Abstract
We have recently shown that preculturing islets with kidney-derived mesenchymal stromal cells (MSCs) improves transplantation outcome in streptozotocin-diabetic mice implanted with a minimal mass of islets beneath the kidney capsule. In the present study, we have extended our previous observations to investigate whether preculturing islets with MSCs can also be used to enhance islet function at the clinically used intraportal site. We have used MSCs derived from adipose tissue, which are more readily accessible than alternative sources in human subjects and can be expanded to clinically efficacious numbers, to preculture islets throughout this study. The in vivo efficacy of grafts consisting of islets precultured alone or with MSCs was tested using a syngeneic streptozotocin-diabetic minimal islet mass model at the clinically relevant intraportal site. Blood glucose concentrations were monitored for 1 month. The vascularization of islets precultured alone or with MSCs was investigated both in vitro and in vivo, using immunohistochemistry. Islet insulin content was measured by radioimmunoassay. The effect of preculturing islets with MSCs on islet function in vitro was investigated using static incubation assays. There was no beneficial angiogenic influence of MSC preculture, as demonstrated by the comparable vascularization of islets precultured alone or with MSCs, both in vitro after 3 days and in vivo 1 month after islet transplantation. However, the in vitro insulin secretory capacity of MSC precultured islets was superior to that of islets precultured alone. In vivo, this was associated with improved glycemia at 7, 14, 21, and 28 days posttransplantation, in recipients of MSC precultured islets compared to islets precultured alone. The area of individual islets within the graft-bearing liver was significantly higher in recipients of MSC precultured islets compared to islets precultured alone. Our experimental studies suggest that preculturing islets with MSCs represents a favorable strategy for improving the efficiency of clinical islet transplantation.
Key words: Diabetes, Islet transplantation, Mesenchymal stromal cells (MSCs), Islet culture
INTRODUCTION
Allogeneic islet transplantation offers the possibility to treat selected patients with type 1 diabetes (T1D), but the limited availability of human islet material is a major obstacle preventing the widespread application of islet transplantation as a therapy for the majority of patients with T1D. This is exacerbated by the dramatic loss of islet cells during pretransplant culture (23,48), as well as significant destruction of islets soon after infusion into the portal vein, which is largely attributed to the instant blood-mediated inflammatory response (4). Since the majority of intraportally transplanted islets fail to engraft adequately (25), most patients require islet infusions from two to four donors (2,42) in order to achieve appropriate blood glucose control. Strategies to reduce the loss of valuable islet material during the clinical islet transplantation procedure will contribute to the achievement of routine single-donor islet transplantation, thereby increasing the availability of transplant material for more patients.
While a number of reports have indicated that fresh islets produce superior transplantation outcomes to cultured islets (24,35,39), it is clear that for clinical purposes, culturing islets is preferable for logistical reasons. A short-term culture period allows time for quality control testing of islet preparations, the initiation of immunosuppressive regimens for transplant recipients, and the shipment of islets to transplant centers (23,49). However, since islet function declines with culture, it is important to develop strategies to maintain or improve the function and quality of islets during this period in vitro prior to transplantation.
Mesenchymal stromal cells (MSCs) secrete an array of trophic factors that are likely to enhance the survival and function of islets during culture. Additionally, MSCs offer a host of immunomodulatory, anti-inflammatory, angiogenic, and regenerative properties that help to improve islet function and survival in vivo. We have recently reported that preculturing islets with kidney-derived MSCs using a direct contact coculture configuration improves islet transplantation outcome at the renal subcapsular site in diabetic mice (39). Although our previous study and other reports (21,28,37) have provided important proof of concept, the experimental models differ from clinical islet transplantation in some important aspects. Thus, although the renal subcapsular site is commonly used for transplantation studies in rodents (31), the morphological remodeling of islets transplanted here differs from that of islets delivered by the more clinically relevant intraportal route. Specifically, at the renal subcapsular site, individual islets fuse to form an aggregated mass of endocrine tissue (6,8,38,40), whereas islets infused into the portal vein disperse throughout the portal vasculature and engraft as single entities. Therefore, in the current study, we investigated whether preculturing islets with MSCs improves the transplantation outcome of islets delivered by the clinically relevant intraportal route. Our previous investigation demonstrated that neither preculture of islets in MSC-conditioned media nor Transwell islet-MSC preculture were sufficient to enhance islet function in vitro, in contrast to a direct contact coculture configuration, where we observed improved islet function both in vitro and in vivo. Thus, we have utilized this direct cell-contact configuration throughout the current study. Additionally, we have utilized murine MSCs derived from adipose tissue, which have good potential for clinical application, as they are readily accessible and can be harvested following liposuction. The overall aim of the current study was therefore to assess the potential benefits of pretreating islets with a clinically accessible population of stromal cells derived from adipose tissue prior to transplantation via the clinically relevant intraportal route.
MATERIALS AND METHODS
Experimental Animals
Male C57Bl/6 mice (Harlan, Huntingdon, UK) aged 8–12 weeks were used as donors and recipients. Mice were made diabetic by intraperitoneal streptozotocin (STZ) injection (180 mg/kg; Sigma-Aldrich, Poole, UK), and those with a nonfasting blood glucose concentration of ≥360 mg/dl were used as recipients. Blood glucose concentrations were determined using a blood glucose meter and strips (Accu-Chek; Roche, Burgess Hill, UK) with blood obtained from a pinprick to the tail. All animal procedures were approved by our institution’s Ethics Committee and carried out under license, in accordance with the UK Home Office Animals (Scientific Procedures) Act 1986.
Isolation of Adipose-Derived MSCs
Adipose-derived MSCs were isolated from male C57Bl/6 mice aged 8–12 weeks. Testicular fat pads were rinsed in Ca2+ and Mg2+-free phosphate-buffered saline (PBS; Sigma-Aldrich) and cut into small pieces. The pieces were digested with collagenase (1 mg/ml; type I; Sigma-Aldrich) at 37°C for 30 min with intermittent shaking. The resultant suspension was passed through a 100-µm cell strainer (SLS, Nottingham, UK) to remove large tissue debris. Cells were pelleted by centrifugation for 10 min at 400 × g. The cell pellet was resuspended in Dulbecco’s modified Eagle’s medium (DMEM; Sigma-Aldrich) supplemented with 1% (v/v) penicillin/streptomycin solution (Gibco BRL, Gaithersburg, MD, USA) and 10% (v/v) fetal calf serum (FCS; Sigma-Aldrich), seeded in 25-cm2 Nunc cell culture flasks (Thermo Fisher Scientific, Loughborough, UK) and incubated at 37°C, 5% CO2. Medium was changed after 24 h to remove any nonadherent cells and floating adipocytes. When cultures reached confluence, cells were trypsinized (0.05% trypsin; Sigma-Aldrich) and subcultured at empirically determined passage ratios until spindle-shaped, adherent cells outgrew other cell types.
Characterization of Adipose-Derived MSCs
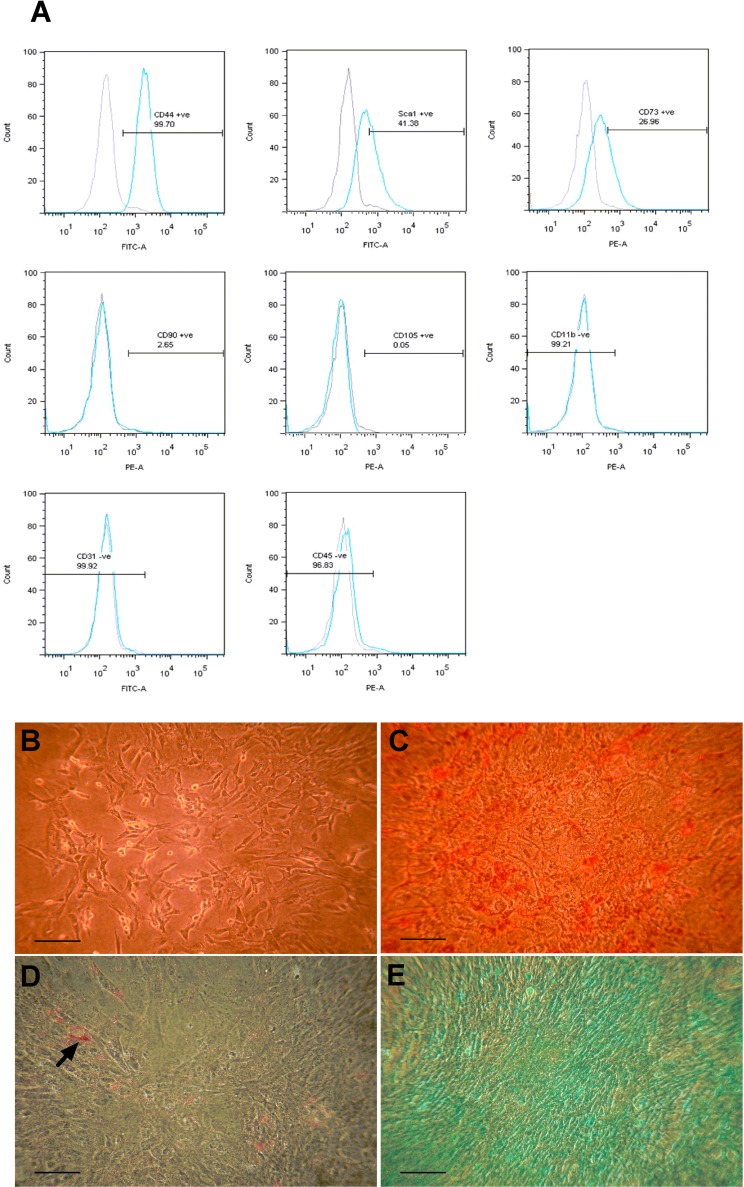
Adipose MSCs were analyzed for the presence of surface markers by flow cytometry. MSCs at passage 8 were trypsinized, resuspended in PBS, and incubated with the following fluorescein isothiocyanate (FITC; 0.025 mg/ml)- or phycoerythrin (PE; 0.01 mg/ml)-conjugated antibodies (1:20): cluster of differentiation 11b (CD11b), CD31, CD44, CD45, CD73, CD90.2, CD105, and stem cell antigen-1 (Sca-1) (BD Pharmingen, San Diego, CA, USA). After 30-min incubation at 4°C, the cells were washed and resuspended in 0.5 ml PBS. Cells were analyzed in a FACS Canto II cytometer (BD Biosciences, San Jose, CA, USA).
Adipose MSCs at passage 9 were assessed for their differentiation capacity along the adipogenic, osteogenic, and chondrogenic lineages. Adipogenic and osteogenic differentiation was performed as previously described (38). Osteogenic differentiation was evaluated by alizarin red (Sigma-Aldrich) detection of mineralized calcium nodule deposition, whereas adipogenic differentiation was evaluated by oil red O (Sigma-Aldrich) staining of lipid droplet formation. Chondrogenic differentiation was induced using micromass cultures before addition of chondrogenic differentiation medium, comprising DMEM supplemented with 10 ng/ml transforming growth factor-β1 (TGF-β1; Miltenyi, Woking, Surrey, UK), 0.1× ITS premix (Invitrogen, Paisley, UK), 100 µM ascorbic acid, 1 µM dexamethasone, and 1.25 µg/ml bovine serum albumin (BSA; Sigma-Aldrich). Micromasses were cultured in this medium for 7 days, followed by 21 days in medium without FBS. Chondrogenic differentiation was evaluated by alcian blue (Sigma-Aldrich) detection of glycosaminoglycan (GAG) deposition.
Islet Isolation and MSC Coculture
Islets were isolated by collagenase digestion (1 mg/ml; type XI; Sigma-Aldrich) followed by density gradient separation (Histopaque-1077; Sigma-Aldrich). After washing with Roswell Park Memorial Institute (RPMI)-1640 medium (Sigma-Aldrich), islets were picked into groups of 100 for culture with or without adipose MSCs for 3 days. We utilized a direct contact monolayer configuration to coculture islets with adipose MSCs, as previously described (39). Briefly, 200,000 adipose MSCs of passage 7–12 were seeded into Nunclon™ 35-mm Petri dishes, forming a confluent monolayer of cells within 12 h. MSCs were cultured in DMEM supplemented with 1% (v/v) penicillin/streptomycin solution supplemented with 10% (v/v) FCS and incubated at 37°C in a humidified atmosphere containing 5% CO2. The medium was changed after 24 h, with removal of nonadherent cells. One hundred freshly isolated islets were then added to each Petri dish allowing direct cell–cell contact between the islets and preseeded MSCs. The medium was replaced with RPMI-1640 [supplemented with 10% (v/v) FCS, 2 mmol/L glutamine (Sigma-Aldrich), and 100 U/ml penicillin/0.1 mg/ml streptomycin]. Control islets were cultured alone as groups of 100, in RPMI-1640, in nontreated 35-mm tissue culture Petri dishes. Islets cultured using the direct contact monolayer configuration formed loose attachments with the MSCs by 3 days but were removed for in vitro analysis or transplantation experiments by gentle pipetting.
Islet Function In Vitro
Insulin secretion in vitro was assessed using static incubations of isolated islets. Islets were preincubated for 2 h in RPMI containing 36 mg/dl glucose (Sigma-Aldrich). Groups of three islets were transferred into 1.5-ml Eppendorf tubes (Star Labs, Ahrensburg, Germany) and incubated at 37°C in a bicarbonate-buffered physiological salt solution (Gey and Gey buffer) containing 2 mmol/L CaCl2 and 0.5 mg/ml BSA (components of, all from Sigma-Aldrich) (15) and either 36 or 360 mg/dl glucose. After 1 h, samples of the incubation medium were taken and stored at −20°C until assayed for insulin content using in-house radioimmunoassay (RIA), as previously described (7,20). For assessment of islet insulin content, islets were pelleted by centrifugation, washed in PBS, lysed in acidified ethanol, and sonicated prior to RIA.
Intraportal Transplantation of Islets
Mice were transplanted intraportally with 250 islets that had been precultured alone or precultured with adipose MSCs. The number of transplanted islets was chosen to act as a minimal islet mass in the control islet-alone group, intending to reverse hyperglycemia in only a proportion of diabetic recipients, to enable detection of treatment-dependent improvements in graft function by increases in the rate of reversal of hyperglycemia. Islets were packed in a 25-gauge butterfly needle in a volume of ≤0.2 ml RPMI. An abdominal incision was made and islets infused into the portal vein with the 25-gauge butterfly needle connected to a Hamilton syringe (Fisher, Pittsburgh, PA, USA). Excess bleeding was prevented by applying a small piece of Spongostan™ film (Ethicon, Norderstedt, Germany) as the needle was removed from the portal vein. The body weight and blood glucose concentrations of recipient mice were monitored every 3 to 4 days. Cure was defined as nonfasting blood glucose concentrations ≤200 mg/dl for at least two consecutive readings, without reverting to hyperglycemia on any subsequent day. The insulin content of the pancreata from all transplanted mice and control nondiabetic, nontransplanted C57BL/6 mice was measured by using an in-house radioimmunoassay, as previously described (7,20).
Immunohistochemistry of Isolated Islets and Islet Grafts
Graft-bearing livers or isolated islets were fixed in 4% (v/v) formalin (Sigma-Aldrich) and paraffin embedded. Sections were stained for β-cells and microvascular endothelial cells (ECs). For CD31 and CD34 staining (detection of ECs), antigen retrieval was required [2 min in 10 mmol/L citric acid solution, pH 6.0 (Sigma-Aldrich) in a pressurized cooker]. Sections were incubated for 1 h at room temperature in the appropriate primary antibody as follows: polyclonal guinea pig anti-insulin antibody (1:1,000; Dako, Ely, UK), with a monoclonal rat anti-CD34 antibody (1:500; AbD Serotec, Kidlington, UK) or with a monoclonal rat anti-CD31 antibody (1:80; Dianova, Hamburg, Germany). Slides were then incubated for 1 h at room temperature with either a goat biotin anti-guinea pig antibody (1:200; Jackson Immunolaboratories, West Grove, PA, USA) or a rabbit biotinylated anti-rat antibody (1:200; Vector Laboratories, Peterborough, UK). Sections were incubated with streptavidin–horseradish peroxidase (Dako) and diaminobenzidine (Dako).
Evaluation of Vascular Density and Islet Graft Morphology
Intraportally transplanted islets were evaluated for size (according to the area of insulin-positive tissue) and integrity (16). Islets were considered intact if they were spheroid with round boundaries or fragmented if they had irregularly shaped borders with uneven boundaries (16). ImageJ software (NIH, Bethesda, MD, USA) was used to determine area and vascular density. The graft endocrine tissue vascular density was determined as the number of CD31+ or CD34+ ECs/mm2 of insulin-positive tissue (consecutive sections stained with insulin antibodies).
Statistical Analysis
Statistical analysis used Student’s t test or ANOVA as appropriate. Two-way repeated measurement ANOVA was used with Bonferroni’s post hoc test to analyze repeated measurements in the same animal at different time points. A Kaplan–Meier survival curve was used to identify differences in the time to cure between groups. A value of p < 0.05 was considered significant. All data are expressed as means ± SEM.
RESULTS
Characterization of Adipose-Derived MSCs
Murine adipose-derived MSCs were assessed for the expression of a panel of cell surface markers associated with MSC phenotype, using flow cytometry. Cells were positive for CD44 (>99%), Sca-1 (>41%), and CD73 (>26%), which are characteristic of murine MSCs. The stromal cells did not express CD90.2 and CD105, which are commonly associated with human MSCs (10). Cells were also negative for CD11b, CD31, and CD45, which are markers of macrophages, ECs, and hematopoietic cells (Fig. 1A). The MSCs demonstrated multilineage differentiation potential in vitro, adopting osteogenic, adipogenic, and chondrogenic cell fates. Prior to differentiation, cells exhibited a characteristic spindle-shaped morphology (Fig. 1B). In contrast to control cells shown in Figure 1B, punctate staining of calcium nodules by alizarin red can be seen in Figure 1C. Conglomerating immature lipid droplets (arrowhead) visualized by oil red O can be seen in Figure 1D, and GAGs uniformly deposited throughout the entire micromass culture were detected by alcian blue staining are shown in Figure 1E.
Figure 1.
Characterization of adipose-derived MSCs. (A) Cell surface marker expression of mouse adipose mesenchymal stromal cells (MSCs) at passage 9. Flow cytometry histograms show the expression levels (blue peaks) of selected markers associated with the characterization of murine MSCs [cluster of differentiation 44 (CD44), stem cell antigen-1 (Sca-1), CD73, CD90, CD105, CD11b, CD31, CD45] compared with negative isotype controls (shaded gray peaks). (B–E) Osteogenic, adipogenic, and chondrogenic differentiation of adipose MSCs at passage 9. Adipose MSCs exhibited characteristic spindle-shaped morphology in culture prior to differentiation (B) and stained positive for alizarin red (C), oil red O, as indicated by arrowhead (D), and alcian blue when treated with osteogenic, adipogenic, and chondrogenic in vitro differentiation protocols, respectively. Scale bars: 100 µm (B, E), 50 µm (C, D).
Preculturing Islets With Adipose-Derived MSCs Enhances Islet Function In Vitro
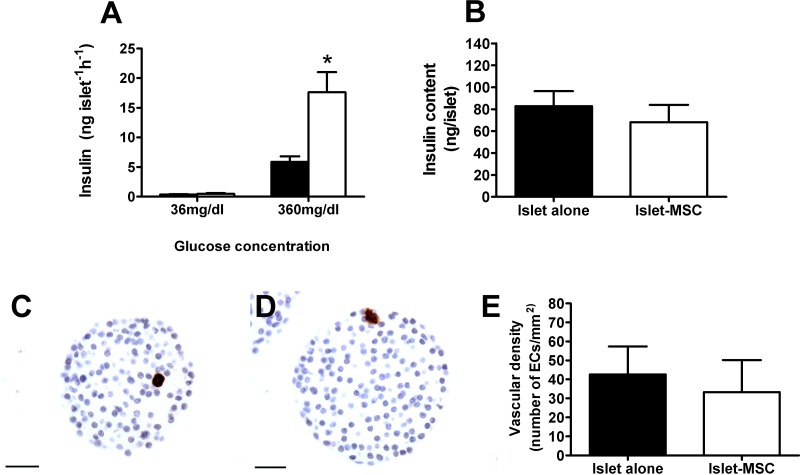
Islet function and intraislet EC number decline during short-term culture. To determine whether preculture with adipose MSCs influenced islet insulin secretory capacity or EC number, islets were maintained in culture in vitro for 3 days either alone or with adipose MSCs using a direct contact monolayer configuration. Glucose-stimulated insulin secretion was potentiated when islets were precultured on the adipose MSC monolayer compared to islets precultured alone (p < 0.05), while basal insulin secretion was similar between culture groups (Fig. 2A). The beneficial influence of adipose MSCs on islet function in vitro was independent of any effect on islet insulin content, which was comparable between the two culture groups (Fig. 2B). In accordance, islet area was also similar between culture groups (11,330 ± 947 and 13,114 ± 1,097 µm2, islets precultured alone and MSC precultured islets, respectively, n = 64–74 islet sections from four mice in each culture group, p > 0.05). There were very few CD31+ ECs present within islet sections of islets precultured alone (Fig. 2C) or in islets that had been precultured on the adipose MSC monolayer for 3 days (Fig. 2D). The ECs that were observed were generally present as single cells, as opposed to contributing to mature intensely immunostained vascular structures, typical of native pancreatic islets, demonstrating that MSC preculture does not prevent the loss and/or dedifferentiation of ECs during short-term culture. Quantification of the number of CD31+ ECs showed that adipose MSCs had no beneficial angiogenic effect in vitro, with a similarly low vascular density observed in islet sections from both culture groups (Fig. 2E). Islet sections from both culture groups were also immunostained with CD34 antibodies as an additional EC marker, which confirmed the lack of any significant intraislet EC survival in both culture groups (37.3 ± 15.4 and 37.1 ± 10.9 CD34+ ECs/mm2, islets precultured alone and islets precultured with MSCs, respectively, n = 64–74 islet sections from four mice in each culture group, p > 0.05), while indicating that a minor subset of intraislet ECs were CD31+, but lacked CD34 expression.
Figure 2.
In vitro islet function and survival of intraislet ECs in adipose MSC precultured islets. (A) Insulin release at 36 mg/dl and 360 mg/dl glucose of 10 replicates of triplicate islets precultured for 3 days with adipose MSCs (white bars) or without MSCs (black bars); *p < 0.05 versus absence of MSCs at the same glucose concentration (two-way ANOVA with Bonferroni post hoc test, n = 4). (B) Preculturing islets with adipose MSCs has no effect on islet insulin content. Insulin content of islets precultured alone (black bar) or with adipose MSCs (white bar) for 3 days; p > 0.2 (Student’s t test, n = 6). (C–E) Preculturing islets with adipose MSCs does not have any angiogenic effect in vitro. (C, D) Immunostaining of endothelial cells (ECs) with CD31 antibodies in islets precultured alone (C) or precultured with adipose MSCs (D) for 3 days. Original magnification: 400×. Scale bars: 25 µm. (E) Vascular density of islets precultured alone (black bar) or with adipose MSCs (white bar) for 3 days; p > 0.05 (Student’s t test, n = 64–74 islet sections from four mice in each culture group).
In Vivo Function of Islets Precultured With Adipose-Derived MSCs Prior to Intraportal Transplantation
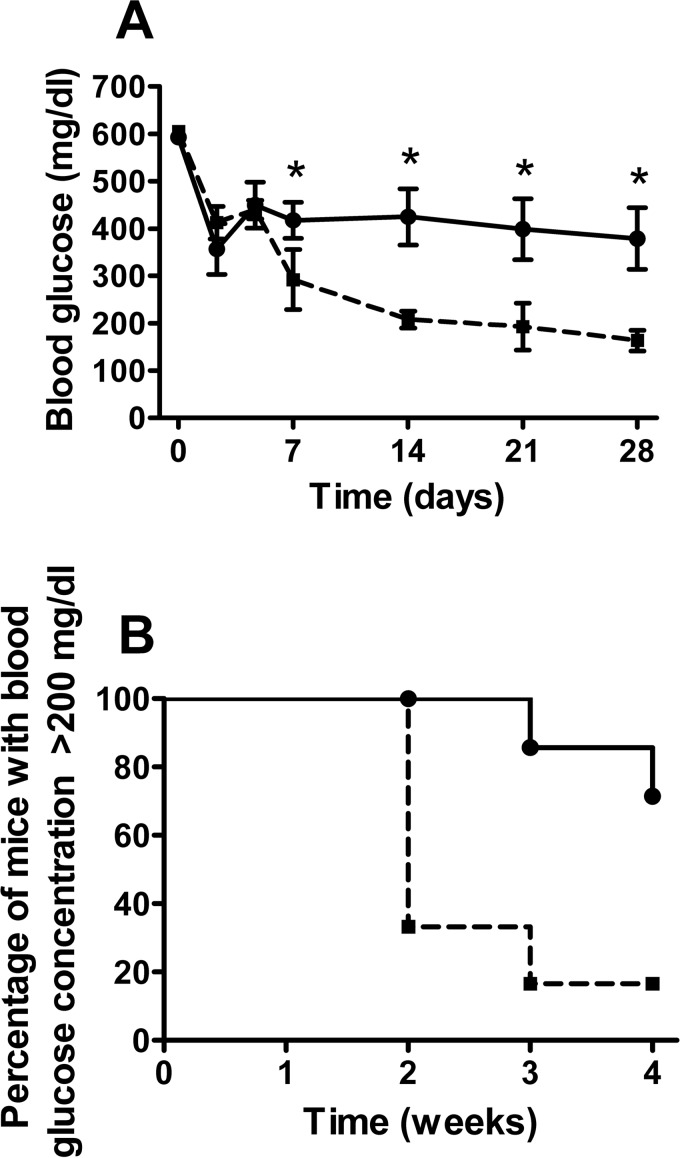
Preculturing islets with adipose MSCs improved graft function in vivo as shown in Figure 3. Average blood glucose concentrations were significantly lower at 7, 14, 21, and 28 days posttransplantation in recipients of adipose MSC precultured islets compared with recipients of islets precultured alone (p < 0.05) (Fig. 3A). At 1 month posttransplantation 5/6 mice transplanted with adipose MSC precultured islets had been cured compared to 2/7 mice transplanted with islets precultured alone (p < 0.05; Fig. 3B). There were no significant differences in the weights of mice in either transplant group on day 0 (24.3 ± 0.9 and 22.5 ± 1.0 g for recipients of islets precultured alone or precultured with MSCs, respectively, n = 6–7, p > 0.2) nor at 1 month after transplantation (26.0 ± 0.8 and 25.5 ± 1.2 g, n = 6–7, p > 0.2). There was no difference in the pancreas insulin content for STZ-diabetic mice transplanted with islets that had been precultured with MSCs or alone (1.2 ± 0.4 and 1.1 ± 0.4 µg/pancreas, p > 0.2, n = 6–7). The insulin content of the STZ pancreata was approximately 1% that of pancreata from control nondiabetic nontransplanted C57BL/6 mice, which had an insulin content of 142.6 ± 24.5 µg/pancreas (n = 5), indicating that endogenous pancreatic β-cell regeneration did not contribute significantly to the maintenance of normoglycemia in cured mice.
Figure 3.
In vivo function of islets precultured with adipose-derived MSCs. (A) Blood glucose concentrations of mice transplanted intraportally with islets precultured alone for 3 days (continuous line) or precultured with adipose MSCs (dashed line); *p < 0.05 versus mice transplanted with MSC precultured islets (RM ANOVA with Bonferroni post hoc test, n = 6–7). (B) Percentage of mice remaining diabetic (blood glucose ≥200 mg/dl) after transplantation as in (A); p < 0.01 (Kaplan–Meier, n = 6–7).
Morphological Assessment of Intraportally Transplanted Islets
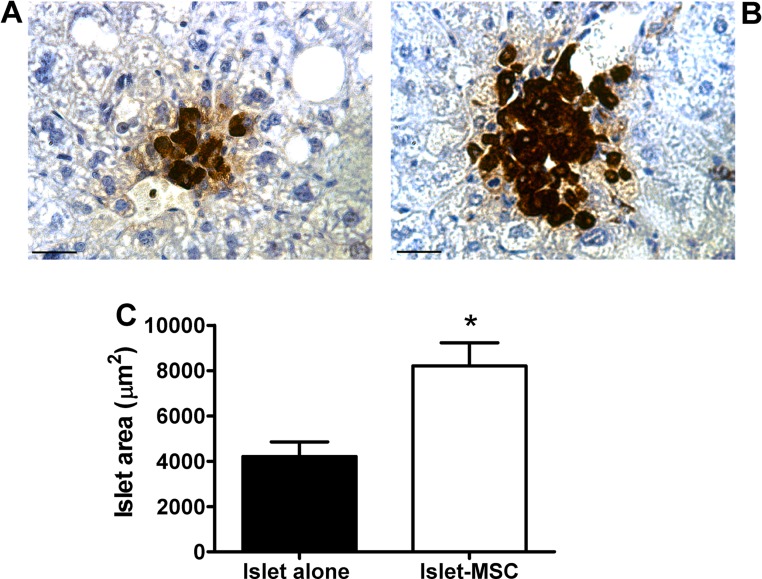
Insulin immunostaining of intraportally transplanted islets at 1 month posttransplantation showed that there were often small clusters of β-cells within the graft-bearing livers of mice transplanted with islets that had been precultured alone (Fig. 4A), whereas larger islets were present in mice that had been transplanted with MSC precultured islets (Fig. 4B). Although a proportion of islets in both transplant groups were considered intact, a large percentage of islets in both transplant groups had disrupted islet borders lacking the spherical morphology typical of endogenous islets (60% and 56% of islets classified as fragmented, for grafts consisting of islets precultured alone and islets precultured with MSCs, respectively). Regardless of islet integrity, the average islet area was approximately twofold higher in the MSC precultured islet grafts compared to islets precultured alone (p < 0.05) (Fig. 4C).
Figure 4.
Morphological assessment of intraportally transplanted islets. (A, B) Representative sections of 1-month grafts consisting of islets precultured alone (A) or with adipose MSCs (B) for 3 days prior to intraportal transplantation. Images are representative of insulin staining for four animals in each transplant group. Original magnification: 400×. Scale bars: 25 µm. (C) Average area of islet graft sections; n = 53–102 graft sections from four animals in each transplant group; *p < 0.05 versus grafts consisting of islets precultured alone (Student’s t test).
Vascularization of Intraportally Transplanted Islets
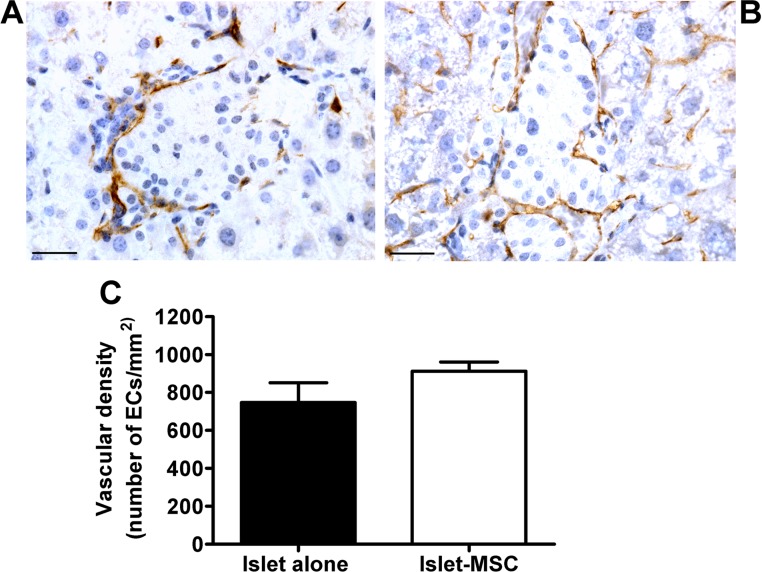
CD31 antibodies were used to immunostain microvascular endothelium in 1-month grafts consisting of islets precultured alone (Fig. 5A) or with adipose MSCs (Fig. 5B). The EC distribution was largely confined to the periphery of islet structures in the grafts of both transplant groups, with extensive areas of endocrine tissue (defined by insulin-positive tissue in consecutive graft sections) devoid of ECs. When the number of ECs was quantified, there was a comparably low vascular density in both transplant groups (Fig. 5C). The lack of beneficial angiogenic effect in vivo when transplanting adipose MSC precultured islets compared to islets precultured alone was confirmed when using CD34 antibodies to immunostain graft sections (740.8 ± 94.1 and 899.4 ± 54.3 CD34+ ECs/mm2, islet-alone and islet-MSC grafts, respectively, p > 0.05, Student’s t test, n = 20–25 graft sections from four animals in each transplant group).
Figure 5.
Vascularization of intraportally transplanted islets. Staining of ECs with CD31 antibodies in 1-month grafts consisting of islets precultured alone (A) or islets precultured with adipose MSCs (B) for 3 days prior to intraportal transplantation. Images are representative of four animals in each transplant group. Original magnification: 400×. Scale bars: 25 µm. (C) Vascular density of grafts consisting of islets precultured alone (black bar) or with MSCs (white bar) for 3 days prior to transplantation; p > 0.05 (n = 20–25 graft sections from four animals in each transplant group).
DISCUSSION
In the current study, we have used a syngeneic minimal islet mass model to determine whether MSC pretreatment improves islet transplantation outcome at the clinically used intraportal site. We have used adipose-derived MSCs, which are readily obtainable from human subjects and have a higher proliferative potential in culture compared to MSCs derived from other tissues (46), making their expansion to clinically efficacious numbers more readily achievable. Our results demonstrate for the first time that preculture with adipose-derived MSCs enhances islet function in vitro, consistent with previous reports utilizing alternative MSC sources (21,28,37,39). We have also shown, for the first time, that preculture with adipose MSCs improved glycemia and curative capacity of islets transplanted via the clinically relevant intraportal route.
The in vivo function of adipose MSC precultured islets correlated with their enhanced insulin secretory function in vitro. Significant reductions in blood glucose concentrations were observed within 7 days of implantation and were maintained for the remainder of the 28-day monitoring period. These rapid and maintained reductions in hyperglycemia induced by MSC precultured islet grafts may reduce β-cell losses in response to glucotoxicity in a hyperglycemic in vivo microenvironment. It has been suggested that blood glucose concentration profiles are an indicator of the degree of islet destruction during the immediate posttransplantation period (41), with blood glucose concentrations falling immediately after intraportal transplantation due to extensive islet cell death and subsequent insulin leakage. Following this, blood glucose concentrations start to rise due to the loss of functional β-cells. A blood glucose profile similar to this was observed in our control mice transplanted with islets precultured alone, consistent with extensive islet cell death in the immediate posttransplantation period. In contrast, in mice receiving MSC precultured islets blood glucose levels continued to decline after 3 days, suggesting that islet survival and/or function was enhanced. In accordance, 28 days after implantation, the islet area in grafts consisting of islets precultured alone was almost twofold lower than that of grafts consisting of MSC precultured islets.
The maintenance of functional β-cell mass in vivo by preculture with MSCs is unlikely to be due to the well-documented anti-inflammatory or immunomodulatory effects of MSCs (1), since few, if any, MSCs were cotransplanted with the islet graft. Similarly, although we (38) and others (12,17,44) have demonstrated beneficial effects of cotransplanted MSCs on islet graft revascularization, this mechanism is unlikely to account for the superior transplantation outcomes demonstrated with MSC precultured islets in the current study. Thus, the presence of adipose MSCs did not prevent the loss of intraislet ECs during the in vitro culture period, nor was MSC preculture associated with any significant differences in graft revascularization, as assessed by the expression of EC markers 28 days after islet implantation.
Our previous histological measurements of intraislet EC survival and graft revascularization at the renal subcapsular site utilized CD34 as an EC marker (39). While the majority of intraislet ECs are generally quiescent in adult rodents (19), a small population of remnant ECs with an angiogenic phenotype persist in cultured islets (26), and it is likely that a subset of ECs with a similar proliferative phenotype are present during the early revascularization process. The rate of islet graft revascularization is implantation site specific, with full revascularization taking approximately 1 month or 3 months for the renal subcapsular (29,30) or intraportal (36) sites, respectively. There is some evidence that CD34 is not a reliable marker for proliferating ECs (13), whereas CD31 has been shown to be expressed by proliferating ECs (3) and by cultured islet ECs (34). Owing to the slower rate of revascularization at the intraportal site, we have therefore utilized both CD31 and CD34 as EC markers in the current study, to ensure that we could detect proliferating ECs. Although we did detect a minor subset of intraislet ECs that were CD31+/CD34−, MSC preculture had no significant effect on any EC population during either in vitro culture or the in vivo engrafting period. At both the renal subcapsular and intraportal transplantation site, codelivery of MSCs with the islets enhances revascularization and maintains islet structure (17,38). This effect was not observed when using an MSC preculture strategy in the current intraportal study, nor in our previous study at the renal subcapsular site (39). Thus, our observations suggest that the proangiogenic effect of MSCs on graft revascularization is dependent on the in vivo presence of MSCs with the transplanted islets. Furthermore, our finding that MSC preculture enhances islet function both in vitro and in vivo, in a manner that is independent of any beneficial angiogenic effect when utilizing both kidney and adipose MSCs, suggests that stromal cells from both tissue sources have similar functional phenotypes.
The beneficial influence of MSCs in islet transplantation protocols may therefore act through independent and additive mechanisms. Notably, our finding that MSCs from different tissue sources help to improve islet function in vitro and in vivo at both the renal subcapsular and intraportal site strengthens an increasing body of evidence to suggest that MSCs may also improve islet function in bioengineered devices. Indeed, we have recently shown that islets coencapsulated with MSCs in alginate microcapsules produced superior transplantation outcomes to islets that are microencapsulated in the absence of MSCs (22). Cotransplantation of MSCs with the islet graft is likely to enhance islet survival and function by local suppression of immune or inflammatory responses (5,9,18,27,43,50), by enhancing the rate of revascularization in a hypoxic microenvironment (12,17,38,44) and by maintaining islet integrity and structure (17,38). However, these benefits are balanced against the potential disadvantages of cotransplanting MSCs with the islets, including increased risk of portal/hepatic thrombus formation because of the procoagulant activity of MSCs (32,45) and the risk of pluripotent MSC populations differentiating along inappropriate lineages (11) or becoming tumorigenic (14). Additionally, although MSCs are generally considered “hypoimmunogenic,” expressing low levels of MHC class II and classical costimulatory molecules (47), there is some evidence to suggest that MSCs may be more immunogenic than was initially thought (33) and therefore that cotransplanting MSCs with the islet graft may increase antigen loading for the graft recipient. The current study demonstrates that preculture with MSCs prior to islet-alone transplantation can confer marked benefits while avoiding these potential disadvantages. The precise mechanism(s) through which preculture with MSCs improves the in vitro and in vivo function of islets in this and previous studies (39) remains unclear. However, the current study extends our previous observations at the experimentally used renal subcapsular site to demonstrate that preculture of islets with MSCs is an efficient strategy for improving transplantation outcome at the clinically used intraportal site.
In summary, the current study has demonstrated that preculturing islets with adipose MSCs enhances islet function in vitro and improves the outcome of islet transplantation via the intraportal route. Given the logistical difficulties of transplanting freshly isolated human islets (49), short-term preculture of islets with MSCs may offer a relatively safe method to improve the quality of islets for transplantation, which would contribute to the routine achievement of single-donor islet transplantation.
ACKNOWLEDGMENTS
We are grateful to Diabetes UK (P. M. Jones and A. J. F. King; 11/0004290; 06/0003387, C. L. Rackham,) and the MRC (P. K. Dhadda) for funding this study. We also thank Dr. Greta Sawyer for her technical assistance. The authors acknowledge financial support from the Department of Health via The National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy’s and St. Thomas’ NHS Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust. The authors declare no conflict of interest.
REFERENCES
- 1. Abdi R.; Fiorina P.; Adra C. N.; Atkinson M.; Sayegh M. H. Immunomodulation by mesenchymal stem cells: A potential therapeutic strategy for type 1 diabetes. Diabetes 57(7):1759–1767; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barton F. B.; Rickels M. R.; Alejandro R.; Hering B. J.; Wease S.; Naziruddin B.; Oberholzer J.; Odorico J. S.; Garfinkel M. R.; Levy M.; Pattou F.; Berney T.; Secchi A.; Messinger S.; Senior P. A.; Maffi P.; Posselt A.; Stock P. G.; Kaufman D. B.; Luo X.; Kandeel F.; Cagliero E.; Turgeon N. A.; Witkowski P.; Naji A.; O’Connell P. J.; Greenbaum C.; Kudva Y. C.; Brayman K. L.; Aull M. J.; Larsen C.; Kay T. W.; Fernandez L. A.; Vantyghem M. C.; Bellin M.; Shapiro A. M. Improvement in outcomes of clinical islet transplantation: 1999–2010. Diabetes Care 35(7):1436–1445; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bencini P. L.; Montagnino G.; Tarantino A.; Alessi E.; Ponticelli C.; Caputo R. Kaposi’s sarcoma in kidney transplant recipients. Arch. Dermatol. 129(2):248–250; 1993. [PubMed] [Google Scholar]
- 4. Bennet W.; Sundberg B.; Groth C. G.; Brendel M. D.; Brandhorst D.; Brandhorst H.; Bretzel R. G.; Elgue G.; Larsson R.; Nilsson B.; Korsgren O. Incompatibility between human blood and isolated islets of Langerhans: A finding with implications for clinical intraportal islet transplantation? Diabetes 48(10):1907–1914; 1999. [DOI] [PubMed] [Google Scholar]
- 5. Berman D. M.; Willman M. A.; Han D.; Kleiner G.; Kenyon N. M.; Cabrera O.; Karl J. A.; Wiseman R. W.; O’Connor D. H.; Bartholomew A. M.; Kenyon N. S. Mesenchymal stem cells enhance allogeneic islet engraftment in nonhuman primates. Diabetes 59(10):2558–2568; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Biarnes M.; Montolio M.; Nacher V.; Raurell M.; Soler J.; Montanya E. Beta-cell death and mass in syngeneically transplanted islets exposed to short- and long-term hyperglycemia. Diabetes 51(1):66–72; 2002. [DOI] [PubMed] [Google Scholar]
- 7. Bjaaland T.; Hii C. S. T.; Jones P. M.; Howell S. L. Role of protein kinase C in arginine-induced glucagon secretion from isolated rat islets of Langerhans. J. Mol. Endocrinol. 1(2):105–110; 1988. [DOI] [PubMed] [Google Scholar]
- 8. Davalli A. M.; Scaglia L.; Zangen D. H.; Hollister J.; Bonner-Weir S.; Weir G. C. Vulnerability of islets in the immediate posttransplantation period. Dynamic changes in structure and function. Diabetes 45(9):1161–1167; 1996. [DOI] [PubMed] [Google Scholar]
- 9. Ding Y.; Xu D.; Feng G.; Bushell A.; Muschel R. J.; Wood K. J. Mesenchymal stem cells prevent the rejection of fully allogenic islet grafts by the immunosuppressive activity of matrix metalloproteinase-2 and -9. Diabetes 58(8):1797–1806; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dominici M.; Le Blanc K.; Mueller I.; Slaper-Cortenbach I.; Marini F.; Krause D.; Deans R.; Keating A.; Prockop D.; Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8(4):315–317; 2006. [DOI] [PubMed] [Google Scholar]
- 11. Duprez I. R.; Johansson U.; Nilsson B.; Korsgren O.; Magnusson P. U. Preparatory studies of composite mesenchymal stem cell islets for application in intraportal islet transplantation. Upsala J. Med. Sci. 116(1):8–17; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Figliuzzi M.; Cornolti R.; Perico N.; Rota C.; Morigi M.; Remuzzi G.; Remuzzi A.; Benigni A. Bone marrow-derived mesenchymal stem cells improve islet graft function in diabetic rats. Transplant. Proc. 41(5):1797–1800; 2009. [DOI] [PubMed] [Google Scholar]
- 13. Fina L.; Molgaard H. V.; Robertson D.; Bradley N. J.; Monaghan P.; Delia D.; Sutherland D. R.; Baker M. A.; Greaves M. F. Expression of the CD34 gene in vascular endothelial cells. Blood 75(12):2417–2426; 1990. [PubMed] [Google Scholar]
- 14. Fiorina P.; Jurewicz M.; Augello A.; Vergani A.; Dada S.; La Rosa S.; Selig M.; Godwin J.; Law K.; Placidi C.; Smith R. N.; Capella C.; Rodig S.; Adra C. N.; Atkinson M.; Sayegh M. H.; Abdi R. Immunomodulatory function of bone marrow-derived mesenchymal stem cells in experimental autoimmune type 1 diabetes. J. Immunol. 183(2):993–1004; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gey G. O.; Gey M. K. Maintenance of human normal cells in continuous culture: preliminary report; cultivation of mesoblastic tumours and normal cells and notes on methods of cultivation. Am. J. Cancer 27:45–76; 1936. [Google Scholar]
- 16. Henriksnas J.; Lau J.; Zang G.; Berggren P. O.; Kohler M.; Carlsson P. O. Markedly decreased blood perfusion of pancreatic islets transplanted intraportally into the liver: Disruption of islet integrity necessary for islet revascularization. Diabetes 61(3):665–673; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ito T.; Itakura S.; Todorov I.; Rawson J.; Asari S.; Shintaku J.; Nair I.; Ferreri K.; Kandeel F.; Mullen Y. Mesenchymal stem cell and islet co-transplantation promotes graft revascularization and function. Transplantation 89(12):1438–1445; 2010. [DOI] [PubMed] [Google Scholar]
- 18. Jacobson S.; Kumagai-Braesch M.; Tibell A.; Svensson M.; Flodström-Tullberg M. Co-transplantation of stromal cells interferes with the rejection of allogeneic islet grafts. Ann. N Y Acad. Sci. 1150(1):213–216; 2008. [DOI] [PubMed] [Google Scholar]
- 19. Johansson M.; Andersson A.; Carlsson P. O.; Jansson L. Perinatal development of the pancreatic islet microvasculature in rats. J. Anat. 208(2):191–196; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jones P. M.; Salmon D. M.; Howell S. L. Protein phosphorylation in electrically permeabilized islets of Langerhans. Effects of Ca2+, cyclic AMP, a phorbol ester and noradrenaline. Biochem. J. 254:397–403; 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jung E. J.; Kim S. C.; Wee Y. M.; Kim Y. H.; Choi M. Y.; Jeong S. H.; Lee J.; Lim D. G.; Han D. J. Bone marrow-derived mesenchymal stromal cells support rat pancreatic islet survival and insulin secretory function in vitro. Cytotherapy 13(1):19–29; 2011. [DOI] [PubMed] [Google Scholar]
- 22. Kerby A.; Jones E. S.; Jones P. M.; King A. J. Co-transplantation of islets with mesenchymal stem cells in microcapsules demonstrates graft outcome can be improved in an isolated-graft model of islet transplantation in mice. Cytotherapy 15(2):192–200; 2013. [DOI] [PubMed] [Google Scholar]
- 23. Kin T.; Senior P.; O’Gorman D.; Richer B.; Salam A.; Shapiro A. M. J. Risk factors for islet loss during culture prior to transplantation. Transpl. Int. 21(11):1029–1035; 2008. [DOI] [PubMed] [Google Scholar]
- 24. King A.; Lock J.; Xu G.; Bonner-Weir S.; Weir G. Islet transplantation outcomes in mice are better with fresh islets and exendin-4 treatment. Diabetologia 48(10):2074–2079; 2005. [DOI] [PubMed] [Google Scholar]
- 25. Korsgren O.; Lundgren T.; Felldin M.; Foss A.; Isaksson B.; Permert J.; Persson N. H.; Rafael E.; Ryden M.; Salmela K.; Tibell A.; Tufveson G.; Nilsson B. Optimising islet engraftment is critical for successful clinical islet transplantation. Diabetologia 51(2):227–232; 2008. [DOI] [PubMed] [Google Scholar]
- 26. Linn T.; Schmitz J.; Hauck-Schmalenberger I.; Lai Y.; Bretzel R. G.; Brandhorst H.; Brandhorst D. Ischaemia is linked to inflammation and induction of angiogenesis in pancreatic islets. Clin. Exp. Immunol. 144(2):179–187; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Longoni B.; Szilagyi E.; Quaranta P.; Paoli G. T.; Tripodi S.; Urbani S.; Mazzanti B.; Rossi B.; Fanci R.; Demontis G. C.; Marzola P.; Saccardi R.; Cintorino M.; Mosca F. Mesenchymal stem cells prevent acute rejection and prolong graft function in pancreatic islet transplantation. Diabetes Technol. Ther. 12(6):435–446; 2010. [DOI] [PubMed] [Google Scholar]
- 28. Lu Y.; Jin X.; Chen Y.; Li S.; Yuan Y.; Mai G.; Tian B.; Long D.; Zhang J.; Zeng L.; Li Y.; Cheng J. Mesenchymal stem cells protect islets from hypoxia/reoxygenation-induced injury. Cell Biochem. Funct. 28(8):637–643; 2010. [DOI] [PubMed] [Google Scholar]
- 29. Mendola J. F.; Goity C.; Fernandez-Alvarez J.; Saenz A.; Benarroch G.; Fernandez-Cruz L.; Gomis R. Immunocytochemical study of pancreatic islet revascularization in islet isograft. Effect of hyperglycemia of the recipient and of in vitro culture of islets. Transplantation 57(5):725–730; 1994. [DOI] [PubMed] [Google Scholar]
- 30. Menger M. D.; Pattenier J.; Wolf B.; Jager S.; Feifel G.; Messmer K. Cryopreservation of islets of Langerhans does not affect angiogenesis and revascularization after free transplantation. Eur. Surg. Res. 24(2):89–96; 1992. [DOI] [PubMed] [Google Scholar]
- 31. Merani S.; Toso C.; Emamaullee J.; Shapiro A. M. Optimal implantation site for pancreatic islet transplantation. Brit. J. Surg. 95(12):1449–1461; 2008. [DOI] [PubMed] [Google Scholar]
- 32. Moll G.; Rasmusson-Duprez I.; von Bahr L.; Connolly-Andersen A. M.; Elgue G.; Funke L.; Hamad O. A.; Lonnies H.; Magnusson P. U.; Sanchez J.; Teramura Y.; Nilsson-Ekdahl K.; Ringdén O.; Korsgren O.; Nilsson B.; Le Blanc K. Are therapeutic human mesenchymal stromal cells compatible with human blood? Stem Cells 30(7):1565–1574; 2012. [DOI] [PubMed] [Google Scholar]
- 33. Nauta A. J.; Westerhuis G.; Kruisselbrink A. B.; Lurvink E. G.; Willemze R.; Fibbe W. E. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood 108(6):2114–2120; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nyqvist D.; Köhler M.; Wahlstedt H.; Berggren P.-O. Donor islet endothelial cells participate in formation of functional vessels within pancreatic islet grafts. Diabetes 54(8):2287–2293; 2005. [DOI] [PubMed] [Google Scholar]
- 35. Olsson R.; Carlsson P. O. Better vascular engraftment and function in pancreatic islets transplanted without prior culture. Diabetologia 48(3):469–476; 2005. [DOI] [PubMed] [Google Scholar]
- 36. Olsson R.; Olerud J.; Pettersson U.; Carlsson P.-O. Increased numbers of low-oxygenated pancreatic islets after intraportal islet transplantation. Diabetes 60(9):2350–2353; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Park K. S.; Kim Y. S.; Kim J. H.; Choi B.; Kim S. H.; Tan A. H.; Lee M. S.; Lee M. K.; Kwon C. H.; Joh J. W.; Kim S. J.; Kim K. W. Trophic molecules derived from human mesenchymal stem cells enhance survival, function, and angiogenesis of isolated islets after transplantation. Transplantation 89(5):509–517; 2010. [DOI] [PubMed] [Google Scholar]
- 38. Rackham C. L.; Chagastelles P. C.; Nardi N. B.; Hauge-Evans A. C.; Jones P. M.; King A. J. Co-transplantation of mesenchymal stem cells maintains islet organisation and morphology in mice. Diabetologia 54(5):1127–1135; 2011. [DOI] [PubMed] [Google Scholar]
- 39. Rackham C. L.; Dhadda P. K.; Chagastelles P. C.; Simpson S. J.; Dattani A. A.; Bowe J. E.; Jones P. M.; King A. J. Pre-culturing islets with mesenchymal stromal cells using a direct contact configuration is beneficial for transplantation outcome in diabetic mice. Cytotherapy 15(4):449–459; 2013. [DOI] [PubMed] [Google Scholar]
- 40. Rackham C. L.; Jones P. M.; King A. J. Maintenance of islet morphology is beneficial for transplantation outcome in diabetic mice. PLoS One 8(2):e57844; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sakata N.; Hayes P.; Tan A.; Chan N. K.; Mace J.; Peverini R.; Sowers L.; Pearce W. J.; Chinnock R.; Obenaus A.; Hathout E. MRI assessment of ischemic liver after intraportal islet transplantation. Transplantation 87(6):825–830; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shapiro A. M. Strategies toward single-donor islets of Langerhans transplantation. Curr. Opin. Organ Transplant. 16(6):627–631; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Solari M. G.; Srinivasan S.; Boumaza I.; Unadkat J.; Harb G.; Garcia-Ocana A.; Feili-Hariri M. Marginal mass islet transplantation with autologous mesenchymal stem cells promotes long-term islet allograft survival and sustained normoglycemia. J. Autoimmunity 32(2):116–124; 2009. [DOI] [PubMed] [Google Scholar]
- 44. Sordi V.; Melzi R.; Mercalli A.; Formicola R.; Doglioni C.; Tiboni F.; Ferrari G.; Nano R.; Chwalek K.; Lammert E.; Bonifacio E.; Borg D.; Piemonti L. Mesenchymal cells appearing in pancreatic tissue culture are bone marrow-derived stem cells with the capacity to improve transplanted islet function. Stem Cells 28(1):140–151; 2010. [DOI] [PubMed] [Google Scholar]
- 45. Stephenne X.; Nicastro E.; Eeckhoudt S.; Hermans C.; Nyabi O.; Lombard C.; Najimi M.; Sokal E. Bivalirudin in combination with heparin to control mesenchymal cell procoagulant activity. PLoS One 7(8):e42819; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Strem B. M.; Hicok K. C.; Zhu M.; Wulur I.; Alfonso Z.; Schreiber R. E.; Fraser J. K.; Hedrick M. H. Multipotential differentiation of adipose tissue-derived stem cells. Keio J. Med. 54(3):132–141; 2005. [DOI] [PubMed] [Google Scholar]
- 47. Tse W. T.; Pendleton J. D.; Beyer W. M.; Egalka M. C.; Guinan E. C. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: Implications in transplantation. Transplantation 75(3):389–397; 2003. [DOI] [PubMed] [Google Scholar]
- 48. Wang R.; Rosenberg L. Maintenance of beta-cell function and survival following islet isolation requires re-establishment of the islet-matrix relationship. J. Endocrinol. 163(2):181–190; 1999. [DOI] [PubMed] [Google Scholar]
- 49. Weir G. C.; Bonner-Weir S. Scientific and political impediments to successful islet transplantation. Diabetes 46(8):1247–1256; 1997. [DOI] [PubMed] [Google Scholar]
- 50. Yeung T. Y.; Seeberger K. L.; Kin T.; Adesida A.; Jomha N.; Shapiro A. M.; Korbutt G. S. Human mesenchymal stem cells protect human islets from pro-inflammatory cytokines. PLoS ONE 7(5):e38189; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]