Supplemental Digital Content is available in the text.
Abstract
Background:
The relationship between arsenic and birth weight is not well understood. The objective was to evaluate the causal relationship between prenatal arsenic exposure and birth weight considering the potential mediation effects of gestational age and maternal weight gain during pregnancy using structural equation models.
Methods:
A prospectively enrolled cohort of pregnant women was recruited in Bangladesh from 2008 to 2011. Arsenic was measured in personal drinking water at the time of enrollment (gestational age <16 weeks, N = 1,140) and in toenails collected ≤1 month postpartum (N = 624) using inductively coupled plasma mass spectrometry. Structural equation models estimated the direct and indirect effects of arsenic on birth weight with gestational age and maternal weight gain considered as mediating variables.
Results:
Every unit increase in natural log water arsenic was indirectly associated with decreased birth weight (β = −19.17 g, 95% confidence interval [CI]: −24.64, −13.69) after adjusting for other risk factors. This association was mediated entirely through gestational age (β = −17.37 g, 95% CI: −22.77, −11.98) and maternal weight gain during pregnancy (β = −1.80 g, 95% CI: −3.72, 0.13). When exposure was modeled using toenail arsenic concentrations, similar results were observed. Every increase in natural log toenail arsenic was indirectly associated with decreased birth weight (β = −15.72 g, 95% CI: −24.52, −6.91) which was mediated through gestational age (β = −13.59 g, 95% CI: −22.10, −5.07) and maternal weight gain during pregnancy (β = −2.13 g, 95% CI: −5.24, 0.96).
Conclusion:
Arsenic exposure during pregnancy was associated with lower birth weight. The effect of arsenic on birth weight appears to be mediated mainly through decreasing gestational age and to a lesser extent by lower maternal weight gain during pregnancy.
Arsenic-contaminated drinking water is a global health concern.1 A naturally occurring element, inorganic arsenic can dissolve in groundwater and lead to human exposure if the contaminated aquifer is used as a source of drinking water or to irrigate crops that accumulate arsenic such as rice.2,3 This is particularly problematic in Bangladesh, where public health interventions intended to reduce the incidence of waterborne disease switched the primary drinking water source from surface water to groundwater.4 It is estimated that 46% of the population in Bangladesh is exposed to arsenic concentrations above the World Health Organization’s drinking water recommendation of 10 μg/L, and 27% are exposed to levels above the Bangladesh government’s recommendation of 50 μg/L.5 There are at least 19 other countries, including Taiwan, Mexico, Chile, Argentina, Vietnam, Laos, India, China, Romania, and the United States, that have groundwater aquifers that are naturally contaminated with arsenic at levels exceeding the World Health Organization drinking water recommendation.6 Many of these aquifers are positioned below densely populated regions, leading to millions of people being chronically exposed to arsenic.1
Bangladesh is also among the top 10 countries with the highest preterm birth rate (<37 weeks of gestation).7 For instance, a large cohort study of 32,126 pregnant women in rural Bangladesh reported a risk of preterm birth of 22.3%,8 and the incidence of low birth weight babies is estimated to be 31%–47%, which is among the highest in the world.9 There are several recommended behavioral, nutritional, clinical, and health systems interventions that have been shown to reduce the preterm birth rate; however, none of these addresses common environmental risk factors, such as arsenic. Many studies have shown that arsenic can cross through the placenta leading to fetal exposure.10–13 There is epidemiologic evidence that exposure to elevated levels of arsenic in drinking water is related to higher rates of spontaneous abortion14,15 and neonatal death.16,17 Several studies have also examined the relationship between arsenic exposure and birth weight, with mixed results. An ecologic study in Taiwan observed that infants (N = 3,872) born into arsenic-exposed villages (ranging from 0.15 μg/L to 3.59 mg/L) were, on average, 29 g (95% CI: 13.6–44.6 g) lighter than infants (N = 14,387) born into nonarsenic-exposed villages (<0.9 μg/L) after adjusting for confounders.18 Whereas a different ecologic study performed in Mongolia observed that infants (N = 9,890) born in arsenic-exposed villages (>100 μg/L) were, on average, 50 g heavier than infants born in nonarsenic-exposed villages (<20 μg/L).16 Prospective cohort studies conducted in Chile and Bangladesh, however, observed a dose-dependent relationship between arsenic measured in drinking water,19 maternal hair,20 or maternal urine21 on lower mean birth weight although the magnitude and precision of the reported relationship differed between studies. Interestingly, the largest of these prospective studies (N = 1,578) only observed a negative relationship between exposure and birth weight when maternal urinary arsenic concentrations were <100 μg/L.21
In addition, arsenic exposure may exacerbate factors that can contribute to low birth weight, including gestational age and maternal health.22 For instance, arsenic exposure is related to increased risk of nausea and vomiting during pregnancy,19 which in turn may decrease maternal weight gain during pregnancy22 and contribute to poor maternal nutritional status, particularly among populations that experience chronic nutritional stress.23–26 Arsenic exposure has also been shown to be associated with increased risk of premature birth.27 Structural equation models (SEMs) provide an opportunity to examine highly intercorrelated factors that may lie on the pathway between arsenic exposure and birth weight, including factors such as maternal weight gain during pregnancy.28,29 This approach has also been widely used to account for gestational age which is a strong predictor of birth weight and has been shown in many studies to be an important intermediate in the causal pathway between an exposure and birth weight.30,31 Therefore, we examined the direct and indirect effects of arsenic exposure on birth weight in a population-based birth cohort recruited in Bangladesh using a causal pathway approach with SEMs. Specifically, we hypothesized that arsenic exposure would be associated with decreased maternal weight gain during pregnancy and decreased gestational age and that these two variables would mediate the effect between arsenic exposure and reduced birth weight.
METHODS
Study Population and Subject Selection
We established a prospective birth cohort in the Sirajdikhan and Pabna Sadar Upazilas of Bangladesh. The objective of this cohort was to observe the effects of chronic moderate arsenic exposure on reproductive outcomes. These districts were selected as the study areas because (1) a national survey conducted by the British Geological Survey indicated that the average concentration of arsenic in the groundwater in these areas was more moderate than other regions in Bangladesh and spanned a wide range of concentrations,5 (2) Dhaka Community Hospital Trust (DCH) operates rural health clinics in these districts that offer prenatal care and promote arsenic awareness by encouraging people to drink water only from wells that comply with the Bangladesh drinking water arsenic standard of ≤50 μg/L, and (3) the clinics serve demographically similar populations.32
Women were eligible to participate in the study if they were 18 years or older with a singleton pregnancy ≤16 weeks’ gestation confirmed by ultrasound at the time of enrollment, planned to continue receiving prenatal care through DCH, had used the same drinking water source for at least the 6 months before enrollment, and intended to live at the same household throughout pregnancy. This analysis used data from participants (N = 1,613) who were enrolled into the cohort between January 2008 to June 2011. After exclusion due to loss of contact with participants (n = 123), study withdrawal (n = 125), stillbirth (n = 75), miscarriage (n = 132), missing drinking water sample at enrollment and/or missing information on environmental tobacco smoke (n = 2), and nonsingleton pregnancy (n = 4) the sample size was 1,153.
Ethical Consideration
Study protocols were approved by the Human Research Committees at Dhaka Community Hospital, Harvard School of Public Health and Oregon State University. The cohort was recruited in villages where DCH has actively engaged in arsenic-awareness campaigns and safe water options were available. All participants were able to request a technician to test their water for arsenic using a field test kit and were given the results immediately. In addition, all participants were informed if their water samples contained arsenic above the Bangladesh standard after analysis by inductively coupled plasma mass spectrometry. Participants were also provided with free prenatal vitamins throughout their pregnancies. Free transportation to a DCH hospital was available to all participants in case of a pregnancy-related emergency. Consent documents were provided to participants in Bengali and read aloud by trained staff. All participants provided consent before participation in the study.
Exposure Assessment
Arsenic was measured in maternal drinking water samples (N = 1,140) at the time of enrollment and has been described previously.22 In brief, nitric acid-preserved water samples were analyzed for total arsenic concentrations by inductively coupled plasma mass spectrometry following US EPA method 200.8 (Environmental Laboratory Services, North Syracuse, NY). The samples that were below the limit of detection of 1 μg As/L (N = 252) were reassigned half the value of the limit of detection for statistical analysis.
Toenail clippings were collected from participants at ≤1 month postpartum. Arsenic concentrations in these nails reflect the cumulative exposure across the prenatal period since it can take several months to up to 1 year for nails to grow to the free edge of the plate where they can be collected.33 Samples were sonicated in 1% Triton X-100 solution (Sigma-Aldrich, Inc., St. Louis, MO) and rinsed repeatedly with Milli-Q water (Millipore Corporation, Billerica, MA) to remove external contamination before microwave acid digestion using Trace Select Ultra Pure nitric acid (HNO3; Sigma-Aldrich, Inc.). Digested samples were diluted with Milli-Q water and analyzed for total arsenic using an inductively coupled plasma mass spectrometer (Perkin-Elmer Model DRC-II 6100, Norwalk, CT). The reported arsenic concentrations were blank-corrected and then further corrected for systemic error by normalizing the sample concentrations using the arsenic concentration of the batch-specific certified human hair reference material (CRM Hair; Shanghai Institute of Nuclear Research, Academia Sinica, China). Of the 641 toenail samples with arsenic measurements available, samples were excluded if the mass was ≤5 mg (n = 3) and/or if the relative standard deviation ≥25% (n = 8). One sample was below the sample limit of detection (which ranged from 0.09–0.7 ng/g) and was reassigned half the value of the limit of detection for statistical analysis. This left a total of 629 samples included in this analysis.
Birth Weight and Covariates
Women were followed throughout their pregnancies with three scheduled clinical visits that occurred at the time of enrollment, approximately 28 weeks gestational age and ≤1 month post-delivery. During these clinical visits, trained interviewers used structured questionnaires to collect sociodemographic, medical, and environmental information. After their first clinical visit which occurred at the time of enrollment, trained health care providers visited participants in their homes once per month to distribute prenatal vitamins, record symptoms, weigh participants, and measure their blood pressure. All births were attended by trained health care workers. Birth weight was measured on a pediatric scale which was calibrated before each measurement and rounded to the nearest 10 g. Length and head circumference were measured using standard protocols. Approximately 46% of birth anthropometry was measured at a hospital or clinic with the remainder occurring at the participant’s home. The same survey instruments and staff were used to collect information in the participants’ home and in the clinic and hospital.
Maternal weight gain over the follow-up period (kg/week) was calculated by subtracting weight obtained before delivery from weight measured at the time of enrollment divided by the amount of weeks of follow up. Birth gestational age (weeks) was estimated from ultrasound measurements collected at the time of enrollment. Other covariates that were considered as potential confounders included infant sex, maternal education (illiterate, primary, or secondary), body mass index (BMI) at the time of enrollment (kg/m2), exposure to secondhand tobacco smoke (yes/no), chewing betel nut (yes/no), birth type (Caesarean/vaginal), birth location (home/clinic-hospital), and maternal age in years (continuous).
Statistical Analysis
Descriptive statistics were computed for all variables. Arsenic concentrations were skewed and, thus, transformed to their natural log. T test or analysis of variance was used to compare mean birth weight across categories of all covariates in bivariate analyses. To evaluate for homogeneity of variances, Levene’s test was performed for all bivariate comparisons, and a histogram of birth weight indicated no gross violations for the normality assumption. Multivariate linear regression models were used to evaluate the association between ln arsenic and birth weight adjusting for other covariates (e.g., infant sex, maternal education, secondhand tobacco smoke exposure, entry BMI, and maternal age). All numerical variables were model as is with the exception of both exposures which were natural log-transformed.
SEMs were used to evaluate the direct relationship between variables (a1, b1, c1, a2, b2, c2) as well as the direct effect of arsenic on birth weight (c′) controlling for all mediators (Figure 1). The potential indirect effect of arsenic exposure through gestational age on birth weight (a1•b1), as well as, the potential indirect effect through maternal weight gain (a2•b2) were calculated. The total effect of arsenic exposure on birth weight mediated through both mediators was calculated (a1•b1 + a2•b2 + c′), as well as the total mediated effect (a1•b1 + a2•b2). The partial and total effects with respect to each mediator on the outcome of birth weight are reported. Furthermore, we assumed the variance for gestational age at birth and maternal weight gain were correlated and modeled them accordingly. After evaluating the proposed mediation pathways, we further adjusted models for the direct effect of the other covariates (e.g., infant sex, maternal education, secondhand tobacco smoke exposure, entry BMI, birth type, birth location, and maternal age; Figure 2). Confidence intervals and standard errors were computed from 10,000 bootstrap samples for all SEM models. Model fit was evaluated using the comparative fit index, the root mean square error of approximation, overall model χ2 P value, the Tucker–Lewis non-normed index and the standardized root mean squared residual. Modification indices based on χ2 improvement were used to optimally tune the final model fit. The R2 for all endogenous variables was also estimated. The maximum likelihood estimation method was used to estimate all parameters. During the model building, statistical significance was evaluated using a cut off value of α ≤ 0.05, and all tests performed were two-tailed. All statistical analyses were performed in STATA (Version 12.1, StataCorp LP, College Station, TX).
FIGURE 1.
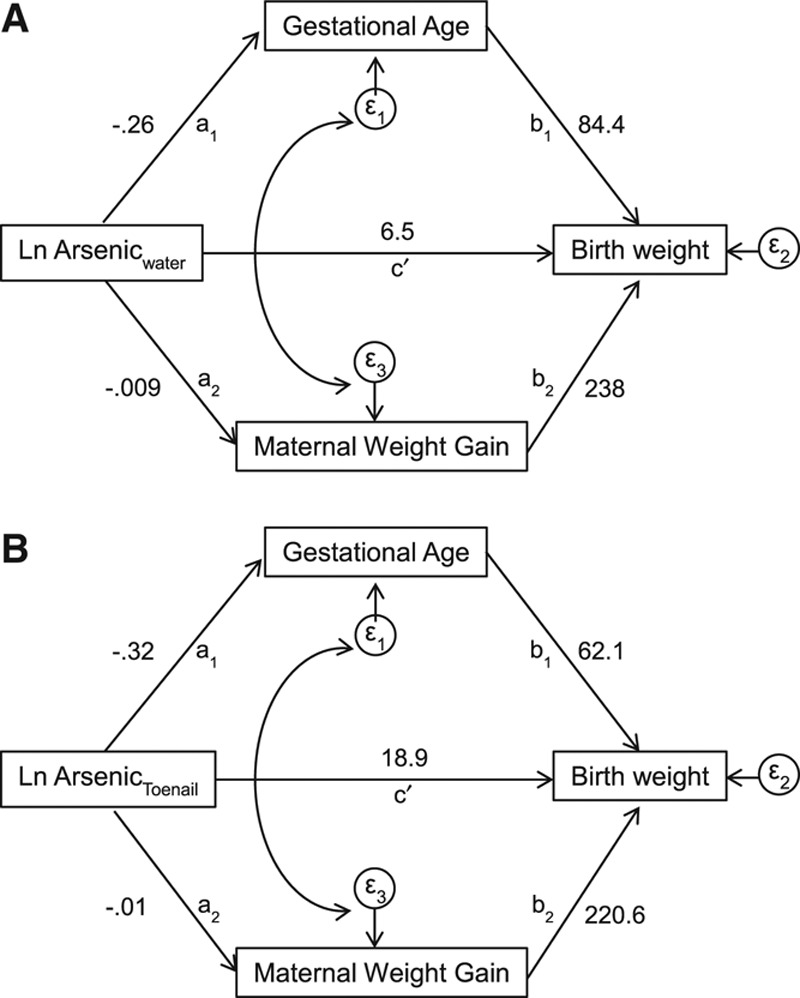
Effect sizes for the initial conceptual SEM model are presented. This model hypothesized that natural log-transformed arsenic water (A) or toenail (B) directly effects (c′) birth weight (g) after adjusting for gestational age (weeks) and maternal weight gain (kg). The partial effects of arsenic on gestational age (a1) and maternal weight gain (a2), as well as, the partial effects of gestational age (b1) and maternal weight gain (b2) on birth weight are presented.
FIGURE 2.
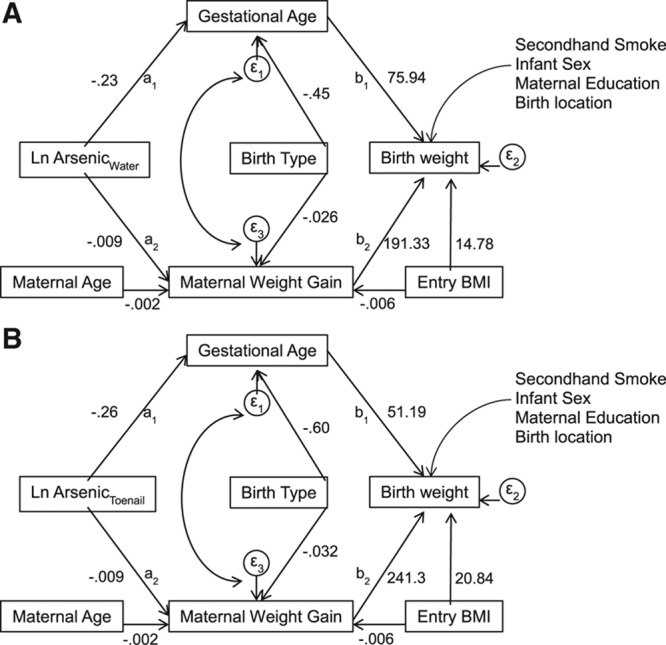
Final SEM models for the indirect effect of log-transformed measured in drinking water arsenic (A) or toenail arsenic (B) on birth weight (g) that is completely mediated through gestational age (weeks) and maternal weight gain (kg) adjusting for other risk factors.
RESULTS
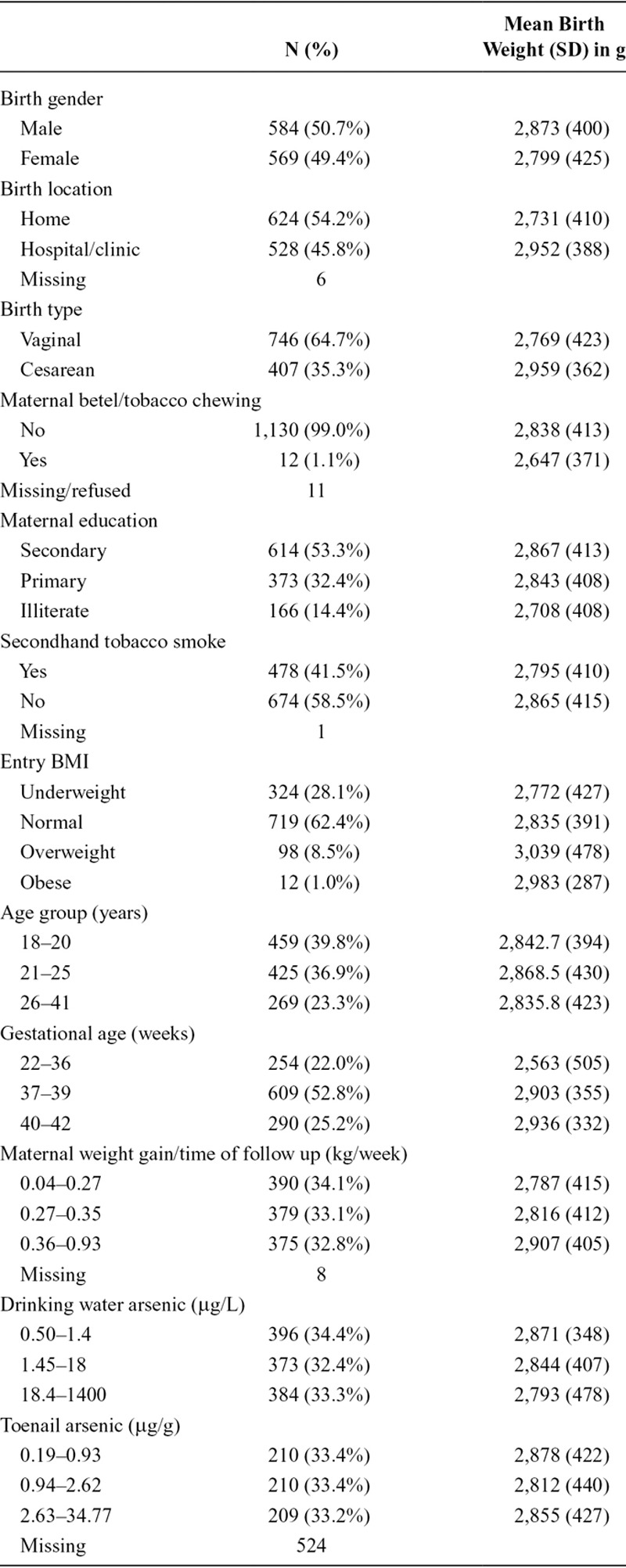
Overall, the average birth weight in this population was 2,836 g (SD: 415 g; range: 800–4,800 g). As anticipated, arsenic exposures were relatively modest with a median concentration of 2.3 μg/L in drinking water at the time of enrollment (interquartile range: 0.9, 36 μg/L) but spanned a wide range (below the limit of detection to 1,400 μg/L). Toenail arsenic was strongly correlated with drinking water exposure (σspearman = 0.49. The median toenail arsenic concentration was of 1.46 μg/g (interquartile range: 0.76, 3.73 μg/g). Other population characteristics and how they are associated with birth weight are presented in Table 1. Birth weight was greater among males, infants not exposed to secondhand tobacco smoke, infants who were born to mothers with higher BMI at study enrollment, infants born to mothers with higher educational attainment, born at a clinic or hospital, Caesarean births, and infants born to mothers who gained the most weight during pregnancy. Increasing birth weight was also strongly associated with gestational age (ρ = 0.41). Parity, expressed as a continuous variable or as a binary variable (uniparous vs. multiparous), was not associated with birth weight.
TABLE 1.
Description of Selected Characteristics and Their Relationship with Birth Weight (N = 1,153)
Initial SEM were developed that included both the direct and indirect effects of arsenic in drinking water and toenail arsenic on birth weight as described in Figure 1. As initially hypothesized, these models showed that arsenic exposure was correlated with shorter gestational age (a1) and reduced maternal weight gain (a2), and in turn, gestational age (b1) and maternal weight gain (b2) were correlated with lower birth weight. In addition, maternal BMI at the time of enrollment was strongly correlated with maternal weight gain (c1) and birth weight (c2; Figure 2). Yet, the estimated direct effect of arsenic on birth weight controlling for both mediators (c′) showed no association. The observed structural relationships were consistent regardless of whether arsenic was measured in drinking water (Figure 1A) or toenail (Figure 1B). These initial models supported the hypothesis that gestational age and maternal weight gain were on the causal pathway between arsenic exposure and birth weight. Contrary to our hypothesis, however, these models indicated no direct effect of arsenic exposure on birth weight. These results suggest that the observed effect of arsenic exposure on birth weight in this population was mediated by its effect on gestational age and maternal weight gain during pregnancy for the follow-up time.
To account for potential confounding, the relationship between arsenic exposure and birth weight was adjusted for the direct effects of the newborn’s sex, mother’s education, environmental tobacco smoke exposure, mother’s age, birth location, birth type, and BMI at enrollment (Figure 2). The modification indices suggested that maternal BMI at time of study enrollment also had an indirect effect on birth weight which was mediated through maternal weight gain during pregnancy. Birth type was only indirectly associated with birth weight through gestational age and maternal weight gain. There was no discernible direct effect of maternal age at enrollment with birth weight. However, age at enrollment had a strong indirect effect on birth weight that was mediated through maternal weight gain. Therefore, the indirect pathways of maternal age and BMI at enrollment were added to all final models.
The results from the fully adjusted SEM that examined the relationship between maternal drinking water arsenic levels and birth weight are presented in Table 2, whereas the results from the fully adjusted SEM that examined the relationship between maternal toenail arsenic levels and birth weight are presented in Table 3. Specifically, arsenic exposure directly reduced gestational age (βwater = −0.23 weeks, 95% CI: −0.28, −0.17; βtoe = −0.26 weeks, 95% CI: −0.40, −0.13) and directly reduced maternal weight gain during pregnancy (βwater = −0.009 kg/week, 95% CI: −0.01, −0.006; βtoe = −0.009 kg, 95% CI: −0.01, −0.0002). In turn, gestational age (weeks) directly increased birth weight (βwater = 75.94 g, 95% CI: 63.46, 88.41; βtoe = 51.19 g, 95% CI: 29.63, 72.74); as did maternal weight gain during pregnancy (kg/week; βwater = 191.33 g, 95% CI: 2.0, 395.26; βtoe = 241.3 g, 95% CI: −85.85, 568.36). Similar to our initial SEM models, there was no direct effect of arsenic exposure on birth weight. These partial direct effects show the negative relationships between arsenic and maternal health characteristics (e.g., gestational age and maternal weight gain during the follow-up period). These maternal characteristics ultimately had a strong positive relationship with birth weight, as did maternal education and BMI at the time of enrollment.
TABLE 2.
Direct and Indirect Effects for the Effect of Natural Log-transformed Arsenic Concentrations in Drinking Water (μg/L) on Birth Weight (g) That Is Mediated by Gestational Age (Weeks) and Maternal Weight Gain (kg) Based on 10,000 Bootstraps Samples (N = 1,140)
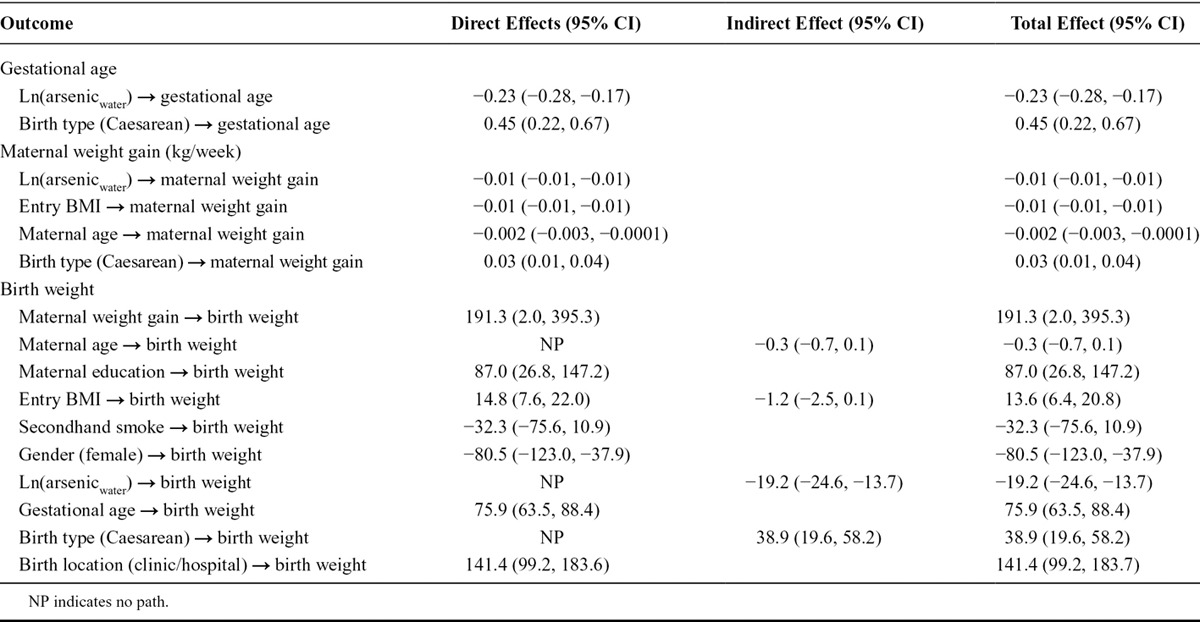
TABLE 3.
Direct and Indirect Effects for the Effect of Natural Log-transformed Arsenic Concentrations in Maternal Toenail (μg/g) on Birth Weight (g) That Is Mediated by Gestational Age (Weeks) and Maternal Weight Gain (kg) Based on 10,000 Bootstraps Sample (N = 624)
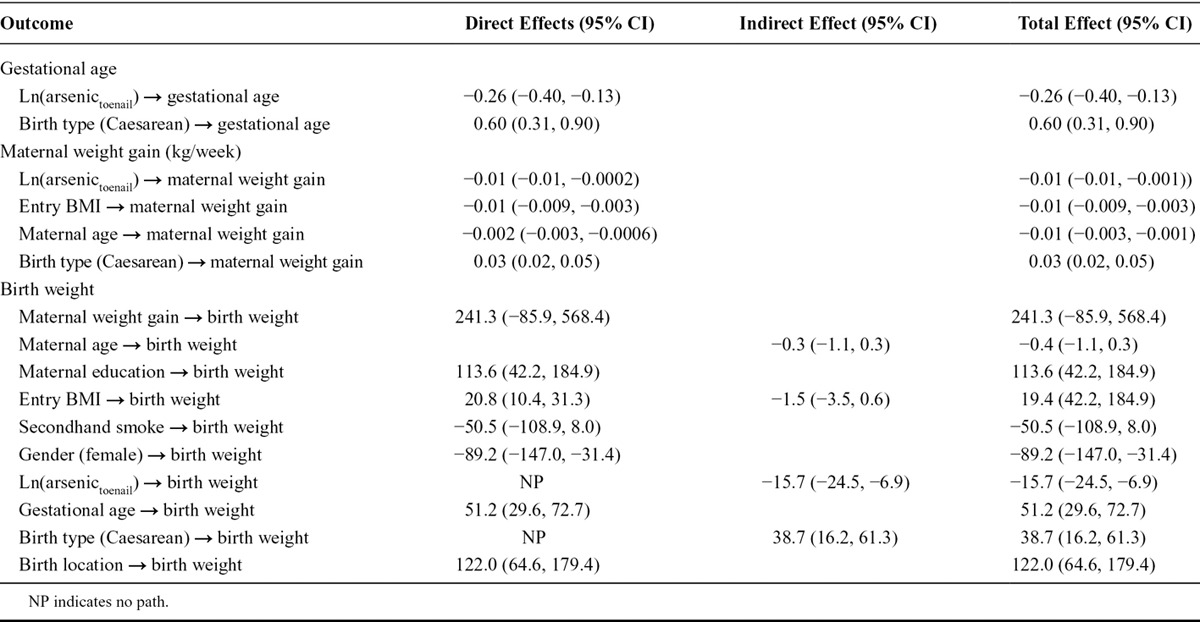
The total mediated effect of arsenic (accounting for the direct and indirect effects) was distributed among the two mediating pathways as described in Table 4. The vast majority of this indirect effect was mediated through birth gestational age (βwater = −17.37 g, 95% CI: −22.77, −11.98; or βtoenail = −13.59 g, 95% CI: −22.10, −5.07) and to a lesser extent through maternal weight gain during pregnancy (βwater = −1.80 g, 95% CI: −3.72, 0.13; or βtoenail = −2.13 g, 95% CI: −5.24, 0.96). Thus, the total indirect effect of arsenic exposure mediated completely through gestational age in weeks and maternal weight gain during pregnancy in kg/week suggested that birth weight would decrease by approximately 16 to 19 g after adjusting for other risk factors for every unit increase in natural log-transformed arsenic in drinking water or maternal toenails. The fit of the final adjusted SEM conformed to all model fit statistics (Tables 5 and 6).
TABLE 4.
Indirect Effects of Natural Log-transformed Arsenic-mediated Through Gestational Age and Mother’s Weight Gain on Birth Weight (g) Based on 10,000 Bootstrap Sample After Adjusting for Infant Sex, Maternal Education, Secondhand Tobacco Smoke Exposure, Entry BMI, Maternal Age, Birth Type, and Birth Location
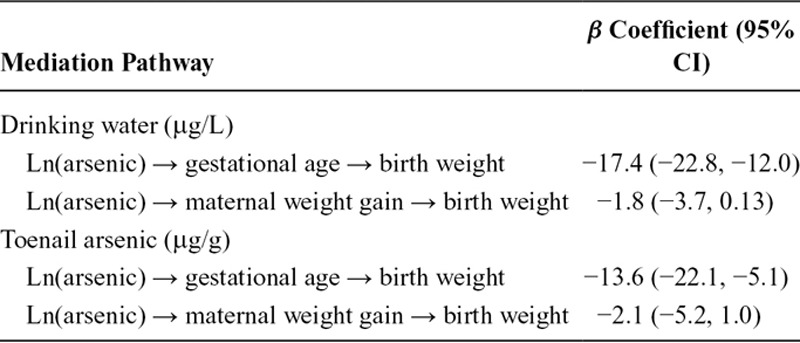
TABLE 5.
Fit Indices for the Final SEM Models (Figure 2) That Describe the Indirect Effect of Arsenic Exposure on Birth Weight That Is Completely Mediated Through Birth Gestational Age and Maternal Weight Gain During Pregnancy
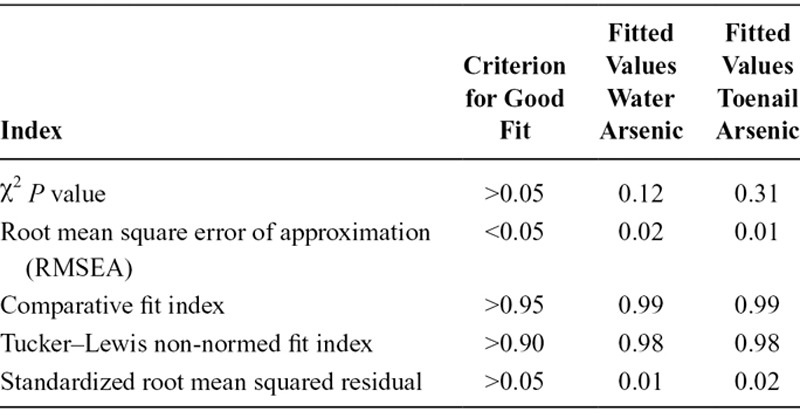
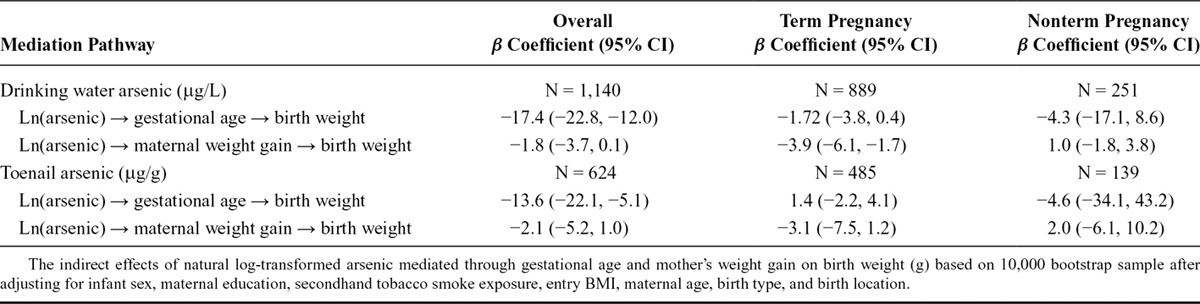
TABLE 6.
A Sensitivity Analysis That Stratifies on Term Pregnancies and Nonterm Pregnancies
DISCUSSION
SEMs provide useful insights into the biologic mechanism underlying life-course epidemiology. In addition, SEMs have been used previously in perinatal epidemiology studies to interpret the causal structure between exposures mediated through gestational age and perinatal outcomes. By integrating an a priori understanding of how arsenic-related reproductive toxicity was influenced by gestational age and maternal weight gain during pregnancy, we constructed SEM that allow testing of the direct, indirect, and total effects of arsenic on birth weight while appropriately controlling for correlated risk factors. This analysis demonstrated that prenatal arsenic exposure was associated with decreased birth weight in a dose-dependent manner. Specifically, we observed that for every doubling in arsenic exposure measured in maternal drinking water or maternal toenails, birth weight decreased by an average of 19 or 15 g, respectively. This effect, however, was completely mediated by gestational age and maternal weight gain during pregnancy, suggesting that these mediators are part of the causal pathway for arsenic-related reproductive toxicity. Moreover, the indirect effect of arsenic was greatest on gestational age and to a lesser extent on maternal weight gain during pregnancy which can indicate maternal health during pregnancy in this rural population.
The results from these SEM are consistent with several epidemiologic studies that show that arsenic exposure is related to decreased birth weight.18,20,21,34 Interestingly, it is only the studies that were conducted in populations with relatively low-level exposure levels that observed negative effects of arsenic on birth weight. It is possible that arsenic’s reproductive toxicity is dose-dependent and causally related to factors that influence fetal growth and survival because high levels of arsenic exposure have been shown to be related to increased rates of spontaneous abortion14,15 and neonatal death.16,17 Our study also shows a strong negative causal relationship between prenatal arsenic exposure and gestational age. It would be useful if future studies examined the relationship between arsenic exposure and gestational age as a continuous variable as well as using a clinical definition of preterm birth (<37 weeks gestational age) to further explore this notion.
Our study has several strengths. We used data from a prospectively enrolled study in which drinking water arsenic exposure was measured in personal drinking water samples early in pregnancy and in maternal toenails providing an estimate of internal dose over the prenatal period. Therefore, the proposed temporal arrangement between the exposure, mediator, and outcome is valid. Our use of two exposure indices also minimized the potential for exposure misclassification since arsenic exposure measured in drinking water at the time of enrollment would reflect the participants initial exposure and arsenic exposure measured in maternal toenails after delivery would provide an integrated measure of exposures that occurred in the prior 9 to 12 months, which would span the entire pregnancy. The arsenic exposures measured in this population are relevant to many other populations that have more modest exposure levels, such as the US. All women received the same level of prenatal health care because DCH is one of the few health care providers in these catchment areas, which would minimize bias and confounding by unmeasured factors related to prenatal care. Also, maternal weight gain was estimated using data collected monthly, although this variable does not include a final maternal weight at delivery and mostly captured weight gain in the second and third trimesters. Gestational age was estimated consistently in this cohort. However, we relied solely on ultrasound measurements to estimate gestational age because few women were able to recall the date of their last menstrual period. Ultrasound measurements are the gold standard for estimating gestational age when taken in early pregnancy but may not be as accurate when used in the second trimester.35 Thus, if gestational age at enrollment into the cohort was associated with arsenic exposure, this could be a source of bias. We carefully examined this issue and saw no correlation between gestational age at enrollment and ln-transformed arsenic in drinking water (Spearman’s ρ = 0.009) or ln-transformed arsenic in toenails (Spearman’s ρ = −0.004). Furthermore, we examined whether women who had arsenic concentrations in their water above and below the Bangladesh drinking water standard of 50 μg/L had different gestational ages at enrollment and found no discernible difference between these groups. In addition, we conducted a sensitivity analysis where we stratified our cohort by gestational age at the time of study enrollment (≤12 weeks of pregnancy compared with 13–16 weeks of pregnancy). Overall the results were consistent between the two strata suggesting that the results for early and late enrollment during pregnancy in this cohort did not influence the estimated overall indirect effects of arsenic observed in adjusted models (eTable 1; http://links.lww.com/EDE/A990). Thus, the potential for bias due to ultrasound-calculated gestational age is most likely minimal. We were also able to control for many other positive and negative confounders in this analysis including maternal BMI at the time of enrollment and maternal age at the time of pregnancy. However, we acknowledge that unmeasured confounding may be present in our analysis since we were unable to adjust for some important covariates such as interpregnancy interval since the vast majority of participants did not provide this information.
This study does have some weaknesses. Namely, there were many missing maternal toenail samples due to a lag in the ongoing laboratory analysis. Toenail samples were randomly selected by the laboratory for analysis and laboratory technicians were blinded to the concentration of arsenic in the participant’s drinking water. However, the subgroup that included toenail measurements had slightly higher arsenic exposure in their drinking water (54.4 vs. 39.98 μg/L) and slightly lower maternal weight gain during pregnancy (0.31 vs. 0.34 kg/weeks) but no difference in birth weight (2,851 vs. 2,860 g) or gestational age (37.9 vs. 37.9 weeks). Since arsenic exposure is negatively associated with maternal weight gain, the estimated mediating effect of maternal weight gain is likely greater in this subset of the population that included toenail arsenic measurements. In addition, we were unable to control for maternal micronutrient deficiencies, although we note that all women were provided with prenatal vitamins throughout their pregnancy and based on monthly interviews with the participants and pill count compliance with taking these vitamins was very good. Also, it is likely that some maternal weight gain during pregnancy was missed given the staggered enrollment into this cohort, the strong correlations between maternal weight gain and gestational age, and the inability to weigh all participants just before birth. We attempted to minimize this misclassification of maternal weight gain by normalizing weight gain by the individual’s follow-up time in the cohort but recognize that there may be error in this term. In addition, maternal BMI at enrollment is also likely to be inflated with regards to gestational age at the time of enrollment. To account for the relationship between gestational age and maternal weight gain, we correlated the residual variance between these two variables that is not explained by arsenic exposure even though we could not incorporate this issue into the causal model structure.
In conclusion, we observed a negative association between prenatal arsenic exposure and birth weight. Taking into account this causal pathway, this toxicological effect was the result of arsenic exposure decreasing gestational age at birth and maternal weight gain during pregnancy.
ACKNOWLEDGMENTS
The authors thank Dr. Alan Acock at Oregon State University for advice on fitting SEM models and model specification.
Supplementary Material
Footnotes
Supported by grants from the US National Institute of Environmental Health Sciences (R01 ES015533, K01 ES017800, P30 ES000210, P30 ES000002, P42 ES016454, and T41 OH008416).
The authors report no conflicts of interest.
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.epidem.com).
REFERENCES
- 1.World Health Organization. Arsenic in Drinking Water. Geneva, Switzerland: World Health Organization; 1999. p. 210. [Google Scholar]
- 2.Amini M, Abbaspour KC, Berg M, et al. Statistical modeling of global geogenic arsenic contamination in groundwater. Environ Sci Technol. 2008;42:3669–3675. doi: 10.1021/es702859e. [DOI] [PubMed] [Google Scholar]
- 3.Ma R, Shen J, Wu J, Tang Z, Shen Q, Zhao FJ. Impact of agronomic practices on arsenic accumulation and speciation in rice grain. Environ Pollut. 2014;194:217–223. doi: 10.1016/j.envpol.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Chowdhury TR, Basu GK, Mandal BK, et al. Arsenic poisoning in the Ganges delta. Nature. 1999;401:545–546; discussion 546. doi: 10.1038/44056. [DOI] [PubMed] [Google Scholar]
- 5.Kinniburgh DG, Smedley PL. British Geological Survey Technical Report WC/00/19. Keyworth, UK: British Geological Survey; 2001. Arsenic contamination of groundwater in Bangladesh. [Google Scholar]
- 6.Smedley PL, Kinniburg DG. A review of the source, behaviour and distribution of arsenic in natural waters. Appl Geochemis. 2002;17:517–568. [Google Scholar]
- 7.Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 8.Shah R, Mullany LC, Darmstadt GL, et al. ProjAHNMo Study Group in Bangladesh. Incidence and risk factors of preterm birth in a rural Bangladeshi cohort. BMC Pediatr. 2014;14:112. doi: 10.1186/1471-2431-14-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hosain GM, Chatterjee N, Begum A, Saha SC. Factors associated with low birthweight in rural Bangladesh. J Trop Pediatr. 2006;52:87–91. doi: 10.1093/tropej/fmi066. [DOI] [PubMed] [Google Scholar]
- 10.Eastman NJ. The arsenic content of the human placenta following arsphenamine therapy. Am J Obstetrics Gynecol. 1931;21:60–64. [Google Scholar]
- 11.Rudge CV, Röllin HB, Nogueira CM, Thomassen Y, Rudge MC, Odland JØ. The placenta as a barrier for toxic and essential elements in paired maternal and cord blood samples of South African delivering women. J Environ Monit. 2009;11:1322–1330. doi: 10.1039/b903805a. [DOI] [PubMed] [Google Scholar]
- 12.DeSesso JM, Jacobson CF, Scialli AR, Farr CH, Holson JF. An assessment of the developmental toxicity of inorganic arsenic. Reprod Toxicol. 1998;12:385–433. doi: 10.1016/s0890-6238(98)00021-5. [DOI] [PubMed] [Google Scholar]
- 13.Kagey BT, Bumgarner JE, Creason JP. Arsenic levels in maternal-fetal tissue sets. Trace Subst Environ Health. 1977;11:252–256. [Google Scholar]
- 14.Milton AH, Smith W, Rahman B, et al. Chronic arsenic exposure and adverse pregnancy outcomes in Bangladesh. Epidemiology. 2005;16:82–86. doi: 10.1097/01.ede.0000147105.94041.e6. [DOI] [PubMed] [Google Scholar]
- 15.Rahman A, Persson LÅ, Nermell B, et al. Arsenic exposure and risk of spontaneous abortion, stillbirth, and infant mortality. Epidemiology. 2010;21:797–804. doi: 10.1097/EDE.0b013e3181f56a0d. [DOI] [PubMed] [Google Scholar]
- 16.Myers SL, Lobdell DT, Liu Z, et al. Maternal drinking water arsenic exposure and perinatal outcomes in inner Mongolia, China. J Epidemiol Community Health. 2010;64:325–329. doi: 10.1136/jech.2008.084392. [DOI] [PubMed] [Google Scholar]
- 17.Hopenhayn-Rich C, Browning SR, Hertz-Picciotto I, Ferreccio C, Peralta C, Gibb H. Chronic arsenic exposure and risk of infant mortality in two areas of Chile. Environ Health Perspect. 2000;108:667–673. doi: 10.1289/ehp.00108667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang CY, Chang CC, Tsai SS, Chuang HY, Ho CK, Wu TN. Arsenic in drinking water and adverse pregnancy outcome in an arseniasis-endemic area in northeastern Taiwan. Environ Res. 2003;91:29–34. doi: 10.1016/s0013-9351(02)00015-4. [DOI] [PubMed] [Google Scholar]
- 19.Hopenhayn C, Ferreccio C, Browning SR, et al. Arsenic exposure from drinking water and birth weight. Epidemiology. 2003;14:593–602. doi: 10.1097/01.ede.0000072104.65240.69. [DOI] [PubMed] [Google Scholar]
- 20.Huyck KL, Kile ML, Mahiuddin G, et al. Maternal arsenic exposure associated with low birth weight in Bangladesh. J Occup Environ Med. 2007;49:1097–1104. doi: 10.1097/JOM.0b013e3181566ba0. [DOI] [PubMed] [Google Scholar]
- 21.Rahman A, Vahter M, Smith AH, et al. Arsenic exposure during pregnancy and size at birth: a prospective cohort study in Bangladesh. Am J Epidemiol. 2009;169:304–312. doi: 10.1093/aje/kwn332. [DOI] [PubMed] [Google Scholar]
- 22.Kile ML, Rodrigues EG, Mazumdar M, et al. A prospective cohort study of the association between drinking water arsenic exposure and self-reported maternal health symptoms during pregnancy in Bangladesh. Environ Health. 2014;13:29. doi: 10.1186/1476-069X-13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Latva-Pukkila U, Isolauri E, Laitinen K. Dietary and clinical impacts of nausea and vomiting during pregnancy. J Human Nutr Dietetics. 2010;23:69–77. doi: 10.1111/j.1365-277X.2009.01019.x. [DOI] [PubMed] [Google Scholar]
- 24.Bang SW, Lee SS. The factors affecting pregnancy outcomes in the second trimester pregnant women. Nutr Res Pract. 2009;3:134–140. doi: 10.4162/nrp.2009.3.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mumbare SS, Maindarkar G, Darade R, Yenge S, Tolani MK, Patole K. Maternal risk factors associated with term low birth weight neonates: a matched-pair case control study. Indian Pediatr. 2012;49:25–28. doi: 10.1007/s13312-012-0010-z. [DOI] [PubMed] [Google Scholar]
- 26.Pike IL. The nutritional consequences of pregnancy sickness: a critique of a hypothesis. Hum Nat. 2000;11:207–232. doi: 10.1007/s12110-000-1011-5. [DOI] [PubMed] [Google Scholar]
- 27.Ahmad SA, Sayed MH, Barua S, et al. Arsenic in drinking water and pregnancy outcomes. Environ Health Perspect. 2001;109:629–631. doi: 10.1289/ehp.01109629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiely JL. Some conceptual problems in multivariable analyses of perinatal mortality. Paediatr Perinat Epidemiol. 1991;5:243–257. doi: 10.1111/j.1365-3016.1991.tb00707.x. [DOI] [PubMed] [Google Scholar]
- 29.VanderWeele TJ, Mumford SL, Schisterman EF. Conditioning on intermediates in perinatal epidemiology. Epidemiology. 2012;23:1–9. doi: 10.1097/EDE.0b013e31823aca5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whitcomb BW, Schisterman EF, Perkins NJ, Platt RW. Quantification of collider-stratification bias and the birthweight paradox. Paediatr Perinat Epidemiol. 2009;23:394–402. doi: 10.1111/j.1365-3016.2009.01053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilcox AJ, Weinberg CR, Basso O. On the pitfalls of adjusting for gestational age at birth. Am J Epidemiol. 2011;174:1062–1068. doi: 10.1093/aje/kwr230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joya SA, Mostofa G, Yousuf J, et al. One solution to the arsenic problem: a return to surface (improved dug) wells. J Health Popul Nutr. 2006;24:363–375. [PMC free article] [PubMed] [Google Scholar]
- 33.Longnecker MP, Stampfer MJ, Morris JS, et al. A 1-y trial of the effect of high-selenium bread on selenium concentrations in blood and toenails. Am J Clin Nutr. 1993;57:408–413. doi: 10.1093/ajcn/57.3.408. [DOI] [PubMed] [Google Scholar]
- 34.Guan H, Piao F, Zhang X, et al. Prenatal exposure to arsenic and its effects on fetal development in the general population of Dalian. Biol Trace Elem Res. 2012;149:10–15. doi: 10.1007/s12011-012-9396-7. [DOI] [PubMed] [Google Scholar]
- 35.Butt K, Lim K Society of Obstetricians and Gynaecologists of Canada. Determination of gestational age by ultrasound. J Obstet Gynaecol Can. 2014;36:171–183. doi: 10.1016/S1701-2163(15)30664-2. [DOI] [PubMed] [Google Scholar]


