Abstract
There is no doubt that the therapeutic efficacy of mesenchymal stem cells (MSCs) needs improvement. SDF-1 (chemokine for MSC homing) and its receptor CXCR4 play a critical role in the migration of MSCs in ischemia. We investigated the effects of the therapeutic application of MSCs transfected to overexpress CXCR4 using an adenoviral construct in the rat stroke model. Both flow cytometry and Western blot analysis indicated that the level of CXCR4 expression was low in naive hMSCs but was consistently high in CXCR4-hMSCs. In vivo migration test using the transwell system showed that the degree of migration was increased in CXCR4-hMSCs compared with the naive hMSCs and was completely blocked by treatment with AMD3100, an antagonist of the CXCR4 receptor. Compared with rats that received naive MSCs, behavioral recovery was more pronounced in rats that received CXCR4-hMSCs (p = 0.023). An immunohistochemistry study using human nuclear antibody (NuMA) showed that the migration of hMSCs in the ischemic boundary zone was increased after 3 days of injection of CXCR4-hMSCs compared with after injection of naive hMSCs. In addition, polymerase chain reaction was performed to assess the biodistribution of human-specific DNA outside the brain after intravenous injection of hMSCs. The expression of human-specific DNA was increased in the lungs of rats receiving naive MSCs, whereas the human-specific DNA expression was increased in the brain of rats receiving CXCR4-hMSCs. Our results indicate that MSCs transfected with the CXCR4 gene expression cassette may be useful in the treatment of cerebral infarction and may represent a new strategy to enhance the efficacy of MSC therapy.
Keywords: Cerebral infarction, Mesenchymal stem cells, Stem cell, SDF-1, Chemokine, CXCR4, Adenovirus
INTRODUCTION
Stroke is a leading cause of death and the most common cause of physical disability in adults. To date, relatively little attention has been devoted in developing methods to restore function after ischemic stroke. Although rehabilitation therapy is important to maximize functional recovery in the early stage after stroke, no definite treatment exists to restore lost brain function after stroke.
Cell therapy is an emerging paradigm in the stroke treatment field, along with acute recanalization therapy and neuroprotective agents, as a regenerative strategy for patients with fixed neurologic deficits. We have recently reported the results of clinical trials using autologous mesenchymal stem cells (MSCs) in patients with severe ischemic stroke (3,21). Our long-term follow-up data (up to 5 years after MSC application) demonstrated that intravenous application of autologous MSCs is feasible and may help recovery after stroke (21). However, there is no doubt that therapeutic efficacy of MSCs needs improvement.
The great potential for improving therapeutic efficacy of stem cell approaches requires further preclinical and clinical trials. Thus, enhancing trophic supports of MSCs may improve therapeutic outcomes with MSCs. Various trophic factors influence neurogenesis in the mature brain (25), and the capacity to release trophic factors is crucial for the beneficial effect of MSCs in cerebral ischemia (8,23,35). Trophic support by MSCs can be enhanced by ischemic preconditioning (15,33), ex vivo treatment of cytokines (10), and genetic modification of MSCs (18,19,30).
Another approach to improve therapeutic efficacy of MSCs is to increase stem cell homing and trophism to target regions. First, blood–brain barrier manipulation may increase trophic factor levels and stem cell numbers in the brain (4). Second, use of an endovascular mode of application to avoid the first pass effect may increase stem cell numbers in specific target regions (17,24,34). We recently reported that endovascular application of MSCs is feasible in patients with neurodegenerative disease (22). However, intra-arterial infusion of autologous MSCs caused small spotty lesions on diffusion-weighted magnetic resonance imaging (DW-MRI), suggesting microembolism, although none of the patients showed neurological deterioration (22). Lastly, upregulation of SDF-1/CXCR4 signaling can increase the number of MSCs in infarcted areas. Among the milieu of chemicals expressed in the injured microenvironment, stromal cell-derived factor-1 (SDF-1 or CXCL12) has emerged as a major attractant of MSCs. SDF-1 and its receptor CXCR4, a CXC chemokine receptor for MSC homing, play a critical role in migration of CXCR4-expressing MSCs. Although SDF-1 protein expression is upregulated in infarcted regions, the level of SDF-1 expression is limited, especially in certain situations, such as chronic stage, less extensive lesions, and younger ages (16,29).
Thus, we investigated the effects of therapeutic application of MSCs transfected to express CXCR4 using an adenoviral construct in the rat stroke model.
MATERIALS AND METHODS
Isolation of Human Bone Marrow Stem Cells and Human MSC Culture
Human MSCs (hMSCs) were obtained from marrow aspirate of the iliac crest of three healthy male volunteers who did not have hematologic disorders or bone marrow suppression (ages 30–40 years) (3). Each 20-ml aspirate was diluted 1:1 with phosphate-buffered saline (PBS) and layered over 20 ml of Ficoll (Ficoll-Paque; Amersham Biosciences, Piscataway, NJ). After centrifugation at 2,000 rpm for 20 min, the mononuclear cell layer was removed from the interface and suspended in PBS. Cells were again centrifuged (1,200 rpm, 5 min) and resuspended in Dulbecco's modified Eagle's medium (DMEM) containing 2 mM l-glutamine, 10% fetal bovine serum (FBS), and 1% penicillin. The cells were incubated at 37°C in 5% CO2 in flasks for 1 day, and nonadherent cells were removed by replacing the medium. After the cultures reached confluence, usually within 1–2 weeks, the cells were harvested with 0.05% trypsin and 0.53 mmol/L EDTA (GIBCO, Gaithersburg, MD) for 5 min at 37°C, replated in a flask, cultured for another 1 week, and harvested. The cells used in these experiments were harvested at either P4 or P5. The protocol was approved by the local institutional review board. Informed consent was given by all donors to use the bone marrow aspirates for research purpose.
Construction of Recombinant Adenovirus and Transfection to MSC
The recombinant adenovirus expressing CXCR4 was constructed on the basis of the system developed by Od260 (Boise, ID). The system comprises the adenoviral genome vector RightZap1.3 and the shuttle vector pZAP1.2 containing the adenoviral left inverted terminal repeat (ITR) and packaging signal (bp 1-358). RightZap1.3 is a 29-kb linear DNA containing 3504 bp-right end of the serotype 5 adenoviral genome, with the fiber knob being replaced with that of serotype 35. An elongation factor 1α (EF1α)-CXCR4 expression cassette was cloned into pZAP1.2 to make pZAP1.2.EF1α-CXCR4, which was then digested with SfiI to release EF1α-CXCR4 together with the adenoviral left ITR and packaging signal Ψ (bp 1-358). The ITRΨ.EF1α-CXCR4 fragment and the linear RightZap1.3 were ligated in the microcentrifuge tube in the presence of Pac I restriction enzyme and then were transfected into HER911E4 using a Lipofectamine 2000 transfection reagent (Invitrogen, Carlsbad, CA) to generate the recombinant adenovirus, Ad5/35EF1α-CXCR4. The amplified adenovirus was purified by CsCl gradient centrifugation. All gradient-purified viral stocks were dialyzed against dialysis buffer [1,000 ml dialysis buffer contains 789 ml double-distilled water, 1 ml 1 mol/L MgCl2, 10 ml 1 mol/L Tris–HCl (pH 7.5), and 200 ml 50% glycerol] for 24 h at 4°C, with three buffer changes. Aliquots of purified and dialyzed viruses were stored at –70°C before use. Ad5/35EF1α-CXCR4 vector was suspended in serum-free DMEM and then added to an aqueous LnCl3 solution to achieve Ln3+ concentration of 0.05 mM and were incubated at room temperature for 5 min. Then, the virus–lanthanide suspension was added to 500, 1,000, or 5,000 virus particles (v.p.) per cell in serum-free DMEM media. After incubation at 37°C for 2 h, the cells were added the same amount of fresh DMEM supplemented with 2× serum and antibiotics. The virus-infected cells were harvested at 96 h and used for flow cytometry and Western blot analysis for dose–response experiment. hMSCs (1×106) infected with 1,000 v.p. per cell were injected into the tail vein of animal models (32).
Characteristics of CXCR4-Overexpressing MSCs
Western Blot Analysis. The naive MSCs and CXCR4-MSCs were prepared after incubation either with or without Ad5/35EF1α-CXCR4 for 96 h. Cells were lysed for 10 min at 4°C in RIPA lysis buffer (50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 0.25% deoxycholic acid, 1% NP-40, 1 mM EDTA; Upstate, NY) supplemented with 1 mM PMSF and complete mini protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN). The samples (40 µg each) were separated by electrophoresis on a Novex® 4–12% Tris-glycine gel (Invitrogen) and transferred to polyvinyldiene difluoride membrane by a transfer apparatus (Bio-Rad, Hercules, CA). The membranes were blotted with 5% nonfat dry milk for 2 h and then incubated with primary antibodies to CXCR4 (1:500; Abcam, Cambridge, MA) overnight. Horseradish peroxidase-conjugated anti-rabbit antibodies (1:3,000; Cell Signaling, Beverly, MA) were used as secondary antibodies. The peroxidase reaction products were visualized by WEST-one™ Western blot detection system (iNtRON, South Korea). The same membrane was then stripped and reprobed with anti-β-actin antibody (1:8,000; Sigma, St. Louis, MO) to determine the total protein abundance using a similar procedure.
Flow Cytometry. The levels of CD105 and 90 (MSC surface markers), CD34 and 45 (MSC negative markers), and CXCR4 expression in MSCs were evaluated by flow cytometry (FACScan; Becton–Dickinson, Rutherford, NJ), as previously described (23). Mouse anti-human CD105 fluorescein isothiocyanate (FITC)-conjugated monoclonal antibody (BD Biosciences, San Jose, CA) was used as a marker for human endoglin, and we used FITC mouse anti-human CD90 (BD), FITC rat anti-mouse CD34, and R-phycoerythrin (R-PE)-conjugated anti-mouse CD45 (BD) for other markers. To compare the level of CXCR4 expression between the naive hMSCs and CXCR4-overexpressing hMSCs, cells were stained with rabbit polyclonal anti-CXCR4 (Santa Cruz, Cambridge, MA), followed by anti-rabbit IgG R-PE (1:400; Molecular Probes, Eugene, OR), according to the manufacturer's instructions.
Migration Test. Migration assays were performed in a transwell system (Corning Life Sciences); the lower side of the transwell filter with 8-µm pore was coated for 1 h at 37°C with 50 µg/ml fibronectin (Sigma). Then, 5×104 hMSCs were placed in the upper chamber, and 600 µl of migration medium with chemotactic factor were placed in the bottom chamber. Migration observed in knockout DMEM alone served as negative control. We evaluated the chemotactic activity of 200 ng/ml SDF-1. To block chemotactic activity via CXCR4 receptor, 50 µg/ml of AMD3100 (Sigma) was used. After 4 h, assays were terminated by removal of the medium from the upper wells, and filters were washed with PBS. Cells remaining on the upper face of the filters were removed with a cotton wool swab. Filters were fixed by submersion in methanol and were stained with toluidine blue (Sigma) solution for 5 min and then air dried. Filters cut out with a scalpel were mounted onto glass slides, placing the lower face on the top. Stained cells in five fields were counted manually under 100-fold magnification using light microscopy. Data were expressed as percentage of cells related to that of the negative control.
In Vivo Assessment of the Effect of CXCR4-Overexpressing MSCs
Animal Model. Rats were subjected to surgery and care according to the guidelines of Laboratory Animal Research Center (LARC; AAALAC International-approved facility) in Samsung Medical Center. Using a face mask, anesthesia was induced in male Sprague–Dawley rats (7–8 weeks, 250–300 g) with 4% isoflurane and maintained with 1.5% isoflurane in 70% N2O and 30% O2. Rectal temperature was maintained at 37.0–37.5°C with heating pads. We induced transient middle cerebral artery occlusion (tMCAo) using a previously described intra-luminal vascular occlusion method (6,39) modified in our laboratory. A 4-0 surgical monofilament nylon suture with a rounded tip was advanced from the left common carotid artery into the lumen of the internal carotid artery until it blocked the origin of the middle cerebral artery. Two hours after tMCAo, reperfusion was allowed by withdrawing the suture until the tip cleared the lumen of the common carotid artery.
Experimental Groups. One day after tMCAo, the animals were randomly divided into three groups (n = 8 for each group): tMCAo + PBS injection (the control group), tMCAo + naive hMSCs injection (the naive MSC group), and tMCAo + CXCR4-overexpressing hMSCs injection (the CXCR4-MSC group). For both the naive MSC and CXCR4-MSC groups, 2 × 106 hMSCs were intravenously injected into the animal models 24 h after tMCAo. Animals were not immunosuppressed after hMSC transplantation.
Functional Tests. In all animals, a battery of functional tests was performed before tMCAo and at 1, 3, 7, and 14 days after tMCAo by an investigator who was blinded to the experimental groups. Using the modified Neurological Severity Score (mNSS), neurological function was graded on a scale of 0–18 (normal score = 0, maximal deficit score = 18). The mNSS is a composite of motor (muscle status and abnormal movement), sensory (visual, tactile, and proprioceptive), reflex, and balance tests (7).
MRI Studies and Measurement of Infarct Volume. MRI was performed on a 7.0-T dedicated animal scanner (Bruker-Biospin, Fallanden, Switzerland) with a 20-cm gradient set capable of providing up to 400 mT/m. A birdcage coil (72 mm i.d.) (Bruker-Biospin, Fallanden, Switzerland) was used for excitation, and a four-channel phased array coil was used for receiving the signal for brain imaging. Diffusion-weighted MRI was obtained from each rat brain using a single-shot echo planar sequence [repetition time (TR)/echo time (TE) = 2820/670 ms, number of experiment (NEX) = 1, echo train length =1,100 µm 3D isotropic resolution, field of view (FOV) = 3.56 × 2.56 × 2.56 cm3, matrix size = 3.56 × 2.56 × 2.56 cm3, b = 0/1,300 s/mm2, slice selection direction = sagittal, and readout direction = head-to-foot] to evaluate the cerebral ischemia. Ten slices with 1-mm-slice thickness were positioned between the olfactory bulb and cerebellum. Rats were placed on a heated circulating water blanket to ensure constant body temperature of 37 ± 1°C. Anesthesia was induced and maintained with 2% isoflurane delivered in a mixture of 70% N2O and 30% O2 via a facemask under constant ventilation monitoring (Small Animal Monitoring & Gating System, SA Instruments, Stony Brook, NY). The ischemic lesion area was calculated from diffusion- and T2-weighted images. For each slice, the higher intensity lesions in T2-WI, where the signal intensity was 1.25-fold higher than in the contralateral brain lesion, were marked as the ischemic lesion area and infarct volume was calculated taking slice thickness (2 mm/slice) into account.
In Vivo Assessment of the Migration of Naive and CXCR4-Overexpressing MSCs
Histological and Immunohistochemical Assessment. Fourteen days after tMCAo, all animals were anesthetized with 2% isoflurane delivered in a mixture of 70% N2O and 30% O2. The rat brains were fixed by transcardial perfusion with saline, followed by perfusion with and immersion in 4% paraformaldehyde solution. The brains were removed quickly and kept in 4% paraformaldehyde solution overnight at 4°C, then placed in a 30% sucrose solution until they sank.
For double-immunofluorescence staining, the tissues were washed three times with PBS; nonspecific binding was blocked with 10% normal horse serum. The tissues were then treated overnight at 4°C with a monoclonal antibody specific for human nuclei matrix antigen (NuMA; Oncogen, Seattle, WA) diluted 1:100 in PBS. Following sequential incubation with anti-mouse Alexa 488 (1:400; Molecular Probes), secondary antibody was bound to the first antibody to NuMA. Cells derived from hMSCs were identified using both morphologic criteria and immunohistochemical staining with NuMA (i.e., present in the human donor cells, but not in rat parenchymal cells).
To visualize the cellular colocalization of NuMA and cell-type-specific markers in the same cells, each coronal slide was treated with the first primary antibody to NuMA, as described above, and then incubated with glial fibrillary acidic protein (GFAP, for astrocytes; Sigma Chemical) and the NeuN (for neurons; Chemicon, Temecula, CA) overnight at 4°C and then incubated with an anti-rabbit Alexa 488 (1:400; Molecular Probes), anti-mouse Alexa 568 (1:400; Molecular Probes), or anti-goat FITC (1:200; Vector Laboratories) antibody for 1 h at room temperature. The sections were washed in PBS, rinsed with double-distilled water, and mounted on slides using an antifade mounting medium (Permafluor; Molecular Probes). In addition, to confirm whether the chemokine SDF-1 induced after tMCAo and its receptor CXCR4 colocalized in the brain, sections were labeled with antibodies to SDF-1 (goat, 1:100, Santa Cruz, CA) and CXCR4 (rabbit, Santa Cruz, CA), and SDF-1 and CXCR4 signals were detected by FITC-conjugated antibodies. The specimens were examined with a Zeiss LSM510 confocal imaging system (Carl Zeiss).
Polymerase Chain Reaction. Polymerase chain reaction was performed to assess the biodistribution of human-specific DNA after intravenous injection of hMSCs. The animals were anesthetized and sacrificed to remove the organs as brain, lung, liver, spleen, and bone marrow. Each organ specimen was obtained at posttreatment 1 day and 3 days from the animal model injected with CXCR4-hMSCs, hMSCs, or vehicle. Genomic DNA was extracted with Qiagen DNeasy® purification kit (Qiagen, Valencia, CA), according to the manufacturer's instructions, and 100 ng per reaction were used for human chromosome 7 alphoid repeats. We used forward (5′-CAA GAA GGC TTC AAA GCA CC-3′) and reverse (5′-TTC ATT GGA ATC GCG AAT AC-3′) primers for human chromosome 7 alphoid repeats. PCR reactions were carried out using the following parameters: 95°C for 2 min, 39 cycles at 95°C for 30 s, 55°C for 30 s, and 72°C for 30 s; final extension was at 72°C for 10 min (9).
Statistical Analysis
Statistical differences between groups were evaluated using the Mann–Whitney U test. We used the Bonferroni correction to account for multiple tests. Values of p < 0.05 were set as statistically significant.
RESULTS
Characteristics of CXCR4-Overexpressing hMSCs
Both flow cytometry analysis and Western blot analysis indicated that the level of CXCR4 expression was low in naive MSCs but was consistently high in MSCs infected with 1,000 v.p. per cell. Thus, CXCR4 was highly expressed in CXCR4-MSCs (87.2 ± 10.1%) compared with naive MSCs (12.8 ± 7.0%) (n = 4, p = 0.001) (Fig. 1A). Expression level of CXCR4 in MSCs with different multiplicity of infection is shown in Figure 1B. The levels of CXCR4 expression were enhanced with the increasing number of virus particles per cell.
Figure 1.
Characteristics of MSCs. (A) Flow cytometry analysis of proportion of mesenchymal stem cells (MSCs) with CXCR4 antigen expression. (B) Western blot analysis for the level of CXCR4 in MSCs infected with different MOI (multiplicity of infection). (C) Flow cytometry analysis of mesenchymal stem cell surface markers.
Flow cytometry analysis indicated that both naive and CXCR4-MSCs had high levels of expression of stem cell markers and did not express CD34 and CD45 antigens (markers for hematopoietic stem cells). No significant difference was found between naive and CXCR4-MSCs (Fig. 1C). Both naive MSCs and CXCR4-MSCs showed normal morphology in light microscopic findings and similar growth rates during cultivation.
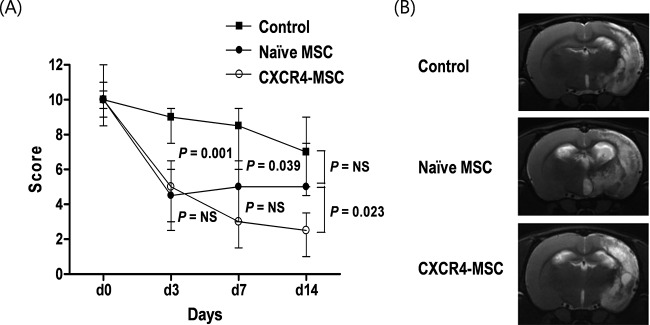
Neurological Functional Testing. At day 3 after tMCAo, both groups that received naive MSCs and CXCR4-MSCs showed much better functional recovery compared with the control group (p = 0.001 and 0.003, respectively). However, at later time points, the degree of improvement was different between the naive MSC and CXCR4-MSC groups; mNSS score was higher in the CXCR4-MSC group than in the naive MSC group at day 7 (p = 0.297), and the difference increased at day 14 after tMCAo (p = 0.023) (Fig. 2A).
Figure 2.
(A) Results of functional tests. Modified neurological severity score (mNSS) before and after transient middle cerebral artery occlusion (tMCAo). (B) MRI findings at 14 days after tMCAo.
An estimate of lesion size was obtained using MRI (Fig. 2B). Although the CXCR4-MSC group showed functional improvement compared with the naive MSC group and control group, the lesion volume was not significantly different between the groups.
In Vitro and In Vivo Migration Testing
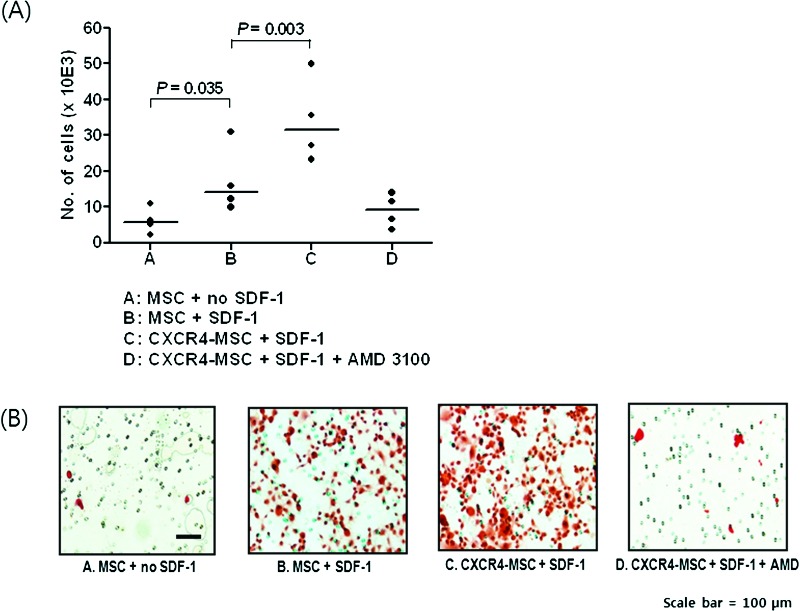
Migration of hMSCs was tested using SDF-1 as a chemokine (Fig. 3). In vitro, compared with knockout DMEM, migration of hMSCs was increased when SDF-1 was used as a chemokine (p = 0.035). The degree of enhanced migration was greater in the CXCR4-MSC group than in the naive MSC group (p = 0.003). Migration of CXCR4-MSC was completely blocked by treatment with the CXCR4 antagonist AMD3100.
Figure 3.
Migration test. In vitro migration test of MSCs under different conditions using a transwell migration plate. The data are summarized in A, and typical antibody staining is shown in B.
An immunohistochemistry study using human nuclear antibody (NuMA) showed that the migration of hMSCs in the ischemic boundary zone was improved after 3 days of injection of CXCR4-MSCs compared with those after injection of naive MSCs (Fig. 4A).
Figure 4.
(A) Immunohistochemistry to assess the migration of human mesenchymal stem cells (hMSCs) in the ischemic boundary zone after 3 days of injection using human nuclear antibody (NuMA). (B) Polymerase chain reaction to assess the biodistribution of human-specific DNA after intravenous injection of hMSCs.
When polymerase chain reaction assessed the biodistribution of human-specific DNA after intravenous injection of hMSCs (Fig. 4B), human-specific DNA was detected throughout the body in both naive and CXCR4-MSC treatment groups. Interestingly, the distribution pattern was quite different between the groups. Specifically, human-specific DNA increased in the lungs of rats receiving naive MSCs, suggesting the first-pass effect, whereas more human-specific DNA accumulated in the brain at 3 days after CXCR4-MSC treatment.
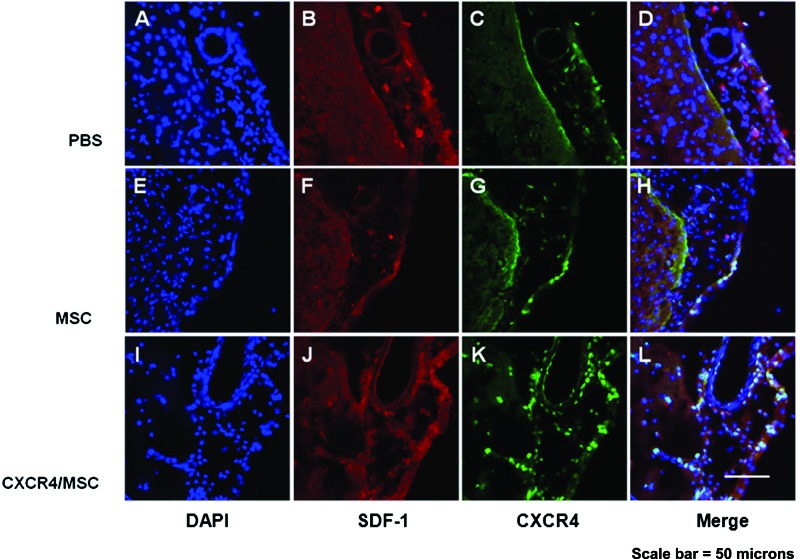
Immunohistochemistry using SDF-1 and CXCR4 showed that CXCR4 expression increased in the CXCR4-MSC group compared with the naive MSC group. In addition, MSCs expressing CXCR4 were colocalized within tissues where SDF-1 was highly expressed (such as in endothelial cells) (Fig. 5). SDF-1-labeled cells were predominant in the ischemic boundary zone, but the SDF-1 protein tissue level was not different between the groups.
Figure 5.
Immunohistochemistry results showing colocalization of SDF-1 and CXCR4-positive human mesenchymal stem cells in the ischemic boundary of ischemic rat brain.
DISCUSSION
Genetically Modified MSCs
Recent experimental studies suggest that gene transduction into MSCs can enhance their therapeutic potential. Human MSCs transfected with adenovirus vector with a brain-derived neurotrophic factor (BDNF) gene increased the level of BDNF in the ischemic brain, reduced lesion volume, and elicited functional improvement compared with naive MSCs (19,30). In addition, other various trophic factor gene-modified MSCs were reported to be associated with improved behavioral recovery in a stroke model, including glial cell-derived neurotrophic factor (GDNF) (18), hepatocyte growth factor (HGF) (42), and placental growth factor (PGF), a vascular endothelial growth factor (26). In addition, application of vascular endothelial growth factor (VEGF)-overexpressing MSCs has also been reported to reduce infarct size in a myocardial infarction model (28). These results suggest that gene-modified cell therapy may be a useful approach for the treatment of stroke.
Beside the trophic support using trophic factor gene-modified MSCs, upregulation of SDF-1/CXCR4 signaling to increase the number of MSCs in infarcted areas can be an important strategy to enhance the efficacy of cell therapy in stroke. Although many chemotactic factors seem to be involved in the processes following tissue injury, SDF-1 was identified as a major stem cell homing factor (13). Binding of SDF-1 to its receptor CXCR4 on both stem and progenitor cells seems to play an essential role in the regulation of bone marrow homing and repopulation as well as mobilization of stem cells into the peripheral blood (20). The level of SDF-1 expression in the ischemic tissues was reduced with time. Therefore, cell therapy using MSCs overexpressing chemokine receptor can be an important strategy to upregulate SDF-1/CXCR4 signaling. To the best of our knowledge, this is the first study focused on the strategy of enhanced migration of adult stem cells through overexpresison of CXCR4 on MSCs using adenovirus.
Reasons for Enhancement of Receptor Rather Than Soluble Factor
There are two ways to increase activity of the SDF-1/CXCR axis for stem cell homing: upregulation of brain concentration of SDF-1 and upregulation of CXCR4 expression on MSCs. Exogenous SDF-1 or upregulation of SDF-1 mRNA or protein expression can be a candidate. However, because the gradient of SDF-1 concentration through the endothelium is an important signal for the stem/progenitor cell homing, perhaps intensive secretion of SDF-1 in the ischemic tissue can direct the flux of cells into ischemic tissue, thus facilitating tissue repair (12). Thus, the increasing serum levels of SDF-1 by exogenous application of SDF-1 may disturb the gradient between specific tissues (i.e., bone marrow, blood, and brain). Moreover, direct application of soluble factors (such as SDF-1 and VEGF) or transplantation of stem cells on which soluble factors were overexpressed or preconditioned have been studied, which may lead to undesirable outcome due to possible systemic effects of these factors (1,33,37–39). Thus, the enhanced expression of CXCR4 on MSCs could be an alternative.
Migration of CXCR4-Overexpressing MSCs to Ischemic Brain Areas
In the present study, assessment of functional outcomes using mNSS revealed that CXCR4-overexpressing MSCs elicited functional improvement compared with the control group and the naive MSC transfected group. As a possible mechanism, our present study demonstrates that enhanced expression of CXCR4 on MSCs promotes migration into the ischemic region, both in vitro and in vivo. The previous studies showed that SDF-1 expression was mainly localized to the ischemic penumbra (16,29). Our immunohistochemistry data showed that CXCR4-overexpressing MSCs were colocalized with the SDF-1-expressing brain cells.
In addition, our PCR result showed that biodistribution of CXCR4-overexpressing MSCs differs from that of naive MSCs, suggesting the possibility to minimize the first passing effect during intravenous application of MSCs.
Lastly, our finding showed the possibility that MSC mobilization can be maintained even when the SDF-1 level in the target tissue (i.e., ischemic brain) was reduced by overexpression of its receptor. Further studies concerning the therapeutic application of CXCR4-overexpressing MSCs are warranted in the chronic rat model, in which the SDF-1 level of ischemic brain is diminished.
Possible Mechanisms of Functional Improvement
In the present study, we primarily focused on the effect of chemokine receptor gene modification of stem cell migrating capacity. Further studies concerning the possible mechanisms of functional improvement are needed. First, MSCs activate endogenous restorative responses in the injured brain, such as stroke-induced neurogenesis (11,23). The number of cells migrated to ischemic areas was determinant for the efficacy of MSCs. The larger cell dose or application of intra-arterial mode to avoid the first passing effect showed more effective in restoration after stroke (7,24,31). Thus, although the differential effect of naive and CXCR4-MSCs on neurogenesis was not evaluated, a larger number of MSCs in the CXCR4-MSC group could be a possible explanation for the better functional outcome.
Second, the presence and degree of apoptotic cell death and inflammation, which are mechanisms of ischemic cell death, were not evaluated in the present study. Neuroprotective effects may be responsible for the beneficial effects of MSCs. Previous studies using trophic factor gene-modified MSCs showed that the levels of trophic factors were increased in the ischemic areas and lesion size was reduced, suggesting neuroprotective effects of trophic factor gene-modified MSCs. However, we failed to show the difference in the infarct size between the groups.
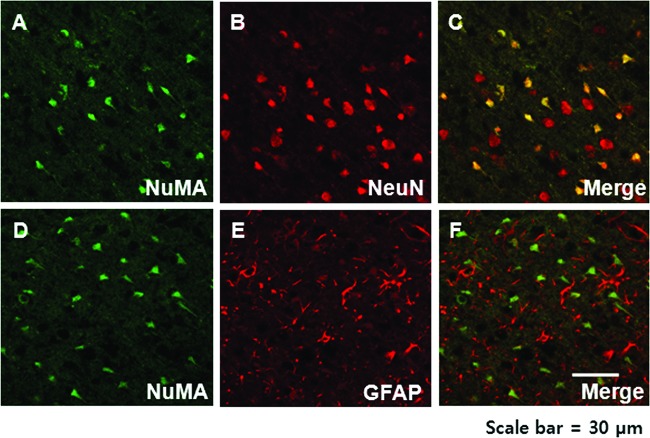
Lastly, pleiotropic effects of the SDF-1/CXCR4 axis have been reported (33). Our immunohistochemistry study showed a more predominant neuronal phenotype of CXCR4-MSCs compared with naive MSCs (Fig. 6). Further studies are needed with various phenotype markers for other CNS cells, such as neuroblast (doublecortin), endothelium, and microglia.
Figure 6.
Immunohistochemistry results demonstrating the neuronal (A–C) but not astrocytic (D–F) phenotype of the human (NuMA-positive) mesenchymal stem cells in the ischemic boundary zone 3 days after injection.
Transduction Using Adenovirus Vector
In this study, we utilized the recombinant adenovirus because recombinant adenovirus vectors are the most favorable delivery vehicle for mammalian gene transfer and are widely used in both vaccine development and gene therapy applications. In addition, we replaced the fiber knob with that of serotype 35, targeting CD46 as a receptor instead of CAR (coxakie-adenovirus receptor) and resulting in the better infectivity to MSC. For eventual clinical translation, the adenoviral vector has several advantages over other vectors, including fewer side effects due to episomal existence inside the cell, efficient transduction of both proliferating and quiescent cells, efficient production to high titers in well-defined cell systems, and high stability, allowing purification and long-term storage (27). In addition, adenoviruses are reportedly effective gene delivery vesicles for bone marrow-derived MSCs (41). A gene therapy product based on an adenovirus vector engineered to express p53 was approved by the State Food and Drug Administration of China in 2003 for the treatment of patients with head and neck squamous cell carcinoma (40).
Perspectives
SDF-1/CXCR axis activates several signaling pathways in the target cells and plays a crucial role in both cell trafficking and interaction with the intercellular environment (33). CXCL12–CXCR4 interaction has been thought to be exclusive, but recent observation suggested that CXCL12 may also bind the CXCR7 receptor (i.e., the second SDF-1 receptor) (2,5). In the focal ischemia rat model, SDF-1 may influence vascular, astroglial, and neuronal function via CXCR7 and mediate cell recruitment to ischemic brain areas via CXCR4 (36). In addition, in experimental autoimmune encephalomyelitis (a demyelinating animal model) CXCR7 expression was maintained on oligodendroglial cells, and CXCL12 stimulation promoted morphological maturation of cultured primary oligodendrocyte precursor cells as well as their myelin expression (14). These findings suggest that activation of the CXCR7 receptor could provide a means to promote reorganization of brain tissue in the diseased or injured central nervous system. Thus, our strategy of SDF-1 receptor overexpression can be used for regulation of a variety of biological processes of the brain via the SDF-1/CXCR7 axis as well as cell recruitment to ischemic brain areas via SDF-1/CXCR4 axis.
Limitations and Conclusions
This study has some limitations. First, we compared only the differential effect of naive MSCs and CXCR4-MSCs on migration; the relationship between the degree of CXCR4 overexpression on MSCs and the number of MSCs migrated to ischemic regions was not assessed quantitatively. Second, the blood–brain barrier was not evaluated in the present study, which could also affect the number of cells that migrate into ischemic brain areas.
Our results indicate that MSCs transfected with the CXCR4 expression cassette may be useful in the treatment of cerebral infarction and may represent a new strategy to enhance the efficacy of MSC therapy.
ACKNOWLEDGMENTS
This study was supported by grants from Mogam Biotechnology Research Institute and Green Cross Corp. (PHO 1080621), Samsung Biomedical Research Institute (SBRI C-B11-131), and the National Research Foundation of Korea, Ministry of Education, Science and Technology (2010-0007979, 2011-0019389). The authors declare no conflict of interest.
REFERENCES
- 1. Askari A.; Unzek S.; Goldman C. K.; Ellis S. G.; Thomas J. D.; DiCorleto P. E.; Topol E. J.; Penn M. S. Cellular, but not direct, adenoviral delivery of vascular endothelial growth factor results in improved left ventricular function and neovascularization in dilated ischemic cardiomyopathy. J. Am. Coll. Cardiol. 43:1908–1914; 2004. [DOI] [PubMed] [Google Scholar]
- 2. Balabanian K.; Lagane B.; Infantino S.; Chow K. Y.; Harriague J.; Moepps B.; Arenzana-Seisdedos F.; Thelen M.; Bachelerie F. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J. Biol. Chem. 280:35760–35766; 2005. [DOI] [PubMed] [Google Scholar]
- 3. Bang O. Y.; Lee J. S.; Lee P. H.; Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann. Neurol. 57:874–882; 2005. [DOI] [PubMed] [Google Scholar]
- 4. Borlongan C. V.; Hadman M.; Sanberg C. D.; Sanberg P. R. Central nervous system entry of peripherally injected umbilical cord blood cells is not required for neuroprotection in stroke. Stroke 35:2385–2389; 2004. [DOI] [PubMed] [Google Scholar]
- 5. Burns J. M.; Summers B. C.; Wang Y.; Melikian A.; Berahovich R.; Miao Z.; Penfold M. E.; Sunshine M. J.; Littman D. R.; Kuo C. J.; Wei K.; McMaster B. E.; Wright K.; Howard M. C.; Schall T. J. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J. Exp. Med. 203:2201–2213; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen H.; Chopp M.; Zhang Z. G.; Garcia J. H. The effect of hypothermia on transient middle cerebral artery occlusion in the rat. J. Cereb. Blood Flow Metab. 12:621–628; 1992. [DOI] [PubMed] [Google Scholar]
- 7. Chen J.; Li Y.; Wang L.; Zhang Z.; Lu D.; Lu M.; Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke 32:1005–1011; 2001. [DOI] [PubMed] [Google Scholar]
- 8. Chen X.; Li Y.; Wang L.; Katakowski M.; Zhang L.; Chen J.; Xu Y.; Gautam S. C.; Chopp M. Ischemic rat brain extracts induce human marrow stromal cell growth factor production. Neuropathology 22:275–279; 2002. [DOI] [PubMed] [Google Scholar]
- 9. Chen Y.; He Z. X.; Liu A.; Wang K.; Mao W. W.; Chu J. X.; Lu Y.; Fang Z. F.; Shi Y. T.; Yang Q. Z.; Chen da Y.; Wang M. K.; Li J. S.; Huang S. L.; Kong X. Y.; Shi Y. Z.; Wang Z. Q.; Xia J. H.; Long Z. G.; Xue Z. G.; Ding W. X.; Sheng H. Z. Embryonic stem cells generated by nuclear transfer of human somatic nuclei into rabbit oocytes. Cell Res. 13:251–263; 2003. [DOI] [PubMed] [Google Scholar]
- 10. Choi Y. J.; Li W. Y.; Moon G. J.; Lee P. H.; Ahn Y. H.; Lee G.; Bang O. Y. Enhancing trophic support of mesenchymal stem cells by ex vivo treatment with trophic factors. J. Neurol. Sci. 298:28–34; 2010. [DOI] [PubMed] [Google Scholar]
- 11. Chopp M.; Li Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol. 1:92–100; 2002. [DOI] [PubMed] [Google Scholar]
- 12. Damas J. K.; Waehre T.; Yndestad A.; Ueland T.; Muller F.; Eiken H. G.; Holm A. M.; Halvorsen B.; Froland S. S.; Gullestad L.; Aukrust P. Stromal cell-derived factor-1alpha in unstable angina: Potential antiinflammatory and matrix-stabilizing effects. Circulation 106:36–42; 2002. [DOI] [PubMed] [Google Scholar]
- 13. Ghadge S. K.; Muhlstedt S.; Ozcelik C.; Bader M. SDF-1alpha as a therapeutic stem cell homing factor in myocardial infarction. Pharmacol. Ther. 129:97–108; 2011. [DOI] [PubMed] [Google Scholar]
- 14. Gottle P.; Kremer D.; Jander S.; Odemis V.; Engele J.; Hartung H. P.; Kury P. Activation of CXCR7 receptor promotes oligodendroglial cell maturation. Ann. Neurol. 68:915–924; 2010. [DOI] [PubMed] [Google Scholar]
- 15. Grayson W. L.; Zhao F.; Bunnell B.; Ma T. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochem. Biophys. Res. Commun. 358:948–953; 2007. [DOI] [PubMed] [Google Scholar]
- 16. Hill W. D.; Hess D. C.; Martin-Studdard A.; Carothers J. J.; Zheng J.; Hale D.; Maeda M.; Fagan S. C.; Carroll J. E.; Conway S. J. SDF-1 (CXCL12) is upregulated in the ischemic penumbra following stroke: Association with bone marrow cell homing to injury. J. Neuropathol. Exp. Neurol. 63:84–96; 2004. [DOI] [PubMed] [Google Scholar]
- 17. Jin K.; Sun Y.; Xie L.; Mao X. O.; Childs J.; Peel A.; Logvinova A.; Banwait S.; Greenberg D. A. Comparison of ischemia-directed migration of neural precursor cells after intrastriatal, intraventricular, or intravenous transplantation in the rat. Neurobiol. Dis. 18:366–374; 2005. [DOI] [PubMed] [Google Scholar]
- 18. Kurozumi K.; Nakamura K.; Tamiya T.; Kawano Y.; Ishii K.; Kobune M.; Hirai S.; Uchida H.; Sasaki K.; Ito Y.; Kato K.; Honmou O.; Houkin K.; Date I.; Hamada H. Mesenchymal stem cells that produce neurotrophic factors reduce ischemic damage in the rat middle cerebral artery occlusion model. Mol. Ther. 11:96–104; 2005. [DOI] [PubMed] [Google Scholar]
- 19. Kurozumi K.; Nakamura K.; Tamiya T.; Kawano Y.; Kobune M.; Hirai S.; Uchida H.; Sasaki K.; Ito Y.; Kato K.; Honmou O.; Houkin K.; Date I.; Hamada H. BDNF gene-modified mesenchymal stem cells promote functional recovery and reduce infarct size in the rat middle cerebral artery occlusion model. Mol. Ther. 9:189–197; 2004. [DOI] [PubMed] [Google Scholar]
- 20. Lapidot T.; Petit I. Current understanding of stem cell mobilization: The roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp. Hematol. 30:973–981; 2002. [DOI] [PubMed] [Google Scholar]
- 21. Lee J. S.; Hong J. M.; Moon G. J.; Lee P. H.; Ahn Y. H.; Bang O. Y. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells 28:1099–1106; 2010. [DOI] [PubMed] [Google Scholar]
- 22. Lee P. H.; Kim J. W.; Bang O. Y.; Ahn Y. H.; Joo I. S.; Huh K. Autologous mesenchymal stem cell therapy delays the progression of neurological deficits in patients with multiple system atrophy. Clin. Pharmacol. Ther. 83:723–730; 2008. [DOI] [PubMed] [Google Scholar]
- 23. Li W. Y.; Choi Y. J.; Lee P. H.; Huh K.; Kang Y. M.; Kim H. S.; Ahn Y. H.; Lee G.; Bang O. Y. Mesenchymal stem cells for ischemic stroke: Changes in effects after ex vivo culturing. Cell Transplant. 17:1045–1059; 2008. [DOI] [PubMed] [Google Scholar]
- 24. Li Y.; Chen J.; Wang L.; Lu M.; Chopp M. Treatment of stroke in rat with intracarotid administration of marrow stromal cells. Neurology 56:1666–1672; 2001. [DOI] [PubMed] [Google Scholar]
- 25. Lichtenwalner R. J.; Parent J. M. Adult neurogenesis and the ischemic forebrain. J. Cereb. Blood Flow Metab. 26:1–20; 2006. [DOI] [PubMed] [Google Scholar]
- 26. Liu H.; Honmou O.; Harada K.; Nakamura K.; Houkin K.; Hamada H.; Kocsis J. D. Neuroprotection by PlGF gene-modified human mesenchymal stem cells after cerebral ischaemia. Brain 129:2734–2745; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lusky M. Good manufacturing practice production of adenoviral vectors for clinical trials. Hum. Gene Ther. 16:281–291; 2005. [DOI] [PubMed] [Google Scholar]
- 28. Matsumoto R.; Omura T.; Yoshiyama M.; Hayashi T.; Inamoto S.; Koh K. R.; Ohta K.; Izumi Y.; Nakamura Y.; Akioka K.; Kitaura Y.; Takeuchi K.; Yoshikawa J. Vascular endothelial growth factor-expressing mesenchymal stem cell transplantation for the treatment of acute myocardial infarction. Arterioscler. Thromb. Vasc. Biol. 25:1168–1173; 2005. [DOI] [PubMed] [Google Scholar]
- 29. Miller J. T.; Bartley J. H.; Wimborne H. J.; Walker A. L.; Hess D. C.; Hill W. D.; Carroll J. E. The neuroblast and angioblast chemotaxic factor SDF-1 (CXCL12) expression is briefly up regulated by reactive astrocytes in brain following neonatal hypoxic-ischemic injury. BMC Neurosci. 6:63; 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nomura T.; Honmou O.; Harada K.; Houkin K.; Hamada H.; Kocsis J. D. I.V. infusion of brain-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Neuroscience 136:161–169; 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Omori Y.; Honmou O.; Harada K.; Suzuki J.; Houkin K.; Kocsis J. D. Optimization of a therapeutic protocol for intravenous injection of human mesenchymal stem cells after cerebral ischemia in adult rats. Brain Res. 1236:30–38; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Palmer G. D.; Stoddart M. J.; Gouze E.; Gouze J. N.; Ghivizzani S. C.; Porter R. M.; Evans C. H. A simple, lanthanide-based method to enhance the transduction efficiency of adenovirus vectors. Gene Ther. 15:357–363; 2008. [DOI] [PubMed] [Google Scholar]
- 33. Pasha Z.; Wang Y.; Sheikh R.; Zhang D.; Zhao T.; Ashraf M. Preconditioning enhances cell survival and differentiation of stem cells during transplantation in infarcted myocardium. Cardiovasc. Res. 77:134–142; 2008. [DOI] [PubMed] [Google Scholar]
- 34. Pendharkar A. V.; Chua J. Y.; Andres R. H.; Wang N.; Gaeta X.; Wang H.; De A.; Choi R.; Chen S.; Rutt B. K.; Gambhir S. S.; Guzman R. Biodistribution of neural stem cells after intravascular therapy for hypoxic-ischemia. Stroke 41:2064–2070; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Savitz S. I.; Rosenbaum D. M.; Dinsmore J. H.; Wechsler L. R.; Caplan L. R. Cell transplantation for stroke. Ann. Neurol. 52:266–275; 2002. [DOI] [PubMed] [Google Scholar]
- 36. Schonemeier B.; Schulz S.; Hoellt V.; Stumm R. Enhanced expression of the CXCl12/SDF-1 chemokine receptor CXCR7 after cerebral ischemia in the rat brain. J. Neuroimmunol. 198:39–45; 2008. [DOI] [PubMed] [Google Scholar]
- 37. Shyu W. C.; Lin S. Z.; Yen P. S.; Su C. Y.; Chen D. C.; Wang H. J.; Li H. Stromal cell-derived factor-1 alpha promotes neuroprotection, angiogenesis, and mobilization/homing of bone marrow-derived cells in stroke rats. J. Pharmacol. Exp. Ther. 324:834–849; 2008. [DOI] [PubMed] [Google Scholar]
- 38. Tang J.; Wang J.; Yang J.; Kong X.; Zheng F.; Guo L.; Zhang L.; Huang Y. Mesenchymal stem cells over-expressing SDF-1 promote angiogenesis and improve heart function in experimental myocardial infarction in rats. Eur. J. Cardiothorac. Surg. 36:644–650; 2009. [DOI] [PubMed] [Google Scholar]
- 39. Wang Y. Q.; Guo X.; Qiu M. H.; Feng X. Y.; Sun F. Y. VEGF overexpression enhances striatal neurogenesis in brain of adult rat after a transient middle cerebral artery occlusion. J. Neurosci. Res. 85:73–82; 2007. [DOI] [PubMed] [Google Scholar]
- 40. Wilson J. M. Gendicine: The first commercial gene therapy product. Hum. Gene Ther. 16:1014–1015; 2005. [DOI] [PubMed] [Google Scholar]
- 41. Wu H.; Lu W.; Mahato R. I. Mesenchymal stem cells as a gene delivery vehicle for successful islet transplantation. Pharm. Res. 28:2098–2109; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhao M. Z.; Nonoguchi N.; Ikeda N.; Watanabe T.; Furutama D.; Miyazawa D.; Funakoshi H.; Kajimoto Y.; Nakamura T.; Dezawa M.; Shibata M. A.; Otsuki Y.; Coffin R. S.; Liu W. D.; Kuroiwa T.; Miyatake S. Novel therapeutic strategy for stroke in rats by bone marrow stromal cells and ex vivo HGF gene transfer with HSV-1 vector. J. Cereb. Blood Flow Metab. 26:1176–1188; 2006. [DOI] [PubMed] [Google Scholar]