Abstract
Introduction
Sodium Nitroprusside has successfully been an excellent choice when considering a decrease in systemic vascular resistance in the critical care setting. However, reflex tachycardia and ventilation-perfusion mismatch are possible side effects of this agent. To maintaining cardiac output, cerebral perfusion pressure, and concurrently drop SVR, low-dose epinephrine or dopamine are viable options. The aim of this paper is to conduct dose-response simulations to identify the equivalent dopamine, epinephrine, and nitroprusside infusion doses to decrease the systemic vascular resistance by 20% and by 40% from baseline resting values.
Methods
Three studies were identified in the literature with reported epinephrine, dopamine, and sodium nitroprusside infusion doses with corresponding systemic vascular resistance responses. Infusion doses were normalized to mcg/kg/min and SVR values were normalized and scaled to the percent decrease (%SVR) in SVR from baseline resting values. The original published studies were mathematically modeled and the Hill equation parameters used for further dose-response simulations of a virtual population. One-hundred patients were simulated various doses resulting in corresponding %SVR responses for each of the three drugs.
Results
Equivalent infusion doses achieving in an approximate 20-25% decrease in SVR, from baseline, were identified for epinephrine, dopamine, and sodium nitroprusside. Moreover, equivalent infusion doses were identified for epinephrine and nitroprusside to decrease the SVR by 40% from baseline.
Conclusion
Even though sodium nitroprusside is traditionally used in decreasing SVR, low doses of dopamine or epinephrine are viable alternatives to patients with contraindications to nitroprusside infusions or who will require prolonged infusions to avoid toxicity. The multiple comparisons procedure-modeling approach is an excellent methodology for dose-finding exercises and has enabled identification of equivalent pharmacodynamic responses for epinephrine, dopamine, and sodium nitroprusside through mathematic simulations.
Keywords: dose response, hypertension, epinephrine, dopamine, nitroprusside, vasodilator
Introduction
Sodium Nitroprusside (SNP) is a nitric oxide releasing drug used in medicine to therapeutically decrease blood pressure in cases of malignant hypertension.1,2 Exogenous administration of nitric oxide elicits effects such as dilating both arterial and venous vessels and adverse effects such as inhibition of platelet aggregation potentially leading to prolonged bleeding in patients; however, despite these effects in SNP maintains high efficacy in the clinical setting.3,4 From a signaling perspective, NO released from an SNP infusion interacts predominately with iron and heme leading to the activation of soluble guanylyl cyclase (cGMP) forming protein kinase G.5 Following a series of reactions, the eventual outcome is a decrease calcium within the cytosol causing reduced smooth muscle contractions within the vasculature. As an endogenous substance, nitric oxide provides the body with antithrombotic, antiatherogenic, and vasodilating effects providing the body with tremendous latitude for the demands of daily life.3–5
The two other vasodilators, at low doses, presented in this paper, are the catecholamines dopamine and epinephrine. At low doses, epinephrine acts via the G-protein coupled β2-adrenergic (ADRB2) receptors causing vasodilation through mediation by Class C L-type calcium channels.6 There is much evidence describing polymorphisms associated with the ADRB2 gene potentially leading to varied clinical outcomes ranging from asthma, chronic obstructive pulmonary syndrome, labor pain and progress, to patient survival in patients receiving beta-blocker therapy after acute coronary syndrome.7–9 Despite these findings, low dose epinephrine stimulates the ADRB2 resulting in vasodilation, providing tremendous inotropic support post cardiac surgery while maintaining cerebral perfusion pressure, amongst other indications.
Dopamine, a precursor to both norepinephrine and epinephrine, stimulates the DA1 and DA2 receptors resulting in vasodilation at low doses.10 Just as with epinephrine, at higher doses, the alpha-adrenergic receptor mediated vasoconstriction predominates elimination the vasodilating actions of the lower doses of both catecholamines. In the catecholamine synthesis pathway, phenylalanine is converted to tyrosine and subsequently dihydroxyphenylalanine, commonly referred to as dopa, an agent able to cross the blood brain barrier.11,12 Further, in the conversion from Dopa to dopamine, Vitamin B6 is the necessary cofactor.13 Similarly, the conversion to dopamine to norepinephrine Vitamin C is the required cofactor. As with all reactions, deficiencies of these cofactors results in build-up of the precursor and decrease in the downstream product. The last conversion from norepinephrine to epinephrine requires S-Adenosyl methionine (SAM) as a cofactor where the formation of methionine is dependent on Vitamin B12, a cofactor, from the conversion from homocysteine to methionine.12,13 Thus, the common theme indicates that nutrition is of sincere importance along the catecholamine synthesis pathway and vitamin deficiencies along the conversion steps may lead to a build-up or depressed levels of dopa, dopamine, norepinephrine, or epinephrine. Moreover, a recent review article summarizes ascorbate's role as a cofactor for enzymes in the catecholamine synthesis pathway and recommends ascorbate-dependent vasopressor support in patients with severe sepsis and septic.14
Dopamine DA1 and DA2 receptors are found in the brain while higher receptor densities are located in the renal vasculature smooth muscle allowing for increased blood flow with low infusion doses of dopamine.15,16 Therefore, the drug effects of nitroprusside, epinephrine, and dopamine have varied mechanisms all allowing for dilation in the human vasculature. What are the equivalent doses allowing for decreasing vascular tone?
The aims of this paper is to use mathematical modeling of established dose-response parameter values to then conduct simulations to identify the equivalent doses of dopamine, epinephrine, and sodium nitroprusside to decrease the systemic vascular resistance by 20% and by 40%. I hypothesize that all three drugs would be capable of achieving a 20% and a 40% decrease in the change from SVR from resting values.
Methods
Literature-Based Data Source
Dose-response data are based from three publications evaluating the effects of epinephrine, dopamine, and sodium nitroprusside on systemic vascular resistance (SVR) as well as other hemodynamic variables.17–19 Results were originally reported in dynes*sec/cm5 and were scaled from the resting/baseline SVR to each respective dose response. Drug doses were all normalized to microgram per kilogram per minute infusion doses. As a result of similar dose ranges, nitroprusside and dopamine results are illustrated on the same dose-response curves; however, epinephrine was reported on a single dose-response curve. Lastly, box and whisker plots depicting the equivalent doses resulting in an approximate 20% decrease in SVR, for all three drugs, and an approximate 40% decrease in SVR for epinephrine and sodium nitroprusside.
PK/PD Modeling and Dose-Response Simulations
PK/PD modeling and simulations were conducted in the R programming language (version 3.2.2, The R Foundation for Statistical Computing, Vienna, Austria).20,21 Specifically, the dose-response simulations were conducted using the Multiple Comparisons Procedure-Modeling package in R.22 This modeling package has been validated by the European Medicines Agency (EMA) Committee for Medicinal Products for Human Use and is used during clinical trials.23 Based on the EMA qualification report, the committee determined the R package an efficient statistical tool for Phase II dose finding studies. The Multiple Comparisons Procedure allows for population dose-response testing and estimation of model uncertainties by enabling researchers to design candidate models in clinical trial design.22,24 Lastly, for all dose-response simulations, a sample size 100 virtual patients (n=100) were created, at each infusion dose.
Equation 1: Pharmacodynamic modeling equation describing the percent decrease in the systemic vascular resistance based on infusion doses of epinephrine, dopamine, or sodium nitroprusside.
Results
The dose-response simulations resulted in 1100 samples for dopamine, 1000 samples for epinephrine, and 1300 samples for nitroprusside. The infusion doses were normalized to mcg/kg/min with the corresponding SVR percent change, from baseline are reported in Table 1. The original Epinephrine dose-response values obtained from the Stratton et al18 in 1984 study that was conducted in 10 healthy participants. This study evaluated the hemodynamic outcomes following infusion of three different epinephrine doses (25, 50, and 100 ng/kg/min). For the original Sodium Nitroprusside data, the values are referenced from the Gerson et al 1982 study in twenty adult patients undergoing elective open heart surgery.17 The original study methods indicate that none of the patients received any drug for at least 11 hours prior to the study period. Therefore, the dose-response simulations are based on a patient population, rather than in healthy participants.
Table 1.
Normalized published Dose-Response data sources used for modeling and simulations. Systemic Vascular Resistance (SVR) responses represent % vasodilation
| Dopamine | Epinephrine | Sodium Nitroprusside | |||
|---|---|---|---|---|---|
| Elkayam et al. (2008) | Stratton et al. (1984) | Gerson et al. (1982) | |||
| Dose | Response | Dose | Response | Dose | Response |
| (mcg/kg/min) | (% SVR) | (mcg/kg/min) | (% SVR) | (mcg/kg/min) | (% SVR) |
| 0 | 100 | 0 | 100 | 0 | 100 |
| 1 | 83 | 0.025 | 69 | 1 | 94 |
| 2 | 82 | 0.050 | 58 | 1.5 | 87 |
| 3 | 81 | 0.100 | 52 | 2 | 82 |
| 10 | 77 | -- | -- | -- | -- |
As with the nitroprusside clinical population, the original dose-response values referenced for the Dopamine data were obtained from the Elkayam et al 2008 study in thirteen patients with a history of congestive heart failure (CHF).19 In these patients, the CHF was due to left ventricular systolic dysfunction with moderate to severe symptoms (New York Heart Association functional class III or IV) with left ventricular ejection fraction ranging from 14% to 32%.19 Moreover, the underlying cause of CHF was coronary artery disease (n=5) and non-ischemic dilated cardiomyopathy (n=8).19 For the original dopamine study, all thirteen patients were treated with diuretics, ten where on ACE inhibitors (n=10), nine were medicated with digoxin (n=9), seven were on beta-blockers (n=7), and lastly, seven were treated with organic nitrates (n=7).19 Therefore, to summarize, healthy participants were evaluated in the epinephrine study, while both the dopamine and nitroprusside dose-response data were collected in patient with cardiovascular conditions.17–19
The R programming language scripts was written creating the resulting model parameters and model diagnostics Akaike Information Criteria (AIC) and fitted Log-likelihood. The results are provided in Table 2. Overall, the Emax model equation adequately described the dose-response data from the overall published studies. The best Log-likelihood fit in decreasing order are: epinephrine, dopamine, and nitroprusside, respectively. The maximum decrease in the percent SVR model parameter (SVRmax) resulted in a very wide standard error for nitroprusside; this finding clinically makes sense due to the powerful vasodilating effects of the drug. At doses less than 2mcg/kg/min dopamine and nitroprusside exhibited a near parallel linear curve, however, at around that 2mcg/kg/min, the population dose-response simulations diverged resulting in dopamine's vasodilation effects ending near 10mcg/kg/min.
Table 2. Calculated Dose-Response modeling parameters and diagnostics.
| Dopamine | Epinephrine | Sodium Nitroprusside | |
|---|---|---|---|
| SVRmax (%) (S.E.) | -22.894 (2.037) | -59.0303 (2.75) | -52.2 (120.54) |
| SVR0 (%) (S.E.) | 99.954 (1.384) | 100.0420 (1.14) | 101.3 (3.89) |
| ID50 (mcg/kg/min) (S.E.) | 0.432 (0.177) | 0.0218 (0.0034) | 4.0 (13.39) |
| Akaike Information Criterion | 20.8632 | 24.6809 | 14.8374 |
| Fitted Log-likelihood | -6.4316 | -3.4187 | -8.3405 |
Figure 1 provides the dose response simulation results for both dopamine and sodium nitroprusside infusions to systemic vascular resistance. There is a clear dose dependent decrease in SVR as epinephrine is infused at doses lower than 0.010mcg/kg/min. It is important to note that the epinephrine dose-response model parameters are valid for doses at or below 0.010mcg/kg/min. Higher simulation doses were attempted and, as expected, an increase in the %SVR was seen with wider shaded confidence interval bands. Therefore, due to the predominate alpha-adrenergic receptor effects at higher epinephrine infusion doses, the mathematical model becomes unstable and is therefore recommended for infusion doses of less than 0.10 mcg/kg/min. However, despite the higher dose simulation findings, the mathematical model allowed for appropriate hypothesis testing and addressing the aims of identify doses resulting in a 20% and 40% decrease in systemic vascular resistance.
Figure 1.

Dose-response simulation results comparing the effect of Dopamine and Sodium Nitroprusside on the percent change, from baseline, of systemic vascular resistance. The solid line describes the population mean while the shaded error bands represent the 95% confidence intervals.
Based on the mathematical simulations, the doses resulting in an equivalent infusion response leading to decreased systemic vascular resistance, by 20-25%, is 5mcg/kg/min for dopamine, 0.015mcg/kg/min for epinephrine, and lastly 2.5mcg/kg/min for sodium nitroprusside. These results are based on the median simulated population response rate and variations with infusion outcomes would potentially be associated with gender and/or genotype status of the Catechol-O-methyltransferase (COMT) and/or Monoamine oxidase (MAO) enzymes when using dopamine or epinephrine.25 Due to the effect on cardiac output by epinephrine and dopamine, cases necessitating maintenance of cerebral perfusion pressure, these two catecholamines are therapeutic alternative vasodilators to sodium nitroprusside.
After analyzing the response rates of the dopamine infusion simulations, the results suggest that the alpha-1 receptor vasoconstriction effects at around 10mcg/kg/min. Therefore, in addressing the second aim, the equivalent dose resulting in a 40% decrease in SVR from baseline is 0.040mcg/kg/min of epinephrine and a maximum infusion dose of 10mcg/kg/min for sodium nitroprusside. As with any drug, population outliers are evident and therefore, careful titration of these aforementioned infusion doses will be critical due in the extreme and rare instances when a 40% drop of SVR from baseline may be desired by the clinical staff. The dopamine simulation dose findings are consistent with the package insert for nitroprusside indicating a maximum infusion dose at 10mcg/kg/min.26
Discussion
This paper described the dose-matched vasodilating properties of epinephrine, dopamine, and sodium nitroprusside based on dose-response simulations. These three drugs, all allowing for the same outcome variable of a decrease in systemic vascular resistance all work with various mechanisms allowing for the same response. These principles highlight the role that endogenous nitric oxide, epinephrine, and dopamine play in maintaining vascular tone and further suggest the possibility to investigate a multi-mechanism approach for drug development to combat treatment resistant hypertension.
Understanding the mechanisms underlying essential hypertension has been an investigated and treatments proposed for several decades using various medical and surgical approaches. The first measurement of blood pressure was accomplished in 1733 by Stephen Hales, an English clergyman27 and has remained key pillar of human health since that time. In the 1930s, nearly 200 years later from the first blood pressure measurement, a common approach to management of hypertension was renal sympathectomy,28 the approach of surgically denervating the kidney. Other approaches at the time were inducing high fevers in patients by using the typhoid bacilli or malaria related substances.29,30 Since then, pharmacological approaches have been quite successful, however, in select patients even individual combination anti-hypertensive medications are of no help. These patients are classified as having essential hypertension and often suffer from the deleterious effects on various organs of the body due to chronically high blood pressures.
Mathematically, that the change in pressure equals the blood flow multiplied by the vascular resistance, all dependent on the vasodilatory capacity of the vessel and are organ-dependent. The brain and kidney have a low vasodilatory capacity and therefore have low vascular tone. In contrast, the heart and skeletal muscle are capable of high vasodilatory capacity resulting in high vascular tone. Thus, the modulation of resting vascular tone varies depending on the organ of interest, which in turn, is influenced by both endogenous substances (i.e. nitric oxide, epinephrine, etc.) and is regulated by sympathetic neurons located in the medulla regulating changes in vascular diameter.
Endogenous and exogenous substances, emotional well-being, environmental influences, and lifestyle factors all result in competing mechanisms resulting in essential hypertension. In this paper, the primary summarized dependent variable was systemic vascular resistance relative to the drug dosing (independent variable). As stated by the EMA report, there is a clear role for the mathematical simulations throughout the drug development and dose-finding process. This exercise clearly exhibited the cost-savings involved using interdisciplinary skills in computer programming, mathematics, and medicine to make recommendations for drug doses. The results are not supporting any of the three vasodilators over another; however, there are clear physiologic benefits to choosing any of the tree vasodilators in the appropriate clinical indication.
Based on the results, the hypothesis-testing results indicate that clearly epinephrine, dopamine, and sodium nitroprusside are able decrease SVR by 20%. However, due to the increased alpha-1 vasoconstriction effects of dopamine near 10mcg/kg/min, a 40% decrease in SVR was not evident based on the dose-response simulations. Rather, a 0.040mcg/kg/min infusion of epinephrine provided the dose-response equivalence results to a 10mcg/kg/min of nitroprusside.
Conclusion
Equivalent pharmacodynamic SVR responses have been identified for epinephrine, dopamine, and sodium nitroprusside using the multiple comparisons procedure-modeling approach. Based on the simulations, low-dose epinephrine or low-dose dopamine are may provide clinicians without access to nitroprusside with more options to decrease patients' SVR and maintain adequate cerebral perfusion pressure, for the appropriate clinical indications.
Figure 2.
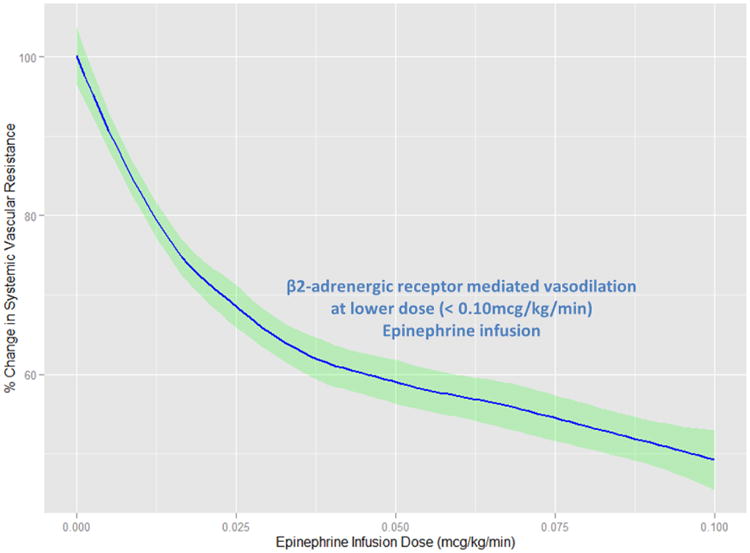
Dose-response simulation results comparing the effect of epinephrine on the percent change, from baseline, of systemic vascular resistance. The solid line describes the population mean while the shaded error bands represent the 95% confidence intervals.
Figure 3.

Approximate equivalent doses resulting in a 20-25% decrease in systemic vascular resistance for Dopamine (5mcg/kg/min), Epinephrine (0.015mcg/kg/min), and Sodium Nitroprusside (2.5mcg/kg/min). The box plots represent 25%, 50% (median), and 75th percentile values, while the whisker lines represent the 10th and 90th percentile results. The dots represent the population outliers.
Figure 4.

Approximate equivalent doses resulting in nearly a 40% decrease in systemic vascular resistance for Epinephrine (0.040mcg/kg/min) and for infusions of Sodium Nitroprusside (10mcg/kg/min). The box plots represent 25%, 50% (median), and 75th percentile values, while the whisker lines represent the 10th and 90th percentile results. The dots represent the population outliers.
Acknowledgments
Funding: Research reported in this publication was supported by National Institute of General Medical Sciences of the National Institutes of Health under award number T32 GM008685.
Abbreviations
- SVR0
effect at baseline dose
- SVRmax
dose resulting in maximum effect
- ED50
50% effective dose
- SVR
systemic vascular resistance
Footnotes
Conflicts of Interests: The author declares no conflict of interests.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Friederich JA, Butterworth JF. Sodium nitroprusside: twenty years and counting. Anesth Analg [Internet] 1995;81(1):152–62. doi: 10.1097/00000539-199507000-00031. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7598246. [DOI] [PubMed] [Google Scholar]
- 2.Immink RV, van den Born BJH, van Montfrans GA, Kim YS, Hollmann MW, van Lieshout JJ. Cerebral hemodynamics during treatment with sodium nitroprusside versus labetalol in malignant hypertension. Hypertension [Internet] 2008;52(2):236–40. doi: 10.1161/HYPERTENSIONAHA.108.110395. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18606905. [DOI] [PubMed] [Google Scholar]
- 3.Saxon a, Kattlove HE. Platelet inhibiton by sodium nitroprusside, a smooth muscle inhibitor. Blood [Internet] 1976;47(6):957–61. Available from: http://www.ncbi.nlm.nih.gov/pubmed/1276477. [PubMed] [Google Scholar]
- 4.Parrish PR, Larson DF. Pharmacological effect of nitroprusside on platelet aggregation. J Extra Corpor Technol [Internet] 1999;31(4):162–8. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med4&NEWS=N&AN=10915472. [PubMed] [Google Scholar]
- 5.Karaki H, Sato K, Ozaki H, Murakami K. Effects of sodium nitroprusside on cytosolic calcium level in vascular smooth muscle. Eur J Pharmacol [Internet] 1988;156(2):259–66. doi: 10.1016/0014-2999(88)90329-9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/3240769. [DOI] [PubMed] [Google Scholar]
- 6.Shen B, Cheng KT, Leung YK, Kwok YC, Kwan HY, Wong CO, et al. Epinephrine-induced Ca2+ influx in vascular endothelial cells is mediated by CNGA2 channels. J Mol Cell Cardiol [Internet] 2008;45(3):437–45. doi: 10.1016/j.yjmcc.2008.06.005. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18621055. [DOI] [PubMed] [Google Scholar]
- 7.Reitman E, Conell-Price J, Evansmith J, Olson L, Drosinos S, Jasper N, et al. β2-adrenergic receptor genotype and other variables that contribute to labor pain and progress. Anesthesiology [Internet] 2011;114(4):927–39. doi: 10.1097/ALN.0b013e318211004e. Available from: /pmc/articles/PMC3063327/?report=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lanfear DE, Jones PG, Marsh S, Cresci S, McLeod HL, Spertus JA. Beta2-adrenergic receptor genotype and survival among patients receiving beta-blocker therapy after an acute coronary syndrome. JAMA [Internet] 2005;294(12):1526–33. doi: 10.1001/jama.294.12.1526. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16189366. [DOI] [PubMed] [Google Scholar]
- 9.Thomsen M, Nordestgaard BG, Sethi aa, Tybjærg-Hansen A, Dahl M. β2-adrenergic receptor polymorphisms, asthma and COPD: two large population-based studies. Eur Respir J [Internet] 2012;39(3):558–66. doi: 10.1183/09031936.00023511. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22075484. [DOI] [PubMed] [Google Scholar]
- 10.Overgaard CB, Dzavík V. Inotropes and vasopressors: review of physiology and clinical use in cardiovascular disease. [cited 2015 Aug 14];Circulation [Internet] 2008 Sep 2;118(10):1047–56. doi: 10.1161/CIRCULATIONAHA.107.728840. Available from: http://circ.ahajournals.org/content/118/10/1047.full. [DOI] [PubMed] [Google Scholar]
- 11.Molinoff PB, Axelrod J. Biochemistry of catecholamines. [cited 2015 Dec 18];Annu Rev Biochem [Internet] 1971 Jan;40:465–500. doi: 10.1146/annurev.bi.40.070171.002341. Available from: http://www.ncbi.nlm.nih.gov/pubmed/4399447. [DOI] [PubMed] [Google Scholar]
- 12.Kruk ZL. Neurotransmitters and Drugs [Internet] Springer; 2014. [cited 2015 Dec 18]. p. 205. Available from: https://books.google.com/books?id=IkLUBwAAQBAJ&pgis=1. [Google Scholar]
- 13.Barile FA. Clinical Toxicology: Principles and Mechanisms. Second. CRC Press; 2010. [cited 2015 Dec 18]. p. 482. Internet. Available from: https://books.google.com/books?id=CJPLBQAAQBAJ&pgis=1. [Google Scholar]
- 14.Carr AC, Shaw GM, Fowler AA, Natarajan R. Crit Care [Internet] 1. Vol. 19. BioMed Central Ltd; 2015. Jan 27, [cited 2015 Dec 3]. Ascorbate-dependent vasopressor synthesis: a rationale for vitamin C administration in severe sepsis and septic shock? p. 418. Available from: http://ccforum.com/content/19/1/418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jose PA, Raymond JR, Bates MD, Aperia A, Felder RA, Carey RM. The renal dopamine receptors. [cited 2015 Dec 18];J Am Soc Nephrol [Internet] 1992 Feb;2(8):1265–78. doi: 10.1681/ASN.V281265. Available from: http://www.ncbi.nlm.nih.gov/pubmed/1627751. [DOI] [PubMed] [Google Scholar]
- 16.Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins; 2001. [cited 2015 Dec 18]. p. 957.p. 2477. Internet. Available from: https://books.google.com/books?id=FVfzRvaucq8C&pgis=1. [Google Scholar]
- 17.Gerson JI, Allen FB, Seltzer JL, Parker FB, Markowitz AH. Arterial and Venous Dilation by Nitroprusside and Nitroglycerin—Is There a Difference? Anesth Analg [Internet] 1982;61(3):256–60. Available from: http://www.anesthesia-analgesia.org/content/61/3/256.abstract. [PubMed] [Google Scholar]
- 18.Stratton JR, Pfeifer MA, Ritchie JL, Halter JB. Hemodynamic effects of epinephrine: concentration-effect study in humans. [cited 2015 Aug 3];J Appl Physiol [Internet] 1985 Apr;58(4):1199–206. doi: 10.1152/jappl.1985.58.4.1199. Available from: http://www.ncbi.nlm.nih.gov/pubmed/3988675. [DOI] [PubMed] [Google Scholar]
- 19.Elkayam U, Ng TMH, Hatamizadeh P, Janmohamed M, Mehra A. Renal Vasodilatory Action of Dopamine in Patients With Heart Failure: Magnitude of Effect and Site of Action. [cited 2015 Aug 3];Circulation [Internet] 2008 Jan 15;117(2):200–5. doi: 10.1161/CIRCULATIONAHA.107.737106. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18172028. [DOI] [PubMed] [Google Scholar]
- 20.R Core Team. R: A Language and Environment for Statistical Computing [Internet] Vienna, Austria: R Foundation for Statistical Computing; 2015. Available from: https://www.r-project.org/ [Google Scholar]
- 21.Team R. R Development Core Team. R A Lang Environ Stat Comput [Internet] 2013 Available from: http://www.mendeley.com/research/r-language-environment-statistical-computing-96/\npapers2://publication/uuid/A1207DAB-22D3-4A04-82FB-D4DD5AD57C28.
- 22.Bornkamp B, Pinheiro J, Bretz F. MCPMod: An R package for the design and analysis of dose-finding studies. J Stat Softw [Internet] 2009;29(7):1–23. Available from: http://cran.uvigo.es/web/packages/MCPMod/vignettes/MCPMod.pdf. [Google Scholar]
- 23.European Medicines Agency Committee for Medicinal Products for Human Use (CHMP) Qualification Opinion of MCP-Mod as an efficient statistical methodology for model-based design and analysis of Phase II dose finding studies under model uncertainty [Internet] London E14 4 HB, United Kingdom: 2014. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_guideline/2014/02/WC500161027.pdf. [Google Scholar]
- 24.Verrier D, Sivapregassam S, Solente AC. Dose-finding studies, MCP-Mod, model selection, and model averaging: Two applications in the real world. Clin Trials [Internet] 2014;11(4):476–84. doi: 10.1177/1740774514532723. Available from: http://ctj.sagepub.com/cgi/doi/10.1177/1740774514532723\nhttp://ctj.sagepub.com/content/11/4/476.full.pdf. [DOI] [PubMed] [Google Scholar]
- 25.Ghimire LV, Kohli U, Li C, Sofowora GG, Muszkat M, Friedman Ea, et al. Catecholamine pathway gene variation is associated with norepinephrine and epinephrine concentrations at rest and after exercise. Pharmacogenet Genomics. 2012;22(4):254–60. doi: 10.1097/FPC.0b013e328350a274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hospira. Product Label - NITROPRESS- sodium nitroprusside injection, solution, concentrate [Internet] DailyMed - U.S. National Library of Medicine; 2014. [cited 2015 Sep 11]. Available from: http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6a44bcac-a0e1-4069-5691-db7b83dbb4b7. [Google Scholar]
- 27.Lewis O. Stephen Hales and the measurement of blood pressure. [cited 2015 Jun 10];J Hum Hypertens [Internet] 1994 Dec;8(12):865–71. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7884783. [PubMed] [Google Scholar]
- 28.Harris SH. Renal Sympathectomy: Its Scope and Limitations: (Section of Urology) [cited 2015 Aug 3];Proc R Soc Med [Internet] 1935 Sep;28(11):1497–510. doi: 10.1177/003591573502801105. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2205239&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Page IH, Taylor RD. Pyrogens in the treatment of malignant hypertension. [cited 2015 Aug 3];Mod Concepts Cardiovasc Dis [Internet] 1949 Oct;18(10):51. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18142381. [PubMed] [Google Scholar]
- 30.Freis ED, Wilkins RW. Effect of pentaquine in patients with hypertension. [cited 2015 Aug 3];Proc Soc Exp Biol Med [Internet] 1947 Apr;64(4):455–8. doi: 10.3181/00379727-64-15829. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20239457. [DOI] [PubMed] [Google Scholar]


