-entries are clearly visualized, and the landing zones can be evaluated. Using IVUS during TEVAR can enable the operator to take measurements of proximal landing zone to check the exact position of the stent graft, and to verify the patency of the branches at the end of the procedure.[13,14]
However, there were only limited studies that had evaluated the feasibility and safety of IVUS-assisted TEVAR, and even fewer comparing IVUS-assisted TEVAR with routine TEVAR (traditional). The actual clinical utility of IVUS-assisted TEVAR for type B-AD remains unclear. In this prospective study, we aimed to evaluate the utility of intraoperative IVUS to assess its impact on TEVAR of “complicated” type B-AD. In addition, aortic remodeling after TEVAR in the two groups was observed by CTA during follow-up.
METHODS
Patient selection
From September 2011 to April 2012, 102 patients presented to our center with type B-AD. Fifty-two patients (51.0%) were defined as having “complicated” type B-AD. The “complicated” type B-AD cases were classified with two or more of the following clinical presentations: (1) Symptomatic visceral malperfusion; (2) lower limb ischemia; (3) multi-barreled AD; (4) great compression of the true lumen (true lumen <10%); (5) large primary entry tear (≥10 mm); (6) refractory hypertension; and (7) recurrent thoracic pain or pain with documented rapid aortic enlargement, interpreted to be impending rupture. Patients were excluded if arterial access was inadequate (<7 mm diameter of iliac or femoral arteries). Five patients were lost to follow-up and were excluded from the data analysis. The inclusion criteria for the IVUS-assisted TEAVR group were patients with acute or chronic renal insufficiency, or allergy to contrast agents, or multi-barreled AD. Of the 47 “complicated” type B-AD cases, 13 patients were selected to receive TEVAR with the assisted IVUS evaluation before and after stent implantation, including hyperacute (2/13), acute (1/13), subacute (4/13), and chronic (6/13) AD, respectively. Thirty-four patients underwent routine TEVAR using angiography alone. Patient demographics, comorbidities, preoperative images, dissection morphology, details of operative strategy, intraoperative events, and postoperative course were recorded. The study received the approval of the Committee for the Protection of Human Subjects of Zhongshan Hospital, Fudan University. All patients participated in the study signed an informed consent document.
Preoperative and intraoperative procedures
Baseline CTA with multiplanar reconstruction was performed to assess the feasibility of endovascular procedure, patency, and origin (true or false lumen) of visceral vessels and cerebral circulation, arterial access, and sizing of stent grafts. The general procedure of TEVAR had been reported previously.[15] The following stent graft systems were used in this series: Talent, Valiant, and Valiant-Captivia (Medtronic, Minneapolis, MN, USA); Zenith TX 2 (Cook, Bjaeverskov, Denmark). IVUS evaluation was performed before and after stent implantation. A final angiogram was performed at the end of TEVAR to confirm accurate endograft fixation at the anticipated location and satisfactory exclusion of the primary entry. If the proximal landing zone (the distance from the origin of the left subclavian artery (LSA) to the primary entry) measured <15 mm, then one of two strategies would be applied to create an extra anchoring area: (1) Intentional coverage of LSA, if the right vertebral artery was patent and the left one was not dominant or (2) revascularization of LSA with bypass or chimney technique. Oversizing was calculated according to the diameter from the adventitia to the adventitia of the proximal landing zone on CTA. If the malperfusion of the aortic branches (iliac or visceral arteries) still existed after TEVAR of the primary entry, endovascular repair would be considered to maintain the target organ perfusion.
All procedures were performed in a hybrid operating room under general anesthesia. If the endovascular procedure was performed percutaneously through a vascular sheath in the femoral artery, then the access site was secured with two proglide devices (Abbott Vascular, Redwood City, CA, USA). Postoperatively, medical management included β-blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, or calcium antagonists administrated alone or in combination to maintain the systolic blood pressure at <140 mmHg. Antiplatelet therapy was recommended for the patients who had undergone bypass, such as the carotid-carotid artery, aorta-carotid artery, or carotid-subclavian artery.
Intravascular ultrasound application
We primarily used the Vision PV 8.2-Fr, 10-MHz catheter (Volcano Therapeutics, Rancho Cordova, CA, USA) for aortic procedures. IVUS catheters were introduced either by percutaneous puncture or by open femoral artery exposure. For aortic procedures, we typically used a 10-Fr or 12-Fr sheath to accommodate the 10-MHz IVUS catheter. The IVUS catheter was loaded onto a 0.035-inch stiff guide wire (e.g., 0.035-inch stiff Terumo or 0.035-inch Amplatz Super Stiff guide wire) and allowed examination of the iliac artery and the entire abdominal and thoracic aorta. Use of a stiff guide wire provided more controlled advancement of the catheter within the lumen, particularly in tortuous or stenotic iliac arteries. A 10-MHz catheter was advanced along the 0.035-inch wire. Once the catheter reached a normal segment of the ascending aorta, we began to withdraw at a speed of approximately 5 mm/s until bifurcation of the abdominal aorta was reached. The morphology of the AD lesion was recorded and analyzed. After deployment of the stent graft, besides the angiography, the same IVUS examination was performed again to evaluate stent apposition and coverage of the dissection entries and patency of any major branches. For some uncertain regions, if the IVUS result was satisfactory, further angiography could be avoided. Along with the previous angiography, further manipulation using balloon angioplasty or a cuff stent graft implantation should be considered.
Definitions and endpoints
According to recent data from the International Registry of Acute Aortic Dissection (IRAD), there were four distinct periods (hyperacute, acute, subacute, and chronic presentation) that define the timing of type B-AD.[16] Malperfusion was defined as decreased perfusion of aortic branches (spinal, iliac, or visceral arteries) that typically lead to paraparesis or paraplegia, lower limb ischemia, abdominal pain, nausea, and diarrhea. Diagnosis of static or dynamic organ malperfusion is corroborated by laboratory markers (bilirubin, amylases, enzymes, creatinine) and imaging data. Refractory hypertension was defined as hypertension persisting despite three or more different classes of antihypertensive therapy at maximal recommended or maximal tolerated doses. Renal function was defined using the chronic kidney disease staging system.
The primary endpoints were AD-related death and AD-related events, including endoleak, aneurysmal dilatation, aortic retrograde dissection, organ ischemia, stent graft-induced new entry (SINE), and spinal cord ischemia.
Follow-up aortic remodeling
Patients were followed up with serial follow-up; CTA imaging was performed at 3, 6, and 12 months and then annually thereafter to assess false lumen status and aortic cross-sectional diameter.
Statistical analysis
Results were analyzed with SPSS software version 20.0 (SPSS Inc., Chicago, IL, USA). Data were assessed for normality and expressed as number (%) for the category and mean ± standard deviation (SD) or median (range) for continuous variables. Two-tailed Student's t-test was used to analyze continuous variables. Categorical variables were compared using Chi-square test or Fisher's exact test. The paired-samples t-test was used for comparison of average diameter values in the landing zone between IVUS measurements and CTA measurements. A P < 0.05 was considered as statistically significant.
RESULTS
Of the 47 patients (females, 8.51%; mean age, 57.38 ± 13.02 years), 13 (27.66%) were selected in the IVUS-assisted TEVAR group, and 34 were selected in the TEVAR group. All the enrolled patients were symptomatic (either chest pain or abdominal pain). Baseline characteristics of the two groups are listed in Table 1. The “complicated” features of these series are shown in Figure 1.
Table 1.
Clinicopathologic and biochemical features of patients with type B-AD undergoing TEVAR
| Variables | IVUS-assisted TEVAR (n = 13) | TEVAR (n = 34) | P |
|---|---|---|---|
| Age (years, mean ± SD) | 48.9 ± 12.2 | 60.6 ± 11.9 | 0.005 |
| Male, n (%) | 11 (84.62) | 24 (70.59) | 0.464 |
| Clinical characteristics, n (%) | |||
| Hypertension | 10 (76.92) | 29 (85.29) | 0.666 |
| PAD | 4 (30.77) | 10 (29.41) | 0.999 |
| Hyperlipidemia | 5 (38.46) | 11 (32.35) | 0.693 |
| Diabetes mellitus | 4 (30.77) | 7 (20.59) | 0.467 |
| Current smoking | 8 (61.54) | 13 (38.24) | 0.666 |
| Marfan syndrome | 1 (7.69) | 1 (2.94) | 0.433 |
| Initial CT findings, n (%) | |||
| Arch involvement | |||
| False lumen located at IAC | 2 (15.38) | 3 (8.82) | 0.607 |
| Pleural fluid | 4 (30.77) | 10 (29.41) | 0.999 |
| Visceral malperfusion | 10 (76.92) | 25 (73.53) | 0.565 |
| SMA | 2 (15.38) | 3 (8.82) | |
| RA | 7 (53.85) | 20 (58.82) | |
| Extremity artery | 1 (7.69) | 2 (5.88) | |
| Large entry tear | 4 (30.77) | 7 (20.59) | 0.706 |
| One-sheet space | 6 (46.15) | 8 (23.53) | 0.163 |
| Multi-barreled | 2 (15.38) | 5 (14.71) | 0.999 |
| Timing of onset, n (%) | 0.905 | ||
| Hyperacute | 2 (15.38) | 5 (14.71) | |
| Acute | 1 (7.69) | 4 (11.76) | |
| Subacute | 4 (30.77) | 12 (35.29) | |
| Chronic | 6 (46.15) | 13 (38.24) | |
| Preoperative laboratory data (mean ± SD) | |||
| BUN (mmol/L) | 6.63 ± 2.71 | 7.41 ± 3.15 | 0.355 |
| Creatinine (μmol/L) | 105.77 ± 25.89 | 81.22 ± 24.77 | 0.001 |
| Hemoglobin (g/L) | 129.8 ± 18.1 | 126.8 ± 22.6 | 0.680 |
| INR | 1.040 ± 0.086 | 1.06 ± 0.13 | 0.279 |
AD: Aortic dissection; TEVAR: Thoracic endovascular aortic repair; PAD: Peripheral artery disease; CT: Computed tomography; IAC: The inner aortic curvature; SMA: Superior mesenteric artery; RA: Renal artery; BUN: Blood urea nitrogen; INR: International normalized ratio; Type B-AD: Type B aortic dissection; IVUS: Intravascular ultrasound; SD: Standard deviation.
Figure 1.
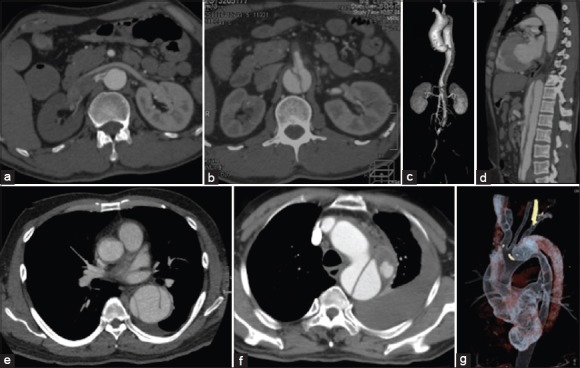
Preoperative computed tomography angiography showed the “complicated” type B aortic dissection. Features of right renal malperfusion with great compression of true lumen (a), with superior mesenteric artery malperfusion (b), with lower limb ischemia (c), with multi-barrel and superior mesenteric artery malperfusion (d), with three barrels (e), with periaortic hematoma and hemorrhagic pleural effusion (f), and with a large tear (g) located in the proximal dissection near the left subclavian artery.
Endograft deployment was successful in all cases. Five cases (5/47) required debranching procedures during the first stage, and endograft repair was performed during the secondary stage. Two of these cases (2/13) were in the IVUS-assisted TEVAR group, and three of these cases (3/34) were in the TEVAR group (P = 0.607). The hybrid procedures with two stages were preferred. The interval time was more than 7 days between the two procedures. In addition, in the IVUS-assisted TEVAR group, one patient [patient 8, Table 2] received TEVAR combined with left common carotid artery (LCCA) chimney stenting to acquire an adequate landing zone.
Table 2.
Presentation and clinical characteristics for IVUS-assisted TEVAR group
| Patient number | Sex/age (years) | Coexisting conditions | Time since diagnosis | Symptoms | Treatment | Follow-up duration |
|---|---|---|---|---|---|---|
| 1 | Male/66 | Hypertension | 35 days | Chest pain | TEVAR (Talent) | 19 months |
| 2 | Male/43 | Hypertension | 67 days | Chest pain, abdominal pain | TEVAR (Zenith) | 34 months |
| 3 | Male/38 | Hypertension | 2 years | Chest pain | TEVAR (Valiant) | 22 months |
| 4 | Male/55 | Marfan syndrome | Postsurgery for 15 years Post-TEVAR for 7 years | Abdominal pain | TEVAR (Valiant) | 21 months |
| 5 | Male/31 | None | 13 days | Chest pain | Aorta-IA + LCCA, TEVAR (Zenith) | 36 months |
| 6 | Male/53 | Hypertension | Post-TEVAR for 5 years | Chest pain | RCCA-LCCA, TEVAR (Valiant) | 32 months |
| 7 | Female/63 | Hypertension | 20 days | Chest pain | TEVAR (Zenith) | 27 months |
| 8 | Male/48 | Hypertension | 14 days | Chest pain | TEVAR (Valiant) + chimney | 29 months |
| 9 | Male/50 | Hypertension | 13 days | Chest pain | TEVAR (Captivia) | 34 months |
| 10 | Male/31 | Hypertension | 1day | Chest pain | TEVAR (Zenith) + SMA stent | 64 days (died) |
| 11 | Male/69 | Hypertension | 16 days | Chest pain | TEVAR (Valiant) | 29 months |
| 12 | Male/47 | Hypertension | 15 days | Chest pain, abdominal pain | TEVAR (Zenith) | 21 months |
| 13 | Female/42 | Hypertension | 14 days | Chest pain | TEVAR (Valiant) | 15 months |
TEVAR: Thoracic endovascular aortic repair; IA: Innominate artery; LCCA: Left common carotid artery; RCCA: Right common carotid artery; SMA: Superior mesenteric artery; IVUS: Intravascular ultrasound.
CTA measurements of the diameter of landing zone ranged from 23.6 to 37.4 mm (mean 30.64 mm) versus 24.7–38.6 mm (mean 31.82 mm) for IVUS in the IVUS-assisted TEVAR group. The average diameter values of IVUS measurements were greater than those estimated by CTA (31.82 ± 4.21 mm vs. 30.64 ± 4.13 mm, P < 0.001). Based on IVUS findings, one case (patient 8) with LCCA partly covered underwent a chimney stent for bailout. One case (patient 10) was found the static ischemia in superior mesenteric artery (SMA) by IVUS after TEVAR, and with a bare stent implantation in SMA. Unfortunately, this patient died of multiple organ dysfunctions at 64 days after the procedure.
The technique success rate was 100%. Postoperative outcomes are analyzed in Table 3. A statistical difference emerged between the IVUS-assisted TEVAR and TEVAR groups for total operative time (89.3 ± 43.3 vs. 116.4 ± 26.7 min; P = 0.013). We also found a statistically significant difference between the two groups regarding the amount of contrast medium (101.3 ± 18.2 vs. 133.7 ± 17.6 ml; P < 0.001). Wound infection and percutaneous access-related complications were not found in the groups.
Table 3.
Comparison of perioperative outcomes between IVUS-assisted TEVAR and TEVAR groups
| Variables | IVUS-assisted TEVAR (n = 13) | TEVAR (n = 34) | P |
|---|---|---|---|
| Stent graft types, n (%) | |||
| Valiant | 6 (46.15) | 15 (44.12) | 0.931 |
| Talent | 1 (7.69) | 2 (5.88) | |
| Captiva | 1 (7.69) | 5 (14.71) | |
| Zenith TX2 | 5 (38.46) | 12 (35.29) | |
| Operation time (min) | 89.3 ± 43.3 | 116.4 ± 26.7 | 0.013 |
| Blood loss (ml) | 87.3 ± 45.0 | 84.6 ± 29.8 | 0.812 |
| Intubation time (min) | 119.69 ± 39.44 | 122.09 ± 29.88 | 0.309 |
| Contrast dosage (ml) | 101.3 ± 18.2 | 133.7 ± 17.6 | 0.001 |
| Postoperative laboratory data | |||
| BUN (mmol/L) | 6.83 ± 2.70 | 7.52 ± 3.20 | 0.457 |
| Creatinine (μmol/L) | 82.38 ± 28.76 | 85.00 ± 27.92 | 0.924 |
| Hemoglobin (g/L) | 125.4 ± 17.3 | 116.5 ± 20.5 | 0.177 |
| INR | 1.06 ± 0.04 | 1.09 ± 0.15 | 0.053 |
| Length of stay (days) | 10.2 ± 2.8 | 12.2 ± 3.8 | 0.087 |
| Follow-up period (months) | 24.7 ± 9.4 | 23.3 ± 8.9 | 0.646 |
Data are shown as n (%) or mean ± SD. TEVAR: Thoracic endovascular aortic repair; BUN: Blood urea nitrogen; INR: International normalized ratio; IVUS: Intravascular ultrasound; SD: Standard deviation.
Follow-up ranged from 15 to 36 months (mean, 24.7 ± 9.4 months) for the IVUS-assisted TEVAR group and from 10 to 35 months (mean, 23.3 ± 8.9 months) for the TEVAR group (P = 0.646). The adverse events during follow-up in the two groups are shown in Table 4. One of the two patients who died in the TEVAR group died of retrograde dissection 6 h after the TEVAR procedure in the ward, and the diagnosis for pericardiocentesis was confirmed. The other patient also died in the hospital after TEVAR after experiencing sudden hypotension, the inability to breathe, and the halting of circulation. The rate of endoleak in the IVUS-assisted TEVAR group was 23.08%. Only one type IA endoleak in this group was found intraoperatively, and it was treated using balloon dilatation and cuff stent graft implantation. This case was closely watched and was free of new endoleak during the follow-up period. The other two cases of type II endoleak were both under surveillance. Compared with the IVUS-assisted TEVAR group for endoleak, the post-CTA showed the presence of type II endoleak in four cases in the TEVAR group (P = 0.377). In the TEVAR group, one case with a type II endoleak and a suspected type IA endoleak was treated by stage. Incidences of aneurysmal dilatation, retrograde dissection, organ ischemia, SINE, and spinal cord ischemia were also analyzed. Among these AD-related adverse events, no statistical difference emerged between the two groups.
Table 4.
Events during the follow-up
| Variables | IVUS-assisted TEVAR (n = 13) n (%) | TEVAR (n = 34) n (%) | P |
|---|---|---|---|
| AD-related death | 1 (7.69) | 2 (5.88) | 0.999 |
| AD-related events | |||
| Endoleak | 3 (23.08) | 4 (11.76) | 0.377 |
| Type IA | 1 (7.69) | 0 (0) | |
| Type IB | 0 (0) | 0 (0) | |
| Type II | 2 (15.38) | 4 (11.76) | |
| Aneurysmal dilatation (false lumen) | 3 (23.08) | 5 (14.71) | 0.667 |
| Retro-dissection | 0 (0) | 1 (2.94) | 0.999 |
| Organ ischemia | 1 (7.69) | 2 (5.88) | 0.999 |
| SINE | 0 (0) | 1 (2.94) | 0.999 |
| Spinal cord ischemia | 0 (0) | 1 (2.94) | 0.999 |
AD: Aortic dissection; TEVAR: Thoracic endovascular aortic repair; SINE: Stent-induced new entry; IVUS: Intravascular ultrasound.
In Patient 11 in the IVUS-assisted TEVAR group, the first entry tear (diameter, 9 mm) was 20 mm distal to the LSA, and CTA demonstrated signs of multi-barrel and multi-entries. With the assistance of intraoperative IVUS, the true lumen was confirmed as soon as possible. Then, a valiant stent graft was successfully implanted. After TEVAR, IVUS imaging showed the enlargement of and return of pulsatile flow to the true lumen with stagnation of flow in the false lumen. Follow-up CTA at 26 months revealed satisfactory exclusion of the primary entry, complete thrombosis of the false lumen, and pleural effusion absorption [Figure 2].
Figure 2.
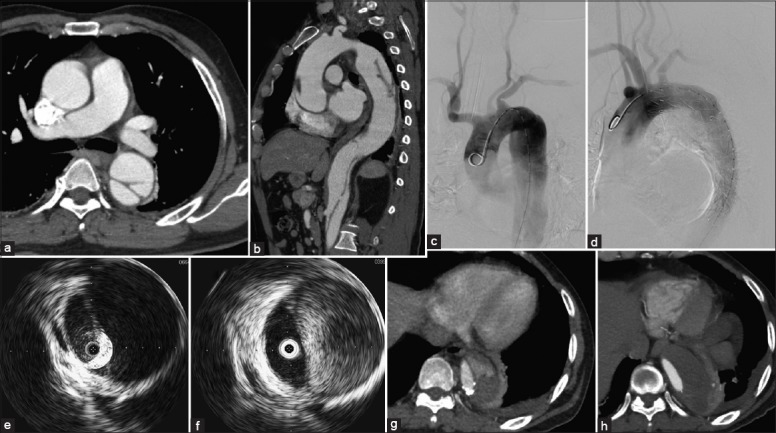
(a and b) Preoperative computed tomography angiography in the intravascular ultrasound-assisted group (patient 11) demonstrated the diameter of the first entry tear was 9 mm and was 20 mm distal to the left subclavian artery. The false lumen located at the outer aortic curvature, whereas the true lumen with great compression at the descending aorta. Fortunately, the superior mesenteric artery generated from the true lumen. The left renal artery from the false lumen was not compromised; the right renal artery has thrombosed. (c-f) With the assistance of intraoperative intravascular ultrasound, the case was successfully repaired with a stent graft (c-d). The flap moved remarkably and the high velocity flow in the false lumen was observed in real-time by the intraoperative IVUS before (e) and after TEVAR (f). (g-h) Post-CTA at 13 months showed partial thrombosis at the end of the stent graft (g), whereas thrombosis was fully formed at 26 months follow-up (h).
DISCUSSION
With the advancements in TEVAR of type B-AD, we tend to take a multimodal approach incorporating the preoperative CTA and intraoperative IVUS to bolster our anatomical knowledge and verify our preoperative understanding in real-time. In the present study, we aimed to evaluate the utility of IVUS to assess its impact on TEVAR for “complicated” type B-AD.
We all know that IVUS is a well-established method for diagnosis of type B-AD and for guiding endovascular treatment. It can provide real-time data during aortic interventions, which is helpful for appropriate diagnosis, morphology analysis, graft selections, and deployment.[13] After deployment of the stent graft, IVUS can identify the location of critical visceral vessels related to re-entry sites and assess pulsatility in the true lumen and false lumen [Figures 3 and 4].[16]
Figure 3.
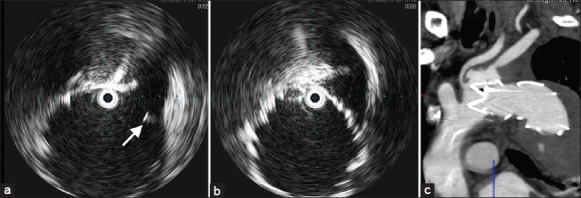
Intravascular ultrasound images after thoracic endovascular aortic repair showed the left common carotid artery was partly covered by the stent graft (a, arrow) and left subclavian artery was totally covered (b). The intraoperative angiography did not show the changes in blood flow in the left common carotid artery, but the intravascular ultrasound images and postoperative computed tomography angiography (c) confirmed the fact.
Figure 4.
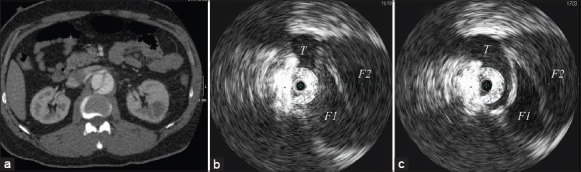
Preoperative computed tomography angiography image (a) demonstrated the renal malperfusion of a three-channeled aortic dissection at the area of the origin of right renal artery. Intravascular ultrasound images before (b) and after (c) thoracic endovascular aortic repair at the same area showed the changes in the three channels and the natural branch flow (T) at the origin of the right renal artery. They also confirmed enlargement of and return of pulsatile flow to the true lumen with stagnation of flow in the false lumen (F1 and F2).
Koschyk et al.[17] compared angiography, TEE, and IVUS intraprocedurally before and after placement of 48 stent grafts in 42 consecutive patients with acute and chronic type B-AD. IVUS and TEE seemed superior to angiography in identifying multiple entries, diagnosing false lumen slow flow and the reperfusion of major branches after stent graft implantation, and detecting incomplete stent apposition. However, TEE was superior to IVUS and angiography for detecting endoleaks.[17] In fact, IVUS is invaluable for providing specialized findings for the complicated morphology AD. Blasco et al.[18] chose pre-established planes to obtain comparable measurements with IVUS and computed tomography (CT). They showed IVUS is a safe procedure without technique-related perioperative complications. On the contrary, Fernandez et al.[19] demonstrated that IVUS measurements, especially those that were not centered along the aortic arch or in a particularly tortuous portion of the aorta, might be less accurate than centerline three-dimensional CT scan measurements. They found that in 66% of measurements, IVUS yielded a value that was larger than the CT scan measurement, and in 11% the difference in measurements was at least 5 mm when compared with CT centerline measurements. Our study was similar to Fernandez et al.'s that IVUS measurements of the diameter of landing zone was greater than those estimated by CTA (P < 0.01).[19] And the measurements of CTA in landing zones ranged from 23.6 to 37.4 mm (mean 30.64 mm) versus 24.7–38.6 mm (mean 31.82 mm) for IVUS. The stent-graft choice mainly depended on the measurements of preoperative CTA, IVUS, and digital subtraction angiography (DSA) information was as the complement to guide our decision-making process.
With the incorporation of the real-time IVUS imaging, we could easily confirm the condition of the proximal of the landing zone, discriminate true and false lumen according to the three layer sign of the true lumen on cross-sectional images, and demonstrate the location and diameter of entries as well as the origins (true or false lumen) of branches. Therefore, one great benefit of IVUS as an adjunct is that it allows contrast use to be significantly limited. This is of specific value in patients with renal insufficiency, which is not uncommonly seen in AD with renal malperfusion. Hoshina et al.[20] reported patients in the IVUS group required fewer intra-arterial contrast agents than those in the non-IVUS group during endovascular aneurysm repair (67 ± 34 ml vs. 123 ± 50 ml; P < 0.01). And, they recommended the routine use of IVUS in EVAR procedures. Similarly, in our study the patients with renal dysfunction were priority to the IVUS-assisted TEVAR group, and the creatinine preoperative between the two groups was statistically different (P < 0.01). Also, the mean amount of contrast medium used in the IVUS-assisted TEVAR group was 101.3 ± 18.2 ml, and in the TEVAR group it was 133.7 ± 17.6 ml. There was a significant difference between the groups (P < 0.01). In the traditional TEVAR group, we usually discriminated the true and false lumen and confirmed the branch arteries perfusion by angiography practice. In fact, it was so difficult to make the true lumen out especially in the multi-barreled AD by angiography that much more dosage of contrast medium was needed in multiple perspectives. On the contrary, IVUS could easily distinguish the true lumen from the false lumen, identify the patency of critical visceral vessels and assess pulsatility in the true lumen and false lumen accurately. Therefore, if IVUS verified a satisfying result, the subsequent angiography could be avoided. Additionally, for patients with “complicated” AD, IVUS may help decrease the operative time. As in the present study, the total operative time between IVUS-assisted TEVAR and TEVAR groups also demonstrated a statistically significant difference (89.3 ± 43.3 vs. 116.4 ± 26.7 min; P = 0.013).
Patients with severe malperfusion syndrome or hemodynamic instability at presentation should be considered at high risk for death.[21,22] IRAD trial data showed much higher in-hospital mortality after medical therapy with refractory hypertension or pain compared with those without these features (35.6% vs. 1.5%; P < 0.001).[23] Ueda et al.[24] reported that detection of the bird-beak configuration was helpful in the prediction of type I or II endoleak after TEVAR (P < 0.01). Similarly, Sueyoshi et al.[25] found that presence of multi-barreled AD on CTA was a powerful predictor of AD-related death compared with double-barreled AD. Imaging features of a large entry tear, a partially thrombosed false lumen, circular configuration of a true lumen, and a false lumen located at the inner aortic curvature on the first CTA were found to be associated with positive aortic growth. Patients with these predictors might benefit from more surveillance or early prophylactic intervention.[26,27,28] In these circumstances, IVUS could assist to provide the full perioperative imaging evaluation for successful endovascular procedures.
Compared with the currently used imaging techniques such as CTA, MRA, TEE, and angiography, IVUS still has the following limitations: (1) It is an invasive procedure that can cause potential damage; (2) because of the high cost, IVUS cannot be used for regular examination for TEVAR or for follow-up;[29] (3) during insertion of the IVUS catheter, inadvertent manipulation may cause new entry or development of AD, especially in complicated morphology AD; (4) when the IVUS catheter crosses the smaller vessels (such as coronary arteries) or severe stenosis, incidences of arterial spasm, dissection, or acute thrombosis may be caused, However, morbidity has been <3%;[30,31] and (5) IVUS accuracy is limited with respect to measurements in tortuous or curved areas, such as the aortic arch.[19]
Admittedly, there were several limitations to the study. Most notably, the limited numbers of patients were not randomized to the different groups. Patients with this high-risk disease often hesitated to this invasive procedure, although IVUS would be very useful in some of hyperacute or acute cases, especially for the renal dysfunction. For another, the protocol for the patient selection was hard to be standard, which also lead to the selection bias in the results. Another variable that should be considered was the five patients (10%) lost to follow-up. If they were assumed to have dead, this would influence the results. The variety of symptoms and procedures within the cohort in our single center also took some bias in the results. However, under such hostile situation, we had enrolled 13 patients in IVUS-assisted TEVAR group and 34 in the control group to evaluate the feasibility and safety of IVUS in TEVAR for “complicated” type B-AD, and most of them were followed up. To the best of our knowledge, the present study first compared the two groups in complicated morphology AD. For these reasons, we only aimed to share our experiences with clinicians to better understand the valuable utility of IVUS.
In conclusion, IVUS played a feasible and safe role in TEVAR and especially benefited patients with “complicated” type B-AD. Nevertheless, IVUS can provide helpful insight regarding the morphology of the dissection, which may influence stent placement and decrease procedural time and contrast dosage. However, we only used preoperative CTA, and DSA in conjunction with IVUS to obtain a picture that was as complete as possible to guide our decision-making process. Therefore, the value of IVUS in evaluation and treatment of AD cannot be overstated.
Financial support and sponsorship
This study was supported by grants from the Key Program of Clinical Medicine from Ministry of Public Health, China (No. 08XD1401200) and the Outstanding Leading Researcher Program of Shanghai (No.2010038).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Peng Lyu and Jian Gao
REFERENCES
- 1.Grabenwöger M, Alfonso F, Bachet J, Bonser R, Czerny M, Eggebrecht H, et al. Thoracic Endovascular Aortic Repair (TEVAR) for the treatment of aortic diseases: A position statement from the European Association for Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Eur J Cardiothorac Surg. 2012;42:17–24. doi: 10.1093/ejcts/ezs107. [DOI] [PubMed] [Google Scholar]
- 2.Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE, Jr, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121:e266–369. doi: 10.1161/CIR.0b013e3181d4739e. [DOI] [PubMed] [Google Scholar]
- 3.Fattori R, Cao P, De Rango P, Czerny M, Evangelista A, Nienaber C, et al. Interdisciplinary expert consensus document on management of type B aortic dissection. J Am Coll Cardiol. 2013;61:1661–78. doi: 10.1016/j.jacc.2012.11.072. [DOI] [PubMed] [Google Scholar]
- 4.Kang SJ, Ahn JM, Kim WJ, Lee JY, Park DW, Lee SW, et al. Intravascular ultrasound assessment of drug-eluting stent coverage of the coronary ostium and effect on outcomes. Am J Cardiol. 2013;111:1401–7. doi: 10.1016/j.amjcard.2013.01.291. [DOI] [PubMed] [Google Scholar]
- 5.Hitchner E, Zayed MA, Lee G, Morrison D, Lane B, Zhou W. Intravascular ultrasound as a clinical adjunct for carotid plaque characterization. J Vasc Surg. 2014;59:774–80. doi: 10.1016/j.jvs.2013.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zacharatos H, Hassan AE, Qureshi AI. Intravascular ultrasound: Principles and cerebrovascular applications. AJNR Am J Neuroradiol. 2010;31:586–97. doi: 10.3174/ajnr.A1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diethrich EB, Irshad K, Reid DB. Virtual histology and color flow intravascular ultrasound in peripheral interventions. Semin Vasc Surg. 2006;19:155–62. doi: 10.1053/j.semvascsurg.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Joan MM, Moya BG, Agustí FP, Vidal RG, Arjona YA, Alija MP, et al. Utility of intravascular ultrasound examination during carotid stenting. Ann Vasc Surg. 2009;23:606–11. doi: 10.1016/j.avsg.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Lee JT, Fang TD, White RA. Applications of intravascular ultrasound in the treatment of peripheral occlusive disease. Semin Vasc Surg. 2006;19:139–44. doi: 10.1053/j.semvascsurg.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Marrocco CJ, Jaber R, White RA, Walot I, DeVirgilio C, Donayre CE, et al. Intravascular ultrasound. Semin Vasc Surg. 2012;25:144–52. doi: 10.1053/j.semvascsurg.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Song TK, Donayre CE, Kopchok GE, White RA. Intravascular ultrasound use in the treatment of thoracoabdominal dissections, aneurysms, and transections. Semin Vasc Surg. 2006;19:145–9. doi: 10.1053/j.semvascsurg.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Pearce BJ, Jordan WD., Jr Using IVUS during EVAR and TEVAR: Improving patient outcomes. Semin Vasc Surg. 2009;22:172–80. doi: 10.1053/j.semvascsurg.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 13.Lee JT, White RA. Basics of intravascular ultrasound: An essential tool for the endovascular surgeon. Semin Vasc Surg. 2004;17:110–8. doi: 10.1053/j.semvascsurg.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 14.von Segesser LK, Marty B, Ruchat P, Bogen M, Gallino A. Routine use of intravascular ultrasound for endovascular aneurysm repair: Angiography is not necessary. Eur J Vasc Endovasc Surg. 2002;23:537–42. doi: 10.1053/ejvs.2002.1657. [DOI] [PubMed] [Google Scholar]
- 15.Fu WG, Shi Y, Wang YQ, Guo DQ, Xu X, Chen B, et al. Endovascular therapy for stanford type B aortic dissection in 102 cases. Asian J Surg. 2005;28:271–6. doi: 10.1016/S1015-9584(09)60359-6. [DOI] [PubMed] [Google Scholar]
- 16.White RA, Donayre CE, Walot I, Kopchok GE. Intraprocedural imaging: Thoracic aortography techniques, intravascular ultrasound, and special equipment. J Vasc Surg. 2006;43(Suppl A):53A–61. doi: 10.1016/j.jvs.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Koschyk DH, Nienaber CA, Knap M, Hofmann T, Kodolitsch YV, Skriabina V, et al. How to guide stent-graft implantation in type B aortic dissection? Comparison of angiography, transesophageal echocardiography, and intravascular ultrasound. Circulation. 2005;112(9 Suppl):I260–4. doi: 10.1161/CIRCULATIONAHA.104.525972. [DOI] [PubMed] [Google Scholar]
- 18.Blasco A, Piazza A, Goicolea J, Hernández C, García-Montero C, Burgos R, et al. Intravascular ultrasound measurement of the aortic lumen. Rev Esp Cardiol. 2010;63:598–601. doi: 10.1016/s1885-5857(10)70122-5. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez JD, Donovan S, Garrett HE, Jr, Burgar S. Endovascular thoracic aortic aneurysm repair: Evaluating the utility of intravascular ultrasound measurements. J Endovasc Ther. 2008;15:68–72. doi: 10.1583/07-2215.1. [DOI] [PubMed] [Google Scholar]
- 20.Hoshina K, Kato M, Miyahara T, Mikuriya A, Ohkubo N, Miyata T. A retrospective study of intravascular ultrasound use in patients undergoing endovascular aneurysm repair: Its usefulness and a description of the procedure. Eur J Vasc Endovasc Surg. 2010;40:559–63. doi: 10.1016/j.ejvs.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 21.Fattori R, Tsai TT, Myrmel T, Evangelista A, Cooper JV, Trimarchi S, et al. Complicated acute type B dissection: Is surgery still the best option?: A report from the International Registry of Acute Aortic Dissection. JACC Cardiovasc Interv. 2008;1:395–402. doi: 10.1016/j.jcin.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Fattori R, Montgomery D, Lovato L, Kische S, Di Eusanio M, Ince H, et al. Survival after endovascular therapy in patients with type B aortic dissection: A report from the International Registry of Acute Aortic Dissection (IRAD) JACC Cardiovasc Interv. 2013;6:876–82. doi: 10.1016/j.jcin.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Trimarchi S, Eagle KA, Nienaber CA, Pyeritz RE, Jonker FH, Suzuki T, et al. Importance of refractory pain and hypertension in acute type B aortic dissection: Insights from the International Registry of Acute Aortic Dissection (IRAD) Circulation. 2010;122:1283–9. doi: 10.1161/CIRCULATIONAHA.109.929422. [DOI] [PubMed] [Google Scholar]
- 24.Ueda T, Fleischmann D, Dake MD, Rubin GD, Sze DY. Incomplete endograft apposition to the aortic arch: Bird-beak configuration increases risk of endoleak formation after thoracic endovascular aortic repair. Radiology. 2010;255:645–52. doi: 10.1148/radiol.10091468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sueyoshi E, Nagayama H, Hayashida T, Sakamoto I, Uetani M. Comparison of outcome in aortic dissection with single false lumen versus multiple false lumens: CT assessment. Radiology. 2013;267:368–75. doi: 10.1148/radiol.12121274. [DOI] [PubMed] [Google Scholar]
- 26.Tolenaar JL, van Keulen JW, Jonker FH, van Herwaarden JA, Verhagen HJ, Moll FL, et al. Morphologic predictors of aortic dilatation in type B aortic dissection. J Vasc Surg. 2013;58:1220–5. doi: 10.1016/j.jvs.2013.05.031. [DOI] [PubMed] [Google Scholar]
- 27.Tolenaar JL, van Keulen JW, Trimarchi S, Jonker FH, van Herwaarden JA, Verhagen HJ, et al. Number of entry tears is associated with aortic growth in type B dissections. Ann Thorac Surg. 2013;96:39–42. doi: 10.1016/j.athoracsur.2013.03.087. [DOI] [PubMed] [Google Scholar]
- 28.van Bogerijen GH, Tolenaar JL, Rampoldi V, Moll FL, van Herwaarden JA, Jonker FH, et al. Predictors of aortic growth in uncomplicated type B aortic dissection. J Vasc Surg. 2014;59:1134–43. doi: 10.1016/j.jvs.2014.01.042. [DOI] [PubMed] [Google Scholar]
- 29.Hu W, Schiele F, Meneveau N, Seronde MF, Legalery P, Caulfield F, et al. Value of intravascular ultrasound imaging in following up patients with replacement of the ascending aorta for acute type A aortic dissection. Chin Med J. 2008;121:2139–43. [PubMed] [Google Scholar]
- 30.Hausmann D, Erbel R, Alibelli-Chemarin MJ, Boksch W, Caracciolo E, Cohn JM, et al. The safety of intracoronary ultrasound. A multicenter survey of 2207 examinations. Circulation. 1995;91:623–30. doi: 10.1161/01.cir.91.3.623. [DOI] [PubMed] [Google Scholar]
- 31.Ramasubbu K, Schoenhagen P, Balghith MA, Brechtken J, Ziada KM, Kapadia SR, et al. Repeated intravascular ultrasound imaging in cardiac transplant recipients does not accelerate transplant coronary artery disease. J Am Coll Cardiol. 2003;41:1739–43. doi: 10.1016/s0735-1097(03)00339-5. [DOI] [PubMed] [Google Scholar]


