Abstract
This study tested whether maternal responsiveness may buffer the child to the effects of maternal depressive symptoms on DNA methylation of NR3C1, 11β-HSD2, and neuroendocrine functioning. DNA was derived from buccal epithelial cells and pre-stress cortisol was obtained from the saliva of 128 infants. Mothers with depressive symptoms who were more responsive and who engaged in more appropriate touch during face-to-face play had infants with less DNA methylation of NR3C1 and 11β-HSD2 compared to mothers with depressive symptoms who were also insensitive. The combination of exposure to maternal depressive symptoms and maternal sensitivity was related to the highest pre-stress cortisol levels whereas exposure to maternal depressive symptoms and maternal insensitivity was related to the lowest pre-stress cortisol levels.
The negative consequences of child exposure to maternal depressive symptoms have been well-documented and range from greater internalizing and externalizing behavior (Brennan, Hammen, Andersen, Bor, Najman, & Williams, 2000; Essex, Klein, Cho, & Kraemer, 2003; Toth, Rogosch, Sturge-Apple, & Cicchetti, 2009), to dysregulated physiological responses to stress (Laurent, Ablow, & Measelle, 2011). Investigating the biological mechanisms involved in this transmission of risk for depression from mother to child has led to a focus on how the neuroendocrine response to stress in mothers with depression may program the infant hypothalamic – pituitary – adrenal (HPA) axis. At present, however, the processes involved in this “programming” are not fully understood.
In brief, the concept of programming is based on epidemiological studies suggesting that an adverse fetal environment resulting in low birth weight in term infants was associated with the development many decades later of adult cardiovascular and metabolic disorders (Barker, 1998; Barker & Osmund, 1986). This increased risk for disease in adulthood was attributed to fetal adjustments to cues from the intrauterine environment, also known as programming (Gluckman, Hanson, Cooper, & Thornburg, 2008; Godfrey & Barker, 2001). Epigenetic mechanisms have been suggested as one explanation underlying programming and such programing may not be limited to the fetal period. Specifically, research with animal models suggest that programming may occur postnatally as the infant adjusts to the quality of the caretaking environment with concomitant epigenetic effects (Liu et al., 1997; Meaney, 2010; Weaver et al., 2004). For instance, using rodent models, Meaney and colleagues demonstrated that rodent offspring deprived of a particular form of maternal caregiving exhibited reduced expression of the glucocorticoid receptor gene via increased DNA methylation in hippocampal tissue (Liu et al., 1997). Determining whether similar programming processes occur in humans could lead to a greater understanding of the molecular basis for the development of infant HPA axis functioning.
Translating this work to humans requires an understanding of whether the quality of the postnatal environment is related to DNA methylation of genes involved in HPA functioning as well as infant cortisol. DNA methylation is the process by which a methyl group is added to individual cytosines in the context of CpG dinucleotides. When this addition occurs in gene promoters, it is most often associated with transcriptional gene silencing, or the reduction of gene activity (Jones & Takai, 2001). Preliminary human evidence indicates that the experience of depression (Conradt et al., 2013; Oberlander et al., 2008) while pregnant, and exposure to childhood abuse (Tyrka et al., 2012) is related to increased methylation of genes involved in the neuroendocrine response to stress, including the glucocorticoid receptor gene (NR3C1) and 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD-2).
The neuroendocrine response to stress is initiated when an individual perceives stress or threat in his/her environment. As a result, limbic brain regions stimulate the release of corticotropin-releasing factor (CRF), which activates the pituitary gland to release adrenocorticotropic hormone (ACTH) which then stimulates cells in the adrenal cortex to release cortisol into the bloodstream (Gunnar & Vazquez, 2006). A negative feedback is initiated whereby glucocorticoids bind to glucocorticoid receptors in the brain, such as the hippocampus, which then inhibits the synthesis and release of CRF (Zhang & Meaney, 2010), thereby shutting down the HPA axis and the release of more cortisol. Therefore, DNA methylation of NR3C1 should result in fewer glucocorticoid receptors for binding cortisol resulting in higher levels of cortisol in the blood. Evidence for this hypothesis comes from the work of Oberlander and colleagues (2008) who found that exposure to prenatal depression was related to greater methylation of NR3C1, which in turn was related to greater cortisol reactivity in infancy (Oberlander et al., 2008).
11β-HSD2 functions to convert maternal cortisol to inert cortisone. DNA methylation of 11β-HSD2 is thought to reduce activity of this gene resulting in greater exposure of the fetus to maternal cortisol. Either increased exposure to glucocorticoids, or inhibition of 11β-HSD-2, results in decreased birth weight, increases in hyperglycemia and hypertension, increased HPA axis reactivity, and increased anxiety in rodent models (Harris & Seckl, 2011). While this preclinical evidence is promising, no studies that we know of have examined relations between DNA methylation of 11β-HSD2 and HPA functioning in humans.
Exposure to maternal depression may be a risk factor for impaired psychophysiological functioning in infancy as some mothers with mood disorders are less sensitive and responsive when interacting with their infants (Beeghly & Tronick, 2011; Campbell et al., 2004). This insensitivity may be a mechanism by which infants of mothers with mood disorders show alterations in the HPA axis. For instance, infants of insensitive mothers with depression and anxiety had higher baseline cortisol (Brennan et al., 2008) compared to their unexposed counterparts. However, to characterize exposure to maternal depressive symptoms as being a risk factor for all children is short-sighted. Maternal depression is a complicated and heterogeneous disorder, with a great deal of variability in the quality of early parenting (Tronick & Weinberg, 1997). Far less attention has been paid to the specific parenting characteristics that may moderate the effect of exposure to maternal depressive symptoms on child outcomes.
The social buffering hypothesis suggests that maternal sensitivity and responsiveness may buffer the child to the effects of early stress, including maternal depression (DiCorcia, & Tronick, 2011; Hostinar, Sullivan, & Gunnar, 2014). An impressive body of research indicates that the HPA response to stress in infants and young children is mitigated in the presence of a sensitive caregiver (Hostinar et al., 2014). In a longitudinal study, women with late, intermittent, or chronic depressive symptoms postnatally and who were less sensitive had preschoolers who were more likely to be insecurely attached in comparison to women with depressive symptoms who were more sensitive (Campbell, Brownell, Hungerford, Spieker, Mohan, & Blessing, 2004). Therefore, maternal sensitivity or responsiveness may buffer infants from the effects of maternal depressive symptoms.
It is unclear what biologic or molecular mechanism might underlie the effects associated with the buffering of stress by caretaking. The animal literature suggests that one such process may be epigenetic in nature, though it remains to be seen whether this research translates to human models. The goal of the present study is to investigate whether maternal depressive symptoms and/or maternal sensitive behaviors and responsiveness are related to DNA methylation of genes involved in the neuroendocrine response to stress and to neuroendocrine functioning in infants. Given the comorbidity of maternal depression and anxiety we also include symptoms of anxiety in our models to determine whether maternal depressive symptoms are related to epigenetic processes above and beyond symptoms of maternal anxiety. We examined maternal sensitive behaviors during the first play phase of the still-face paradigm as we were interested in observing mother/infant interactions during baseline, or more typical conditions. Furthermore, our goal was to understand whether maternal behavior may be related to epigenetic processes. Our first aim was to examine the relations between maternal depressive symptoms and maternal sensitive behaviors and responsiveness and DNA methylation of NR3C1 and 11β-HSD2. Our second aim was to examine the main effects of maternal depressive symptoms, maternal sensitive behaviors and responsiveness, and DNA methylation of NR3C1 and 11β-HSD2 on pre-stress cortisol and cortisol reactivity. Our third aim was motivated by the social buffering hypothesis. Specifically, we tested interactions between maternal depressive symptoms and maternal sensitive behavior and responsiveness on DNA methylation of NR3C1 and 11β-HSD2, pre-stress cortisol, and cortisol reactivity.
Method
Participants
Mothers and their 4 month-old infants were recruited from an existing cohort of infants born of average weight for gestational age following approval from the Women and Infants Hospital of Rhode Island and Dartmouth College IRBs. Only singleton, full-term (>= 37 weeks GA) infants were included in the study. Other exclusion criteria were maternal age <18 years, a life-threatening medical complication of the mother, and congenital or chromosomal abnormality of the infant. Data collection took place between June, 2011-December, 2013. Most of the participants were Caucasian (72.7%), with 12.5% African American, 3.1% Hispanic, 1.6% Asian, .8% American Indian, and 9.3% identifying themselves as “other” (see Table 1 for additional sample characteristics). Mother’s mean age was 30.5 years (range = 18–40 years). The sample included 128 infants (64 female) with an average age of 19.1 weeks (range = 13–26 weeks). All mothers gave written informed consent.
Table 1.
Participant Characteristics
| Demographic variable | Mean (range) or % |
|---|---|
| Household income | |
| Maternal employment status: work full-time | 49.2% |
| Maternal employment: part-time work | 18.8% |
| Household income: $0–24,999 | 20.0% |
| Household income: $25,000–49,999 | 22.6% |
| Household income: >$50,000 | 57.4% |
| Caucasian | 72.7% |
| African American | 12.5% |
| Hispanic | 3.1% |
| Asian | 1.6% |
| American Indian | .8% |
| “Other” | 9.3% |
| Maternal age | 30.5 years (18–40 years) |
| Infant sex: Female | 50% |
| Infant age | 19.1 weeks (13–26 weeks) |
Measures
Maternal Symptoms of Depression
Maternal symptoms of depression were assessed by the Center for Epidemiologic Studies-Depression scale (CES-D; Radloff, 1977), a 20-item self-report measure designed to assess for symptoms of depression in the past week. It is considered to be a reliable and a valid indicator of maternal depression in postpartum women (Conradt, Manian, & Bornstein, 2012). The α was 0.99.
Maternal symptoms of anxiety
Maternal symptoms of anxiety were assessed with the Beck Anxiety Inventory (Beck & Steer, 1997), a 21-item self-report inventory. The alpha was 0.99.
Maternal sensitivity and responsiveness
Maternal sensitivity and responsiveness were assessed using a coding scheme adapted from (Gunning, Fiori-Cowley, & Murray, 1999) and included 4 scales. Maternal acceptance included the willingness and ability of the mother to follow her infant’s lead; demandingness (reverse-scored) was defined as the degree to which the mother required her infant to behave in a certain way; responsiveness was operationalized as both the mother’s awareness of her infant’s signals and her response to them (regardless of the appropriateness of the response), and appropriate touch was defined as the mother’s ability to touch her infant in a gentle and affectionate manner as opposed to a more intrusive style.
Maternal sensitivity and responsiveness were coded every 30 seconds during a 2-minute face-to-face play episode by coders trained to reliability against a set of 10 training tapes coded by 3 experts in the field of maternal sensitivity. The first play episode was part of the face-to-face still-face paradigm (Tronick et al., 1978), which includes three episodes: a 2-minute play episode, a 2-minute still-face episode in which mothers are asked to be unresponsive to their infant, and a 2-minute reunion episode. The modification by Haley and Stansbury (2003) was conducted, which includes an additional second still-face and reunion episode. Only the first 2-minute play episode was used in this study due to our interest in measuring maternal sensitivity to non – distress. Coders then coded an additional 20% of tapes for reliability. The intra-class correlations were .78 for accepting, .90 for demandingness, .95 for responsiveness, and .83 for touch. Each score within each maternal sensitivity and responsiveness domain was significantly and positively correlated (r’s ranged from .40–.60 for accepting, .26–.43 for demandingness, .42–.67 for responsiveness, and .46–.68 for touch). The values were therefore averaged to create a single score. We then ran a principal components analysis to reduce the number of variables tested in analyses. Two factors emerged which accounted for 80.5% of the variance, and all sensitivity and responsiveness variables had factor loadings greater than .64. The first factor was the responsiveness/appropriate touch factor and the second factor was the accepting/non-demanding factor. These two factors were employed in our analyses.
Cortisol
Pre-stress cortisol samples were taken from infants upon entry into the laboratory and two post-stress cortisol samples were taken following the still-face paradigm (Tronick et al., 1978). Following Haley & Stansbury (2003), the first post-stress saliva sample was taken 30 minutes after the end of the first still-face episode and the second post-stress saliva sample was taken 40 minutes after the end of the first still-face episode. Salivary cortisol was collected from the infant using a small sponge that was swabbed in the infant’s mouth until it became saturated with saliva. The swab was then placed into a storage vial and frozen until analyzed. If infants ate or drank 30 minutes prior to sample collection their mouths were first swabbed with a wet paper towel. Samples were analyzed by Salimetrics (Arizona) for analysis.
Buccal sample collection, DNA, and bisulfite modification
Buccal- derived DNA was collected from saliva samples following the still-face paradigm using the Oragene-DNA saliva collection system. Buccal cells were taken from the infants’ cheeks using a small swab. The swabs were then placed in a collection tube and sealed, releasing a stabilizing solution into the collected sample to allow for processing of the sample at a later period. Batches of sample collection tubes were sent to Dartmouth College for DNA isolation. DNA was isolated from the collection tubes following the Oragene methods. Purified DNA was quantified using a ND-1000 spectrophotometer (Nanodrop, Wilmington, DE), and DNA samples (500 ng) were bisulfite-modified using the EZ DNA Methylation Kit (Zymo Research, CA, USA.) and stored at −20°C.
Bisulfite pyrosequencing DNA methylation analysis
NR3C1
Of the 13 CpG sites in the NR3C1 exon 1F promoter region, our primary interest was in sites 1–3, which have previously showed variability in DNA methylation associated with maternal depression and cortisol response in infant cord blood. Pyrosequencing, which allows for quantitative assessment of DNA methylation in short sequence regions, was performed on PCR product amplified from bisulfite modified DNA as described previously (Conradt et al., 2013).
The primers for amplification were Forward: 5′-TTT TTT TTT TGA AGT TTT TTT A-3′ and Reverse: 5′-Biotin-CCC CCA ACT CCC CAA AAA-3′. The first sequencing primer was designed to sequence the first five CpG sites (5′-GAG TGG GTT TGG AGT-3′), and the second sequencing primer was designed to sequence the following eight CpG sites (5′-AGA AAA GAA TTG GAG AAA TT-3′) for a total of 13 sites sequenced.
11β-HSD-2
Pyrosequencing was performed on PCR product amplified from bisulfite-modified DNA as described in (citation blinded for review) based on the region sequenced and displaying differential methylation in human placenta from Alikhani-Koopaei and colleagues (2004). Amplification primers were HSD11B2-F, 5′-GGAAGTGGGGTTGTGYGTTTTTAGGTTTAAGTT -3′ and HSD11B2-R, 5′-biotin-ATACCCTTTACTAATCRCACCACC-3′ (IDT Inc., Coralville, IA), and the sequencing primer designed to interrogate 4 CpG sites HSD11B2-seq, 5′-GGGGTAGAGATTTTAAGAA -3′.
For both NR3C1 and HSD11B2, the percent methylation at each CpG site was quantified using the Pyro Q-CpG software, version 1.0.11 (Qiagen). For both assays, bisulfite conversion controls were included on each sequencing read. In order for the sample’s methylation extent to be called, the bisulfite conversion rate must be >93%, and for all samples examined the conversion rate was >95%. All assays were performed in triplicate on the same bisulfite converted DNA template on all samples, and if any of the repeats differed by >10% those assays on that sample were repeated. To prevent batch effects from bisulfite treatments interfering with the analysis, samples were randomized across batches.
Missing Data
There were 128 infants with complete 11β-HSD2 methylation and maternal sensitivity and responsiveness data. Of these, 9 children had missing NR3C1 methylation data due to insufficient saliva volume needed for testing, 6 had missing cortisol data because the quantity of saliva was insufficient (n = 5) or because their cortisol values were extreme outliers (n = 1). One participant had missing CES-D data.
There were no significant differences in maternal sensitive behaviors or responsiveness between infants with and without missing NR3C1 methylation data (p’s > .21) or maternal depression among infants with and without missing cortisol data (t (126) = −.48, p = .63). Infants with missing NR3C1 methylation values had mothers with significantly greater symptoms of depression, t (126) = −2.26, p= .03. Tests for birth and demographic differences between infants with and without missing data revealed that there were no differences in birth weight, gestational age, ethnicity, education level, or maternal age among infants with and without missing NR3C1 methylation data (all p’s > .15) or missing cortisol data (all p’s > .10).
We controlled for False Discovery among the 10 tests of interaction using the Benjamini and Hochberg (1995) procedure. This method was used to determine the percent of findings which could be a false discovery. Instead of a corrected p-value, a q-value is obtained, which represents the proportion of tests below which are false positives. As is standard in the epigenetic literature, we chose a q-value of .10. In the results we present both the p-value and q-values.
Results
Descriptive Statistics
Data were examined for outliers and violations of normality. In addition to examining outliers among individual variables, we checked the assumption that the error term residuals should be normally distributed by looking at Normal p-p plots of regression standardized residuals and found that residuals were normally distributed. The raw cortisol values (μg/dL) and 11β-HSD2 methylation scores were positively skewed and normalized using a log transformation. Outliers above or below 3SD in all 3 samples and the difference scores were winsorized by replacing the value with the value at 3SD (<1% of values were affected).
Table 2 includes the means, standard deviations, and correlations among our variables of interest. There were no significant associations between maternal depressive symptoms or DNA methylation of either gene. Greater levels of maternal accepting and non-demanding behavior were related to greater methylation of 11β-HSD2 CpG1. Greater levels of maternal sensitive behaviors (both factors) were related to lower levels of cortisol at the first post-stress sample, but not cortisol reactivity (difference score of cortisolpost-stress 1 or 2 − cortisolpre-stress). Greater levels of DNA methylation of NR3C1 CpG1 were related to lower levels of cortisol at the first and second post-stress sample, but not cortisol reactivity.
Table 2.
Means, Standard Deviations, and Correlations of Variables of Interest
| Variable | M | SD | 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | 12. | 13. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Maternal symptoms of depression | 9.01 | 8.63 | --- | ||||||||||||
| 2. Maternal symptoms of anxiety | 6.25 | 8.06 | .76*** | --- | |||||||||||
| 3. Maternal responsiveness and appropriate touch factor | .00 | 1.00 | .07 | .07 | --- | ||||||||||
| 4. Maternal accepting and non-demanding factor | .00 | 1.00 | −.17 | −.004 | .13 | --- | |||||||||
| 5. NR3C1 CpG1 | 1.09 | 1.35 | .01 | −.02 | −.09 | .04 | --- | ||||||||
| 6. NR3C1 CpG2 | 1.15 | 1.46 | .05 | −.03 | −.07 | −.05 | .51*** | --- | |||||||
| 7. NR3C1 CpG3 | 1.98 | 1.95 | .09 | .16 | −.02 | .08 | .04 | −.10 | --- | ||||||
| 8. 11β-HSD2 CpG1 | .66 | .76 | −.09 | .08 | .03 | .21* | −.03 | −.05 | .22** | --- | |||||
| 9. 11β-HSD2 CpG2 | 1.30 | 1.37 | .04 | .06 | −.05 | −.05 | −.03 | −.07 | −.05 | .29*** | --- | ||||
| 10. 11β-HSD2 CpG3 | .81 | 1.04 | .01 | −.04 | −.05 | −.10 | −.13 | −.10 | −.01 | .16 | .65*** | --- | |||
| 11. 11β-HSD2 CpG4 | 3.26 | .82 | .11 | .05 | −.08 | .04 | −.06 | .16 | .09 | .45*** | .41*** | .51*** | --- | ||
| 12. Pre-stress cortisol (μg/dl) | .22 | .19 | .14 | .05 | −.13 | −.14 | −.05 | −.11 | −.05 | −.12 | −.09 | −.13 | −.10 | --- | |
| 13. Post-stress cortisol sample 1 (μg/dl) | .24 | .26 | .08 | .004 | −.15 | −.43*** | −.16 | −.15 | −.06 | .02 | −.03 | −.04 | .04 | .55*** | --- |
| 14. Post-stress cortisol sample 2 (μg/dl) | .22 | .18 | −.07 | −.08 | −.11 | −.33*** | −.19* | −.10 | −.05 | −.01 | .02 | .03 | .11 | .14 | .73*** |
Note:
p <.05;
p <.01;
p <.001
Covariates
Because of the diurnal rhythm of cortisol, all assessments took place in the morning between 8:00–11:30AM (range = 8:11AM – 11:20AM). We examined whether the time of each of the three assessments were associated with each measure of cortisol (e.g. whether time of the pre-stress measurement was correlated with the pre-stress cortisol value). Time of measurement was not significantly related with the time-specific measurement of cortisol (all p’s > .35). We also examined whether either infant or maternal prescription and/or non-prescription steroid medication, or maternal use of caffeine impacted cortisol concentrations. Steroid use within the last twelve hours by either mother or infant was not significantly associated with the cortisol values (all p’s > .40), and neither was maternal consumption of caffeine that morning (p’s >.11). If infants had eaten less than 30 minutes prior to cortisol sampling their mouths were rinsed with water. As nap times may also affect cortisol values we examined whether time of nap and/or time of awakening affected cortisol. Neither was related to our cortisol values (p’s > .18).
We also examined covariates that may be related to DNA methylation of 11β-HSD2, NR3C1, or cortisol. These covariates include birth weight, gestational age, ethnicity, and sex. None of these covariates were significant predictors of DNA methylation of 11β-HSD2, NR3C1, or cortisol (all p’s >.08).
Aim 1: Main effects of maternal sensitive behaviors and responsiveness and depressive symptoms on DNA methylation of 11β-HSD2 and NR3C1
We tested the main effects of maternal sensitive behaviors (responsiveness/appropriate touch factor and accepting/non-demanding factor, entered separately), and maternal depressive symptoms on DNA methylation of 11β-HSD2 CpG sites 1–4 and NR3C1 CpG sites 1–3 in infants. Out of 14 regressions tested, we found 1 main effect. Greater levels of the accepting/non-demanding factor were related to greater methylation of 11β-HSD2 CpG1, b = .23, p = .02, q = .007.
Aim 2: Main effects of maternal sensitive behaviors and responsiveness and depressive symptoms on pre-stress cortisol and cortisol reactivity
We again tested the main effects of maternal sensitive behaviors (responsiveness/appropriate touch factor and accepting/non-demanding factor, entered separately), and maternal depressive symptoms on pre-stress cortisol and cortisol reactivity in infants (outcomes tested separately). Out of 21 regressions tested, no main effects emerged.
Aim 3: Test of maternal sensitive behavior as a moderator of the effect of maternal depressive symptoms on DNA methylation of 11β-HSD2 and NR3C1and cortisol
We next tested the hypothesis that the effect of maternal depressive symptoms on DNA methylation of 11β-HSD2 and NR3C1 may depend upon maternal sensitive behaviors. In other words, we examined whether these sensitive behaviors buffered, or were moderators of, the effect of maternal depressive symptoms on DNA methylation of 11β-HSD2 and NR3C1. Our regression models included maternal depressive symptoms, the maternal responsiveness/appropriate touch factor and the maternal accepting/non-demanding factor entered as main effects in step 1, the interaction between maternal responsiveness/appropriate touch and maternal depressive symptoms, and the maternal accepting/non-demanding factor entered in step 2 of all models. Ten outcomes were tested separately: 4 CpG sites for 11β-HSD2, 3 for NR3C1, and our 3 cortisol outcomes (pre-stress and the 2 reactivity measures). These results are reported in Table 3 below.
Table 3.
Hierarchical Regression Predicting DNA Methylation and Pre-stress Cortisol
| Predictors | β step 1 | β step 2 | R2 | F |
|---|---|---|---|---|
| Outcome: DNA methylation of 11β-HSD2 CpG3 | ||||
|
| ||||
| Responsiveness/appropriate touch factor | −.05 | −.22 | ||
| Accepting/non-demanding factor | .04 | .09 | ||
| Maternal depressive symptoms | .02 | .06 | ||
| Maternal anxious symptoms | −.05 | −.20 | ||
| Maternal depressive symptoms x accepting/non-demanding factor | --- | −.13 | ||
| Maternal depressive symptoms x responsiveness/appropriate touch factor | --- | −.26* | ||
| ΔR2 = .06 | .07 | 3.09* | ||
|
| ||||
| Outcome: DNA methylation of 11β-HSD2 CpG4 | ||||
|
| ||||
| Responsiveness/appropriate touch factor | −.11 | −.32** | ||
| Accepting/non-demanding factor | .09 | .16 | ||
| Maternal depressive symptoms | .14 | .19 | ||
| Maternal anxious symptoms | −.01 | .02 | ||
| Maternal depressive symptoms x accepting/non-demanding factor | --- | −.19 | ||
| Maternal depressive symptoms x responsiveness/appropriate touch factor | --- | −.30** | ||
| ΔR2 = .08 | .10 | 4.73** | ||
|
| ||||
| Outcome: NR3C1 CpG2 | ||||
|
| ||||
| Responsiveness/appropriate touch factor | −.09 | −.33* | ||
| Accepting/non-demanding factor | .02 | −.01 | ||
| Maternal depressive symptoms | .30* | .39** | ||
| Maternal anxious symptoms | −.21 | −.20 | ||
| Maternal depressive symptoms x accepting/non-demanding factor | --- | .03 | ||
| Maternal depressive symptoms x responsiveness/appropriate touch factor | --- | −.34* | ||
| ΔR2 = .05 | .11 | 2.57 | ||
|
| ||||
| Outcome: pre-stress cortisol | ||||
|
| ||||
| Responsiveness/appropriate touch factor | −.09 | .13 | ||
| Accepting/non-demanding factor | −.14 | −.10 | ||
| Maternal depressive symptoms | .02 | −.07 | ||
| Maternal anxious symptoms | .05 | .06 | ||
| Maternal depressive symptoms x accepting/non-demanding factor | --- | −.08 | ||
| Maternal depressive symptoms x responsiveness/appropriate touch factor | --- | .37** | ||
| ΔR2 = .09 | .12 | 4.89* | ||
Note:
p<.05,
p<.01
In the first model, only the interaction between maternal depressive symptoms and the maternal responsiveness/appropriate touch factor was a significant predictor of 11β-HSD2 CpG3, p = .04, q = .04. We used the online computational tools provided by Preacher, Curran and Bauer (2006; http://www.quantpsy.org/interact/mlr2.htm) to clarify the nature of this interaction. The simple slopes of maternal responsiveness and maternal depressive symptoms were computed at 1 standard deviation above and below their respective means. As seen in Figure 1a, there were no differences in DNA methylation of 11β-HSD2 CpG3 among infants whose mothers scored high on responsiveness/appropriate touch, regardless of the number of depressive symptoms the mother endorsed (b = −1.55, p = .12). The highest levels of DNA methylation of 11β-HSD2 CpG3, however, were found among infants of mothers who were less responsive and with high depressive symptoms (b = 2.09, p = .04).
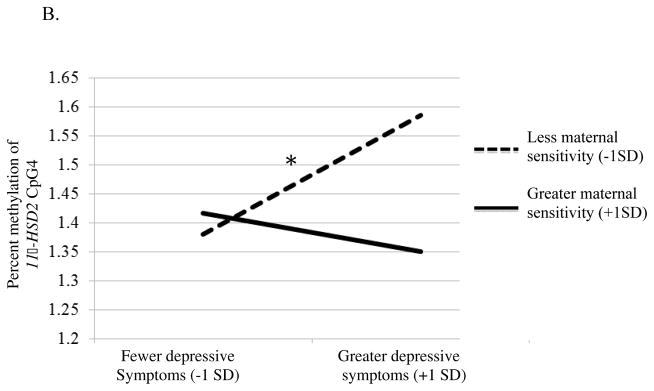
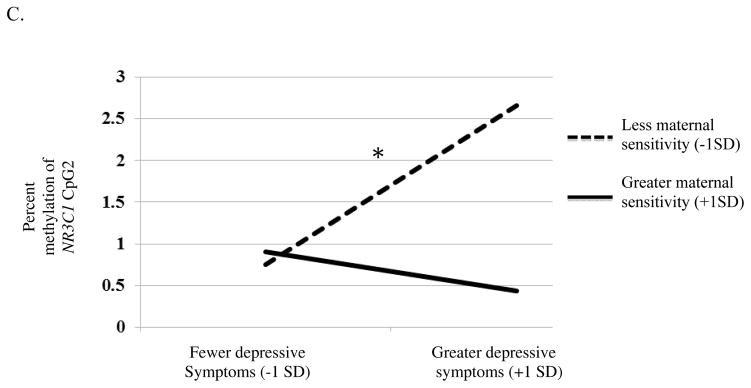
Figure 1.
Interactions between maternal depressive symptoms and maternal sensitivity on 11β-HSD2 CpG3 (A), 11β-HSD2 CpG4 (B), NR3C1 CpG2 (C), pre-stress cortisol (D). Simple slopes were tested at ± 1SD from the mean; * p <.05; ** p <.01; *** p <.001.
In the second model we examined DNA methylation of 11β-HSD2 CpG4 and NR3C1 CpG2 (tested separately). There was a main effect of maternal depressive symptoms and the responsiveness/appropriate touch factors on DNA methylation of 11β-HSD2 CpG4, p = .03, q = .03, and NR3C1 CpG2, p = .01, q = .02 (Table 3). This main effect, however, was qualified by a significant interaction between maternal responsiveness/appropriate touch and maternal depressive symptoms. Again, a test of simple slopes revealed no differences in DNA methylation of 11β-HSD2 CpG4, (b = −1.02, p = .31; Figure 1b) or NR3C1 CpG2 (b = −.96, p = .34; Figure 1c) among infants whose mothers were more responsive, regardless of depressive symptom severity. Infants with the highest levels of DNA methylation of 11β-HSD2 CpG4 (b = 3.27, p = .001) or NR3C1 CpG2 (b = 2.83, p = .01), however, had mothers who were both less responsive and who reported greater depressive symptoms.
In our final model the same predictors were used to test pre-stress cortisol as our outcome (Table 3). While there were no significant main effects, there was a significant interaction of maternal depressive symptoms and the responsiveness/appropriate touch factor, p = .003, q = .01. Simple slopes testing revealed infants of mothers who had lower levels of responsiveness/appropriate touch and higher levels of depressive symptoms had the lowest pre-stress cortisol levels (b= −2.43, p = .02; Figure 1d). Infants of mothers who had higher levels of responsiveness/appropriate touch and higher levels of maternal depression had the highest pre-stress cortisol levels (b = 2.70, p = .01).
Discussion
Decades of research with animals have demonstrated that the quality of maternal care may be protective in the face of environmental challenge. What biologic mechanism underlies this process is unknown though animal studies suggest that epigenetic mechanisms may be at play. This study was an attempt to determine if similar effects could be observed in humans. These initial findings provide some support for the hypothesis that maternal responsiveness may buffer infants from the effects of maternal depressive symptoms. This could suggest that epigenetic processes are sensitive to environmental input. These findings are similar to those of Meaney and colleagues and could have translational implications by suggesting that particular forms of maternal caregiving is related to less methylation of genes involved in HPA axis functioning to humans (Meaney, 2010).
There was a significant positive correlation between DNA methylation of 11β-HSD2 CpG1 and the accepting/non-demanding factor, which was not expected. There are no known transcription factor (proteins that regulate the transcription, or the first step in gene expression, of genes) binding sites on 11β-HSD2 CpG1, and thus it is difficult to interpret why maternal behavior would be associated, in the opposite direction, with methylation at this site. For instance, CpG site 4 is the binding site for transcription factor GATA1 (Armstrong, Lesseur, Conradt, Lester, & Marsit, 2014). GATA1 is involved in the regulation of the immune response (Yamagata et al., 1995) and may be a more important site for regulation of the neuroendocrine response to stress than is CpG 1. Methylation at site 4 could decrease GATA1 binding and subsequent transcription, which may ultimately interfere with HPA axis regulation. This process could explain why we found relations between maternal responsiveness and maternal depressive symptoms in this site implicated in GATA1 binding. In previous work examining 11β-HSD2 from placenta samples, we also found relations between maternal prenatal depression exposure and methylation at CpG site 4, but not CpG1 (Conradt et al., 2013). Therefore, it may be that some CpG sites play a stronger role in HPA axis regulation, and subsequent neuroendocrine/behavior relations than others, because of their proximity to transcription factor binding sites.
It was only by examining maternal depressive symptoms that the effect of maternal sensitive behavior on DNA methylation and HPA axis functioning became clear. Mothers with depressive symptoms who were more responsive and engaged in more appropriate touch during face-to-face play had infants with less DNA methylation compared to mothers with depressive symptoms who were also less sensitive. This interaction emerged for three of the seven CpG sites tested and thus appears to be a robust effect. Furthermore, the combination of exposure to maternal depressive symptoms and maternal responsiveness was related to the highest pre-stress cortisol levels whereas exposure to maternal depressive symptoms and maternal unresponsiveness was related to the lowest pre-stress cortisol levels. The false discovery rates were low, indicating that our results likely represent true discoveries. However, like all findings from initial studies, our results should be replicated in an independent sample.
These results could be interpreted in favor of the social buffering hypothesis as maternal sensitive behavior may buffer the effects that exposure to maternal depression has on genes that regulate the infant HPA axis and on the HPA axis itself. Even in the face of maternal depressive symptoms, having a mother who is responsive and engages in appropriate touch during play may dampen HPA axis activity via decreased methylation of genes involved in the neuroendocrine response to stress. Furthermore, DNA methylation outcomes were similar between infants whose mothers were more responsive, regardless of the mother’s report of her own depressive symptoms. While these data are preliminary, they could suggest that having a responsive caregiver may buffer infants to the exposure of maternal depressive symptoms. Put another way, infants do not know the diagnosis or symptom levels of their mother; they only know what they experience.
Exposure to maternal depressive symptoms at 4 months could be a proxy for exposure to prenatal maternal depression, which may program the infant HPA axis in utero. It is possible that, exposure to prenatal maternal depression is related to increased glucocorticoid exposure, as some adults with depression hypersecrete and exhibit prolonged elevations in cortisol (Parker et al., 2003), and their offspring tend to have higher cortisol levels (Field et al., 2004), though other work finds null results (Huot et al., 2004). Indeed, in previous work we have found that exposure to prenatal maternal depression is related to more DNA methylation of NR3C1 and 11β-HSD2 (Conradt et al., 2013). Therefore, at birth, these infants may exhibit greater cortisol levels compared to infants who are not exposed to maternal depression, and may be more reactive to stress. By contrast, more responsive caregiving and greater infant capacity for self- buffering may lead to demethylation of genes involved in HPA axis functioning. Indeed, Meaney and colleagues (Liu et al., 1997) found that at postnatal day 1, all of the rats exhibited hypermethylation of specific CpG sites on exon 1F of NR3C1 and it was the experience of receiving high levels of licking and grooming that led to demethylation; perhaps a similar process is occurring in humans.
We found interaction effects for the factor that included maternal appropriate touch, but not for the accepting factor. This research was informed by animal models suggesting that maternal licking and grooming is related to the expression of genes regulating the HPA axis, and we expected that maternal sensitivity is a good proxy for this licking and grooming behavior in rats. As others have argued, licking, grooming, and maternal sensitivity reflect species-specific parenting practices, both of which are involved in the offspring response to stress, and buffer HPA axis reactivity in infancy (Loman & Gunnar, 2010). In humans, for instance, studies have found relations between maternal caregiving and infant stress reactivity, over and above the effects of infant negative temperament (Conradt & Ablow, 2010; Hane & Fox, 2006). Gusella, Muir and Tronick (1988) found that maternal holding of the infant during the still-face, even when the infant was in an infant seat, reduced negative affect compared to infants not touched. Our research suggests that appropriate touch in human mothers may be a better proxy for rat licking and grooming than global measures of maternal sensitivity per se. There is also a large literature suggesting that touch dampens the stress response and reduces cortisol levels (Field, Hernandez-Reif, Diego, Schanberg, & Kuhn, 2004), negative affect (Feldman, Weller, Sirota, & Eidelman, 2003), and stress in preterm infants (Hernandez-Reif, Diego, & Field, 2007). Future research may even include measures of appropriate touch during a feeding interaction to determine whether touch further dampens the HPA response to stress via DNA methylation.
Though we find support for the social buffering hypothesis, alternative explanations are still warranted. For instance, maternal depression could moderate the effect of maternal sensitivity on DNA methylation and neuroendocrine functioning. These findings could then be interpreted in light of a “dual risk” framework by which the combination of exposure to maternal depression and maternal insensitivity is related to the poorest outcomes. It may also be that epigenetic factors could be related to increased behavioral reactivity, which in turn could affect maternal responsiveness. On the other hand it is critical to consider the infant’s capacity to cope or buffer him or herself from the stress. In addition, we need to keep in mind that the effects of depression, maternal sensitivity, reactivity and methylation along with other processes are on-going dynamic processes which may change or maintain initial effects.
This research has several limitations that should be noted. Given the small sample size, these are initial findings and need to be replicated in an independent sample. Second, all measures assessed were concurrent and thus we cannot imply direction of effect with these data. While animal models using cross-fostering paradigms to determine direction of effect found that maternal licking and grooming does indeed drive DNA methylation effects, this type of design cannot be conducted in humans due to obvious ethical implications (but see Nelson, Fox, & Zeanah, 2000, for their intervention with children reared in orphanages). Relatedly, there was a lack of independence between our measures of maternal sensitive behaviors and cortisol reactivity. It is important that replication studies assess cortisol reactivity in settings different from assessments of maternal behavior. In addition, the percent methylation values for our outcomes were low, but are consistent with our prior work and work from independent laboratories (e.g., Oberlander et al., 2008) where effects of maternal prenatal depression exposure were found on NR3C1 CpG 2. While this information gives us more confidence that we are identifying a meaningful relation between depression, responsiveness, and DNA methylation, the presences of low methylation may also represent cellular heterogeneity. Future work should therefore consider how to account for this heterogeneity. Our sample size was also restricted due to missing NR3C1 data for women with depressive symptoms. Given that we found significant effects of maternal depressive symptoms on NR3C1 CpG2 on this “milder” (e.g., less depressed) portion of the sample highlights the more robust nature of the findings. In addition, increasing variability in socio-economic status and/or risk for clinical depression will be important in future work to determine whether maternal sensitivity may buffer infants against exposure to clinical levels of depression and describe the epigenetic pathways involved.
Acknowledgments
We would like to thank Gilda Ferro, Joyce Lee, Erica Oliveira, and Susan Capobianco for their hard work in recruitment of subjects and the support and staff of the Brown Center for the Study of Children at Risk.
Footnotes
Conflict of Interest: The authors report no conflict of interest.
Financial Disclosure: This study was supported by the National Institute of Mental Health R01MH094609 (to C.J.M.) and a Career Development Award from the National Institute on Drug Abuse 7K08DA038959-02 (to E.C.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health, National Institute on Drug Abuse, or the National Institutes of Health.
References
- Alikhani-Koopaei R, Fouladkou F, Frey FJ, Frey BM. Epigenetic regulation of 11β-hydroxysteroid dehydrogenase type 2 expression. The Journal of clinical investigation. 2004;114(8):1146–1157. doi: 10.1172/JCI21647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJP. Mothers, babies and health in later life. Elsevier Health Sciences; 1998. [Google Scholar]
- Barker DJP, Osmond C. Infant mortality, childhood nutrition and ischaemic heart disease in England and Wales. The Lancet. 1986;1:1077–1081. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Manual for Beck Depression Inventory. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- Beeghly M, Tronick E. Early Resilience in the Context of Parent-Infant Relationships: A Social Developmental Perspective. Infant Mental Health: Relationship Issues, Social-Emotional Development, and Pediatric Practice, Special Issue of Current Problems in Pediatric and Adolescent Health Care. 2011;41:197–201. doi: 10.1016/j.cppeds.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PA, Hammen C, Andersen MJ, Bor W, Najman JM, Williams GM. Chronicity, severity, and timing of maternal depressive symptoms: relationships with child outcomes at age 5. Developmental psychology. 2000;36(6):759. doi: 10.1037//0012-1649.36.6.759. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Pargas R, Walker EF, Green P, Jeffrey Newport D, Stowe Z. Maternal depression and infant cortisol: influences of timing, comorbidity and treatment. Journal of Child Psychology and Psychiatry. 2008;49(10):1099–1107. doi: 10.1111/j.1469-7610.2008.01914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell SB, Brownell CA, Hungerford A, Spieker SJ, Mohan R, Blessing JS. The course of maternal depressive symptoms and maternal sensitivity as predictors of attachment security at 36 months. Development and psychopathology. 2004;16(02):231–252. doi: 10.1017/s0954579404044499. [DOI] [PubMed] [Google Scholar]
- Cohn JF, Matias R, Tronick EZ, Connell D, Lyons-Ruth K. Face-to-face interactions of depressed mothers and their infants. In: Tronick EZ, Field T, editors. Maternal depression and infant disturbance (New directions for child development, No. 34) San Francisco: Jossey-Bass; 1986. pp. 31–44. [DOI] [PubMed] [Google Scholar]
- Conradt E, Ablow J. Infant physiological response to the still-face paradigm: Contributions of maternal sensitivity and infants’ early regulatory behavior. Infant Behavior and Development. 2010;33(3):251–265. doi: 10.1016/j.infbeh.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Conradt E, Lester BM, Appleton AA, Armstrong DA, Marsit CJ. The roles of DNA methylation of NR3C1 and 11β-HSD2 and exposure to maternal mood disorder in utero on newborn neurobehavior. Epigenetics. 2013;8(12):1321–1329. doi: 10.4161/epi.26634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradt E, Manian N, Bornstein MH. Screening for depression in the postpartum using the Beck Depression Inventory II: What logistic regression reveals. Journal of reproductive and infant psychology. 2012;30(5):427–435. doi: 10.1080/02646838.2012.743001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCorcia J, Tronick E. Quotidian resilience: Exploring mechanisms that drive resilience from a perspective of everyday stress and coping. Neuroscience and Biobehavioral Reviews. 2011;35:1593–1602. doi: 10.1016/j.neubiorev.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Klein MH, Cho E, Kraemer HC. Exposure to maternal depression and marital conflict: Gender differences in children’s later mental health symptoms. Journal of the American Academy of Child & Adolescent Psychiatry. 2003;42(6):728–737. doi: 10.1097/01.CHI.0000046849.56865.1D. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Thomas Boyce W, Hertzman C, Lam LL, Armstrong JM, Neumann S, Kobor MS. Epigenetic vestiges of early developmental adversity: childhood stress exposure and DNA methylation in adolescence. Child Development. 2013;84(1):58–75. doi: 10.1111/j.1467-8624.2011.01641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R, Weller A, Sirota L, Eidelman AI. Testing a family intervention hypothesis: the contribution of mother-infant skin-to-skin contact (kangaroo care) to family interaction, proximity, and touch. Journal of Family Psychology. 2003;17(1):94. [PubMed] [Google Scholar]
- Field T, Diego M, Dieter J, Hernandez-Reif M, Schanberg S, Kuhn C, Bendell D. Prenatal depression effects on the fetus and the newborn. Infant Behavior and Development. 2004;27(2):216–229. [Google Scholar]
- Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. New England Journal of Medicine. 2008;359(1):61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey KM, Barker DJ. Fetal programming and adult health. Public Health Nutrition. 2001;4(2b):611–624. doi: 10.1079/phn2001145. [DOI] [PubMed] [Google Scholar]
- Gunning M, Fiori-Cowley A, Murray L. The global ratings of mother-infant-interaction-scoring manual. 1999. [Google Scholar]
- Gusella J, Muir D, Tronick E. The effect of manipulating maternal behavior during an interaction on three and six month-olds’ affect and attention. Child Development. 1988;59:1111–1124. doi: 10.1111/j.1467-8624.1988.tb03264.x. [DOI] [PubMed] [Google Scholar]
- Haley DW, Stansbury K. Infant Stress and Parent Responsiveness: Regulation of Physiology and Behavior During Still-Face and Reunion. Child development. 2003;74(5):1534–1546. doi: 10.1111/1467-8624.00621. [DOI] [PubMed] [Google Scholar]
- Hane AA, Fox NA. Ordinary variations in maternal caregiving influence human infants’ stress reactivity. Psychological Science. 2006;17(6):550–556. doi: 10.1111/j.1467-9280.2006.01742.x. [DOI] [PubMed] [Google Scholar]
- Harris A, Seckl J. Glucocorticoids, prenatal stress and the programming of disease. Hormones and behavior. 2011;59(3):279–289. doi: 10.1016/j.yhbeh.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Hernandez-Reif M, Diego M, Field T. Preterm infants show reduced stress behaviors and activity after 5 days of massage therapy. Infant Behavior and Development. 2007;30(4):557–561. doi: 10.1016/j.infbeh.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MC, Sangra M, French KL, Whittle IR, Paterson J, et al. 11beta-Hydroxysteroid dehydrogenase type 2 protects the neonatal cerebellum from deleterious effects of glucocorticoids. Neuroscience. 2006;137:865–873. doi: 10.1016/j.neuroscience.2005.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Sullivan RM, Gunnar MR. Psychobiological mechanisms underlying the social buffering of the hypothalamic–pituitary–adrenocortical axis: A review of animal models and human studies across development. Psychological bulletin. 2014;140(1):256. doi: 10.1037/a0032671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PA, Takai D. The role of DNA methylation in mammalian epigenetics. Science. 2001;293(5532):1068–1070. doi: 10.1126/science.1063852. [DOI] [PubMed] [Google Scholar]
- Laurent HK, Ablow JC, Measelle J. Risky shifts: How the timing and course of mothers’ depressive symptoms across the perinatal period shape their own and infant’s stress response profiles. Development and psychopathology. 2011;23(02):521–538. doi: 10.1017/S0954579411000083. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277(5332):1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Loman MM, Gunnar MR. Early experience and the development of stress reactivity and regulation in children. Neuroscience & Biobehavioral Reviews. 2010;34(6):867–876. doi: 10.1016/j.neubiorev.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ. Epigenetics and the biological definition of gene× environment interactions. Child development. 2010;81(1):41–79. doi: 10.1111/j.1467-8624.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008;3(2):97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interaction effects in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics. 2006;31:437–448. [Google Scholar]
- Radloff LS. The CES-D scale a self-report depression scale for research in the general population. Applied psychological measurement. 1977;1(3):385–401. [Google Scholar]
- Toth SL, Rogosch FA, Sturge-Apple M, Cicchetti D. Maternal depression, children’s attachment security, and representational development: An organizational perspective. Child development. 2009;80(1):192–208. doi: 10.1111/j.1467-8624.2008.01254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronick E, Als H, Adamson L, Wise S, Brazelton TB. The infant’s response to entrapment between contradictory messages in face-to-face interaction. Journal of the American Academy of Child psychiatry. 1978;17(1):1–13. doi: 10.1016/s0002-7138(09)62273-1. [DOI] [PubMed] [Google Scholar]
- Tronick EZ, Weinberg MK. Depressed mothers and infants: Failure to form dyadic states of consciousness. In: Murray L, Cooper P, editors. Postpartum Depression and Child Development. Vol. 1 New York: Guilford Press; 1997. pp. 54–81. [Google Scholar]
- Tyrka AR, Price LH, Marsit C, Walters OC, Carpenter LL. Childhood adversity and epigenetic modulation of the leukocyte glucocorticoid receptor: preliminary findings in healthy adults. PLoS One. 2012;7(1):e30148. doi: 10.1371/journal.pone.0030148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Meaney MJ. Epigenetic programming by maternal behavior. Nature neuroscience. 2004;7(8):847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]