Abstract
The specific properties of mesenchymal stem cells (MSCs) in oral tissues still remain unknown though their existence has been previously reported. We collected gingiva, dental pulp, and periodontal ligament tissues from removed teeth and isolated MSCs. These MSCs were compared in terms of their yields per tooth, surface epitopes, and differentiation potentials by patient-matched analysis. For in vivo calcification analysis, rat gingival and dental pulp cells mounted on β-tricalcium phospateTCP were transplanted into the perivertebral muscle of rats for 6 weeks. Gingival cells and dental pulp cells showed higher yield per tooth than periodontal ligament cells (n=6, p<0.05). Yields of periodontal ligament cells were too low for further analysis. Gingival and dental pulp cells expressed MSC markers such as CD44, CD90, and CD166. Gingival and dental pulp cells obtained phenotypes of chondrocytes and adipocytes in vitro. Approximately 60% of the colonies of gingival cells and 40% of the colonies of dental pulp cells were positively stained with alizarin red in vitro, and both gingival and dental pulp cells were calcified in vivo. We clarified properties of MSCs derived from removed teeth. We could obtain a high yield of MSCs with osteogenic potential from gingiva and dental pulp. These results indicate that gingiva and dental pulp are putative cell sources for hard tissue regeneration.
Keywords: Mesenchymal stem cells (MSCs), Gingiva, Dental pulp, Periodontal ligament, Yields, Differentiation
INTRODUCTION
Mesenchymal stem cells (MSCs) are a heterogeneous population and are defined as being derived from mesenchymal tissue and by their functional capacity to form colonies and to differentiate into bone, cartilage, and adipose cells in vitro (23). These cells participate in tissue homeostasis, remodeling, and repair by ensuring replacement of mature cells that are lost during the course of physiological turnover, injury, or disease (2). MSCs can be isolated from various mesenchymal tissues, and MSCs contain common features independent of their origin, but an increasing number of reports describe their distinguishing properties dependent on origin.
MSCs can be obtained from oral tissues such as gingiva (21,25), dental pulp (1,4,12), and periodontal ligament (1,5,11,17,22). These tissues can be obtained from a removed tooth and are useful for regenerative medicine if they are efficient cell sources for MSCs. However, the optimal cell source for MSCs in a removed tooth remains unknown because gingival, dental pulp, and periodontal ligament cells have not been compared from the standpoint of the properties of MSCs.
In this study, we collected gingiva, dental pulp, and periodontal ligament from removed teeth and isolated MSCs with the same method. MSCs derived from gingiva, dental pulp, and periodontal ligament were compared in terms of their yields per tooth, surface epitopes, and differentiation potentials. Our results demonstrate the optimal cell source of MSCs in the removed tooth and indicate one possible application for regeneration therapy.
MATERIALS AND METHODS
Tissue Harvest and Cell Preparation
This study was approved by our institutional review board, and informed consent was obtained from all patients. Twelve human healthy impacted third molars, removed by orthodontic treatment, were used for this study. The age of the subjects ranged between 20 and 40 years. Gingiva, dental pulp, and periodontal ligament were collected from the removed molars. As Somerman et al. reported previously, the remaining gingival tissues from the cervical area of the root surface were curetted away with care to avoid contamination of the periodontal ligament tissues by gingival connective tissues. Furthermore, periodontal ligament tissues attached to the middle third of the root were mainly curetted and corrected to reduce contamination with both gingival and apical tissue (19). The corrected tissues were minced by scalpel, washed in 1 ml of phosphate-buffered saline (PBS) containing 100 units/ml penicillin amphotericin B (Invitrogen) three times to prevent oral normal bacterial and fungal contamination, and then digested with 3 mg/ml of type 5 collagenase (Sigma-Aldrich, St. Louis, MO) in αMEM (α-modified Eagle's medium; Invitrogen) for 2 h at 37°C. Then, the cells were passed through a 70-µm nylon filter (Becton Dickinson Bioscience, Bedford, MA) to remove undigested debris, counted with a hemocytometer, and cultured in a complete culture medium [αMEM containing 10% fetal bovine serum (Invitrogen), 100 units/ml penicillin, 100 µg/ml streptomycin, and 250 ng/ml amphotericin B)] at a density of 1×103, 1×104, 1×105 cells/60 cm2 dish (Nalge Nunc International, Rochester, NY) for 14 days. Three dishes were stained with 0.5% crystal violet, and colonies larger than 2 mm in diameter were counted. The optimal initial cell density was determined based on the following criteria: 1) the colony size was not affected by colony-to-colony contact inhibition and 2) the largest number of colonies was obtained. The cells were harvested with 0.25% trypsin and 1 mm Ethylenediaminetetraacetic acid [EDTA, (Invitrogen)] at 37°C for 5 min and were then counted with a hemocytometer to determine the total yields of cells per tooth at passage 0. The cells were replated, cultured, and harvested with trypsin-EDTA every 14 days at optimal initial cell density, and passage 2 cells were used for further analysis.
Surface Epitopes
Two million passage 2 cells were suspended in 1 ml PBS with antibodies, incubated for 30 min at 4°C, then resuspended in 1 ml of PBS. Fluorescein isothiocyanate (FITC)-, or phycoerythrin (PE)-, coupled antibodies against cluster of differentiation 34 (CD34), CD45, CD90 and CD146 were from Becton Dickinson (Franklin Lakes, NJ); CD44 and CD117 were from eBioscience (San Diego, CA); CD105 and CD166 were from Ancell Corporation (Bayport, MN). For the isotype control, FITC- or PE-coupled nonspecific mouse IgG (Becton Dickinson) was substituted for the primary antibody. Cell fluorescence was evaluated by FACSCalibur instrument (Becton Dickinson), and data were analyzed using CellQuest software (Becton Dickinson).
Synovial MSCs
Human synovium was harvested from three male donors (20, 23, and 30 years old) during an operation for anterior cruciate ligament reconstruction of the knee. The synovium was minced and digested with 3 mg/ml collagenase for 3 h, and then the tissues were filtered with a 70-µm nylon filter. The nucleated cells were plated at 1×104 cells/60 cm2 dish, cultured for 14 days, and replated at 1×105 cells/150 cm2 dish. The cells were cultured every 14 days, and passage 2 cells were used for further analysis (15).
Chondrogenesis
Passage 2 cells (2.5×105) were placed in a 15-ml polypropylene tube (Falcon, Bedford, MA) and pelleted by centrifugation at 450×g for 10 min. The pellets were cultured for 21 days in chondrogenic media, which contained 1000 ng/ml bone morphogenetic protein 7 (BMP-7; Stryker Biotech, Hopkinton, MA), in addition to high- glucose Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10 ng/ml transforming growth factor (TGF)-β3 (R&D Systems, Minneapolis, MN), 100 nM dexamethasone (Wako, Tokyo, Japan), ascorbate-2-phosphate (Sigma-Aldrich), l-proline (Sigma-Aldrich), pyruvate (Sigma-Aldrich), and insulin-transferrin-selenium premix (Becton Dickinson) (15).
For type 2 collagen immunostain, sections were deparaffinized, washed in PBS, and then pretreated with 0.4 mg/ml proteinase K (DAKO, Carpinteria, CA) in Tris-HCl buffer for 15 min at room temperature for antigen retrieval. The tissue sections were incubated with mouse monoclonal anti-human type 2 collagen antibody (1:200 dilution; Daiichi Fine Chemical, Toyama, Japan) for 1 h at room temperature. After extensive washes with PBS, the sections were incubated with biotinylated horse anti-mouse IgG secondary antibody (Vector Laboratories) for 30 min at room temperature. Immunostaining was detected with the Vectastain ABC reagent (Vector Laboratories) followed by diaminobenzidine staining.
Adipogenesis
One hundred passage 2 cells were plated in 60-cm2 dishes and cultured in complete medium for 14 days. Then the medium was switched to adipogenic medium, which consisted of complete medium supplemented with 10−7 M dexamethasone, 0.5 mm isobutyl-1-methyl xanthine (Sigma-Aldrich), and 100 µm indomethacin (Wako) for 21 days. The cells were fixed in 4% paraformaldehyde and stained with fresh oil red O solution (Sigma-Aldrich). After taking pictures of the dishes, they were overstained with crystal violet (13).
Osteogenesis In Vitro
One hundred passage 2 cells were plated in 150-cm2 dishes and cultured in complete medium for 14 days. Then the medium was switched to osteogenic medium, which consisted of complete medium supplemented with 1 nM dexamethasone, 10 mM β-glycerol phosphate (Wako), and 50 µg/ml ascorbate-2-phosphates for 21 days. The dishes were stained with 0.5% alizarin red solution (Sigma-Aldrich). After taking pictures of the dishes, they were overstained with crystal violet (13).
Quantitative PCR
For chondrogenesis, 2.5×105 cells were pelleted and cultured in chondrogenic medium for 21 days. Undifferentiated 2×106 cells and eight pellets were used for RNA collection. For adipogenesis and osteogenesis, undifferentiated 1×105 cells were initially plated on a 60-cm2 dish and cultured in complete medium for 14 days. Then the medium was switched to adipogenic and osteogenic medium, and the cells were cultured for 21 days. Four dishes before and after the inductions were used for RNA collection. Total RNA was prepared by using TRIzol Reagent (Invitrogen). Pellets were homogenized before preparation. Total RNA was purified by RNeasy Mini kit (QIAGEN, Valencia, CA). One microgram of total RNA was reverse transcribed into first-strand cDNA with a Transcriptor High Fidelity cDNA Synthesis Kit (Roche Diagnostics, Manheim, Germany).
Quantitative plymerase chain reaction (PCR) analysis was conducted on a LightCycler® 480 Real-Time PCR System (Roche Diagnostics) using a FastStart TaqMan® Probe Master Kit (Roche Diagnostics) for 45 cycles with denaturation at 95°C for 15 s and annealing at 60°C for 60 s. Each procedure was repeated three times. The amounts of mRNA were calculated as relative quantities in comparison to that of β-actin mRNA. PCR primers were as follows:
| Aggrecan: | 5′-CCTCCCCTTCACGTGTAAAA-3′ (forward), |
| 5′-GCTCCGCTTCTGTAGTCTGC-3′ (reverse); | |
| Adipsin: | 5′-TCCAAGCGCCTGTACGAC-3′ (forward), |
| 5′-GTGTGGCCTTCTCCGACA-3′ (reverse); | |
| Runx2: | 5′-CACCATGTCAGCAAAACTTCTT-3′ (forward), |
| 5′-TCACGTCGCTCATTTTGC-3′ (reverse); | |
| Osteocalcin: | 5′-TGAGAGCCCTCACACTCCTC-3′ (forward), |
| 5′-CCTCCTGCTTGGACACAAAG-3′ (reverse); | |
| β-actin: | 5′-ATTGGCAATGAGCGGTTC-3′ (forward), |
| 5′-TGAAGGTAGTTTCGTGGATGC-3′ (reverse). |
Runx2, Runt-related transcription factor 2.
Osteogenesis In Vivo
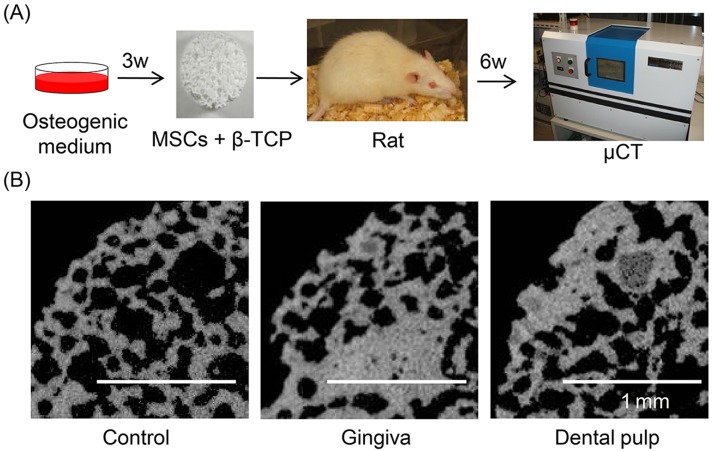
This study was conducted according to the protocol approved by the Animal Committee of Tokyo Medical and Dental University. Both maxillary and mandibular incisors were obtained from inbred Fisher 344 rats at 8 weeks old (Charles River) after sacrifice by carbon dioxide. Gingiva and dental pulp cells were prepared and cultured in osteogenic medium as described previously. Then the cell suspensions of 1×106 cells in 100 µl PBS were seeded into β-tricalcium phosphate (β-TCP) discs (porosity 75%, 5 mm diameter, 3 mm height, provided by HOYA, Tokyo, Japan) with 27-G needles. The discs were partially soaked with 5 ml osteogenic medium described above on 60-cm2 culture dishes and incubated overnight at 37°C, under 5% CO2 and saturated water vapor. β-TCP discs with 100 µl PBS only and without cells were served as controls. The discs were implanted in perivertebral muscles of rats under anesthesia with pentobarbital. After 6 weeks, β-TCP discs were removed and analyzed with a microCT ScanXmate-E090 system (Comscan) and Tri3D Bon (Ratoc System Engineering, Tokyo).
Statistics
Comparisons of total yields per tooth among gingiva, dental pulp, and periodontal ligament were performed using the Steel-Dwass multiple comparison test after the Kruskal-Wallis test. Comparisons of means between groups were performed using paired Student's t-test for the oil red O-positive colony rate, the alizarin red-positive colony rate, and quantitative PCR analysis. Differences at p<0.05 were considered significant.
RESULTS
Yields of Gingival, Dental Pulp, and Periodontal Ligament Cells
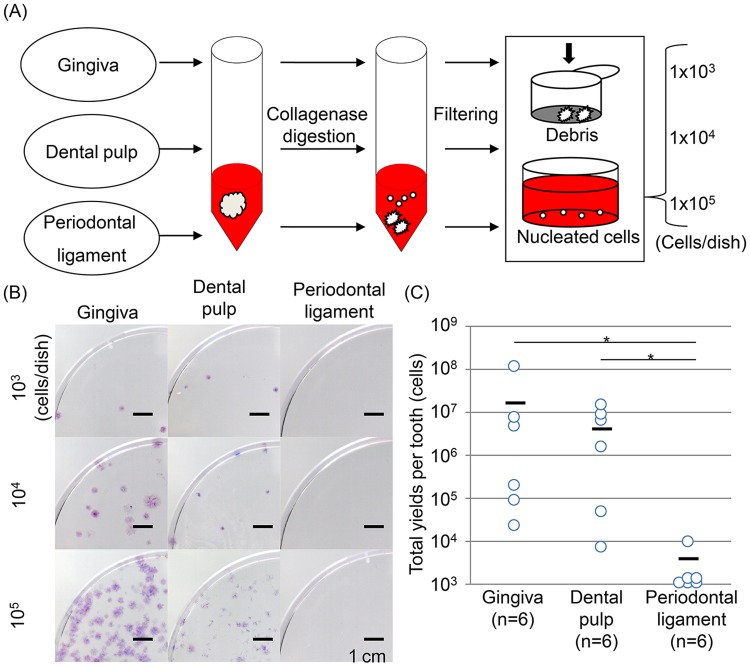
When cells were plated at a relatively low density (1×103 cells/60 cm2; Fig. 1A), gingival cells and dental pulp cells formed a few colonies, but periodontal ligament cells formed no colonies (Fig. 1B). The colony number increased according to the number of nucleated cells plated. Gingival cells and dental pulp cells showed higher yield per tooth than periodontal ligament cells (Fig. 1C). Yields of periodontal ligament cells were too low to perform further analysis.
Figure 1.
Properties of cells derived from gingiva, dental pulp, and periodontal ligament. (A) Preparation of cells. (B) Colony-forming ability. (C) Yields of cells derived from gingiva, dental pulp, and periodontal ligament cells per tooth after 14-day incubation (n=6, p=0.004 by the Kruskal-Wallis test and *p<0.05 by the Steel-Dwass multiple comparison test).
Surface Epitopes
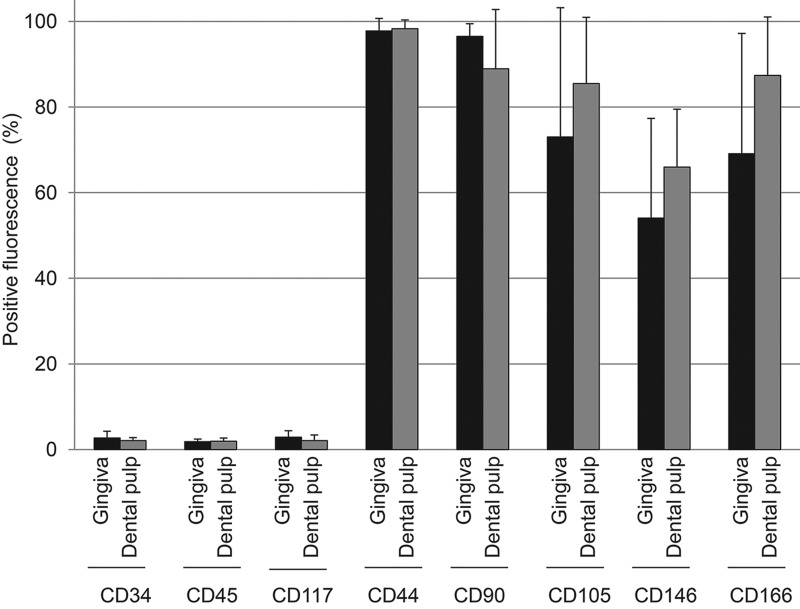
The positive rates for CD34, CD45, and CD117 were less than 3% on both gingival and dental pulp cells (Fig. 2). In contrast, the positive rates for CD44 were more than 97%, for CD90, CD105, and CD166 they were more than 60%, and for CD146 they were around 60%. There were no obvious differences of positive rates for surface epitopes we examined between gingival cells and dental pulp cells.
Figure 2.
Expression of cell surface epitopes on gingival and dental pulp cells by flow cytometric analysis. Percentage positivity (%) is displayed as mean±standard deviation based on six donors.
Chondrogenic Potential
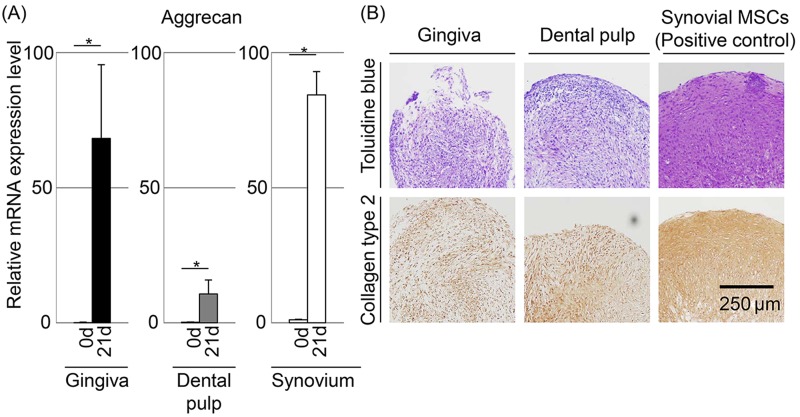
After chondrogenic induction, the mRNA expression level of aggrecan, one of the chondrocyte-specific genes, increased significantly in both gingival and dental pulp cells (Fig. 3A). Both of these cells produced cartilage matrix positively stained with toluidine blue and immunostained with collagen type 2 (Fig. 3B). However, chondrogenic potentials of both gingival and dental pulp cells were inferior to those of synovial MSCs, which have a high chondrogenic potential by histological analyses (6,14).
Figure 3.
Chondrogenic potential of gingival and dental pulp cells. Synovial MSCs were used as a positive control. (A) Aggrecan mRNA expression by quantitative-PCR. The values are displayed as mean±standard deviation of triplicates and are representative of three independent experiments (n=3 samples per group). *p<0.05 by paired Student's t test. (B) Histologies stained with toluidine blue and immunostained with collagen type 2.
Adipogenic Potential
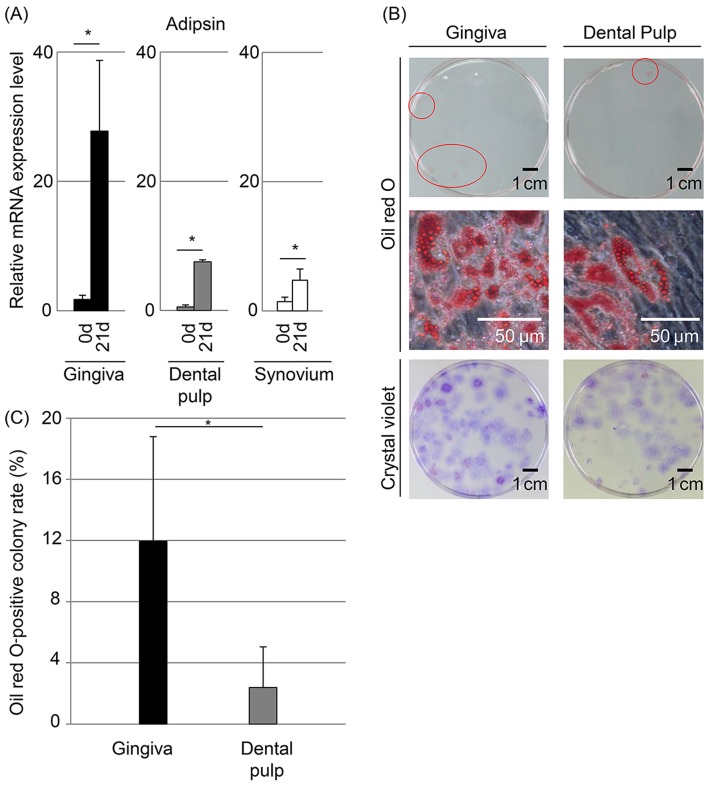
After culturing in adipogenic medium, the mRNA expression level of adipsin, one of the adipocyte-specific genes, was increased significantly in both gingival and dental pulp cells (Fig. 4A). Though some colonies were stained with oil red O in both gingival and dental pulp cells, the number of colonies positive for oil red O was low (Figs. 4B and C). The oil red O-positive colony rate was higher in gingival cells (12.0%) than in dental pulp cells (2.4%) (p<0.05).
Figure 4.
Adipogenic potential of gingival and dental pulp cells. (A) Adipsin mRNA expression by quantitative PCR. The values are displayed as mean±standard deviation of triplicates and are representative of three independent experiments (n=3 samples per group). *p<0.05 by paired Student's t test. (B) Colony-forming cells stained with oil red O and crystal violet. Oil red O-positive colonies are indicated with red circles at the top row. (C) Oil red O-positive colony rate. The values are displayed as mean±standard deviation (n=3). *p<0.05 by paired Student's t test.
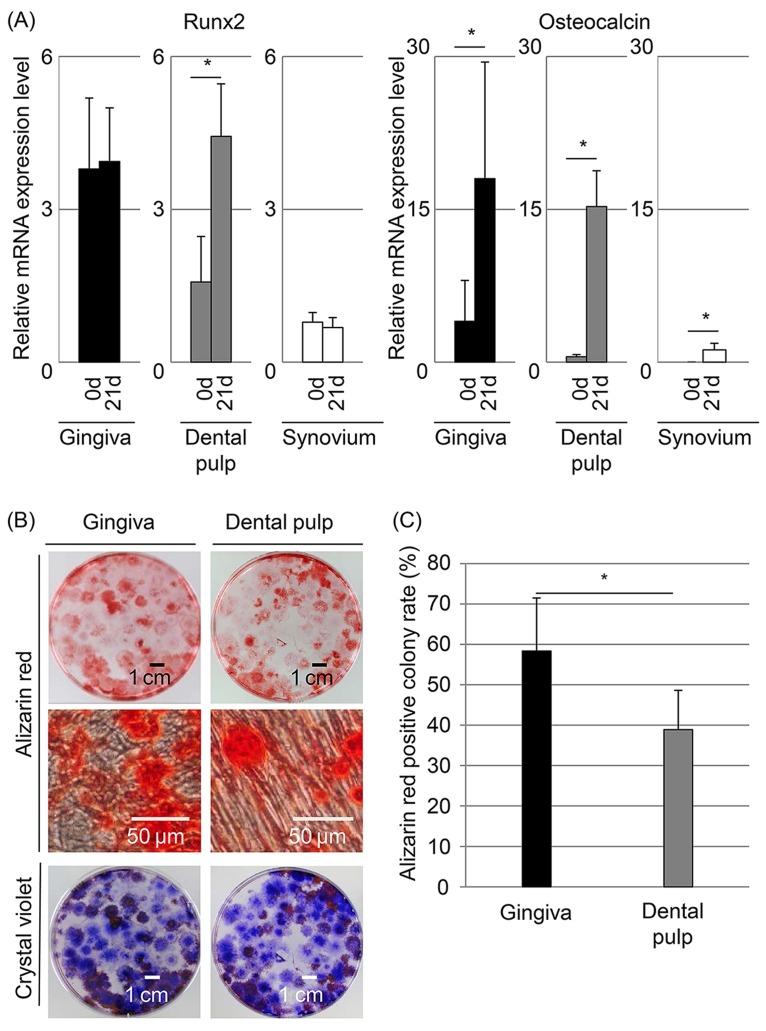
Osteogenic Potential In Vitro and In Vivo
The mRNA expression level of Runx2, an early marker for osteogenesis, was higher in dental pulp cells at 21 days than at 0 day (Fig. 5A). The mRNA expression level of osteocalcin, a late marker for osteogenesis, increased significantly in both gingival and dental pulp cells after osteogenic induction. Approximately 60% of the colonies of gingival cells and 40% of the colonies of dental pulp cells were positively stained with alizarin red (Figs. 5B and C). The alizarin red-positive colony rate was higher in gingival cells than in dental pulp cells (p<0.05). Finally, in vivo osteogenic potentials of gingival and dental pulp cells were examined (Fig. 6A). At 6 weeks after implantation, the calcified area was larger in discs implanted with both gingival and dental pulp cells than in the control disc (Fig. 6B).
Figure 5.
Osteogenic potential of gingival and dental pulp cells. (A) Runt-related transcription factor 2 (Runx2) and osteocalcin mRNA expression by quantitative PCR. The values are displayed as mean±standard deviation of triplicates and are representative of three independent experiments (n=3 samples per group). *p<0.05 by paired Student's t test. (B) Colony-forming cells stained with alizarin red and crystal violet. (C) Alizarin red-positive colony rate. The values are displayed as mean±standard deviation (n=4). *p<0.05 by paired Student's t test.
Figure 6.
In vivo osteogenic potential of gingival and dental pulp cells after 6 weeks of implantation. (A) Protocol. (B) Microcomputed tomography images of implants.
DISCUSSION
A considerable amount of retrospective data is available that describes putative MSCs; however, there is still very little knowledge available that documents the properties of MSCs, especially in their native environment. Although the precise identity of MSCs remains a challenge, we herein define an MSC as being derived from mesenchymal tissue and by its functional capacity both to self-renew and to generate a number of differentiated progeny (9). Since the earliest work by Friedenstein (3), the standard assay used to identify MSCs is the colony-forming unit fibroblast assay, which identifies adherent, spindle-shaped cells that proliferate to form colonies. By this method, we isolated colony-forming cells from gingiva and dental pulp. We also demonstrated the in vitro multipotentialities of these cells. Therefore, we referred to the cells from gingiva and dental pulp studied in this article as MSCs.
To compare properties of MSCs derived from gingiva, dental pulp, and periodontal ligament strictly, we used patient-matched samples to avoid donor variations. Also, we controlled initial cell density to avoid colony-to-colony contact inhibition because colony-to-colony contact inhibition affects proliferation potential, surface epitopes, and differentiation potential (16). The optimal initial cell density was 1×105 cells/60 cm2 dish for gingiva and dental pulp cells according to the criteria described above. No remarkable contact inhibition was observed at this cell density. Furthermore, cells were compared at the same passage because differentiation potential decreases after every passage (24).
We first revealed that the yield of periodontal ligament cells per tooth was much lower than the yields of gingival and dental pulp MSCs. We could not harvest a sufficient number of periodontal ligament cells from a tooth after a 14-day culture in five donors among six donors to perform further analyses with periodontal ligament cells. Previously, several reports described MSCs derived from periodontal ligament (5,11,17). We suppose that only limited batches of periodontal ligament cells among a considerable number of samples were targeted for the studies. In our opinion, periodontal ligament was not suitable as an MSC source for regenerative medicine because of low yields.
Epitope profiles of gingival MSCs and dental pulp MSCs were similar, in that the rate of positivity for CD34, CD45, and CD117 (hematopoietic stem cell markers) was low and the rate of positivity for CD44 (hyaluronan receptor), CD90 (Thy-1), CD105 (SH2), and CD166 (activated leukocyte cell adhesion molecule) was high. These results coincide with the phenotypic properties of bone marrow MSCs, synovial MSCs, and other MSCs (10,14,15). In this study, we examined only popular surface epitopes, and it would be intriguing to identify novel epitopes that are distinguished specifically among gingival, dental pulp, and other MSCs.
After chondrogenic induction, both gingival and dental pulp MSCs showed chondrogenic characteristics. However, histological analyses demonstrated that chondrogenic potentials of gingival and dental pulp MSCs were lower than those of synovial MSCs, which have high chondrogenic potential (6,14). This difference might have been due to the chondrogenic medium, for which the optimal combination of cytokines was determined by synovial MSCs (18).
After culturing in adipogenic medium, adipocyte-specific gene expression increased, and some colonies were stained with oil red O in both gingival and dental pulp MSCs. These findings indicate that both gingival and dental pulp MSCs had adipogenic potential. However, the oil red O-positive colony rate seemed to be extremely lower in comparison to that of bone marrow MSCs and other MSCs reported previously (14), showing low adipogenic potential of gingival and dental pulp MSCs.
Osteogenesis-related genes increased in both gingival and dental pulp MSCs after osteogenic induction in vitro. The alizarin red-positive colony rate of both gingival and dental pulp MSCs was also comparable to those in other MSCs reported previously (14). Furthermore, both gingival and dental pulp MSCs increased the calcified area of the β-TCP disc after implantation into the muscle of rats. These results indicate that one of the characteristics of gingival and dental pulp MSCs is high osteogenic potential.
When we compare gingiva and dental pulp as a cell source for MSCs, gingiva is more appealing because of its easy accessibility. Gingiva can be harvested without removal of tooth. The high healing capability of gingiva at the donor site is also advantageous.
In this study, we demonstrated the usefulness of gingiva as a cell source for bone regeneration. Zhang et al. also reported the usefulness of gingiva as an MSC source, especially for its immunomodulatory effect in a mouse colitis model (25). However, Lin et al. negatively reported that gingival cells showed poor differentiational potential though they possessed proliferative efficiency to some extent (7). Other studies also reported lower osteogenic potential of gingival cells in comparison to that of periodontal ligament cells (19). The difference may be due to the procedure of preparation for gingival cell.
CONCLUSION
We clarified properties of MSCs derived from removed teeth. We were able to obtain a high yield of MSCs with osteogenic potential from gingiva and dental pulp. Our results indicate that gingiva and dental pulp are putative cell sources for hard tissue regeneration.
ACKNOWLEDGMENTS
We thank Miyoko Ojima for his expert help with histological analyses and Izumi Nakagawa for his help with laboratory management. This study was supported by “the Project for Realization of Regenerative Medicine” and “the Global Center of Excellence (GCOE) Program” by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. Recombinant human bone morphogenetic protein-7 was kindly provided by Stryker Biotech. β-TCP discs were kindly provided by HOYA Corporation. The authors declare no conflicts of interest.
REFERENCES
- 1. Ballini A.; De Frenza G.; Cantore S.; Papa F.; Grano M.; Mastrangelo F.; Tete S.; Grassi F. R. In vitro stem cell cultures from human dental pulp and periodontal ligament: New prospects in dentistry. Int. J. Immunopathol. Pharmacol. 20:9–16; 2007. [DOI] [PubMed] [Google Scholar]
- 2. De Bari C.; Dell'accio F. Mesenchymal stem cells in rheumatology: A regenerative approach to joint repair. Clin. Sci. 113:339–348; 2007. [DOI] [PubMed] [Google Scholar]
- 3. Friedenstein A. J. Precursor cells of mechanocytes. Int. Rev. Cytol. 47:327–359; 1976. [DOI] [PubMed] [Google Scholar]
- 4. Gronthos S.; Mankani M.; Brahim J.; Robey P. G.; Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 97:13625–13630; 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gronthos S.; Mrozik K.; Shi S.; Bartold P. M. Ovine periodontal ligament stem cells: Isolation, characterization, and differentiation potential. Calcif. Tissue Int. 79: 310–317; 2006. [DOI] [PubMed] [Google Scholar]
- 6. Koga H.; Muneta T.; Nagase T.; Nimura A.; Ju Y. J.; Mochizuki T.; Sekiya I. Comparison of mesenchymal tissues-derived stem cells for in vivo chondrogenesis: Suitable conditions for cell therapy of cartilage defects in rabbit. Cell Tissue Res. 333:207–215; 2008. [DOI] [PubMed] [Google Scholar]
- 7. Lin N. H.; Menicanin D.; Mrozik K.; Gronthos S.; Bartold P. M. Putative stem cells in regenerating human periodontium. J. Periodontal Res. 43:514–523; 2008. [DOI] [PubMed] [Google Scholar]
- 8. Marynka-Kalmani K.; Treves S.; Yafee M.; Rachima H.; Gafni Y.; Cohen M. A.; Pitaru S. The lamina propria of adult human oral mucosa harbors a novel stem cell population. Stem Cells 28:984–995; 2010. [DOI] [PubMed] [Google Scholar]
- 9. McKay R. Stem cells in the central nervous system. Science 276:66–71; 1997. [DOI] [PubMed] [Google Scholar]
- 10. Mochizuki T.; Muneta T.; Sakaguchi Y.; Nimura A.; Yokoyama A.; Koga H.; Sekiya I. Higher chondrogenic potential of fibrous synovium- and adipose synovium-derived cells compared with subcutaneous fat-derived cells: Distinguishing properties of mesenchymal stem cells in humans. Arthritis Rheum. 54:843–853; 2006. [DOI] [PubMed] [Google Scholar]
- 11. Nagatomo K.; Komaki M.; Sekiya I.; Sakaguchi Y.; Noguchi K.; Oda S.; Muneta T.; Ishikawa I. Stem cell properties of human periodontal ligament cells. J. Periodontal Res. 41:303–310; 2006. [DOI] [PubMed] [Google Scholar]
- 12. Pierdomenico L.; Bonsi L.; Calvitti M.; Rondelli D.; Arpinati M.; Chirumbolo G.; Becchetti E.; Marchionni C.; Alviano F.; Fossati V.; Staffolani N.; Franchina M.; Grossi A.; Bagnara G. P. Multipotent mesenchymal stem cells with immunosuppressive activity can be easily isolated from dental pulp. Transplantation 80:836–842; 2005. [DOI] [PubMed] [Google Scholar]
- 13. Sakaguchi Y.; Sekiya I.; Yagishita K.; Ichinose S.; Shinomiya K.; Muneta T. Suspended cells from trabecular bone by collagenase digestion become virtually identical to mesenchymal stem cells obtained from marrow aspirates. Blood 104:2728–2735; 2004. [DOI] [PubMed] [Google Scholar]
- 14. Sakaguchi Y.; Sekiya I.; Yagishita K.; Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: Superiority of synovium as a cell source. Arthritis Rheum. 52:2521–2529; 2005. [DOI] [PubMed] [Google Scholar]
- 15. Segawa Y.; Muneta T.; Makino H.; Nimura A.; Mochizuki T.; Ju Y. J.; Ezura Y.; Umezawa A.; Sekiya I. Mesenchymal stem cells derived from synovium, meniscus, anterior cruciate ligament, and articular chondrocytes share similar gene expression profiles. J. Orthop. Res. 27:435–441; 2009. [DOI] [PubMed] [Google Scholar]
- 16. Sekiya I.; Larson B. L.; Smith J. R.; Pochampally R.; Cui J. G.; Prockop D. J. Expansion of human adult stem cells from bone marrow stroma: Conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells 20:530–541; 2002. [DOI] [PubMed] [Google Scholar]
- 17. Seo B. M.; Miura M.; Gronthos S.; Bartold P. M.; Batouli S.; Brahim J.; Young M.; Robey P. G.; Wang C. Y.; Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364:149–155; 2004. [DOI] [PubMed] [Google Scholar]
- 18. Shirasawa S.; Sekiya I.; Sakaguchi Y.; Yagishita K.; Ichinose S.; Muneta T. In vitro chondrogenesis of human synovium-derived mesenchymal stem cells: Optimal condition and comparison with bone marrow-derived cells. J. Cell. Biochem. 97:84–97; 2006. [DOI] [PubMed] [Google Scholar]
- 19. Somerman M. J.; Archer S. Y.; Imm G. R.; Foster R. A. A comparative study of human periodontal ligament cells and gingival fibroblasts in vitro. J. Dent. Res. 67:66–70; 1988. [DOI] [PubMed] [Google Scholar]
- 20. Tang L.; Li N.; Xie H.; Jin Y. Characterization of mesenchymal stem cells from human normal and hyperplastic gingiva. J. Cell. Physiol. 226:832–842; 2011. [DOI] [PubMed] [Google Scholar]
- 21. Tomar G. B.; Srivastava R. K.; Gupta N.; Barhanpurkar A. P.; Pote S. T.; Jhaveri H. M.; Mishra G. C.; Wani M. R. Human gingiva-derived mesenchymal stem cells are superior to bone marrow-derived mesenchymal stem cells for cell therapy in regenerative medicine. Biochem. Biophys. Res. Commun. 393:377–383; 2010. [DOI] [PubMed] [Google Scholar]
- 22. Trubiani O.; Scarano A.; Orsini G.; Di Iorio D.; D’Arcangelo C.; Piccirilli M.; Sigismondo M.; Caputi S. The performance of human periodontal ligament mesenchymal stem cells on xenogenic biomaterials. Int. J. Immunopathol. Pharmacol. 20:87–91; 2007. [DOI] [PubMed] [Google Scholar]
- 23. Uccelli A.; Moretta L.; Pistoia V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 8:726–736; 2008. [DOI] [PubMed] [Google Scholar]
- 24. Yoshimura H.; Muneta T.; Nimura A.; Yokoyama A.; Koga H.; Sekiya I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 327:449–462; 2007. [DOI] [PubMed] [Google Scholar]
- 25. Zhang Q.; Shi S.; Liu Y.; Uyanne J.; Shi Y.; Le A. D. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J. Immunol. 183:7787–7798; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]