Abstract
Pancreatic islet transplantation has received widespread attention as a promising treatment for type 1 diabetes. However, islets for transplantation are subject to damage from a number of sources, including ischemic injury during removal and delivery of the donor pancreas, enzymatic digestion during islet isolation, and reperfusion injury after transplantation in the recipient. Here we found that protein fractions secreted by mesenchymal stem cells (MSCs) were capable of activating preserved islets. A conditioned medium from the supernatant obtained by culturing adipose tissue MSCs (derived from wild-type Lewis rats) was prepared for 2 days in serum-free medium. Luc-Tg rat islets to which an organ preservation solution was added were then incubated at 4°C with fractions of various molecular weights prepared from the conditioned medium. Under the treatment with some of the fractions, by 4 days the relative luminescence intensities (representative of the ATP levels of the cold-preserved islets) had increased to over 150% of their initial values. Our novel system may be able to restore isolated islets to the condition they were in before transport, culture, and transplantation.
Keywords: Preservation of islets, Mesenchymal stem cells (MSCs), Transgenic rat, Living image
INTRODUCTION
Implantation of functional islets is a potential cure for diabetes, but the availability of high-quality islets for transplantation is critical to success. Recent progress in clinical islet transplantation has resulted in less diabetogenic immunosuppression, and the ability to prepare highly viable islets in sufficient quantities makes transplantation an attractive treatment option for selected patients with type 1 diabetes mellitus (7,22,29,31,33). The yield and quality of isolated islets determine whether the donor organ is more suitable for clinical islet transplantation or for research (7,22,29). One major disadvantage of preserving islets is that, in culture, loss of tissue mass occurs over time (7). Therefore, the development of suitable preservation solutions and systems is essential.
Several approaches have been used in attempts to reverse the disease process of type 1 diabetes. For example, the generation of insulin-producing cells has been attempted (2,4,12,18,23,34). However, these insulin-producing cells have not yet been used in the treatment of diabetes, and more studies are needed. In addition, efforts have been made to improve islet preservation. To inhibit the gradual deterioration of islets during shipment, a gas-permeable bag has been developed to maintain a stable temperature and stable O2 and CO2 pressures (13,14). This system can be used to carry transplantable islets from a remote place over a 48-h period. Moreover, we have developed a multiwell, whole-cell, luciferase-based viability assay for assessing islet viability (25). Using this assay system, we have examined the effects of common organ preservation solutions on the viability of preserved islets (36) and have found that islet viability is halved after a few days.
Previous reports have revealed that transplantation of mesenchymal stem cells (MSCs) in mice and rats has functional benefits, in part because of these cells’ ability to produce large numbers and volumes of bioactive factors (3,17). MSCs have the capacity for self-renewal and multipotency and can differentiate into bone, fat, and cartilage cells (11,28). Cell-based therapy is now viewed as an important tool in regenerative medicine (6,30).
Here we identified factors that are secreted from MSCs and directly activate preserved islets. This novel system will help to elucidate the precise molecular mechanisms of pancreatic commitment and could be useful in treating diabetes by the transplantation of preserved islets.
MATERIALS AND METHODS
Adipose Tissue-Derived MSC Preparation and Culture
Male wild-type LEW rats weighing between 260 and 310 g were purchased from Charles River (Breeding Laboratories, Kanagawa, Japan). Wild-type LEW rat-derived adipose tissue (AT), obtained from the inguinal region, was minced with scissors and scalpels into pieces less than 3 mm in diameter and then subjected to isolation of AT-MSCs. Briefly, after gentle shaking of the minced tissue with an equal volume of phosphate-buffered saline (PBS; Takara, Kyoto, Japan), the mixture was separated into two phases. After being washed with PBS, the upper phase (containing stem cells, adipocytes, and blood) was enzymatically dissociated with 0.075% collagenase (type 1) (Wako, Tokyo, Japan) in PBS for 1.5 h at 37°C with gentle shaking. The dissociated tissue was mixed with an equal volume of minimum essential medium α modification (MEMα; Gibco-BRL, Tokyo, Japan), supplemented with 10% fetal bovine serum (FBS; Gibco), and incubated for 10 min at room temperature. The solution was separated into two phases. The lower phase was centrifuged at 1,200 rpm (rotor size: 7 cm) for 5 min at 20°C. The resulting isolated AT-MSCs were seeded onto 100-mm tissue culture dishes (Thermo Scientific, Tokyo, Japan) and cultured in MEMα supplemented with 10% FBS, and these cells have the same MSC-specific characteristics and morphology as other tissue-derived MSCs (3,17). When the cells were 70–80% confluent, they were harvested with 0.05% trypsin-EDTA (Invitrogen, Tokyo, Japan), replated at 2.0 × 104 cells/cm2, and cultured for 5 days. AT-MSCs between the fifth and eighth passages were used for the experiments.
Making the Conditioned Medium
To analyze the factors secreted by the MSCs, we prepared a conditioned medium. AT-MSCs derived from wild-type LEW rats were plated onto 30 100-mm dishes. When they had reached confluence, the cells were washed with PBS and incubated in serum-free MEMα. After 2 days, the supernatant was collected and then centrifuged, filtered, and concentrated at 12,000 rpm (rotor size: 7 cm) using Amicon Ultra centrifugal filters (Millipore, Tokyo, Japan; molecular weights: 3, 10, 30, 50, and 100 kDa).
Isolation and Culture of Pancreatic Islets
All experiments were performed in accordance with the Jichi Medical University Guide for Laboratory Animals. The Luciferase-transgenic rats (Luc-Tg rats) were established in our laboratory as described previously (10,35). Pancreases were removed when the rats were aged 8 weeks and dissected into 2- to 3-mm segments. The islets were isolated by collagenase (Wako, Tokyo, Japan) digestion, as described previously (21,25). The islets were then selected by handpicking under an inverted light microscope. Isolated islets were cultured for 16–24 h in Roswell Park Memorial Institute (RPMI) 1640 medium with 10% FBS and 1% penicillin–streptomycin solution (all Gibco).
Islet Viability Assay in Preservation Solution
Freshly isolated Luc-Tg rat islets were plated in 12-well tissue culture plates (20 islets per well, n = 4 plates; Nunc, Tokyo, Japan) and incubated in preservation solution [extracellular-type trehalose-containing Kyoto (ET-Kyoto) solution, Otsuka Pharmaceutical Factory, Inc., Tokushima, Japan] at 4°C for 24 h. Viable islets were detected by the analysis of luciferase gene expression activity using an in vivo imaging system (IVIS; Xenogen, Alameda, CA, USA) with the addition of 22 μl (2.29 mg/ml) of a luciferase-based reagent (D-luciferin, Wako). In this system, a noninvasive charge-coupled device camera is used to detect bioluminescence emitted from D-luciferin, which reacts with firefly luciferase in living animals and cells.
To detect islet activation, we used a luciferase-based cell viability assay that detects ATP levels in viable cells; we previously have described the use of this assay to assess the viability of Luc-Tg rat organs or tissues (15,25). Serum-free conditioned medium was prepared from supernatant derived from the culture of wild-type LEW rat-derived AT-MSCs for 2 days. Fresh Luc-Tg rat islets were cultured in a CO2 incubator for 3 days in RPMI 1640 medium containing 5% FBS (controls); the conditioned medium was added to two experimental groups, one of which received heat treatment at 37°C. During the experiment, the media were not refreshed.
Dithizone (DTZ) Staining
Islets were then tested for their specificity by DTZ staining. DTZ staining was carried out by adding 10 ml DTZ stock solution (Wako) to islets suspended in 1 ml Krebs-Ringer bicarbonate buffer (pH 7.4) with HEPES (10 mM) (KRBH; Wako) and incubated at 37°C for 10–15 min. The stained islets appeared bright red under the microscope.
Statistical Analysis
Data are represented as means ± SEM. Results were analyzed by using a two-tailed Student’s t test. A value of p < 0.05 was considered significant.
RESULTS
Effect of MSC-Conditioned Medium on Islet Activation
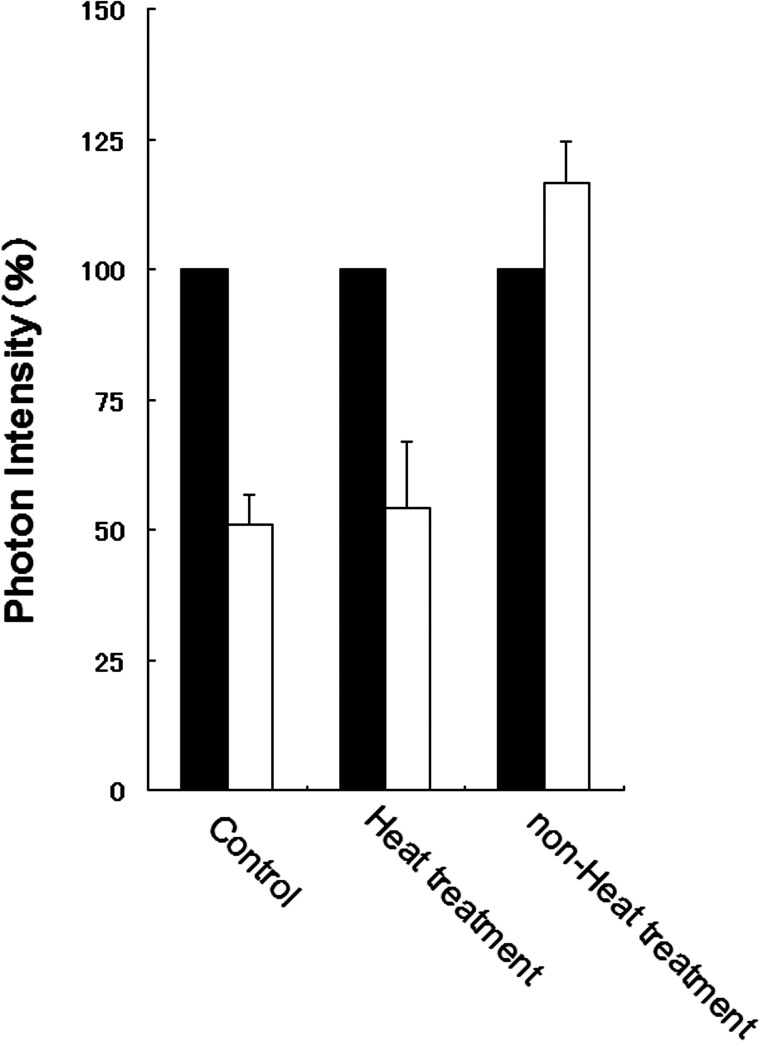
At the first, we investigated whether or not islet-activating factors are included in the MSC-conditioned medium. The photon intensity emitted from the islets was treated with conditioned medium, but no heat treatment had increased at 3 days (Fig. 1). In contrast, like the controls, the islets treated with both conditioned medium and heat showed an approximately 50% decrease in photon intensity at the end of 3 days of culture. This result suggested that a protein or proteins secreted from the MSCs acted as an islet-activating factor.
Figure 1.
Comparison of changes in luminescence intensity of islets under culture conditions after addition of medium conditioned with mesenchymal stem cells. Black bars, day 0; white bars, after 3 days of culture.
Analysis of Islet Activation Factors From MSC-Secreted Fractions
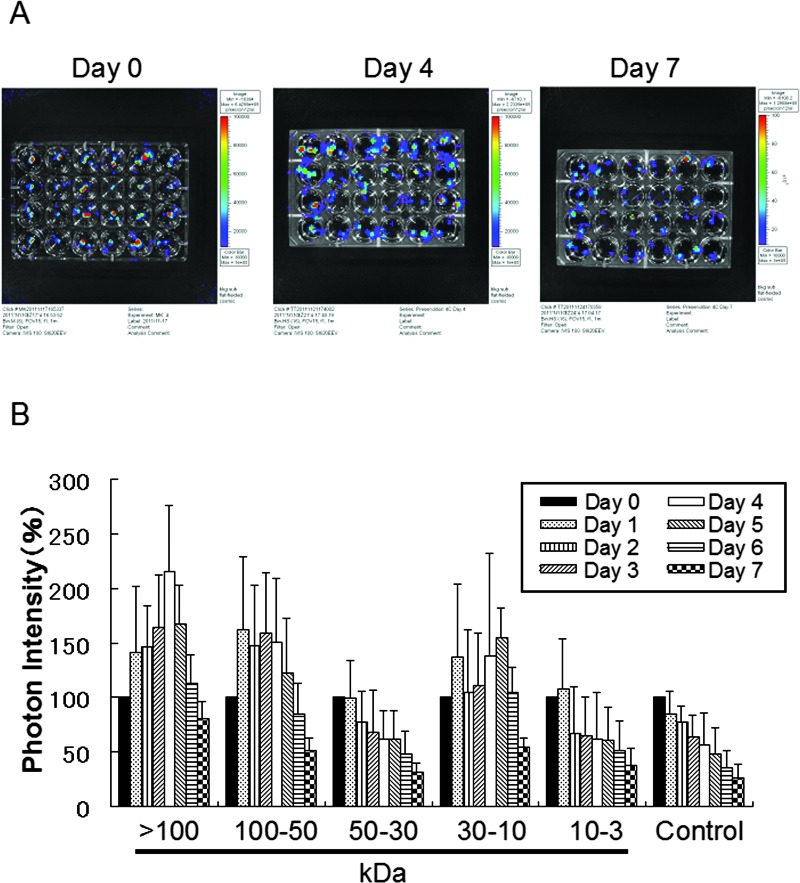
Next, we investigated which fractions derived from the MSC-conditioned medium appeared to activate the preserved islets (Fig. 2). During the experiment, the preservation solution was not refreshed. The photon intensity of each group receiving a fraction of the conditioned medium changed over time at 4°C (Fig. 2A). Photon intensity was quantified as color images. By comparison with the controls, the fractions were classified into two groups in terms of their effects on preserved Luc-Tg rat islets: an effective group (>50 and 10–30 kDa) and an ineffective group (30–50 and 3–10 kDa) (Fig. 2B). Peak activation of islets was found at 4 or 5 days, and photon intensity decreased after the peak. At 4 days, the relative photon intensities of the preserved samples receiving the >50 or 10–30 kDa fractions of the conditioned medium had increased to over 150% of their initial values. These results suggested that the fractions of >50 and 10–30 kDa secreted by the MSCs were superior in their activation of the preserved islets.
Figure 2.
Comparison of changes in luminescence intensity of islets in ET-Kyoto organ preservation solution after addition of medium conditioned with various fractions from mesenchymal stem cells (MSCs). (A) Photographs of Luc-Tg rat-derived islets in preservation solution treated with various fractions from the MSC-conditioned medium. From the left column on the plate, >100 kDa fraction, 50–100 kDa, 30–50 kDa, 10–30 kDa, 3–10 kDa, and 0 kDa (control). (B) Bioluminescence imaging using an in vivo imaging system to assess cell viability in the islets. Samples were exposed to extracellular-type trehalose-containing Kyoto (ET-Kyoto) organ preservation solution at 4°C. Data are representative of four independent experiments.
Characterization of Activated Islets
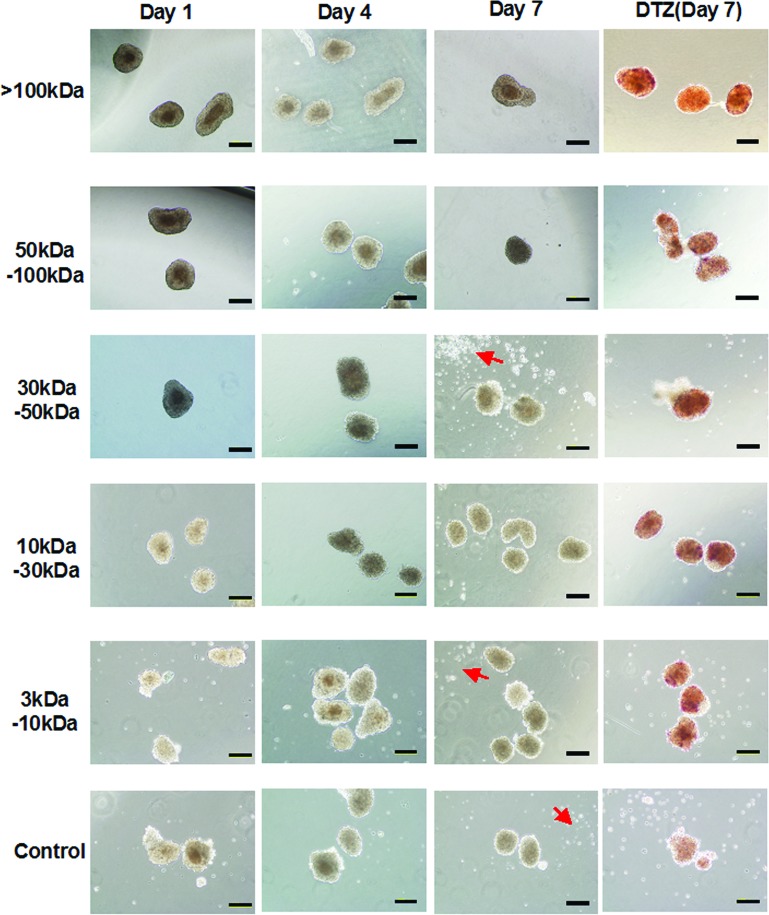
Finally, under a microscope, we examined the morphology of islets that had been preserved with ET-Kyoto solution at 4°C and then treated with the various fractions from the conditioned medium (Fig. 3). There were no obvious morphological changes at 4 days. However, by day 7, the samples that had received the 3–10 and 30–50 kDa fractions showed collapse of islet conformation, as did the controls. Additionally, these fractions’ preservation islets were scattered by many islet-derived dissolution cells (Fig. 3, arrow). After cultivation, some islets were immediately separated and treated with DTZ. Before preservation of islets, all the islets stained with DTZ became crimson red. No other cell types were observed with the islets, indicating that only the islets were present in the preparation. However, DTZ staining was negative in only the control islets after 7 days (Fig. 3, right column). Insulin-producing ability was consistent with the relative photon intensities of the preserved islets (data not shown). The limited fractions secreted by the MSCs, therefore, were capable of maintaining the conformation of preserved islets and activating their function.
Figure 3.
Microscopic morphology and dithizone (DTZ) staining of isolated Luc-Tg rat islets in ET-Kyoto organ preservation solution after addition of medium conditioned with various fractions from mesenchymal stem cells. DTZ staining was performed on the last day. Arrows point out islet-derived dissolution cells. Scale bar: 200 μm.
DISCUSSION
Pancreatic islet transplantation has received widespread attention as a promising treatment for type 1 diabetes. In 2000, Shapiro suggested that the use of a corticosteroid-free immunosuppression therapy, the Edmonton Protocol, would enable insulin independence for a median of 11.9 months (32). In the protocol, islets are infused into the portal system, where they may embolize in the liver. Successful transplantation normally requires 6,000–9,000 islets/kg human body weight, suggesting that one recipient needs 500,000–1,000,000 fresh islets; the islet diameter should be >150 μm (5). Although a single normal human pancreas may have more than 1,000,000 islets, the most successful isolation yields only 400,000 islets. Therefore, a recipient typically requires two to four pancreases for islet transplantation, and the islets need to be transplanted as soon as possible (19).
Glucose-sensing and insulin-signaling pathways have been shown to play important roles in insulin secretion as well as β-cell growth and survival. Glucose plays an essential role in control of the secretary activity of β-cells. Metabolism of glucose leads to an increase in the ATP-to-ADP ratio, membrane depolarization, Ca2+ influx, and stimulation of insulin secretion (1). Recently, the luciferase-based viability assay described here utilizes firefly luciferase to detect intracellular ATP levels in viable cells (25,35,36). We have already established that the Luc-Tg rat system with modern optical imaging offers a new platform for a better understanding of transplantation (10).
Islets for transplantation are subject to damage from many sources, including ischemic injury during removal and delivery of the donor pancreas, enzymatic digestion for islet isolation, and reperfusion injury after transplantation into the recipient. Cell culture conditions for islet transplantation should provide sufficient oxygen and nutrients to allow the islets to recover from the damage incurred during isolation and thus minimize islet loss. Miki and colleagues and Yamamoto and colleagues have reported that medium containing recombinant human proteins may be beneficial for pretransplant culture of islet cells (24,37). In fact, most islet transplant centers transplant cultured islets (8,9,20). In this study, we presented that persevered islet was activated of function and viability using MSC-secreted fractions. We think that candidate effectors of degenerate islets are likely to be hepatocyte growth factor (HGF), insulin-like growth factor (IGF), and tumor necrosis factors (TNFs), since these cytokines were previously suggested as activating factors of pancreas and/or β-cells (16,26,27), and this will be confirmed in future studies. To suppress islet deterioration and thus increase success rates, new approaches to islet transplantation will be required in the future.
CONCLUSION
We found here that proteins secreted from MSCs were able to activate preserved islets. By using these factors, it should be possible to restore islets to the condition they were in before culture, transport, and transplantation. This is important for the shipping of fractions for research and is even more important for shipping entire islet clinical preparations.
ACKNOWLEDGMENTS
We thank all the staff of the Division of Bioimaging Sciences, Center for Molecular Medicine, Jichi Medical University. Eiji Kobayashi is a Chief Scientific Advisor to Otsuka Pharmaceutical Co., Ltd. There are no patents, products in development, or marketed products to declare. The position held by E. Kobayashi does not alter the authors’ adherence to all of the Cell Medicine policies on sharing data and materials, as described in detail online in the Guide for Authors. The other authors declare no competing financial interests.
REFERENCES
- 1. Ashcroft F. M.; Rorsman P. Electrophysiology of the pancreatic β-cell. Progr. Biophys. Molec. Biol. 54:87–143; 1998. [DOI] [PubMed] [Google Scholar]
- 2. Assady S.; Maor G.; Amit M.; Itskovitz-Eldor J.; Skorecki K. L.; Tzukerman M. Insulin production by human embryonic stem cells. Diabetes 50:1691–1697; 2001. [DOI] [PubMed] [Google Scholar]
- 3. Banas A.; Teratani T.; Yamamoto Y.; Tokuhara M.; Takeshita F.; Osaki M.; Kawamata M.; Kato T.; Okochi H.; Ochiya T. IFATS collection: In vivo therapeutic potential of human adipose tissue mesenchymal stem cells after transplantation into mice with liver injury. Stem Cells 26:2705–2712; 2008. [DOI] [PubMed] [Google Scholar]
- 4. Blyszczuk P.; Czyz J.; Kania G.; Wagner M.; Roll U.; St-Onge L.; Wobus A. M. Expression of Pax4 in embryonic stem cells promotes differentiation of nestin-positive progenitor and insulin-producing cells. Proc. Natl. Acad. Sci. USA 100:998–1003; 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burridge P. W.; Shapiro A. M.; Ryan E. A.; Lakey J. R. Future trends in clinical islet transplantation. Transplant. Proc. 34:3347–3348; 2002. [DOI] [PubMed] [Google Scholar]
- 6. Busch S. A.; van Crutchen S. T. J.; Deans R. J.; Ting A. E. Mesenchymal stromal cells as a therapeutic strategy to support islet transplantation in type 1 diabetes mellitus. Cell Med. 2:43–53; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fraga D. W.; Sabek O.; Hathaway D. K.; Gaber A. O. A comparison of media supplement methods for the extended culture of human islet tissue. Transplantation 65:1060–1066; 1998. [DOI] [PubMed] [Google Scholar]
- 8. Froud T.; Ricordi C.; Baidal D. A.; Hafiz M. M.; Ponte G.; Cure P.; Pileggi A.; Poggioli R.; Ichii H.; Khan A.; Ferreira J. V.; Pugliese A.; Esquenazi V. V.; Kenyon N. S.; Alejandro R. Islet transplantation in type 1 diabetes mellitus using cultured islets and steroid-free immunosuppression: Miami experience. Am. J. Transplant. 5:2037–2046; 2005. [DOI] [PubMed] [Google Scholar]
- 9. Goto M.; Eich T. M.; Felldin M.; Foss A.; Källen R.; Salmela K.; Tibell A.; Tufveson G.; Fujimori K.; Engkvist M.; Korsgren O. Refinement of the automated method for human islet isolation and presentation of a closed system for in vitro islet culture. Transplantation 78:1367–1375; 2004. [DOI] [PubMed] [Google Scholar]
- 10. Hakamata Y.; Murakami T.; Kobayashi E. “Firefly rats” as an organ/cellular source for long-term in vivo bioluminescent imaging. Transplantation 81:1179–1184; 2006. [DOI] [PubMed] [Google Scholar]
- 11. Ho S. T. B.; Tanavde V. M.; Hui J. H.; Lee E. H. Upregulation of adipogenesis and chondrogenesis in MSC serum-free culture. Cell Med. 2:27–41; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hori Y.; Rulifson I. C.; Tsai B. C.; Heit J. J.; Cahoy J. D.; Kim S. K. Growth inhibitors promote differentiation of insulin-producing tissue from embryonic stem cells. Proc. Natl. Acad. Sci. USA 99:16105–16110; 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ichii H.; Sakuma Y.; Pileggi A.; Fraker C.; Alvarez A.; Montelongo J.; Szust J.; Khan A.; Inverardi L.; Naziruddin B.; Levy M. F.; Klintmalm G. B.; Goss J. A.; Alejandro R.; Ricordi C. Shipment of human islets for transplantation. Am. J. Transplant. 7:1010–1020; 2007. [DOI] [PubMed] [Google Scholar]
- 14. Ikemoto T.; Matsumoto S.; Itoh T.; Noguchi H.; Tamura Y.; Jackson A. M.; Shimoda M.; Naziruddin B.; Onaca N.; Yasunami Y.; Levy M. F. Assessment of islet quality following international shipping of more than 10,000 km. Cell Transplant. 19:731–741; 2010. [DOI] [PubMed] [Google Scholar]
- 15. Iwai S.; Kikuchi T.; Kasahara N.; Teratani T.; Yokoo T.; Sakonju I.; Okano S.; Kobayashi E. Impact of normothermic preservation with extracellular type solution containing trehalose on rat kidney grafting from a cardiac death donor. PLoS One. 7:e33157; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Izumida Y.; Aoki T.; Yasuda D.; Koizumi T.; Suganuma C.; Saito K.; Murai N.; Shimizu Y.; Hayashi K.; Odaira M.; Kusano T.; Kushima M.; Kudano M. Hepatocyte growth factor is constitutively produced by donor-derived bone marrow cells and promotes regeneration of pancreatic β-cells. Biochem. Biophys. Res. Commun. 333:273–282; 2005. [DOI] [PubMed] [Google Scholar]
- 17. Kanazawa H.; Fujimoto Y.; Teratani T.; Iwasaki J.; Kasahara N.; Negishi K.; Tsuruyama T.; Uemoto S.; Kobayashi E. Bone marrow-derived mesenchymal stem cells ameliorate hepatic ischemia reperfusion injury in a rat model. PLoS One 29:e19195; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim D.; Gu Y.; Ishii M.; Fujimiya M.; Qi M.; Nakamura N.; Yoshikawa T.; Sumi S.; Inoue K. In vivo functioning and transplantable mature pancreatic islet-like cell clusters differentiated from embryonic stem cells. Pancreas 27:e34–41; 2003. [DOI] [PubMed] [Google Scholar]
- 19. Kin T.; Murdoch T. B.; Shapiro A. M.; Lakey J. R. Estimation of pancreas weight from donor variables. Cell Transplant. 15:181–185; 2006. [DOI] [PubMed] [Google Scholar]
- 20. Kin T.; Senior P.; O’Gorman D.; Richer B.; Salam A.; Shapiro A. M. Risk factors for islet loss during culture prior to transplantation. Transpl. Int. 21:1029–1035; 2008. [DOI] [PubMed] [Google Scholar]
- 21. Lacy P. E.; Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes 16:35–39; 1967. [DOI] [PubMed] [Google Scholar]
- 22. Lakey J. R.; Warnock G. L.; Rajotte R. V.; Suarez-Alamazor M. E.; Ao Z.; Shapiro A. M.; Kneteman N. M. Variables in organ donors that affect the recovery of human islets of Langerhans. Transplantation 61:1047–1053; 1996. [DOI] [PubMed] [Google Scholar]
- 23. Lumelsky N.; Blondel O.; Laeng P.; Velasco I.; Ravin R.; McKay R. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science 292:1389–1394; 2001. [DOI] [PubMed] [Google Scholar]
- 24. Miki A.; Narushima M.; Okitsu T.; Takeno Y.; Soto-Gutierrez A.; Rivas-Carrillo J. D.; Navarro-Alvarez N.; Chen Y.; Tanaka K.; Noguchi H.; Matsumoto S.; Kohara M.; Lakey J. R.; Kobayashi E.; Tanaka N.; Kobayashi N. Maintenance of mouse, rat, and pig pancreatic islet functions by coculture with human islet-derived fibroblasts. Cell Transplant. 15:325–334; 2006. [PubMed] [Google Scholar]
- 25. Negishi K.; Teratani T.; Iwasaki J.; Kanazawa H.; Kasahara N.; Lefor A. T.; Uemoto S.; Fujimoto Y.; Kobayashi E. Luminescence technology in preservation and transplantation for rat islet. Islets 3:111–117; 2011. [DOI] [PubMed] [Google Scholar]
- 26. Ortis F.; Pirot P.; Naamane N.; Kreins A. Y.; Rasschaert J.; Moore F.; Théâtre E.; Verhaeghe C.; Magnusson N. E.; Chariot A.; Orntoft T. F.; Eizirik D. L. Induction of nuclear factor-κB and its downstream genes by TNF-α and IL-1β has a pro-apoptotic role in pancreatic β–cells. Diabetologia 51:1213–1225; 2008. [DOI] [PubMed] [Google Scholar]
- 27. Park S. M.; Hong S. M.; Sung S. R.; Lee J. E.; Kwon D. Y. Extracts of Rehmanniae radix, Ginseng radix and Scutellariae radix improve glucose-stimulated insulin secretion and β-cell proliferation through IRS2 induction. Genes Nutr. 2:347–351; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pittenger M. F.; Mackay A. M.; Beck S. C.; Jaiswal R. K.; Douglas R.; Mosca J. D.; Moorman M. A.; Simonetti D. W.; Craig S.; Marshak D. R. Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147; 1999. [DOI] [PubMed] [Google Scholar]
- 29. Ponte G. M.; Pileggi A.; Messinger S.; Alejandro A.; Ichii H.; Baidal D. A.; Khan A.; Ricordi C.; Goss J. A.; Alejandro R. Toward maximizing the success rates of human islet isolation: Influence of donor and isolation factors. Cell Transplant. 16:595–607; 2007. [DOI] [PubMed] [Google Scholar]
- 30. Seo J. H.; Jang I. K.; Kim H.; Yang M. S.; Lee J. E.; Kim H. E.; Eom Y-W.; Lee D-H.; Yu J. H.; Kim J. Y.; Kim H. O.; Cho S-R. Early immunomodulation by intravenously transplanted mesenchymal stem cells promotes functional recovery in spinal cord injured rats. Cell Med. 2:55–67; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shapiro A. M.; Lakey J. R.; Ryan E. A.; Korbutt G. S.; Toth E.; Warnock G. L.; Kneteman N. M.; Rajotte R. V. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 343:230–238; 2000. [DOI] [PubMed] [Google Scholar]
- 32. Shapiro A. M.; Ricordi C.; Hering B. J.; Auchincloss H.; Lindblad R.; Robertson R. P.; Secchi A.; Brendel M. D.; Berney T.; Brennan D. C.; Cagliero E.; Alejandro R.; Ryan E. A.; DiMercurio B.; Morel P.; Polonsky K. S.; Reems J. A.; Bretzel R. G.; Bertuzzi F.; Froud T.; Kandaswamy R.; Sutherland D. E.; Eisenbarth G.; Segal M.; Preiksaitis J.; Korbutt G. S.; Barton F. B.; Viviano L.; Seyfert-Margolis V.; Bluestone J.; Lakey J. R. International trial of the Edmonton protocol for islet transplantation. N. Engl. J. Med. 355:1318–1330; 2006. [DOI] [PubMed] [Google Scholar]
- 33. Shapiro A. M.; Ryan E. A.; Lakey J. R. Islet cell transplantation. Lancet 358:S21; 2001. [DOI] [PubMed] [Google Scholar]
- 34. Soria B.; Roche E.; Berná G.; León-Quinto T.; Reig J. A.; Martín F. Insulin-secreting cells derived from embryonic stem cells normalize glycemia in streptozotocin-induced diabetic mice. Diabetes 49:157–162; 2000. [DOI] [PubMed] [Google Scholar]
- 35. Teratani T.; Kobayashi E. In vivo bioimaging of rats for translational research in cell/tissue transplantation. Cell Med. 3:3–11; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Teratani T.; Matsunari H.; Kasahara N.; Nagashima H.; Kawarasaki T.; Kobayashi E. Islets from rats and pigs transgenic for photogenic proteins. Curr. Diabetes Rev. 8:382–389; 2012. [DOI] [PubMed] [Google Scholar]
- 37. Yamamoto T.; Mita A.; Ricordi C.; Messinger S.; Miki A.; Sakuma Y.; Timoneri F.; Barker S.; Fornoni A.; Molano R. D.; Inverardi L.; Pileggi A.; Ichii H. Prolactin supplementation to culture medium improves β-cell survival. Transplantation 89:1328–1335; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]