Abstract
Background
Drusen are amorphous yellowish deposits beneath the sensory retina. People with drusen, particularly large drusen, are at higher risk of developing age‐related macular degeneration (AMD). The most common complication in AMD is choroidal neovascularisation (CNV), the growth of new blood vessels in the centre of the macula. The risk of CNV is higher among people who are already affected by CNV in one eye.
It has been observed clinically that laser photocoagulation of drusen leads to their disappearance and may prevent the occurrence of advanced disease (CNV or geographic atrophy) associated with visual loss.
Objectives
To examine the effectiveness and adverse effects of laser photocoagulation of drusen in AMD.
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (2015, Issue 7), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to August 2015), EMBASE (January 1980 to August 2015), Latin American and Caribbean Health Sciences Literature Database (LILACS) (January 1982 to August 2015), the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 3 August 2015.
Selection criteria
Randomised controlled trials (RCTs) of laser treatment of drusen in AMD in which laser treatment had been compared with no intervention or sham treatment. Two types of trials were included. Some trials studied one eye of each participant (unilateral studies); other studies recruited participants with bilateral drusen and randomised one eye to photocoagulation or control and the fellow eye to the other group.
Data collection and analysis
Two review authors independently selected studies and extracted data. We pooled data from unilateral and bilateral studies using a random‐effects model. For the bilateral studies, we estimated the within‐person correlation coefficient from one study and assumed it was valid for the others.
Main results
The update of this review found two additional studies, totaling 11 studies that randomised 2159 participants (3580 eyes) and followed them up to two years, of which six studies (1454 participants) included people with one eye randomised to treatment and one to control. Studies were conducted in Australia, Europe and North America.
Overall, the risk of bias in the included studies was low, particularly for the larger studies and for the primary outcome development of CNV. Photocoagulation did not reduce the development of CNV at two years' follow‐up (odds ratio (OR) 1.07, 95% confidence interval (CI) 0.79 to 1.46, 11 studies, 2159 participants (3580 eyes), high quality evidence). This estimate means that, given an overall occurrence of CNV of 8.3% in the control group, we estimated an absolute risk reduction by no more than 1.4% in the laser group, according to the lower CI limit. Only two studies investigated the effect on the development of geographic atrophy and could not show a difference, but estimates were imprecise (OR 1.30, 95% CI 0.38 to 4.51, two studies, 148 participants (148 eyes), low quality evidence).
Among secondary outcomes, photocoagulation led to drusen reduction (OR 9.16, 95% CI 6.28 to 13.4, three studies, 570 participants (944 eyes), high quality evidence) but was not shown to limit loss of 3 or more lines of visual acuity (OR 0.99, 95% CI 0.81 to 1.22, nine studies, 2002 participants (2386 eyes), moderate quality evidence).
In a subgroup analysis, no difference could be shown for conventional visible (eight studies) versus subthreshold invisible (four studies) photocoagulation for the primary outcomes (P value = 0.29). The effect in the subthreshold group did not suggest a relevant benefit (OR 1.27, 95% CI 0.82 to 1.98). No study used micropulse subthreshold photocoagulation.
No other adverse effects (apart from development of CNV, geographic atrophy or visual loss) were reported.
Authors' conclusions
The trials included in this review confirm the clinical observation that laser photocoagulation of drusen leads to their disappearance. However, treatment does not result in a reduction in the risk of developing CNV, and was not shown to limit the occurrence of geographic atrophy or visual acuity loss.
Ongoing studies are being conducted to assess whether the use of extremely short laser pulses (i.e. nanosecond laser treatment) cannot only lead to drusen regression but also prevent neovascular AMD.
Plain language summary
Laser treatment of drusen to prevent progression to advanced age‐related macular degeneration
Review question We reviewed the evidence about the effect of laser treatment of the centre of the retina in people with macular drusen to prevent the occurrence of the more advanced type of age‐related macular degeneration (AMD).
Background Drusen are yellowish deposits that can be seen in the macula (the centre of the retina) in a larger proportion of people as they get older. People with drusen, particularly extensive large drusen, are at higher risk of developing AMD. The most common complications in AMD are the growth of new blood vessels in the centre of the macula (called choroidal neovascularisation (CNV), also known as 'wet AMD') and loss of retinal cells or photoreceptors in the macula (called geographic atrophy). It has been observed clinically that making very small burns around the macula with laser light (laser photocoagulation) makes drusen disappear. Laser photocoagulation of drusen has thus been proposed as a way to prevent the development of CNV and geographic atrophy. More recently, subthreshold photocoagulation has been used to cause invisible laser burns and achieve drusen reduction with less damage to the retinal structure.
Search date The evidence is current to 3 August 2015.
Study characteristics This review included data from 11 trials conducted in Australia, Europe and North America. The studies followed up 2159 participants with drusen (3580 eyes) to two years, of which six studies (1454 participants) included people with one eye randomised to treatment and one to control. Four studies (850 eyes) used subthreshold photocoagulation.
Study funding sources Three out of four studies using laser subthreshold photocoagulation were sponsored by the laser producer.
Key results These studies showed that laser photocoagulation of drusen leads to their disappearance. However, laser photocoagulation of drusen did not reduce the risk of developing CNV, which was about 10% at three years in untreated participants. A smaller number of studies reported on the development of geographic atrophy, that is, atrophy in the centre of the macula, but these studies were inconclusive and the effect of laser treatment of drusen on the development of geographic atrophy was uncertain. The risk of visual loss was similar in treated and untreated groups. There was no suggestion that a benefit may exist with subthreshold photocoagulation.
Quality of the evidence The overall quality of the evidence was high regarding failure to prevent CNV, but it was low for prevention of atrophy due to the small number of participants for whom this outcome was assessed.
Summary of findings
Summary of findings for the main comparison. Summary of findings table: photocoagulation of drusen versus control.
| Outcomes at two years | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (eyes, studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Control | Photocoagulation | ||||
| Development of CNV | 83 per 1000 | 89 per 1000 (67 to 117) | OR 1.07 (0.79 to 1.46) | 2159 (3580 eyes, 11 studies) | ⊕⊕⊕⊕ high1 |
| Low risk population (people with bilateral drusen) | |||||
| 50 per 1000 | 54 per 1000 (40 to 73) | ||||
| High risk population (people with CNV in the fellow eye) | |||||
| 250 per 1000 | 268 per 1000 (199 to 365) | ||||
| Development of geographic atrophy | 66 per 1000 | 84 per 1000 (26 to 241) | OR 1.3 (0.38 to 4.51) | 148 (148 eyes, 2 studies) | ⊕⊕⊝⊝ low2 |
| Visual loss of 2‐3+ lines of visual acuity | 150 per 1000 | 149 per 1000 (122 to 183) | OR 0.99 (0.81 to 1.22) | 2002 (3486 eyes, 9 studies) | ⊕⊕⊕⊝ moderate3 |
| Loss of ≥ 0.3 log units of contrast sensitivity | 119 per 1000 | 100 per 1000 (26 to 309) | OR 0.82 (0.20 to 3.31) | 82 (82 eyes, 1 study) | ⊕⊕⊝⊝ low2 |
| Reading speed in words/minute | The mean reading speed in words/minute in the control groups was 100 words/minute | The mean reading speed in words/minute in the intervention groups was 12.5 lower (7.2 lower to 32.2 higher) | ‐ | 44 (44 eyes, 1 study) | ⊕⊕⊝⊝ low2 |
| Drusen reduction | 107 per 1000 | 522 per 1000 (428 to 614) | OR 9.16 (6.28 to 13.4) | 570 (944 eyes, 3 studies) | ⊕⊕⊕⊕ high4,5 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CNV: choroidal neovascularisation; OR: odds ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1Allocation sequence generation and allocation concealment and masking of visual acuity outcome assessors was achieved in half or less of them; however, the larger studies in this meta‐analysis were good quality. Other quality items were not a problem for the primary outcome (no downgrade). 2Small study yielding wide 95% confidence intervals (‐2 for imprecision). 3Visual acuity examiners were masked in less than half of studies (‐1 for risk of bias). 4The three studies included in this analysis had low risk of bias (no downgrade). 5Estimates were heterogeneous but they both suggest a strong effect (no downgrade).
Background
Description of the condition
Age‐related macular degeneration (AMD) is the leading cause of vision loss in industrialised countries (Klein 2004; Vingerling 1996). Early AMD is characterised by focal or diffuse depositing of extracellular material between the retinal pigment epithelium (RPE) and Bruch's membrane, forming drusen (focal deposits) or basal laminar deposits (diffuse deposits) (Bressler 1994; Sarks 1999; Young 1987). This process is associated with progressive degeneration of PRE and photoreceptor cells (Guidry 2002; Phipps 2003; Young 1987). Advanced AMD is characterised by the development of geographic atrophy (characterising the non‐exudative AMD form) or choroidal neovascularisation (CNV; characterising the exudative AMD). When the fovea, which represents the centre of vision, is involved by atrophic or exudative manifestations of AMD, a severe visual loss results.
Advanced AMD has a prevalence that increases markedly with age (EDPRG 2004; Owen 2003). In the US, advanced AMD prevalence is 3.5% in white men and 4% in white women at 75 to 79 years (EDPRG 2004). Based on one systematic review of UK studies, Owen 2003 reported that there were approximately 214,000 people with visual impairment caused by AMD. In this study, the ratio of neovascular AMD to geographic atrophy was about 2 : 1, such as in Smith 2001. Ten years later, Owen 2012 estimated 513.000 prevalent cases of late AMD, of which 263,000 were neovascular AMD. Rudnicka 2015 conducted a systematic review and estimated 293.000 incident cases of late AMD each year in the U.S.A.
Drusen results from deposition of the photoreceptors debris, which are composed of lipofuscin and membranous deposits. Drusen appear when sufficient material has been deposited, clinically characterised by amorphous yellowish deposits beneath the sensory retina. Four main types of drusen can be detected in the retina. Hard drusen are discrete, yellow, nodular deposits, smaller than 50 μm in diameter. Basal laminar drusen are tiny, whitish, multiple deposits with a 'starry night' appearance. Soft drusen are yellowish deposits with poorly defined margins, tending to coalesce, and are usually larger than 50 μm. Crystalline drusen are discrete, calcific, refractile deposits. Drusen characteristics associated with a high risk of progression to exudative AMD (high‐risk drusen) include: soft drusen, more than five, larger size (greater than 63 μm), drusen confluence and associated hyperpigmentation.
The cumulative incidence of new exudative or atrophic lesions in eyes initially free of advanced AMD has been estimated as 8.6% at one year, 16.4% at two years and 23.5% at three years (Holz 1994). Focusing on the CNV incidence, the results of a prospective investigation of people with exudative manifestation in one eye and drusen in the fellow eye has shown that the risk of developing CNV peaks at four years, dissipating thereafter, whereas there is a slightly increased incidence of geographic atrophy in the longer term (Sarraf 1999). Moreover, the five‐year risk of CNV occurrence in the fellow eye of people who have already experienced CNV in the first eye, varies from 7% to 87% depending on the co‐existence of four main risk factors (presence of five or more drusen, focal hyperpigmentation, one or more large drusen and systemic hypertension) (MPSG 1997).
Drusen can spontaneously disappear in people with AMD, generally leaving atrophic lesions. More specifically, the Waterman study has reported that disappearance of large drusen occurred in 16/47 (34%) participants in one five‐year longitudinal study (Bressler 1995).
Description of the intervention
Laser treatment is based on the release of a powerful beam of light that, combined with ophthalmic equipment and lenses, can be precisely focused on the retina to treat some diseases. The laser energy causes a certain amount of controlled damage to the tissues in order to obtain the desired effect. Small laser burns are usually employed to obliterate or destroy abnormal blood vessels or other lesions in the eye.
Several observers noted that laser application can lead to drusen being resorbed in the macular area (Cleasby 1979; Gass 1973; Gross‐Jendroska 1998; Wetzig 1994). Owing to the risk of vision loss associated with the presence of high‐risk drusen, laser application was proposed as an intervention to prevent progression to advanced AMD. Laser burns are applied to the retina, either directly to the drusen or following pre‐defined patterns. Argon, krypton, dye or diode lasers have been used with varying levels of energy (achieving from not visible to faint or intense whitish retinal lesions). The spot size used varies between 100 and 200 μm and number of spots from less than 10 to nearly 300.
Subthreshold laser therapy is a more recent application of laser energy to retinal diseases. The theoretical advantage of subthreshold laser is based on minimising the damage to the retinal tissue by ensuring the energy spreads as little as possible. This aim can be achieved by reducing the duration of laser exposure and operates with a sub‐visible clinical endpoint. As a result, no laser scar is produced in the retina, obtaining at the same time equivalent clinical effects of conventional laser treatment (Sivaprasad 2010). Several studies have shown that subthreshold laser photocoagulation can be a viable option for several disorders, including diabetic macular oedema (Lavinsky 2011; Luttrull 2012), macular oedema secondary to branch retinal vein occlusion (Parodi 2006; Parodi 2008), and macular drusen. Subthreshold photocoagulation encompasses a range of techniques, among which micropulse subthreshold photocoagulation delivers light energy with very short impulses that are absorbed by the RPE only, aiming to spare the neurosensory retina.
How the intervention might work
The mechanisms of laser‐induced drusen regression are only speculative. Laser treatment may lead to an increased clearance of debris by choroidal phagocytic cells or macrophages. Alternatively, laser application may improve egress of drusen material through a thinner or more permeable Bruch's membrane, with the consequent reduction of its outflow resistance. Laser effect may manifest by triggering retinal pigment epithelial proliferation leading to the production and release of cytokines and growth factors. These molecules may be able to modify the biochemical process underlying the clinical manifestations of the retinal disorder, rather than simply destroying drusen, and may also act on the drusen remote from the site of the laser energy application (Abdelsalam 1999; Frennesson 1998; Pauleikhoff 1990a; Pauleikhoff 1990b). Histopathological examinations in animal models have shown that phagocytic cells, probably derived from choriocapillaris pericytes, can remove drusenoid material after laser photocoagulation (Duvall 1985). Protrusion of choroidal endothelial cell processes into Bruch's membrane are enhanced by laser photocoagulation and may play a part in the clearance of debris from the Bruch's membrane (Guymer 2001).
Micropulse laser delivery tries to achieve photostimulation rather than photocoagulation (Luttrull 2012). However, the mechanism of photocoagulation itself is poorly known, as reported above, thus we also include micropulse photocoagulation in this review. Picosecond laser treatment has been attempted to achieve drusen reduction and prevent CNV, a treatment that was defined "retinal rejuvenation therapy (2RT)" (Guymer 2014). Using very short laser pulses (3 ns), an insult caused by steam production around melanosomes can be confined to the RPE inducing a highly selective and discrete non‐thermal injury. It has been hypothesised that a 3‐ns laser could induce migration of RPE cells and release of matrix metalloproteinases, improving the hydraulic conductivity of Bruch's membrane. The hope is to achieve a prophylactic treatment of early AMD without the potential harmful effects seen with traditional thermal lasers.
Why it is important to do this review
AMD is a major public health problem in developed economies where the life expectancy is greatest (but of no significance in poorer countries with a life expectancy of under 65 years of age). Several investigations about health‐related and vision‐targeted features have shown that AMD is associated with decreased quality of life (Brown 2006; Chakravarthy 2005; Hassell 2006; Maguire 2004; Mangione 1999).
Although people with drusen experience few visual symptoms, once advanced AMD is present, visual loss is generally irreversible. It has been shown that people with drusen who take antioxidant supplementation are less likely to lose 15 or more letters of visual acuity over the follow‐up (AREDS 2001), even though this benefit was considered modest in people with moderate to severe signs of the disease (Evans 2012). Antioxidants have not been shown beneficial in the primary prevention of AMD (Chong 2007). Thus, the identification of a prophylactic treatment able to reduce the complications related to AMD may be highly beneficial.
Laser treatment of drusen appeared to provide positive results in observational studies (Cleasby 1979; Gass 1973; Gross‐Jendroska 1998; Sigelman 1991; Wetzig 1994). A systematic review is necessary to ensure that all the evidence on this intervention is considered objectively. People with AMD and their carers need to have recommendations as to the possible benefits or harms of this intervention.
Objectives
To examine the effectiveness and adverse effects of laser photocoagulation of drusen in AMD.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomised controlled trials (RCTs) of laser treatment of drusen in AMD.
Types of participants
People with retinal drusen associated with AMD in one or both eyes.
Types of interventions
We included trials in which laser treatment for retinal drusen was compared with no intervention or sham treatment. We considered a variety of different laser sources and photocoagulation techniques.
Types of outcome measures
Primary outcomes
Progression of AMD as measured by the development of CNV.
Progression of AMD as measured by the development of geographic atrophy.
Secondary outcomes
Loss of visual acuity (LogMAR (logarithm of the Minimum Angle of Resolution) values);
Changes in contrast sensitivity;
Drusen reduction;
Changes in reading ability;
Vision‐related quality of life.
Visual acuity is generally measured using a standard chart, the ETDRS (Early Treatment of Diabetic Retinopathy Study) chart and scored in letters. There are five letters per line in this chart. We extracted both dichotomous outcomes, such as moderate (3 lines or 15 ETDRS letters) and severe (6 lines or 30 ETDRS letters) visual loss and continuous measures (mean visual acuity) when possible. Whenever no ETDRS values were used, we converted visual acuity to LogMAR for pooling data.
Contrast sensitivity is generally measured with the Pelli‐Robson chart. Scores are collected in letters or as logarithm of contrast sensitivity. We used the logarithm of contrast sensitivity for pooling data when feasible. We extracted both continuous and dichotomous measures if possible. For dichotomous data, we recorded the proportion of participants with a change of at least 0.3 (corresponding to a two‐fold change) or 0.6 log‐units (corresponding to a four‐fold change).
In the protocol, we planned to evaluate drusen reduction considering the number of eyes showing at least a 50% reduction of drusen area from the baseline aspect. However, data were sparsely reported and, therefore, we modified the protocol to allow an extraction based on the investigators' definition.
We converted reading ability measures to LogMAR for reading acuity, whereas we considered reading speed as the logarithm of the number of words read in one minute.
Timing of outcome assessment
We assessed outcomes at 24 months, where data were available.
Adverse effects
We recorded adverse effects as documented in the included trials but noted that the main complication of laser was visual loss, especially due to CNV, which is considered under Primary outcomes and Secondary outcomes.
Microperimetry could be used to measure retinal sensitivity in laser‐treated perimacular areas in future studies, especially if the aim is to document the presumed absence of damage expected with subthreshold micropulse or nanosecond laser treatment. Thus, we will extract such measure if available in future updates.
Search methods for identification of studies
Electronic searches
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (2015, Issue 7), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to August 2015), EMBASE (January 1980 to August 2015), Latin American and Caribbean Health Sciences Literature Database (LILACS) (January 1982 to August 2015), the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 3 August 2015.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), EMBASE (Appendix 3), LILACS (Appendix 4), ISRCTN (Appendix 5), ClinicalTrials.gov (Appendix 6), and the ICTRP (Appendix 7).
Searching other resources
We searched the reference lists of retrieved articles for details of further relevant studies. We did not handsearch journals or conference proceedings specifically for this review.
Data collection and analysis
Selection of studies
Two review authors independently assessed the titles and abstracts resulting from the electronic searches for inclusion. We obtained copies of all relevant or potentially relevant trials and assessed these according to the Criteria for considering studies for this review. The review authors were not masked as to the names of authors, institutions, journal of publication or results when making their assessments. We resolved disagreements about whether a trial should be included by discussion and consensus. In cases where additional information was needed before a decision was made whether to include a trial, we obtained this information by contacting the authors.
Data extraction and management
We recorded information about the methods used in the trial on a form including details of participants, details of intervention, outcomes and other information. Two review authors independently extracted the data for the primary outcomes, secondary outcomes and adverse effects onto paper forms. Since the double‐entry facility is no longer available in Review Manager 5, one review author extracted data and entered them into Review Manager 5 for the update (RevMan 2014), and another review author checked the entries for errors and inconsistencies.
Assessment of risk of bias in included studies
Two review authors independently assessed the included trials for bias according to the methods described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). With the update of review management software, we assessed risk of bias using the tool set out in Higgins 2011a.
Sequence generation: the method used to generate the allocation sequence, to assess whether it should have produced comparable groups.
Allocation concealment: the method used to conceal the allocation sequence, to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment.
Masking of personnel and outcome assessors: the assessments were made for each main class of outcomes (i.e. anatomic versus functional outcomes) and we considered whether all measures used, if any, to mask study personnel from knowledge of which intervention a participant received were adequate.
Incomplete outcome data: the assessments were made for each main class of outcomes (i.e. anatomic versus functional outcomes) when possible and were based on the description of the completeness of outcome data, including attrition and exclusions from the analysis and their causes, if they were reported.
Selective outcome reporting: the possibility of selective outcome reporting, such as found when some measures were obtained, as declared in the methods section or in protocols, but not reported in the results section.
We used the following grading:
low risk of bias: plausible bias unlikely to seriously alter the results;
unclear risk of bias: plausible bias that raises some doubt about the results;
high risk of bias: plausible bias that seriously weakens confidence in the results.
If the information available in the published trial reports was inadequate to assess any of the above items of the risk of bias assessment, we contacted the trial authors for clarification. If they did not respond within a reasonable period, we classified the trial based on the available information. When studies did not report any concealment approach, we considered adequacy unclear. We also assessed the impact of any assumptions made in this regard in a sensitivity analysis.
We considered a trial to have conducted an intention‐to‐treat analysis only if it included all participants who were randomised including those randomised but not treated and excluded after randomisation for other reasons.
Measures of treatment effect
We evaluated development of CNV and geographic atrophy on the basis of the percentage of their occurrence over the follow‐up. We assessed visual acuity loss taking into consideration the loss of 3 or more lines of visual acuity, which corresponds to a doubling of the visual angle if visual acuity is measured using a logMAR chart.
We planned to evaluate drusen reduction considering the number of eyes showing at least a 50% reduction of drusen area from the baseline aspect. However, data were sparsely reported and, therefore, we modified the protocol to allow an extraction based on the investigators' definition.
Dichotomous data
Dichotomous data included, for example, progression of CNV or geographic atrophy, or loss of 3 or more lines of visual acuity. In the protocol, we stated that we would have used the risk ratio (RR) as our preferred measure of effect since we anticipated that the event rate would be greater than 10%. We actually found that the event rate was lower than this threshold in bilateral studies. Furthermore, to be able to manage data from unilateral and bilateral studies, we had to manipulate them using formulas proposed by Elbourne 2002, which only apply to odds ratios (OR). Little difference is expected between RRs and ORs in this review, since the crude event rate was less than 10% in bilateral studies and less than 25% in unilateral studies, and also because the pooled OR was close to 1.
Continuous data
Continuous data included, for example, reading speed. We used the mean difference (MD), unless the outcomes were measured on different scales in which case we used the standardised mean difference as the measure of effect.
Unit of analysis issues
Some trials identified a 'study eye' and randomised participants to treatment group. Other trials randomised the eye to treatment and compared it with the other eye in the same person. We were careful to consider these trials separately at the data collection and extraction stage.
We did two sets of analyses for the primary outcome 'development of CNV'. First, we pooled all the data ignoring the fact that the data from the bilateral studies were not independent. Second, we then did a sensitivity analysis assuming an intra‐class correlation coefficient (ICC) of 0.5 for the development of CNV and 0.22 for visual acuity loss. We adjusted the standard errors using the methods given in Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b) and Elbourne 2002. See Appendix 7 for more details on the method used.
We used the generic inverse variance facility in Review Manager 5 to enter the data for the sensitivity analysis (RevMan 2014).
Dealing with missing data
In the event that data were missing, we contacted the authors of the studies in an attempt to obtain more information. On the basis of the data we could collect, we first did an available case analysis. We recorded the amount of missing data in the included studies as shown in Table 2. At the time the protocol for this review was prepared, we planned that if missing data should prove to be a problem in the constituent studies, we would consider doing a sensitivity analysis considering outcome in the people lost to follow‐up as either 'all OK' or 'all not OK' to see the range within which the true result might lie. This did not prove necessary at this stage. According to further guidance available in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a), missing outcome data are not a problem if loss to follow‐up is both balanced in the study arms of parallel arm studies and causes of loss are documented and judged to be unrelated to outcome in both study arms. When these conditions are not satisfied, a study can still be at low risk of bias if the outcome frequency is about 50% and loss to follow‐up is below 10% in both arms (Higgins 2011a).
1. Primary analysis data including deaths and missing cases.
| Study | Photocoagulation | Observation | Risk of bias due to incomplete outcome data | ||||||
| F | S | D | M | F | S | D | M | ||
| CAPT | 41 | 967 | 25 | 19 | 50 | 958 | 25 | 19 | Low (bilateral) |
| CNVPT | 12 | 34 | 2 | 11 | 13 | 34 | 3 | 11 | Low |
| DLS bilateral | 12 | 91 | 0 | 2 | 7 | 96 | 0 | 2 | Low (bilateral) |
| DLS unilateral | 27 | 72 | 0 | 0 | 15 | 70 | 0 | 0 | Low |
| Figueroa 1994 | 0 | 30 | 0 | 0 | 1 | 29 | 0 | 0 | Low (bilateral) |
| Frennesson 1995 | 0 | 17 | 0 | 2 | 4 | 15 | 0 | 0 | Low |
| Frennesson 2009 | 7 | 67 | NA | NA | 5 | 68 | NA | NA | Low (see Results) |
| Laser to Drusen Study 1995 | 6 | 34 | 0 | 7 | 11 | 31 | 0 | 10 | Low |
| Little 19951 | 3 | 24 | NA | NA | 5 | 22 | NA | NA | Low (bilateral) |
| Olk 1999 bilateral | 3 | 28 | 2 | 10 | 3 | 62 | 4 | 5 | Low (bilateral) |
| Olk 1999 unilateral2 | 4 | 17 | NA | 6 | 7 | 19 | NA | 4 | Low |
| PTAMD bilateral 2009 | 24 | 197 | NA | 419 | 20 | 200 | NA | 419 | Low (bilateral) |
| PTAMD unilateral 20023 | 13 | 50 | 5.5 | 55.5 | 9 | 62 | 5.5 | 43.5 | High |
The assessment of the risk of bias due to incomplete outcome data is based on the graphical presentation in Figure 1 based on the methods described in Appendix 8. In the updated version of this review, we considered missing data as at no risk of bias in bilateral studies because a participant with paired treatment and control eyes is missed.
F: failures (choroidal neovascularisation development), S: successes, D: deaths, M: missing of unknown cause, NA: not available.
1Only last visit follow‐up available and no information on when choroidal neovascularisation developed in cases with event. 2Deaths were not reported and all missing data were coded as missing of unknown cause. 3Deaths were provided overall (n = 11 at 2 years) and were equally split between assignment groups. Data at 1 or 3 years were available and midpoints were used.
Because our primary outcomes were relatively rare in the complete case analysis in this review, and there were missing data of unreported cause, there was potential for bias due to incomplete outcome data in this review. In the updated version of this review, we considered missing data as at no risk of bias in bilateral studies because a participant with paired treatment and control eyes is missed. For each unilateral study, we assessed the risk of bias using methods described in Appendix 8, based on the users' written function 'metamiss' in Stata 13.1 (StataCorp 2013) to conduct sensitivity analyses on primary outcome meta‐analysis results by making different assumptions on informative missingness odds ratios (IMORs) across studies according to White 2008.
Finally, in the updated version of this review, we used the user written command extfunnel in Stata to assess the impact of a new study on the meta‐analysis according to Langan 2012.
Assessment of heterogeneity
Before carrying out a meta‐analysis, we assessed heterogeneity by examining the characteristics of the study, the forest plot of the results of the studies and the I2 statistic to assess the presence of statistical heterogeneity.
Assessment of reporting biases
We investigated small‐study bias using contour enhanced funnel plot (Peters 2008), and assessed significance of funnel asymmetry using Harbord's test (Harbord 2006).
Data synthesis
We planned to perform a meta‐analysis if there were sufficient trials available without substantial heterogeneity. We used a random‐effects model unless there were three or fewer trials in which case we used a fixed‐effect model. We compared fixed‐effect and random‐effects models to see how robust the results were.
Subgroup analysis and investigation of heterogeneity
We conducted the following subgroup analyses for the primary outcome 'development of CNV':
type of laser treatment, mainly laser wavelength;
clinically visible burns versus sub‐threshold laser treatment.
Studies were duplicated when there were three arms and two different laser wavelengths were compared, and study arms were collapsed when unilateral and bilateral studies were published simultaneously.
Sensitivity analysis
We considered the results of between‐person and within‐person trials separately. We had planned to consider the effect of excluding poor quality studies, if there were sufficient studies. This was not done.
The methods for this review were published in the protocol (Parodi 2007).
Summary of findings
We prepared Table 1 as per guidance given in Chapter 11 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011), and graded the overall quality of the evidence for each outcome using GRADE (GRADEpro 2015).
Results
Description of studies
Results of the search
The original searches identified 111 reports of studies. We excluded Sarks 1999 and Sigelman 1991 because the treatment groups were not randomly allocated. Overall, nine trials were considered suitable for inclusion in the review of which two included both a unilateral and a bilateral arm with data available for both (DLS; Olk 1999), and four were only bilateral (CAPT; Figueroa 1994; Little 1995; PTAMD bilateral 2009). One study was published in abstract form only and the investigators supplied unpublished data for inclusion in this review (Laser to Drusen Study 1995).
An update search run in August 2015 identified a further 174 references (Figure 2). The Trials Search Co‐ordinator removed 60 duplicates and screened the remaining 114 references, of which 59 were not relevant to the scope of the review. We reviewed the remaining 55 references and discarded 49 reports as not relevant. We obtained six full‐text reports for potential inclusion in the review and included two new studies in the review (Frennesson 2009; PTAMD bilateral 2009). We excluded studies by Huang 2011 and Guymer 2014, see Characteristics of excluded studies table for reasons. We also added two ongoing studies, Beaumont 2011 and NCT01790802. Beaumont 2011 was only published as an abstract and the authors confirmed results still had to be published. NCT01790802 aimed to treat 250 participants with nanosecond laser treatment, starting in November 2011 with estimated study completion in June 2017.
2.
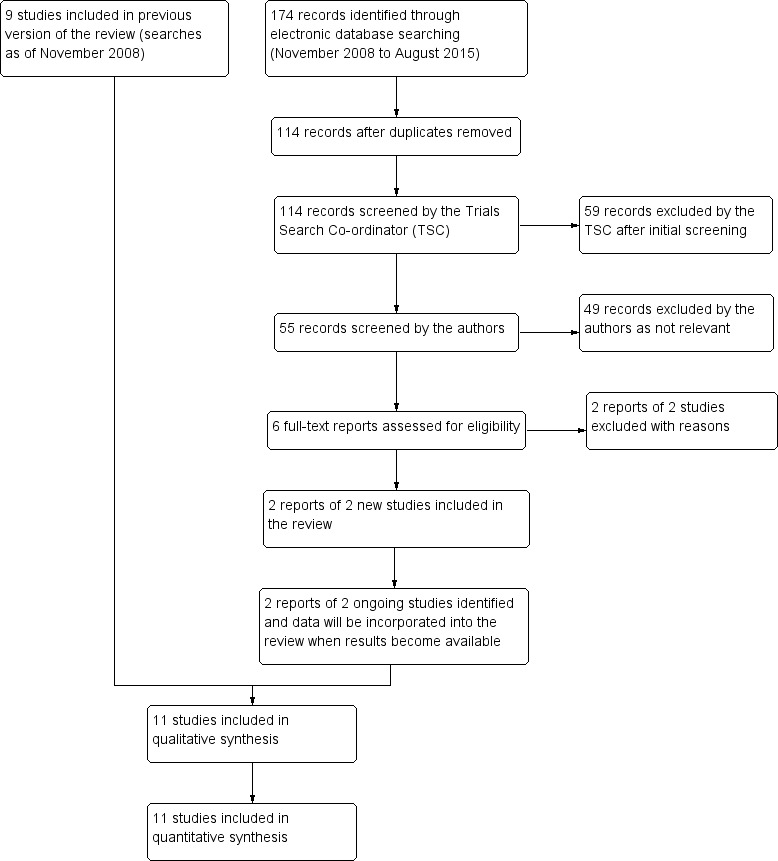
Study flow diagram.
Included studies
See Characteristics of included studies table.
Types of studies
The study design was different across studies. Four studies included one eye of each participant (Frennesson 1995; Frennesson 2009; Laser to Drusen Study 1995; PTAMD unilateral 2002), and we refer to them as 'unilateral' studies. Four studies adopted a paired design whereby both eyes of the participant were included in the study, one eye randomly allocated to photocoagulation or control and the fellow eye to the other group (CAPT; Figueroa 1994; Little 1995; PTAMD bilateral 2009), and we refer to them as 'bilateral' studies. Three more studies included both a unilateral and a bilateral arm (CNVPT; DLS; Olk 1999). However, CNVPT did not report results from the bilateral study arm except for the number of participants with a gain of 1 or more lines of visual acuity at one year in an early report and, therefore, we could not extract data on this arm.
Types of participants
The 11 trials randomised 3629 people. The studies took place in the US (CAPT; CNVPT; Laser to Drusen Study 1995; Little 1995; Olk 1999; PTAMD bilateral 2009; PTAMD unilateral 2002), Sweden (Frennesson 1995; Frennesson 2009), Denmark (Frennesson 2009), Finland (Frennesson 2009), the UK (DLS), Germany (DLS), Australia (DLS), and Spain (Figueroa 1994).
The mean age of the participants was approximately 70 years. The majority of participants were women in all studies (range 54% to 70%).
All studies recruited participants presenting medium (greater than 63 μm) or large (greater than 125 μm) drusen with pigmentary changes. CNVPT, DLS, Figueroa 1994, and Frennesson 2009 enrolled participants with bilateral macular drusen in the bilateral arm and participants with neovascular AMD in one eye and macular drusen in the fellow eye in a unilateral study. Little 1995, Olk 1999, Frennesson 1995, PTAMD bilateral 2009, and CAPT enrolled participants with macular drusen in both eyes.
Types of interventions
Table 3 gives details of the laser treatment employed in the different studies. Six studies employed argon laser, three diode laser and two dye laser. Laser spot size varied from 50 to 200 μm. The duration of each burn ranged from 0.05 seconds to 0.2 seconds. The number of laser spots included was between one and 100. PTAMD unilateral 2002 and PTAMD bilateral 2009 used subthreshold photocoagulation using an 810‐nm diode laser in all treated participants. Frennesson 2009 used subthreshold photocoagulation a using argon green laser. Olk 1999 used subthreshold photocoagulation in a random subset of treated eyes. Subthreshold photocoagulation was obtained by lowering the power that achieves standard photocoagulation and no study used micropulse photocoagulation.
2. Characteristics of the intervention and control in each study.
| Study ID | Laser type | Parameters | Control |
| CAPT | Argon | 100 μm spot size/0.1 sec/60 burns | Observation |
| CNVPT | Argon | 100 μm spot size/0.1 sec/ laser‐20 protocol in 85% of cases | Observation |
| DLS | Argon green/yellow dye | 200 μm spot size/0.2 sec/12 burns | Observation |
| Figueroa 1994 | Argon | 100 μm spot size/0.1 sec/ temporal fovea or grid pattern | Observation |
| Frennesson 1995 | Argon | 200 μm spot size/0.05 sec/temporal horseshoe‐shaped area | Observation |
| Frennesson 2009 | Argon green | 200 μm spot size/0.05 sec/˜100 spots on and between drusen | Observation |
| PTAMD bilateral 2009 | Diode | 125 μm spot size/0.1 sec/grid of 48 lesions | Observation |
| Laser to Drusen Study 1995 | Yellow dye | 50 μm spot size/0.1 sec/variable number | Observation |
| Little 1995 | Dye 577‐620 nm | 100‐200 μm spot size/0.05‐0.1 sec | Observation |
| Olk 1999 | Diode | 125 μm spot size/0.2 sec/grid of 48 burns | Observation |
| PTAMD unilateral 2002 | Diode | 125 μm spot size/0.1 sec/grid of 48 lesions | Observation |
sec: second.
Primary outcomes
Six bilateral studies or study arms (CAPT; DLS; Figueroa 1994; Little 1995; Olk 1999; PTAMD bilateral 2009), and seven unilateral studies or study arms (CNVPT; DLS; Frennesson 1995; Frennesson 2009; Laser to Drusen Study 1995; Olk 1999; PTAMD unilateral 2002) presented data on the outcome 'development of CNV'.
We stated in the protocol that we aimed to obtain data at two years. However, we used three‐year data for three studies that reported the outcome with more detail at this time point (DLS; Frennesson 1995; PTAMD bilateral 2009). For PTAMD bilateral 2009, the number of events were calculated applying the percentage estimated from Kaplan‐Mayer survival curves to complete cases; Little 1995 and Frennesson 2009 used the last visit at a mean of 3.2 years (Little 1995) and 3.7 years (Frennesson 2009).
CAPT and Olk 1999 did not report crude data at two years, but presented survival curves, from which we extracted graphically the proportion of people with CNV and atrophy at two years using the number of eyes followed up in each group to compute standard errors. Most bilateral studies provided marginal data only (i.e. a frequency tabulation that ignores the paired nature of data), but we could extract and use a correlation coefficient from DLS as shown in Appendix 7.
Among bilateral studies, we could extract paired data on development of CNV from Little 1995, but we considered that this study was too small to estimate the correlation coefficient reliably. CAPT provided marginal data, but the P value was obtained from a Cox proportional hazards model, not from a McNemar Chi2 test; thus, we did not use the method shown in Appendix 7.
There was poor reporting of the primary outcome 'development of geographic atrophy'. Data from Laser to Drusen Study 1995 were obtained from the authors. Data from survival curves could be estimated from the unilateral arm of CNVPT; cross‐tabulations were constructed using the number of complete cases who did not develop CNV because, in the absence of a clear reporting of the total number of eyes at each step of the survival curve, we considered that eyes with a neovascular lesion may have complex fundus changes preventing a precise assessment of geographic atrophy.
Secondary outcomes
Loss of visual acuity was the only secondary outcome that could be extracted for most studies. Particularly, DLS, CAPT, Figueroa 1994, and PTAMD bilateral 2009 presented bilateral data and DLS, Olk 1999, DLS, PTAMD unilateral 2002, and CNVPT presented unilateral data. Most studies provided marginal data, but we could extract a correlation coefficient from Little 1995 and DLS and use it as shown in Appendix 7 to obtain correct standard errors. Frennesson 2009 only reported mean visual acuity and dichotomous data on visual loss could not be obtained.
Only CNVPT presented contrast sensitivity and reading ability data.
Most studies analysed drusen reduction. It was possible to extract data on this outcome from two unilateral studies and one bilateral study. For CNVPT, we extracted data graphically from a survival curve using the number of eyes followed up in each group to generate a cross‐tabulation of the eyes with a 50% or more drusen area reduction among treated and control eyes. Two studies gave the approximate percentages with apparent drusen reduction: PTAMD unilateral 2002 at 18 months and PTAMD bilateral 2009 at two years. We used the number of participants still followed minus those who developed CNV as the total number in each group for generating the 2 x 2 table. We could not extract data on drusen reduction from the other studies. In fact, CAPT and Little 1995 were bilateral studies but reported marginal data only. Olk 1999 provided pooled data only for unilateral and bilateral cases. Frennesson 1995 provided means and standard deviations but used Snellen values to compute them, which is incorrect, and data had a very skewed distribution. Thus, we decided not to use data from this study. DLS did not report drusen reduction.
None of the studies reported quality of life data.
Other comparisons
Olk 1999 also compared subthreshold (i.e. yielding non‐visible laser burns) photocoagulation with observation. We obtained marginal data from the bilateral study arm, but no estimate of the intraindividual correlation, together with data from the unilateral study arm.
Excluded studies
See Characteristics of excluded studies table.
We excluded four studies: Guymer 2014 was not an RCT, Huang 2011 as the investigator reported that treatment was randomised, but then the participant could choose which eye would receive laser treatment; Sarks 1999, which was a comparative but non‐randomised study and Sigelman 1991, which was a case report.
Sivagnanavel 2004 and Beaumont 2011 have only been published as an abstract. In particular, Beaumont 2011 treated 121 participants with subthreshold photocoagulation and used the fellow eye as control, finding that drusen disappeared and that there was a small benefit of borderline significance on visual acuity; the contact author reported treatment assignment to either eye was randomised and the study was ongoing and unpublished. We are still trying to contact Sivagnanavel 2004, but have not as yet received further information and so have placed this study under Studies awaiting classification.
Risk of bias in included studies
See 'Risk of bias' tables in Characteristics of included studies table and Figure 3.
3.
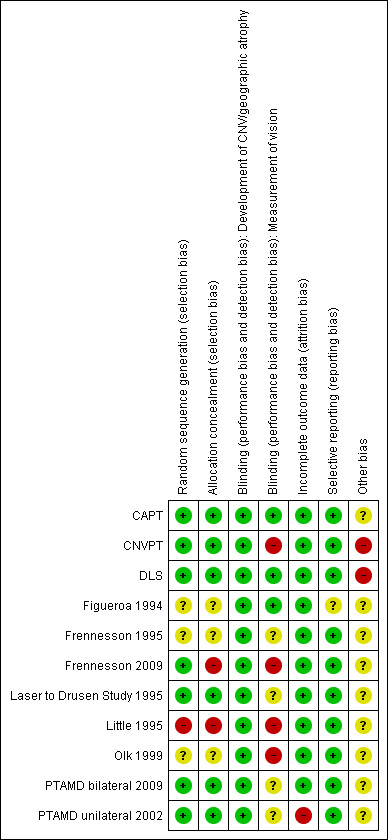
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Slightly more than half of the trials reported adequate methods to generate and conceal the allocation sequence.
Blinding
Participants were not masked (blinded) since a sham procedure was never adopted. We acknowledge that it is not possible to mask outcome assessors to anatomic outcomes because laser scars are visible around the macula, unless subthreshold photocoagulation is used and effective. However, masking of functional outcome assessors can be achieved in theory, but was rarely so, or reported as such, in these studies. We think that development of CNV is a sufficiently objective diagnosis to be classified as having low risk of bias despite lack of masking of outcome assessors. On the contrary, vision outcomes such as visual acuity and contrast sensitivity can easily be measured by a masked assessor, and lack of masking can introduce bias because the procedure is operator dependent.
Incomplete outcome data
Table 2 shows events and non‐events of complete cases, number of deaths and number of missing participants in the treatment and control arms. We used these data to assess the impact of incomplete outcome data only in unilateral studies. In fact, in the updated version of this review, we considered missing data as at no risk of bias in bilateral studies because a participant with paired treatment and control eyes is missed.
Figure 4 shows the potential impact of missing data using the method described in Appendix 8. Based on this analysis, only PTAMD unilateral 2002 was at high risk of bias for missing data.
4.
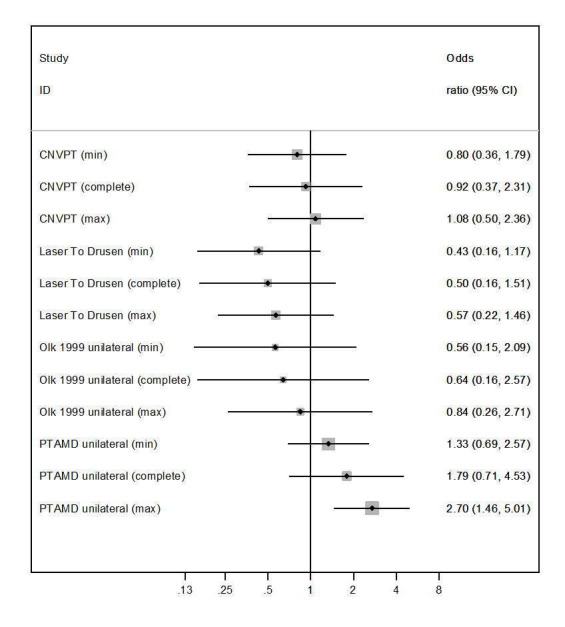
Photocoagulation versus control, outcome: Development of CNV at two years (see Figure 5; CNVPT; Laser to Drusen Study 1995; Olk 1999; PTAMD unilateral 2002). Graphical assessment of the risk of bias due to incomplete outcome data in unilateral studies with missing data reported (see Appendix 8). The minimum and maximum odds ratio change, compared to complete or available cases, is assessed graphically and subjectively taking into account its 95% CI. CI: confidence interval; max: maximum; min: minimum.
Frennesson 2009 was a unilateral study, but we could only obtain the number of events at the end of follow‐up (about 3.5 years) and could not extract missing data at two years. Thus, we scored the study at unclear risk of bias for incomplete outcome data.
Selective reporting
Most studies reported the development of CNV and visual acuity, which are the key outcomes in this study, so selective reporting was not a problem in this review.
Other potential sources of bias
One trial was stopped early because an interim analysis suggested a harmful effect of photocoagulation (DLS).
The laser producers sponsored three out of four studies using laser subthreshold photocoagulation (Olk 1999; PTAMD bilateral 2009; PTAMD unilateral 2002).
Effects of interventions
See: Table 1
Primary outcomes
Development of choroidal neovascularisation
Pooling the results, as seen in Figure 5, showed that photocoagulation did not reduce the development of CNV at two years' follow‐up (OR 1.07, 95% CI 0.79 to 1.46, 11 studies, 2159 participants (3580 eyes), high quality evidence; Analysis 1.1). This estimate means that, given an overall occurrence of CNV of 8.3% in the control group, we estimated an absolute risk reduction of no more than 1.4% in the laser group, which is sufficient to exclude a benefit, or an increase of more than 2.8%, which is sufficient to exclude an important harm in this low risk population.
5.
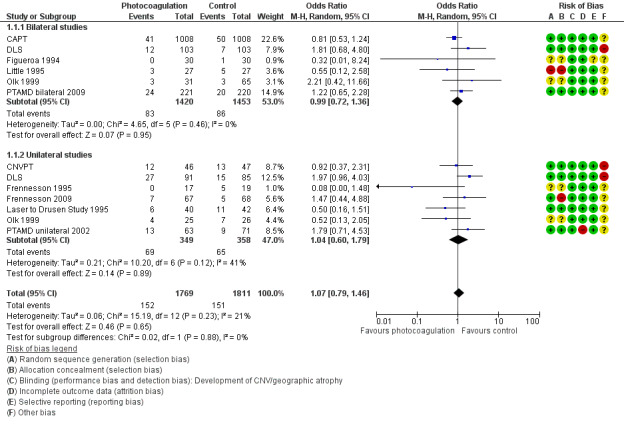
Forest plot of comparison: 1 Photocoagulation versus control, outcome: 1.1 Development of choroidal neovascularisation (two studies provided data on both unilateral and bilateral participants).
1.1. Analysis.
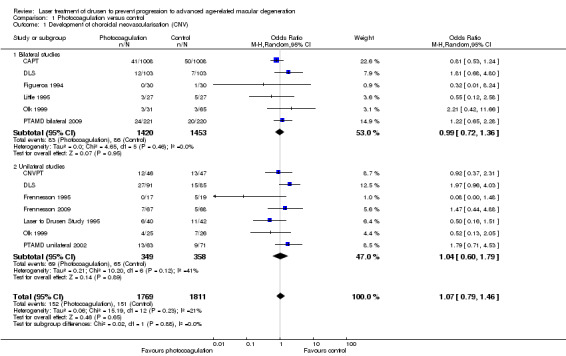
Comparison 1 Photocoagulation versus control, Outcome 1 Development of choroidal neovascularisation (CNV).
A sensitivity analysis assuming moderate correlation (0.5) of the outcome within individuals increased the heterogeneity of bilateral studies to an I2 value of 52%, but Analysis 1.2 shows that the conclusions would not change.
1.2. Analysis.
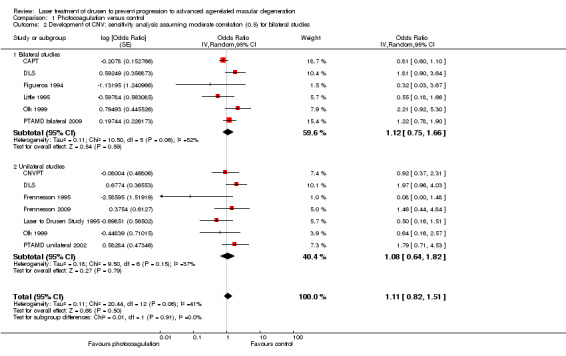
Comparison 1 Photocoagulation versus control, Outcome 2 Development of CNV: sensitivity analysis assuming moderate correlation (0.5) for bilateral studies.
Figure 6 presents a graphical exploration of small‐study bias in a contour‐enhanced funnel plot. In this analysis, we pooled data from bilateral and unilateral studies when they were based on a similar protocol and published simultaneously. Although there were two small studies in the area of larger effect, the lower‐left corner of the plot, the Harbord test did not suggest statistical significance of funnel plot asymmetry (P value = 0.444).
6.
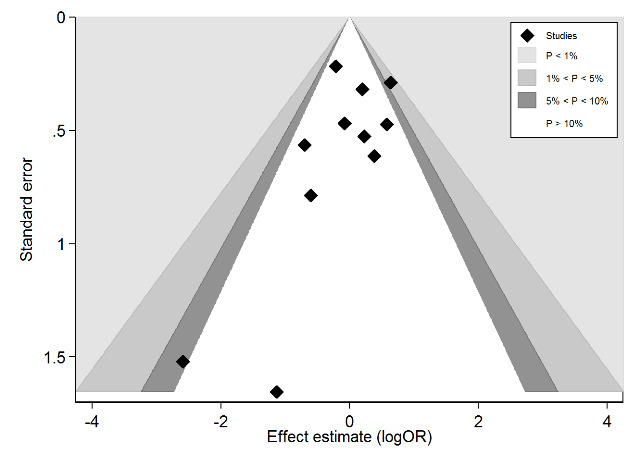
Contour enhanced funnel‐plot investigating small study bias for the primary outcome 'development of choroidal neovascularisation or geographic atrophy'. Shaded areas are areas of statistical significance as explained in the legend.
Development of geographic atrophy
We could extract data on the development of atrophy from only two small studies (CNVPT; Laser to Drusen Study 1995). Analysis demonstrated no benefit or harm using photocoagulation for development of geographic atrophy (OR 1.30, 95% CI 0.38 to 4.51; Analysis 1.3). The quality of the evidence was low because of imprecision.
1.3. Analysis.
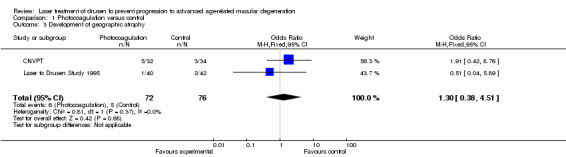
Comparison 1 Photocoagulation versus control, Outcome 3 Development of geographic atrophy.
One bilateral study presented marginal data on development of geographic atrophy. Specifically, CAPT reported that 1.9% of treated eyes compared to 1.4% of control eyes of 1008 participants developed atrophy at two years, but due to the paired nature, we could not extract and analyse these data.
Secondary outcomes
Visual acuity
Four bilateral studies and five unilateral studies allowed the extraction of data on the risk of visual loss of 3 or more lines of visual acuity at two years (a value of 2 or more lines was available in Olk 1999 and PTAMD bilateral 2009) (Analysis 1.4). The analysis demonstrated no benefit or harm with photocoagulation (OR 0.99, 95% CI 0.81 to 1.22). The quality of this evidence was moderate because visual acuity examiners were masked in less than half of studies.
1.4. Analysis.
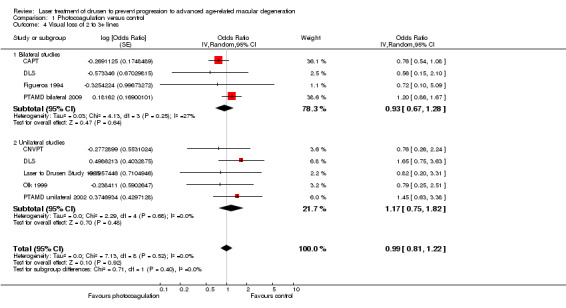
Comparison 1 Photocoagulation versus control, Outcome 4 Visual loss of 2 to 3+ lines.
Contrast sensitivity
We obtained data on contrast threshold from the authors of Laser to Drusen Study 1995. There was a large uncertainty of the estimates (low quality evidence; Analysis 1.5) and analysis demonstrated no effect of photocoagulation.
1.5. Analysis.
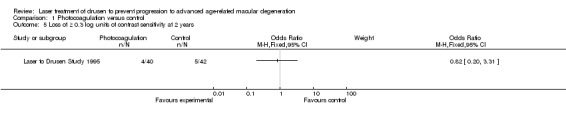
Comparison 1 Photocoagulation versus control, Outcome 5 Loss of ≥ 0.3 log units of contrast sensitivity at 2 years.
CAPT also reported on contrast sensitivity, but this was a paired study and the data could not be analysed since an estimate of the correlation coefficient was not obtained. The authors reported marginal data at five years, which indicated that 212/888 (23.9%) treated eyes and 182/887 (20.5%) observed eyes required twice as much contrast (corresponding to a loss of 0.3 log 10 units or more of contrast sensitivity) to read letters.
Reading ability
We obtained data on reading speed from the authors of Laser to Drusen Study 1995 for about 50% of the participants included in this small study. Analysis found no statistically significant difference between photocoagulation and observation (low quality evidence due to imprecision; Analysis 1.6).
1.6. Analysis.
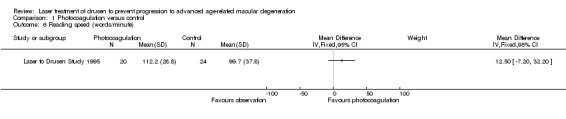
Comparison 1 Photocoagulation versus control, Outcome 6 Reading speed (words/minute).
CAPT also reported marginal data on reading ability expressed as critical print size (i.e. the character print's size below which a person's reading speed slows down). The authors reported marginal data at five years, which indicated that 260/879 (29.6%) treated eyes and 249/878 (28.4%) observed eyes required a print size twice as large (0.3 LogMAR or 3 lines) or could not read even the largest print size.
Drusen reduction
We extracted data on drusen reduction as defined by the investigators from two unilateral studies (CNVPT; PTAMD unilateral 2002), and one bilateral study (PTAMD bilateral 2009), at approximately two years (Analysis 1.7). All three studies found an apparent drusen reduction in treated eyes compared to control eyes with a cumulative OR of 9.16 (95% CI 6.28 to 13.37, 944 eyes, high quality evidence).
1.7. Analysis.
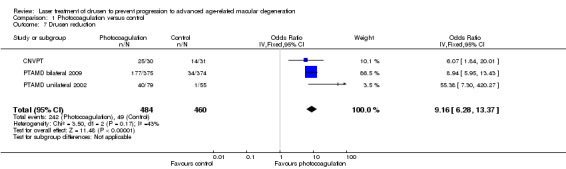
Comparison 1 Photocoagulation versus control, Outcome 7 Drusen reduction.
Among bilateral studies, others presented marginal data suggesting that photocoagulation caused drusen resorption, but we could not extract these data since an estimate of the within‐person correlation was not obtained. Specifically, CAPT found that 34.3% of treated eyes versus 8.6% of control eyes of 1008 participants had a 50% drusen reduction at two years. Figueroa 1994 reported that 29/30 treated eyes versus 2/30 control eyes had drusen reduction, on average after three months. Little 1995 reported that 17/27 treated eyes had drusen resorption by six months compared to 5/27 observed eyes by one year.
Other studies reported data suggesting drusen disappearance using photocoagulation compared to observation, but we could not extract data for various reasons (means and standard deviations suggesting skewed data (Frennesson 1995; Frennesson 2009), pooled data from unilateral and bilateral study arms (Olk 1999), or data not available (DLS)).
Quality of life
None of the studies reported quality of life.
Subgroup analysis
We conducted subgroup analyses for the primary outcomes only (development of CNV and geographic atrophy).
Type of laser
We conducted a subgroup analysis comparing argon, diode and dye laser, pooling data from unilateral and bilateral studies when they had been conducted and reported simultaneously using similar methods. We excluded DLS since it used two types of laser and no separate data were available.
Although there was some subgroup heterogeneity (I2 = 53%), the P value for subgroup differences did not reach significance (P value = 0.12), and none of the groups showed a significant increase or decrease of the risk of CNV (Analysis 1.8).
1.8. Analysis.
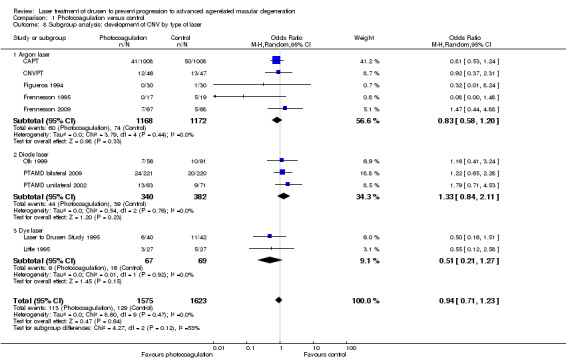
Comparison 1 Photocoagulation versus control, Outcome 8 Subgroup analysis: development of CNV by type of laser.
Visible versus subthreshold photocoagulation
In the update of this review, we included two more studies adopting subthreshold photocoagulation (Frennesson 2009; PTAMD bilateral 2009). Thus, we could conduct a subgroup analysis comparing visible, standard photocoagulation (eight studies, 2870 eyes) with subthreshold photocoagulation (four studies, 950 eyes) (Analysis 1.9). We could not show statistically significant differences between the two groups (I2 = 9%, P value = 0.29) and the OR in the subthreshold group excluded a large benefit (OR 1.27, 95% CI 0.82 to 1.98).
1.9. Analysis.
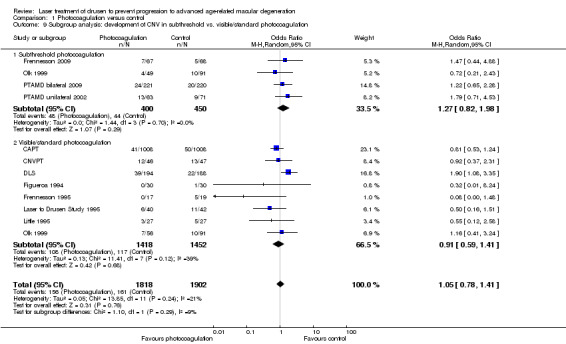
Comparison 1 Photocoagulation versus control, Outcome 9 Subgroup analysis: development of CNV in subthreshold vs. visible/standard photocoagulation.
Adverse effects
We considered adverse effects such as development of CNV, development of geographic atrophy and visual loss above.
Only one trial formally considered additional adverse effects. They noted that, "There were no reports of burns applied to the foveal avascular zone, breaks in Bruch's membrane, or haemorrhages at the initial or 1‐year treatment" (CAPT).
Olk 1999 noted a juxtafoveal scar in one eye (out of 63 eyes) treated with visible burns.
Potential impact of a new study
As explained in the Data synthesis section, we assessed the potential impact of a new study on the meta‐analysis according to Langan 2012. Figure 1 shows that it is unlikely that further research will change the results of this review, since they should be either favourable studies and much larger than those included, or smaller but with favourable effects far exceeding those observed in this review. This is compatible with the fact that no trials were published after 2009.
1.
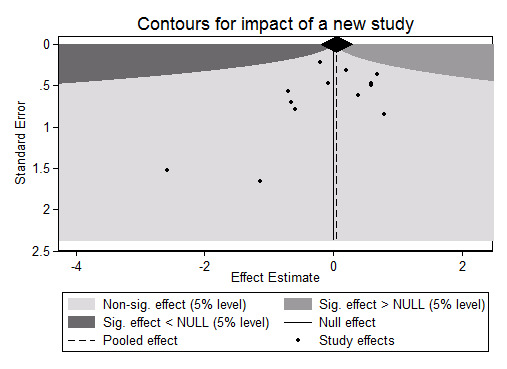
Potential impact of a new study on the meta‐analysis using the command 'extfunnel' in Stata (see Data synthesis and Langan 2012). sig: significant.
Discussion
AMD is a disease characterised by an enormous social burden. The availability of a therapeutic approach able to reduce the incidence of the major complications (i.e. CNV and atrophy) would be extremely welcome. Several authors have recorded that in their experience the use of laser can result in reabsorption of macular drusen (Cleasby 1979; Figueroa 1994; Gass 1973; Wetzig 1994). As yet, it is unclear whether drusen reduction can lead to clinical benefits, including improvement or stabilisation of visual acuity, delayed or reduced CNV, or harms such as the onset of atrophy.
Summary of main results
In the update of this review, we identified two new trials, leading the total number to 11 studies, in which 2159 participants (3580 eyes) were randomised to laser treatment of drusen or observation and followed up to at least two years. These trials confirmed the clinical observation that laser photocoagulation of drusen was able to cause their disappearance. However, there was no evidence that this loss of drusen resulted in any benefit in terms of the development of CNV or geographic atrophy or prevention of visual acuity loss. The results of the present review indicated that the prophylactic laser treatment of drusen is ineffective as a means for delaying the progression of AMD and preventing visual loss. A clinically relevant benefit can be excluded for people at medium risk of CNV, which was about 9% within two years in our primary meta‐analysis, based on the primary outcomes. Among the secondary outcomes, the CI of the visual loss outcome also tended to exclude important harms.
The two new studies used near‐infrared subthreshold photocoagulation, totalling four studies with 539 eyes of different people treated with this technique. The meta‐analytic OR and its 95% CI also did not suggest potential for benefit. However, no study included in this review used micropulse subthreshold photocoagulation, since photocoagulation was used with low power but continuous laser light delivery.
Overall completeness and applicability of evidence
Some of these trials adopted a paired study design (six studies, 1454 participants), which rendered the analysis of the data difficult. Moreover, only a few studies reported data on secondary outcomes, especially contrast sensitivity and reading ability. Despite these limitations, the studies included in this review were conducted in different countries and follow‐up length was enough to be able to record long‐term effects of this intervention.
Quality of the evidence
Overall, we judged the evidence included for the primary outcome 'development of CNV' represent high quality of evidence (i.e. that we are reasonably certain that the estimate of effect represents the true value). We downgraded the judgement of quality to low for the other primary outcome 'development of geographic atrophy' because there were only two studies with few participants and, therefore, the effect estimates were very imprecise.
Potential biases in the review process
One source of bias in this review may be the pooling of unilateral and bilateral studies based on assumptions about the statistical correlation of within‐person data. To try to counteract this potential shortcoming, we not only used the information available from some studies, suggesting very low correlation for the primary outcome 'occurrence of CNV', but also used a mean correlation as a sensitivity analysis, which did not change the conclusions of our review.
Authors' conclusions
Implications for practice.
Even though drusen area reduction can be achieved through laser treatment, this review suggests that this intervention is not associated with improved outcomes for the patients, based on meta‐analyses of studies, which, overall, had a low risk of bias.
Implications for research.
The results of this review suggest there is no need to conduct more research on photocoagulation directed to drusen in people with AMD, in accordance with the fact that no further trials were published after 2009. We acknowledge that the evidence on different lasers sources and strategies, such as the subthreshold one, is more limited, yet our findings do not seem to differ for this subtype of laser photocoagulation, if it is obtained with low‐power continuous laser light, such as in the included studies, which did not use micropulse photocoagulation.
A study is ongoing to investigate the effect of nanosecond laser treatment to drusen.
What's new
| Date | Event | Description |
|---|---|---|
| 3 August 2015 | New citation required and conclusions have changed | Issue 10, 2015: Conclusions changed from "unable to show difference" to "treatment ineffective" for the primary outcome development of CNV. |
| 3 August 2015 | New search has been performed | Issue 10, 2015: Updated searches yielded two new trials that met the inclusion criteria (Frennesson 2009; PTAMD bilateral 2009). |
History
Protocol first published: Issue 2, 2007 Review first published: Issue 3, 2009
| Date | Event | Description |
|---|---|---|
| 9 March 2008 | Amended | Converted to new review format. |
Acknowledgements
Thank you to Barbara S. Hawkins, PhD; Marta M. Gilson, PhD; Susan Vitale, PhD; Neil M. Bressler, MD and Susan B. Bressler, MD for providing unpublished data on the Laser to Drusen Study 1995 study. Also thanks to Dr. Christina Frennesson, Dr. Thomas R. Friberg and Dr. Ying‐xiang Huang for providing additional data on included and excluded studies.
The Cochrane Eyes and Vision Group (CEVG) created and executed the search strategies.
We thank José María Ruiz‐Moreno and Alicja Rudnicka for their comments on this review and Stephen Gichuhi and Barbara Hawkins for their comments on the protocol for this review.
We gratefully acknowledge the contribution of David Brandon, Harold Burton, Peter Dyson, Jan Elson and Brenda Rogers of the CEVG AMD Consumer Panel for their comments on the abstract and plain language summary.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Retinal Drusen #2 drusen* #3 (#1 OR #2) #4 MeSH descriptor Lasers #5 laser* #6 MeSH descriptor Laser Coagulation #7 photocoagulat* #8 (#4 OR #5 OR #6 OR #7) #9 (#3 AND #8)
Appendix 2. MEDLINE (Ovid) search strategy
1. randomized controlled trial.pt. 2. (randomized or randomised).ab,ti. 3. placebo.ab,ti. 4. dt.fs. 5. randomly.ab,ti. 6. trial.ab,ti. 7. groups.ab,ti. 8. or/1‐7 9. exp animals/ 10. exp humans/ 11. 9 not (9 and 10) 12. 8 not 11 13. exp retinal drusen/ 14. drusen$.tw. 15. or/13‐14 16. exp lasers/ 17. laser$.tw. 18. exp laser coagulation/ 19. photocoagulat$.tw. 20. or/16‐19 21. 13 and 20 22. 12 and 21
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville (Glanville 2006).
Appendix 3. EMBASE (Ovid) search strategy
1. exp randomized controlled trial/ 2. exp randomization/ 3. exp double blind procedure/ 4. exp single blind procedure/ 5. random$.tw. 6. or/1‐5 7. (animal or animal experiment).sh. 8. human.sh. 9. 7 and 8 10. 7 not 9 11. 6 not 10 12. exp clinical trial/ 13. (clin$ adj3 trial$).tw. 14. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 15. exp placebo/ 16. placebo$.tw. 17. random$.tw. 18. exp experimental design/ 19. exp crossover procedure/ 20. exp control group/ 21. exp latin square design/ 22. or/12‐21 23. 22 not 10 24. 23 not 11 25. exp comparative study/ 26. exp evaluation/ 27. exp prospective study/ 28. (control$ or prospectiv$ or volunteer$).tw. 29. or/25‐28 30. 29 not 10 31. 30 not (11 or 23) 32. 11 or 24 or 31 33. exp drusen/ 34. drusen$.tw. 35. or/33‐34 36. exp laser/ 37. laser$.tw. 38. exp laser coagulation/ 39. photocoagulat$.tw. 40. or/36‐39 41. 35 and 40 42. 32 and 41
Appendix 4. ISRCTN search strategy
drusen AND laser
Appendix 5. ClinicalTrials.gov search strategy
drusen AND laser
Appendix 6. ICTRP search strategy
drusen AND laser
Appendix 7. Estimate of the correlation coefficient of the measurements within participants in bilateral studies
Elbourne 2002 provided a method for conducting meta‐analyses of studies using paired data, such as cross‐over studies or studies on paired organs. In this appendix, we showed how we adjusted the marginal measurements, that is, with eyes as the unit of analysis extracted from bilateral studies by the intraindividual correlation coefficient extracted from other studies in order to obtain correct standard errors of the odds ratio.
We found both marginal and paired analyses in DLS. Data were limited to the primary outcome 'development of choroidal neovascularisation (CNV)' and to the secondary outcome 'loss of visual acuity'. In particular, Table 4 in DLS presented marginal data on CNV occurrence, our primary outcome, and visual loss while displaying P values obtained with the McNemar test, which is based on the Chi2 distribution and is adequate for paired data. In particular, 12/103 laser‐treated eyes and 7/103 fellow eyes developed CNV and the McNemar P value was 0.2253. The marginal P value using the Chi2 test would have been 0.2286. We considered that the ratio of the z‐values corresponding to these paired and marginal P values (1.2039 (paired) and 1.1907 (marginal)) could be used to adjust the standard errors of the marginal logOR of CNV occurrence for laser‐treated eyes compared to controls. The inverse ratio of these two z‐values was 0.9782, implying that no adjustment of the marginal logOR standard error was needed for the DLS data. Because the marginal logOR variance was 0.4976, its value adjusted for the correlation between eyes was 0.4867, the difference between the two being twice the covariance (which was 0.0054). From these data, the correlation coefficient could be calculated to be only 0.0451 (i.e. 0.0054*square root(12*7*96*91)/103, using the method shown in Elbourne 2002). An issue concerning this correlation coefficient imputation is whether the coverage achieved by the McNemar test is acceptable given the possibility of cells with counts close to nil in paired 2 x 2 tables from medium size studies such as this when events are not common.
Given the negligible effect of the correlation between eyes of the same participant for the CNV development outcome in DLS, we used marginal data from bilateral studies as if eyes were independent units.
Using the same method for visual acuity loss, the ratio of the marginal and paired logOR standard errors was 0.8143, resulting in a correlation coefficient of 0.2290. Therefore, for this outcome, we decided to use the inverse variance method and adjust the marginal logOR standard error by 1.2280 (the reciprocal of the previous ratio).
We obtained a different estimate of the correlation between eyes for the CNV outcome from Little 1995. Using the formulas provided by Elbourne 2002, the correlation coefficient was 0.69 in this small data‐set using the last follow‐up examination to assess the risk of CNV occurrence. Using Elbourne 2002 notations, the numbers to calculate this value would be: s = 23, t = 2, u = 0, v = 2, hence a = 25, b = 23, c = 2, d = 4. However, this was a very small study and was expected to estimate correlation imprecisely and also to be affected by approximations due to low cell counts, for which common formulas for 2 x 2 tables do not hold. Thus, we did not use this type of estimate of the correlation coefficient.
Finally, we decided to conduct a sensitivity analysis for the outcome 'development of CNV' using a moderate correlation between eyes of 0.5 to correct standard errors of the marginal odds ratio.
Appendix 8. Methods used to deal with incomplete outcome data
We used the following approaches to take into account the impact of missing data. In the updated version of this review, we considered missing data as at no risk of bias in bilateral studies because a participant with paired treatment and control eyes is missed. Thus, we only considered losses in unilateral studies.
We used Stata 13.1 software (StataCorp 2013) users' written function 'metamiss' assuming random uncorrelated opposite informative missingness odds ratios (IMORs) for treatment and controls (1/2 and 2; 2 and 1/2). We assumed additional uncertainty about log(IMOR) by setting its prior standard deviation at 1, which will result in larger 95% confidence intervals and, finally, in less weight on studies with numerous missing data. Finally, we assumed uncorrelated IMORs of treatment and control groups when setting the 'metamiss' command. White 2008 provides the underlying theory and a link to download 'metamiss'.
The results of these sensitivity meta‐analyses on the primary analysis occurrence of CNV are shown and discussed in unilateral studies.
Data and analyses
Comparison 1. Photocoagulation versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Development of choroidal neovascularisation (CNV) | 11 | 3580 | Odds Ratio (M‐H, Random, 95% CI) | 1.07 [0.79, 1.46] |
| 1.1 Bilateral studies | 6 | 2873 | Odds Ratio (M‐H, Random, 95% CI) | 0.99 [0.72, 1.36] |
| 1.2 Unilateral studies | 7 | 707 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.60, 1.79] |
| 2 Development of CNV: sensitivity analysis assuming moderate correlation (0.5) for bilateral studies | 11 | Odds Ratio (Random, 95% CI) | 1.11 [0.82, 1.51] | |
| 2.1 Bilateral studies | 6 | Odds Ratio (Random, 95% CI) | 1.12 [0.75, 1.66] | |
| 2.2 Unilateral studies | 7 | Odds Ratio (Random, 95% CI) | 1.08 [0.64, 1.82] | |
| 3 Development of geographic atrophy | 2 | 148 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.38, 4.51] |
| 4 Visual loss of 2 to 3+ lines | 8 | Odds Ratio (Random, 95% CI) | 0.99 [0.81, 1.22] | |
| 4.1 Bilateral studies | 4 | Odds Ratio (Random, 95% CI) | 0.93 [0.67, 1.28] | |
| 4.2 Unilateral studies | 5 | Odds Ratio (Random, 95% CI) | 1.17 [0.75, 1.82] | |
| 5 Loss of ≥ 0.3 log units of contrast sensitivity at 2 years | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6 Reading speed (words/minute) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7 Drusen reduction | 3 | 944 | Odds Ratio (IV, Fixed, 95% CI) | 9.16 [6.28, 13.37] |
| 8 Subgroup analysis: development of CNV by type of laser | 10 | 3198 | Odds Ratio (M‐H, Random, 95% CI) | 0.94 [0.71, 1.23] |
| 8.1 Argon laser | 5 | 2340 | Odds Ratio (M‐H, Random, 95% CI) | 0.83 [0.58, 1.20] |
| 8.2 Diode laser | 3 | 722 | Odds Ratio (M‐H, Random, 95% CI) | 1.33 [0.84, 2.11] |
| 8.3 Dye laser | 2 | 136 | Odds Ratio (M‐H, Random, 95% CI) | 0.51 [0.21, 1.27] |
| 9 Subgroup analysis: development of CNV in subthreshold vs. visible/standard photocoagulation | 11 | 3720 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.78, 1.41] |
| 9.1 Subthreshold photocoagulation | 4 | 850 | Odds Ratio (M‐H, Random, 95% CI) | 1.27 [0.82, 1.98] |
| 9.2 Visible/standard photocoagulation | 8 | 2870 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.59, 1.41] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
CAPT.
| Methods | Method of allocation: treatment assignments were generated using a randomly permuted block method, stratified by clinical centre and using a randomly chosen block size. A member of the CAPT Co‐ordinating Centre reviewed an eligibility checklist with the local ophthalmologist and clinic co‐ordinator during a teleconference before disclosing which of the 2 eyes was assigned to laser treatment Masking: masked VA examiners. Unclear if participants and care providers were masked. Not reported if anatomic outcomes assessors were masked (i.e. Photograph Reading Centre), but masking was unlikely to be achieved since photocoagulation generates visible scars Exclusions after randomisation: none reported Losses to follow‐up: during 5 years of follow‐up, 5891 (97.2%) visits were completed of the 6061 6‐month and annual visits scheduled for surviving CAPT participants. This percentage was relatively stable over time Unusual study design: bilateral or paired study, i.e. 1 eye randomised to treatment or control and the fellow eye to the other study arm |
|
| Participants | Country: US Number randomised: 1052 participants Enrolment period: May 1999 to March 2001 Age: mean 71 years Sex: 637 women (60.6%) Inclusion criteria: at least 10 drusen of size ≥ 125 μm within 3000 μm of FAZ centre; BCVA: 20/40 or more; aged ≥ 50 years Exclusion criteria: CNV or serous retinal PED in either eyes; geographic atrophy within 500 μm of FAZ centre; any ocular disease that might affect VA |
|
| Interventions | Treatment: 60 burns in a grid pattern using a 100‐μm spot size, 0.1‐second duration and power to achieve a barely visible lesion. The burns were applied within an annulus between 1500 and 2500 μm from the FAZ centre Control: observation |
|
| Outcomes | Primary: loss of ≥ 15 letters Secondary: change in VA; change in contrast sensitivity; change in critical print size; incidence of late AMD (CNV, serous PED, geographic atrophy) |
|
| Notes | Since 2001, the participants were informed of the AREDS results and were left free to consume antioxidants Supported by the National Eye Institute, Bethesda, Maryland (grant nos: EY012211, EY012261, EY012279) COI declaration: the Manuscript Writing Team had no COI with regard to the material presented in the article |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly permuted block method used, stratified by clinical centre and using a randomly chosen block size |
| Allocation concealment (selection bias) | Low risk | Eligibility assessed before randomisation and central allocation by telephone |
| Blinding (performance bias and detection bias) Development of CNV/geographic atrophy | Low risk | Unmasked study, but CNV occurrence was sufficiently objective as a diagnosis to be considered unbiased |
| Blinding (performance bias and detection bias) Measurement of vision | Low risk | Masked VA examiners, unclear if care providers were masked. Participants could not be masked since no sham procedure was mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | See Appendix 8. Throughout 5 years of follow‐up, 5891 (97.2%) visits were completed of the 6061 6‐month and annual visits scheduled for surviving CAPT participants. This percentage was relatively stable over time. In the updated version of this review, we considered missing data as no risk of bias in bilateral studies because a participant with paired treatment and control eyes is missed |
| Selective reporting (reporting bias) | Low risk | Development of CNV and atrophy, as well as loss of ≥ 3 lines of VA were well defined and relevant outcomes |
| Other bias | Unclear risk | Unclear |
CNVPT.
| Methods | BILATERAL: method of allocation: right eye randomly assigned to either laser treatment or observation. Left eye assigned to alternate treatment UNILATERAL: random allocation to laser treatment or observation Stratified by clinical centre and study (bilateral/unilateral) and blocked using a randomly selected block size. Issued over telephone from central location Masking: participant: no; provider: unclear; outcome: no for fundus features; yes for VA Exclusions after randomisation: not reported Losses to follow‐up: among participants alive at 12 months, 57/57 were examined in the laser group and 58/61 in the observation group. At 2 years, 46/57 (80.7%) treated eyes compared to 47/58 (81%) control eyes were still followed. However, causes of loss to follow‐up other than death were not reported |
|
| Participants | Country: US in 15 clinical centres Enrolment period: October 1994 to December 1996 BILATERAL: number randomised: 156 participants (312 eyes). Age: mean 71 years. Sex: 61% women UNILATERAL: number randomised: 120 participants. Age: mean 73 years. Sex: 63% women in treatment group; 59% women in control group Inclusion criteria: aged ≥ 50 years with colour stereo photographs and a fluorescein angiogram of both eyes taken within 14 days of enrolment, free of any condition that would preclude 2 years' follow‐up. No exudative AMD. Study eye: > 10 large drusen (> 63 μm) within 3000 μm of the FAZ with VA of 20/40 or better and no evidence of current or past CNV BILATERAL: no exudative AMD in both eyes UNILATERAL: no evidence of current or past CNV. Exudative AMD in fellow (non‐study) eye Exclusion criteria: evidence of serous PED ≥ 1 MPS disc area, geographic atrophy within 500 μm of the centre of the FAZ, myopia (≥ 8 dioptres spherical equivalent), previous laser treatment to the retina, severe non‐proliferative or proliferative diabetic retinopathy or diabetic macular oedema, progressive ocular disease |
|
| Interventions | Treatment: low‐intensity laser treatment. 3 different laser treatment protocols: 1. Laser 20: 20 laser burns, 100 μm in diameter, in a pattern of 3 rows placed between the 12 and 6 o'clock positions beyond the temporal perimeter of the FAZ. The desired intensity of the burns was a grey‐white lesion. Direct application of laser burns to drusen to be avoided. Whenever the area of drusen had not been reduced by ≥ 50% at 6 months of enrolment, a second treatment was applied nasal to the fovea in a mirror image of the first treatment. During the last 6 months of enrolment, a second laser treatment protocol was adopted that specified 24 laser burns, 100 μm in diameter in a circular pattern of 2 rows surrounding the macular drusen Control: observation of fellow eyes |
|
| Outcomes | VA (EDTRS); contrast threshold (Pelli Robson); reading ability (MN Read charts) Development of CNV, development of geographic atrophy, disappearance of drusen (stereoscopic colour photographs of the macular and disc of each eye and fluorescein angiogram) |
|
| Notes | Enrolment in these pilot studies was suspended after recommendation by the Data and Safety Monitoring Committee (DSMC) because there was a higher incidence of CNV within 12 months of study enrolment in laser‐treated eyes than in observed eyes, predominantly in the Fellow Eye Study Furthermore, data from the bilateral study arm were reported at 12 months but not thereafter Supported by an unrestricted gift from Research to Prevent Blindness, New York, NY, to the University of Pennsylvania; gifts to the Macular Degeneration Research Fund, Department of Ophthalmology, University of Pennsylvania, Philadelphia, PA; grants from the Macula Foundation, New York, NY; Research Foundation of the University of Pennsylvania, Philadelphia, PA; and Mackall Trust, New York, NY; and grant R21 EY11275 from the National Eye Institute, National Institutes of Health, Bethesda, MD COI declaration: none of the authors have a proprietary interest in this study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified by clinical centre and study (bilateral/unilateral) and blocked using a randomly selected block size |
| Allocation concealment (selection bias) | Low risk | Issued over the telephone from central location |
| Blinding (performance bias and detection bias) Development of CNV/geographic atrophy | Low risk | Unmasked study, but CNV occurrence was sufficiently objective as a diagnosis to be considered unbiased |
| Blinding (performance bias and detection bias) Measurement of vision | High risk | Participant and outcome assessors were not masked, unclear if care providers were masked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | See Results, Appendix 8, Figure 4. UNILATERAL: 81% followed at 2 years in both study arms; loss to follow‐up was balanced but causes of loss were not reported |
| Selective reporting (reporting bias) | Low risk | Development of CNV and atrophy, as well as loss of ≥ 3 lines of VA were well defined and relevant outcomes |
| Other bias | High risk | Enrolment in these pilot studies was suspended under recommendation by the Data and Safety Monitoring Committee (DSMC) because there was a higher incidence of CNV within 12 months of study enrolment in laser‐treated eyes than in observed eyes, predominantly in the Fellow Eye Study |
DLS.
| Methods | Method of allocation: randomisation was conducted with a computerised weighted coin method in the Research and Development office. The randomisation assignment was provided by telephone, and the clinic co‐ordinator printed the randomisation assignment on the participant's baseline form. The clinical investigator was then informed of the randomisation allocation. All study eyes of eligible participants in the UNILATERAL group were randomised. The study eye was randomised to laser treatment or no laser treatment. All right eyes of eligible participants in the BILATERAL group were randomised to laser treatment or no laser treatment; the fellow eye received the alternate treatment Masking: participant: unclear; provider: unclear; outcome assessor: masked VA examiner Exclusions after randomisation: none reported Losses to follow‐up: UNILATERAL: at 3 years, VA was obtained in 73/92 (80.7%) laser‐treated eyes vs. 66/85 (77.6%) control eyes. Development of CNV was recorded in 91/92 treated eyes and 85/85 control eyes. BILATERAL: VA obtained in 72/105 participants at 3 years, and CNV development assessed in 103/105 eyes at 3 years Unusual study design: some participants had both eyes randomised (BILATERAL group) and within‐person correlation was taken into account |
|
| Participants | Country: UK BILATERAL: number randomised: 105 participants (210 eyes). Age: 70.1 years (range: 52 to 100). Sex: 31 men/74 women UNILATERAL: number randomised: 177 participants. Age: 72 years (range: 54 to 87). Sex: 80 men/97 women Inclusion criteria: drusen with/without focal RPE hyperpigmentation in the study eye and CNV in the fellow eye; BCVA at least 6/12 (20/40); aged at least 50 years Exclusion criteria: geographic atrophy in either eye; any other eye disease able to influence VA; allergy to fluorescein |
|
| Interventions | Treatment: argon green/yellow dye laser with 200‐μm spot size, 0.2 second duration and the lowest energy to produce a very faint burn; overall 12 burns: 4 burns placed 750 μm from FAZ centre (12, 3, 6, 9 o'clock), and 8 burns 1500 μm from FAZ centre (12, 1.30, 3, 4.30, 6, 7.30, 9. 10.30, 12 o'clock); drusen treated directly if they were coincident with protocol treatment allocation Control: observation |
|
| Outcomes | Proportion of participants who developed CNV; VA | |
| Notes | Protocol of treatment revised after 23 months: 12 burns (0.2 seconds to 200‐μm spot size) placed in circular pattern at 1000 μm from FAZ centre Supported in part by Deutsche Forschungsgemeinschaft (DFG GR 1007/3‐1 and Ho 1926/1‐2) and the Deutsche Akademischer Austauschdienst ARC IX‐95/32 (MG) COI declaration: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated method |
| Allocation concealment (selection bias) | Low risk | The clinical investigator was informed of the randomisation allocation by the co‐ordinator by telephone after eligibility was assessed |
| Blinding (performance bias and detection bias) Development of CNV/geographic atrophy | Low risk | Unmasked study, but CNV occurrence was sufficiently objective as a diagnosis to be considered unbiased |
| Blinding (performance bias and detection bias) Measurement of vision | Low risk | Masked VA examiners. Participants cannot be masked since no sham procedure was mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | See Results, Appendix 8. Losses to follow‐up were balanced but causes were not reported; no risk of bias given the paired study design for the BILATERAL study arm |
| Selective reporting (reporting bias) | Low risk | Development of CNV and atrophy, as well as loss of ≥ 3 lines of VA were well defined and relevant outcomes |
| Other bias | High risk | The trial was stopped early after an interim analysis suggested that laser treatment induced CNV in treated eyes of participants in the unilateral group |
Figueroa 1994.
| Methods | Method of allocation: not reported. 1 eye of participants with bilateral drusen was assigned to treatment and the fellow eye to control Masking: not reported if participants and providers, but participants could not be masked since there was no sham procedure. VA examiners were masked Exclusions after randomisation: none reported Losses to follow‐up: since they reported on results at last examination (mean follow‐up 3 years), assessing the impact of loss to follow‐up was difficult Unusual study design: paired or bilateral study; authors also reported on a parallel case series of people with CNV in 1 eye who were all treated in the fellow eye |
|
| Participants | Country: Spain Number randomised: 30 participants (60 eyes) Age: 69 years (range: 62 to 74) Inclusion criteria: AMD with large confluent soft drusen involving the fovea Exclusion criteria: not specified |
|
| Interventions | Treatment: green argon laser; 0.1 mW, 0.1 seconds, 100‐μm spot; laser spot on drusen in the temporal fovea, or grid pattern if drusen > 300 μm Control: observation Duration: mean 3 years (range: 1.5 to 5) |
|
| Outcomes | Occurrence of CNV, reduction of drusen, VA | |
| Notes | Drusen resolution possible also for drusen located far from the laser application Supported in part by National Institutes of Health grant NEI EY12769 and 5 P30 EY 01583, the Vivian Simkins Lasko Research Fund, the Nina C. Mackall Trust, and an unrestricted grant from Research to Prevent Blindness, New York, NY COI declaration: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Development of CNV/geographic atrophy | Low risk | Unmasked study, but CNV occurrence was sufficiently objective as a diagnosis to be considered unbiased |
| Blinding (performance bias and detection bias) Measurement of vision | Low risk | Masked visual examiner |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | See Results, Appendix 8. Data at mean follow‐up were reported. Since 12/30 participants were followed for < 3 years, it was difficult to assess the impact of this type of reporting. However, in the updated version of this review, we considered missing data as no risk of bias in bilateral studies because a participant with paired treatment and control eyes is missed |
| Selective reporting (reporting bias) | Unclear risk | Development of CNV and atrophy, as well as loss of ≥ 3 lines of VA were well defined and relevant outcomes |
| Other bias | Unclear risk | Unclear |
Frennesson 1995.
| Methods | Method of allocation: not reported; in 5 participants with both eyes eligible the eye with better VA was randomised Masking: participant: unclear; provider: unclear; outcome: unclear Exclusions after randomisation: none reported Losses to follow‐up: 2/19 participants in the treated group vs. 0/19 in the control group lost to follow‐up at 3 years Unusual study design |
|
| Participants | Country: Sweden Number randomised: 38 participants Age: 71.6 years (SD 6.5) treated participants; 68.5 years (SD 6.2) control participants Inclusion criteria: soft drusen; VA at least 0.8 Exclusion criteria: CNV, PED, pigmentary clumping, macular atrophy, haemorrhage, any other eye disorder that could affect VA |
|
| Interventions | Treatment: argon green laser with 200‐μm spot size, 0.05 seconds' duration, power to produce a barely visible lesion. Treatment with a temporal horse shoe‐shaped area extending to the vascular arcades, with direct treatment of the drusen Control: observation Duration: 3‐8 years |
|
| Outcomes | Anatomic: mean drusen area, development of CNV. Functional: Snellen VA; colour vision (Farnsworth panel D‐15); central visual field (Humphrey 10‐2) | |
| Notes | The study was supported by grants from the Swedish Medical Research Council (Project No 12X‐734), from the Research Committee of the County of Östergötland and from Synfrämjandet's Research Foundation COI declaration: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Development of CNV/geographic atrophy | Low risk | Unmasked study, but CNV occurrence was sufficiently objective as a diagnosis to be considered unbiased |
| Blinding (performance bias and detection bias) Measurement of vision | Unclear risk | Not reported. Participants could not be masked since no sham procedure was mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | See Results, Appendix 8. 2/19 (11%) participants in the treated group vs. 0/19 in the control group lost to follow‐up at 3 years; causes of loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Development of CNV and atrophy, as well as loss of ≥ 3 lines of VA were well defined and relevant outcomes |
| Other bias | Unclear risk | Unclear |
Frennesson 2009.
| Methods | Method of allocation: randomisation generated as a permuted block design; the randomisation was delivered from Linkoping University Hospital. Enrolling doctors were not masked to treatment allocation (personal communication) Masking: participant: yes; provider: no; outcome: no (personal communication) Outcome: incidence of CNV, VA Follow up: mean 3.7 years (range 1‐7.5 years) Exclusions after randomisation: none reported Losses to follow‐up: two‐thirds of participants were followed up to 4 years, with losses balanced across groups Unusual study design: nothing reported |
|
| Participants | Country: Sweden, Denmark, Finland Number randomised: 135 participants Age: mean 70.4 years Inclusion criteria: people with soft drusen with or without mild pigmentary changes; VA ≥ 0.8 (20/25) in the study eye, aged ≥ 50 years Exclusion criteria: including pigmentary clumping, PED, CNV, haemorrhage or macular atrophy, and any other ophthalmological disease in the study eye that might possibly influence the outcome |
|
| Interventions | Treatment: laser treatment (subthreshold or barely visible laser spots). About 100 mild argon green laser spots with a size of 200 µm and a duration of 0.05 seconds Unspecified control, possibly observation only |
|
| Outcomes | VA, occurrence of CNV | |
| Notes | The study was supported by grants from the Health Research Council in the South‐East Region of Sweden, Crown Princess Margareta's Foundation for the Visually Handicapped and Synframjandet’s Research Foundation COI information: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, permuted block design |
| Allocation concealment (selection bias) | High risk | Randomisation was delivered from Linkoping University Hospital. Enrolling doctors were not masked to treatment allocation |
| Blinding (performance bias and detection bias) Development of CNV/geographic atrophy | Low risk | Participants masked and doctors unmasked, but CNV occurrence was sufficiently objective as a diagnosis to be considered unbiased |
| Blinding (performance bias and detection bias) Measurement of vision | High risk | Care providers were unmasked. Participants could not be masked since no sham procedure was mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Mean follow‐up time was about 3.5 years and two‐thirds of participants were followed up to 4 years, with losses balanced across groups. Study authors reported causes of missingness were death or illness in 5 of 6 cases at 2 years |
| Selective reporting (reporting bias) | Low risk | Main relevant outcome measure were reported |
| Other bias | Unclear risk | Unclear |
Laser to Drusen Study 1995.
| Methods | Method of allocation: computer‐generated randomisation list with randomly selected block sizes. Allocation groups: observation vs. laser (1 : 1), laser further divided (1 : 1) in temporal vs. nasal and temporal treatment Masking: participant: unclear; provider: unclear; outcome: unclear Exclusions after randomisation: none reported Losses to follow‐up: 7/47 (15%) of treatment group and 10/52 (19%) of control group seen at 2 years |
|
| Participants | Country: US Number randomised: 99 participants Age: mean 74 years (SD 6.6), range 55 to 84 years Sex: 69.7% women Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Laser wavelength: dye yellow laser (577 nm) or infrared diode (very early ‐ was discontinued). Number of burns: various, 2 scatter patterns described below; spot size: 50 μm; duration: 0.1 seconds; intensity: very light grey burn (just visible); no treatment within 500 μm of foveal centre and beyond 3000 μm from foveal centre; scatter burns approximately 2‐3 burn widths apart, trying to avoid placing burns directly over focal clumps of hyperpigmentation. Do not have to place directly on drusen, but in placing scatter, small placement changes (< 50 μm) should be done to centre spot on drusen Pattern 1: (temporal = 180 degree) ‐ not placed in nasal portion of macula (vertical line intersects foveal centre) Pattern 2: (temporal and nasal = 360 degree) ‐ burns placed in scatter both nasal and temporal portion of macula (exclusive of central macula within 500 μm of foveal centre and not beyond 3000 μm of foveal centre) |
|
| Outcomes | Development of CNV; VA; information on other outcomes not available | |
| Notes | Randomisation changed ‐ originally 1 : 1 (laser vs. observation), then laser group randomised 1 : 1 (infrared diode vs. yellow dye) ‐ each colour laser was randomised 1:1 (temporal vs. temporal and nasal) The red diode laser arm was stopped early (probably December 1995) Pilot study nature ‐ so some clinical centres did not do all tests (reading, contrast) ‐ not all clinical photographs graded Funding source unknown |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated. Randomly selected block size (Marta M Gilson, personal communication) |
| Allocation concealment (selection bias) | Low risk | Serially numbered sealed opaque envelopes. Co‐ordinator had to fill out checklist ‐ document eligibility ‐ then open sequentially numbered envelope, record date opened, time opened, participant number, name code and sign the form (2 copies ‐ keep 1, and fax other to co‐ordinating centre within 24 hours of opening). Faxed forms were later mailed to co‐ordinating centre (Marta M Gilson personal communication) |
| Blinding (performance bias and detection bias) Development of CNV/geographic atrophy | Low risk | Participants: unclear; care providers: ophthalmologists (applying laser) were not masked; care providers ‐ co‐ordinators: unclear; outcome assessors: Photograph Reading Centre graders were to be masked, but it was possible that some of the laser scars may have unmasked the graders (Marta M Gilson, personal communication) |
| Blinding (performance bias and detection bias) Measurement of vision | Unclear risk | VA examiners: unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | See Results, Appendix 8, Figure 4. 7/47 (15%) of treatment group and 10/52 (19%) of control group lost at 2 years. No information on reasons for loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Outcomes selected by review author |
| Other bias | Unclear risk | Unclear |
Little 1995.
| Methods | Method of allocation: after participants eligibility was ascertained and participant consent was obtained, 1 eye was randomised to photocoagulation treatment; the right eye was assigned to treatment if participant's birth date was an odd month, the left if it was an even month Masking: participant: unclear; provider: unclear; outcome assessor: unclear Exclusions after randomisation: none reported Losses to follow‐up: a minimum 1‐year follow‐up was obtained (mean 3.2 years) Unusual study design: paired study |
|
| Participants | Country: US Number randomised: 27 participants (54 eyes) Age: mean 69.7 years Sex: 9 men/18 women Inclusion criteria: symmetrical drusen; minimum drusen size 100 μm; at least 20 drusen or 10 drusen + 2 drusen at least 500 μm in diameter; drusen within 500 μm from foveola; VA at least 20/60 Exclusion criteria: PED; atrophy; subretinal fluid, haemorrhage, exudate; any other eye disorder which could affect VA |
|
| Interventions | Treatment: 577‐ to 620‐nm wavelength laser with 100‐200 μm spot size, 0.05‐0.1 seconds' duration, 100‐200 power. Direct treatment of the drusen Control: observation Duration: 1‐ to 6‐year follow‐up |
|
| Outcomes | Snellen VA; colour vision (Farnsworth panel D‐15 colour‐test); central visual field with Humphrey 10‐2 | |
| Notes | No COI for any author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | After participants eligibility was ascertained and participant consent was obtained, 1 eye was randomised to photocoagulation treatment; the right eye was assigned to treatment if person's birth date was an odd month, the left if it was an even month |
| Allocation concealment (selection bias) | High risk | See above, the enrolling researcher could have foreseen which eye would have been treated. Nonetheless, this can be irrelevant since both eyes of each participant were included, i.e. there was no risk of confounding |
| Blinding (performance bias and detection bias) Development of CNV/geographic atrophy | Low risk | Unmasked study, but CNV occurrence was sufficiently objective as a diagnosis to be considered unbiased |
| Blinding (performance bias and detection bias) Measurement of vision | High risk | Not reported. Participants could not be masked since no sham procedure was mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Unclear: only last visit data reported, thus being impossible to reconstruct the pattern of missing data; 4/27 participants were followed for ≥ 1 year but < 2 years. However, in the updated version of this review, we considered missing data as no risk of bias in bilateral studies because a participant with paired treatment and control eyes is missed |
| Selective reporting (reporting bias) | Low risk | Development of CNV and atrophy, as well as loss of ≥ 3 lines of VA were well defined and relevant outcomes |
| Other bias | Unclear risk | Unclear |
Olk 1999.
| Methods | Method of allocation: not reported; BILATERAL: 1 eye was assigned to treatment and 1 eye to observation. UNILATERAL: 1 eye eligible that eye was assigned to either treatment or observation. BILATERAL/UNILATERAL: eyes assigned to treatment were further randomised to either 'visible' or 'subthreshold' treatment Masking: participant: unclear; provider: unclear; outcome: unclear Exclusions after randomisation: 25/152 participants (35 eyes) were enrolled initially in the pilot study but subsequently determined to be ineligible for various reasons, mainly violation of inclusion criteria Losses to follow‐up: at 24 months, 33 eyes had missed visits: 9 eyes (4 observation, 2 visible, 3 subthreshold) were in deceased participants, 14 eyes were in the observation group, and 10 eyes were in the treatment group (5 eyes, visible; 5 eyes, subthreshold) Unusual study design: some eyes |
|
| Participants | Country: US Number randomised: BILATERAL: 77 participants (154 eyes) with both eyes eligible. UNILATERAL: 75 participants (75 eyes) with 1 eye eligible (unilateral study arm), that eye was assigned to either treatment or observation Enrolment period: July 1994 to June 1996 Sex: 152 participants enrolled; 57 men, 95 women Age: mean 74.5 years, range 54‐88 years Inclusion criteria: aged > 50 years; diagnosis of AMD with ≥ 5 large (≥ 63 µm), soft drusen within 2250 µm of the centre of the FAZ in both eyes (bilateral study arm) or in 1 eye (unilateral study arm) if the fellow eye had evidence of exudative AMD; and VA of ≥ 20/63 on the ETDRS chart in all eligible eyes Exclusion criteria: exudative macular degeneration in either eye for bilateral participants and in both eyes for unilateral participants; other ocular diseases |
|
| Interventions | Eyes were treated with a slit‐lamp integrated diode photocoagulator using 810‐nm wavelength (IRIS Medical OcuLight SLx; IRIDEX Corp., Mt. View, CA). 48 diode laser lesions of 125 mm were applied in 4 concentric circles outside the FAZ in a scatter or grid pattern between 750 and 2250 mm from the centre of the fovea. Test spot laser lesions were applied to the retina nasal to the optic nerve using 200‐millisecond duration, and the power was increased to produce a mild grey lesion (visible burn). For eyes assigned to visible treatment, this intensity was then applied in a grid pattern as described above. For eyes assigned to subthreshold treatment, the energy needed for the visible test burn was kept constant, but the duration was halved to 100 milliseconds and treatment then carried out. Only 1 laser treatment was applied to each eye throughout the duration of the study | |
| Outcomes | Anatomic: reduction of drusen, development of CNV. Functional: VA | |
| Notes | Within‐person correlation of outcomes in the bilateral arm not analysed and reported Supported in part by grants from IRIS Medical, Mountain View, CA (producer of the laser used in the study), and The University of Pittsburgh Eye and Ear Foundation, Pittsburgh, PA COI declaration: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Development of CNV/geographic atrophy | Low risk | Unmasked study, but CNV occurrence was sufficiently objective as a diagnosis to be considered unbiased |
| Blinding (performance bias and detection bias) Measurement of vision | High risk | Not reported. Participants could not be masked since no sham procedure was mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | See Results, Appendix 8 and Figure 4. Losses to follow‐up: at 24 months, 33 eyes had missed visits: 9 eyes (4 observation, 2 visible, 3 subthreshold) were in deceased participants, 14 eyes were in the observation group, and 10 eyes were in the treatment group (5 eyes, visible; 5 eyes, subthreshold). Causes of loss to follow‐up other than death were not reported. In the updated version of this review, we considered missing data as no risk of bias in bilateral studies because a participant with paired treatment and control eyes is missed. Thus, only losses in unilateral arm was considered |
| Selective reporting (reporting bias) | Low risk | Development of CNV and atrophy, as well as loss of ≥ 3 lines of VA were well defined and relevant outcomes |
| Other bias | Unclear risk | Unclear |
PTAMD bilateral 2009.
| Methods | Method of allocation: study eyes were assigned randomly to either treatment or observation by a computer‐generated, centre‐specific, variable block size randomisation at a 1 : 1 ratio. These random assignments were concealed in opaque envelopes that were opened only upon enrolment of an eligible person who gave consent Masking: participant: unclear; provider: unclear; outcome: unclear Participant: 1278 eyes of 639 participants Outcome: development of CNV and change in best‐corrected VA Exclusions after randomisation: none reported Losses to follow‐up: 374/639 (54.3%) participants followed to 2 years Unusual study design: paired study |
|
| Participants | Country: US Number randomised: 1278 eyes of 639 participants Enrolment period: April 1996 to March 2000 Mean age: 73.0 years (SD 2.5) Inclusion criteria: aged ≥ 50 years. Eligible eye must have had BCVA of ≥ 20/63 on the ETDRS chart in both eyes; AMD with ≥ 5 drusen that were ≥ 63 μm in diameter and were located within 2250 μm of the centre of the fovea; unilateral participants must have had 1 eye ineligible due to vision loss that was attributed to advanced AMD Exclusion criteria: other ocular disease causing visual loss |
|
| Interventions | Eyes randomised to treatment received a single‐session treatment of a grid of 48 diode laser lesions of 125 μm in diameter. Laser treatment was applied in an annular grid that extended from 0.5 (750 μm) to 2.0 (3000 μm) disc diameters from the centre of the FAZ. A slit lamp‐based diode laser photocoagulation system (IRIS Medical, Mountain View, CA) emitting energy at 810 nm was used to deliver the laser treatment. Laser lesions were placed in a subthreshold manner by first delivering test spot(s) of 200‐millisecond duration placed outside of the macula at a low power (e.g. 200 mW) and then incrementally increasing the power in small (50 mW) increments until a faint grey (threshold) lesion could be detected visually through the treatment lens. While the power setting was left unchanged, the pulse duration was reduced to a 100‐millisecond interval to achieve an invisible subthreshold lesion. Laser lesions were then scattered within the annular grid as defined above, beginning by placing 12 spots in a given quadrant and then proceeding to adjacent quadrants to complete the treatment pattern. The drusen were not targeted specifically or preferentially. If a visible lesion was produced while the annular grid treatment was performed, the power setting was reduced to achieve subthreshold lesions with the remainder | |
| Outcomes | Anatomic: drusen reduction, development of CNV. Functional: VA | |
| Notes | Supported by IRIDEX Corporation, Mountain View, CA (the producer of the laser used in the study); the Eye and Ear Foundation of Pittsburgh, Pittsburgh, PA; Research to Prevent Blindness, Inc., New York, NY and unrestricted funds from several participating centres COI declaration: the authors had no financial or proprietary interest in the materials presented |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, centre‐specific, variable block size randomisation at a 1 : 1 ratio |
| Allocation concealment (selection bias) | Low risk | These random assignments were concealed in opaque envelopes that were opened only upon enrolment of an eligible person who gave consent |
| Blinding (performance bias and detection bias) Development of CNV/geographic atrophy | Low risk | Unmasked study, but CNV occurrence was sufficiently objective as a diagnosis to be considered unbiased |
| Blinding (performance bias and detection bias) Measurement of vision | Unclear risk | Not reported, masking of care providers and photograph graders might be achieved since subthreshold photocoagulation should not generate visible scars. Participants cannot be masked since no sham procedure was mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Large proportion of participants lost to follow‐up, but this was unlikely to bias effect estimates since this was a paired study. In the updated version of this review, we considered missing data as no risk of bias in bilateral studies because a participant with paired treatment and control eyes is missed |
| Selective reporting (reporting bias) | Low risk | Development of CNV and atrophy, as well as loss of ≥ 3 lines of VA were well defined and relevant outcomes |
| Other bias | Unclear risk | Unclear |
PTAMD unilateral 2002.
| Methods | Method of allocation: study eyes were assigned randomly to either treatment or observation by a computer‐generated, centre‐specific, variable block size randomisation at a 1 : 1 ratio. These random assignments were concealed in opaque envelopes that were opened only upon enrolment of an eligible person who gave consent Masking: participant: unclear; provider: unclear; outcome: unclear Exclusions after randomisation: not reported Losses to follow‐up: at 1 year, 184/244 (75%) participants followed (5 deaths), 92 treated eyes and 99 control eyes followed. At 3 years, 124/244 (51%) participants followed (20 deaths), 64 treated eyes and 55 control eyes followed Unusual study design: another arm of the study included participants with both eyes eligible, but this report deals with unilateral participants only |
|
| Participants | Country: US Number randomised: 244 participants Age: mean 75.4 years for treated participants, 75.1 years for observed participants Gender (% women): 59.3 treated participants, 61.5 observed participants Inclusion criteria: aged ≥ 50 years. Eligible eye must have had BCVA of ≥ 20/63 on the ETDRS chart; AMD with ≥ 5 drusen that were 63 μm in diameter and were located within 2250 μm of the centre of the fovea; unilateral participants must have had 1 eye ineligible due to vision loss that was attributed to advanced AMD Exclusion criteria: other ocular disease causing visual loss |
|
| Interventions | Eyes randomised to treatment received a single‐session treatment of a grid of 48 diode laser lesions of 125 μm in diameter. Laser treatment was applied in an annular grid that extended from 0.5 (750 μm) to 2.0 (3000 μm) disc diameters from the centre of the FAZ. A slit lamp‐based diode laser photocoagulation system (IRIS Medical, Mountain View, CA) emitting energy at 810 nm was used to deliver the laser treatment. Laser lesions were placed in a subthreshold manner by first delivering test spot(s) of 200‐millisecond duration placed outside of the macula at a low power (e.g. 200 mW) and then incrementally increasing the power in small (50 mW) increments until a faint grey (threshold) lesion could be detected visually through the treatment lens. While the power setting was left unchanged, the pulse duration was reduced to a 100‐millisecond interval to achieve an invisible subthreshold lesion. Laser lesions were then scattered within the annular grid as defined above, beginning by placing 12 spots in a given quadrant and then proceeding to adjacent quadrants to complete the treatment pattern. The drusen were not targeted specifically or preferentially. If a visible lesion was produced while the annular grid treatment was performed, the power setting was reduced to achieve subthreshold lesions with the remainder | |
| Outcomes | Anatomic: drusen reduction, development of CNV. Functional: VA | |
| Notes | Supported by IRIDEX Corporation, Mountain View, CA (the producer of the laser used in the study); the Eye and Ear Foundation of Pittsburgh, Pittsburgh, PA; Research to Prevent Blindness, Inc., New York, NY and unrestricted funds from several participating centres COI declaration: the authors had no financial or proprietary interest in the materials presented |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, centre‐specific, variable block size randomisation |
| Allocation concealment (selection bias) | Low risk | Random assignments were concealed in opaque envelopes that were opened only upon enrolment of an eligible person who gave consent |
| Blinding (performance bias and detection bias) Development of CNV/geographic atrophy | Low risk | Unmasked study, but CNV occurrence was sufficiently objective as a diagnosis to be considered unbiased |
| Blinding (performance bias and detection bias) Measurement of vision | Unclear risk | Not reported, masking of care providers and photograph graders might be achieved since subthreshold photocoagulation should not generate visible scars. Participants could not be masked since no sham procedure was mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | See Results, Appendix 8, Figure 4. Survival analysis used. Losses to follow‐up: at 1 year, 184/244 (75%) participants followed (5 deaths), 92 treated eyes and 99 control eyes followed. At 3 years, 124/244 (51%) participants followed (20 deaths), 64 treated eyes and 55 control eyes followed. Causes of loss other than death were not reported |
| Selective reporting (reporting bias) | Low risk | Development of CNV and atrophy, as well as loss of ≥ 3 or more lines of VA were well defined and relevant outcomes |
| Other bias | Unclear risk | Unclear |
AMD: age‐related macular degeneration; AREDS: Age‐Related Eye Disease Study; BCVA: best‐corrected visual acuity; CNV: choroidal neovascularisation; COI: conflict of interest; ETDRS: Early Treatment Diabetic Retinopathy Study; FAZ: foveal avascular zone; MPS: Macular Photocoagulation Study; PED: pigment epithelial detachment; RPE: retinal pigment epithelial; SD: standard deviation; VA: visual acuity; vs.: versus.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Guymer 2014 | Non‐randomised study assessing a novel, ultra‐low energy nanosecond laser (retinal rejuvenation therapy) to slow progression of early age‐related macular degeneration. Drusen reduction was achieved in 44% of treated eyes and 22% of untreated fellow eyes |
| Huang 2011 | Paired controlled study (10 participants): 1 eye randomly assigned to laser, the fellow eye to control. However, authors reported that participants could have chosen which eye had to receive laser, so unclear whether randomisation was maintained |
| Sarks 1999 | Comparative study but no randomisation |
| Sigelman 1991 | Case report |
Characteristics of studies awaiting assessment [ordered by study ID]
Sivagnanavel 2004.
| Methods | Prospective, double masked, randomised controlled trial at King's College Hospital, London, UK |
| Participants | People with subfoveal choroidal neovascularisation from age‐related macular degeneration in 1 eye and significant drusen (> 5 large drusen or > 20 small drusen) in the fellow eye |
| Interventions | Drusen photocoagulation by means of diode laser using large spot size, low energy and long duration (4200 μm x 400 mW x 60 seconds); control group received sham treatment (laser with no energy) |
| Outcomes | Fundus changes measured with photography, visual acuity, contrast sensitivity and colour contrast sensitivity recorded every 3 months |
| Notes | — |
Characteristics of ongoing studies [ordered by study ID]
Beaumont 2011.
| Trial name or title | Prophylactic Laser Photocoagulation of Drusen in Early Age‐Related Macular Degeneration |
| Methods | Paired controlled study, contact author reported random assignment |
| Participants | Quote: "121 consecutive patients with large, ill defined drusen within the perifoveal zone of both maculae were studied prospectively" |
| Interventions | Quote: "One eye was treated with sub‐threshold intensity photocoagulation to the drusen, sparing the fovea. The fellow eye served as the control" |
| Outcomes | Quote: "The mean follow‐up duration was 65.0 ± 24.4 months. The treated eyes lost a mean of 0.2 ± 2.5 logMAR lines, compared to 0.9 ± 2.7 lines in the control group (p = 0.051). CNV [choroidal neovascularisation] developed in 5 (4.1%) of the treated eyes and 10 (8.3%) of the control eyes, while GA [geographic atrophy] developed in 12 (9.9%) of the treated and 8 (6.6%) of the control eyes (p = 0.291)." |
| Starting date | Unknown |
| Contact information | H. Kwon Kang, Retina & Vitreous Centre, Sydney, NSW, Australia |
| Notes | Reported as ongoing and unpublished by contact author |
NCT01790802.
| Trial name or title | Laser Intervention in Early Age‐Related Macular Degeneration Study (LEAD) |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental: active laserTwelve 2RT nanosecond laser shots in 2 arcs of 6 shots superiorly and 6 shots inferiorly, inside the retinal vascular arcades at an approximate distance from the fovea of 3000 μm, with approximately 1 laser spot diameter between them Sham comparator: sham laser procedure. To simulate laser application the maximum illumination button will be briefly pressed by the operating physician at each of the 12 locations described above where and when the laser would normally be applied. The laser remains in standby mode preventing accidental laser firing |
| Outcomes | Primary outcomes:
Secondary outcomes:
Other outcomes: reversal of early clinical indicators of AMD (time frame: 36 months), reversal of early clinical indicators of AMD (drusen area) Improvements in visual acuity (time frame: 36 months) |
| Starting date | Estimated enrolment: 250 Study start date: November 2011 Estimated study completion date: June 2017 Estimated primary completion date: June 2017 (final data collection date for primary outcomes) |
| Contact information | Centre for Eye Research Australia ‐ Royal Victorian Eye & Ear Hospital East Melbourne, Victoria, Australia, 3002 Emily EA Caruso, B Orth & OphSc +61 3 9929 emily.caruso@unimelb.edu.au |
| Notes |
AMD: age‐related macular degeneration; BCVA: best‐corrected visual acuity; CNV: choroidal neovascularisation; GA: geographical atrophy; FAF: fundus autofluorescence; MAIA: macular integrity assessment; OCT: optical coherence tomography; RPE: retinal pigment epithelial.
Differences between protocol and review
The protocol was originally published in 2007 and since that time there have been considerable developments in Cochrane methodology including assessment of risk of bias and preparation of 'Summary of findings' tables and grading of the overall quality of evidence using GRADE. We have incorporated these developments in the review.
We have also made the following specific changes from the protocol.
In the protocol, drusen reduction was planned to be evaluated considering the number of eyes showing at least a 50% reduction of drusen area from the baseline aspect. However, data were sparsely reported and, therefore, we modified the protocol to allow an extraction based on the investigators' definition.
In the protocol, we planned to use the risk ratio as the main effect measure but we used the odds ratio because this made it easier to adjust for within‐person correlation. See section 'Measures of treatment effect'.
In the 2015 update, we decided that the risk of bias due to missing data was low in bilateral trials, since a pair of treatment and control eyes would be lost, which would be unlikely to alter the odd ratio significantly. Thus, we simplified the sensitivity analysis of missing data as described in Appendix 8.
Contributions of authors
Conceiving the review: JE.
Designing the review: MBP, JE, GV.
Co‐ordinating the review: GV, MBP, JE.
Data collection for the review.
Designing search strategies: Cochrane Eyes and Vision Group.
Undertaking searches: Cochrane Eyes and Vision Group.
Screening search results: MBP, JE, GV, DB, MM.
Organising retrieval of papers: Cochrane Eyes and Vision Group.
Screening retrieved papers against inclusion criteria: MBP, JE, GV, DB, MM.
Appraising quality of papers: MBP, JE, GV, DB, MM.
Extracting data from papers: GV, DB, MM, MBP.
Writing to authors of papers for additional information: JE, GV, DB, MM.
Obtaining and screening data on unpublished studies: JE, GV, DB, MM.
Data management for the review.
Entering data into Review Manager 5: GV, DB, MM.
Analysis of data: GV, DB, MM, JE, MBP.
Interpretation of data.
Providing a methodological perspective: GV, JE, MBP.
Providing a clinical perspective: MBP, GV, DB, MM.
Providing a policy perspective: JE, MBP.
Providing a consumer perspective: AMD Consumer Panel.
Writing the review: GV, MBP, JE, DB, MM.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute of Health Research (NIHR), UK.
- Richard Wormald, Co‐ordinating Editor for the Cochrane Eyes and Vision Group (CEVG) acknowledges financial support for his CEVG research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
- The NIHR also funds the CEVG Editorial Base in London.
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS, or the Department of Health.
The contribution of the IRCCS Fondazione Bietti in this paper was supported by the Italian Ministry of Health and by Fondazione Roma, Italy.
Declarations of interest
None known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
CAPT {published data only}
- Alexander J, Elsner KS, Javornik NB, Whittock ER, Brightwell‐Arnold M, Duncan JL, et al. Characteristics of exudation in patients with bilateral large drusen. Investigative Ophthalmology and Visual Science 2004;45:ARVO E‐abstract 2989. [Google Scholar]
- Complications of Age‐Related Macular Degeneration Prevention Trial Research Group. Laser treatment in patients with bilateral large drusen: the complications of age‐related macular degeneration prevention trial. Ophthalmology 2006;113(11):1974‐86. [DOI] [PubMed] [Google Scholar]
- Complications of Age‐Related Macular Degeneration Prevention Trial Study Group. The Complications of Age‐Related Macular Degeneration Prevention Trial (CAPT): rationale, design and methodology. Clinical Trials 2004;1(1):91‐107. [DOI] [PubMed] [Google Scholar]
- Figueroa M, Schocket LS, DuPont J, Metelitsina TI, Grunwald JE. Effect of laser treatment for dry age related macular degeneration on foveolar choroidal haemodynamics. British Journal of Ophthalmology 2004;88(6):792‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa M, Schocket LS, DuPont J, Metelitsina TI, Grunwald JE. Long‐term effect of laser treatment for dry age‐related macular degeneration on choroidal hemodynamics. American Journal of Ophthalmology 2006;141(5):863‐7. [DOI] [PubMed] [Google Scholar]
- Grunwald JE, Figueroa M, Metelitsina TI, Schocket LS, DuPont JC. Effect of CAPT laser treatment on choroidal hemodynamics in age‐related macular degeneration (AMD). Investigative Ophthalmology and Visual Science 2004;45:ARVO E‐abstract 3133. [Google Scholar]
- Maguire M, Complications of Age‐Related Macular Degeneration Prevention Trial Research Group. Baseline characteristics, the 25‐Item National Eye Institute Visual Functioning Questionnaire, and their associations in the Complications of Age‐Related Macular Degeneration Prevention Trial (CAPT). Ophthalmology 2004;111(7):1307‐16. [DOI] [PubMed] [Google Scholar]
- Sbarbaro JA, Maguire MG, CAPT Study Group. Annual changes in refractive error over 3 years among eyes with early AMD. Investigative Ophthalmology and Visual Science 2004;45:ARVO E‐abstract 3055. [Google Scholar]
- Stoltz RA, Ying GS, Alexander J, CAPT Study Group. Fundus features of untreated eyes with visual acuity loss in CAPT. Investigative Ophthalmology and Visual Science 2004;45:ARVO E‐abstract 1389. [Google Scholar]
- Ying GS, Liu C, Maguire MG, CAPT Research Group. Night‐vision symptoms as a risk factor for vision loss and choroidal neovascularization in the Complications of Age‐related Macular Degeneration Prevention Trial (CAPT). Investigative Ophthalmology and Visual Science 2005;46:ARVO E‐abstract 204. [Google Scholar]
CNVPT {published data only}
- Anonymous. Choroidal neovascularization in the Choroidal Neovascularization Prevention Trial. The Choroidal Neovascularization Prevention Trial Research Group. Ophthalmology 1998;105(8):1364‐72. [DOI] [PubMed] [Google Scholar]
- Anonymous. Laser treatment in eyes with large drusen. Short‐term effects seen in a pilot randomized clinical trial. Choroidal Neovascularization Prevention Trial Research Group. Ophthalmology 1998;105(1):11‐23. [PubMed] [Google Scholar]
- Choroidal Neovascularization Prevention Trial Research Group. Laser treatment in fellow eyes with large drusen: updated findings from a pilot randomized clinical trial. Ophthalmology 2003;110(5):971‐8. [DOI] [PubMed] [Google Scholar]
- Fine SL, Maguire MG, Javornik NB, Ho AC, CNVPT Research Group. The Choroidal Neovascularization Prevention Trial (CNVPT): 4‐year results. Investigative Ophthalmology and Visual Science 2001;42:ARVO Abstract 676. [Google Scholar]
- Ho AC, Maguire MG, Yoken J, Lee MS, Shin DS, Javornik NB, et al. Laser‐induced drusen reduction improves visual function at 1 year. Choroidal Neovascularization Prevention Trial Research Group. Ophthalmology 1999;106(7):1367‐73. [DOI] [PubMed] [Google Scholar]
- Ho AC, Yoken J, Lee MS, Shin DS, Javornik NB, Maguire MG, et al. Laser‐induced drusen reduction improves visual function at one year. American Academy of Ophthalmology 1998:127. [DOI] [PubMed] [Google Scholar]
- Kaiser RS, Berger JW, Maguire MG, Ho AC, Javornik NB. Choroidal Neovascularization Prevention Trial Study Group. Laser burn intensity and the risk for choroidal neovascularization in the CNVPT Fellow Eye Study. Archives of Ophthalmology 2001;119(6):826‐32. [DOI] [PubMed] [Google Scholar]
- Prenner JL, Rosenblatt BJ, Tolentino MJ, Ying GS, Javornik NB, Maguire MG, et al. Risk factors for choroidal neovascularization and vision loss in the fellow eye study of CNVPT. Retina 2003;23(3):307‐14. [DOI] [PubMed] [Google Scholar]
- Regillo CD. Laser treatment in fellow eyes with large drusen: updated findings from a pilot randomized clinical trial. Evidence‐Based Eye Care 2003;4(4):204‐5. [DOI] [PubMed] [Google Scholar]
- Rosenbaltt BJ, Prenner JL, Tolentino M, Ying G, Javornik NB, Maguire MG, et al. Predictors of visual acuity loss in the fellow eye study of the Choroidal Neovascularization Prevention Trial (CNVPT). Investigative Ophthalmology and Visual Science 2001;42:ARVO E‐abstract 675. [Google Scholar]
DLS {published data only}
- Kertes PJ. Prophylactic laser treatment hastens choroidal neovascularization in unilateral age‐related maculopathy: final results of the drusen laser study ‐ commentary. Evidence‐Based Ophthalmology 2006;7(3):135‐7. [DOI] [PubMed] [Google Scholar]
- Owens SL, Bunce C, Brannon AJ, Wormald R, Bird AC, Drusen Laser Study Group. Prophylactic laser treatment appears to promote choroidal neovascularisation in high‐risk ARM: results of an interim analysis. Eye 2003;17(5):623‐7. [DOI] [PubMed] [Google Scholar]
- Owens SL, Bunce C, Brannon AJ, Xing W, Chisholm IH, Gross M, et al. Prophylactic laser treatment hastens choroidal neovascularization in unilateral age‐related maculopathy: final results of the drusen laser study. American Journal of Ophthalmology 2006;141(2):276‐81. [DOI] [PubMed] [Google Scholar]
- Owens SL, Guymer RH, Gross‐Jendroska M, Bird AC. Fluorescein angiographic abnormalities after prophylactic macular photocoagulation for high‐risk age‐related maculopathy. American Journal of Ophthalmology 1999;127(6):681‐7. [DOI] [PubMed] [Google Scholar]
Figueroa 1994 {published data only}
- Figueroa MS, Regueras A, Bertrand J. Laser photocoagulation to treat macular soft drusen in age‐related macular degeneration. Retina 1994;14(5):391‐6. [DOI] [PubMed] [Google Scholar]
- Figueroa MS, Regueras A, Bertrand J, Aparicio MJ, Manrique MG. Laser photocoagulation for macular soft drusen. Updated results. Retina 1997;17(5):378‐84. [DOI] [PubMed] [Google Scholar]
Frennesson 1995 {published data only}
- Frennesson C, Nilsson SE. Prophylactic laser treatment in early age related maculopathy reduced the incidence of exudative complications. British Journal of Ophthalmology 1998;82(10):1169‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frennesson CI. Prophylactic laser treatment in early age‐related maculopathy: an 8‐year follow‐up in a randomized pilot study shows a reduced incidence of exudative complications. Acta Ophthalmologica Scandinavica 2003;81(5):449‐54. [DOI] [PubMed] [Google Scholar]
- Frennesson IC, Nilsson SE. Effects of argon (green) laser treatment of soft drusen in early age‐related maculopathy: a 6 month prospective study. British Journal of Ophthalmology 1995;79(10):905‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frennesson IC, Nilsson SE. Laser photocoagulation of soft drusen in early age‐related maculopathy (ARM). The one‐year results of a prospective, randomised trial. European Journal of Ophthalmology 1996;6(3):307‐14. [DOI] [PubMed] [Google Scholar]
Frennesson 2009 {published and unpublished data}
- Frennesson CI, Bek T, Jaakkola A, Nilsson SE. Prophylactic Laser Treatment Study Group. Prophylactic laser treatment of soft drusen maculopathy: a prospective, randomized Nordic study. Acta Ophthalmologica 2009;87(7):720‐4. [DOI] [PubMed] [Google Scholar]
Laser to Drusen Study 1995 {unpublished data only}
- Bressler SB, Vitale S, Hawkins BS, Alexander J, Orr PR, Schachat AP, et al. Laser to Drusen Trial: an assessment of short term safety within randomized, prospective, controlled clinical trial. Investigative Ophthalmology and Visual Science 1995;36:ARVO E‐abstract 1028. [Google Scholar]
Little 1995 {published data only}
- Little HL, Showman J. A pilot randomized, controlled study on the effect of laser photocoagulation of confluent soft macular drusen. American Academy of Ophthalmology 1995:120. [DOI] [PubMed] [Google Scholar]
- Little HL, Showman JM, Brown BW. A pilot randomized controlled study on the effect of laser photocoagulation of confluent soft macular drusen. Ophthalmology 1997;104(4):623‐31. [DOI] [PubMed] [Google Scholar]
Olk 1999 {published data only}
- Olk RJ, Friberg TR, Stickney KL, Akduman L, Wong KL, Chen MC, et al. Therapeutic benefits of infrared (810‐nm) diode laser macular grid photocoagulation in prophylactic treatment of nonexudative age‐related macular degeneration: two‐year results of a randomized pilot study. Ophthalmology 1999;106(11):2082‐90. [DOI] [PubMed] [Google Scholar]
PTAMD bilateral 2009 {published and unpublished data}
- Friberg TR, Brennen PM, Freeman WR, Musch DC, PTAMD Study Group. Prophylactic treatment of age‐related macular degeneration report number 2: 810‐nanometer laser to eyes with drusen: bilaterally eligible patients. Ophthalmic Surgery, Lasers and Imaging 2009;40(6):530‐8. [DOI] [PubMed] [Google Scholar]
PTAMD unilateral 2002 {published data only}
- Friberg TR, Musch DC, Lim JI, Morse L, Freeman W, Sinclair S, et al. Prophylactic treatment of age‐related macular degeneration. Report number 1: 810‐nanometer laser to eyes with drusen. Unilaterally eligible patients. Ophthalmology 2006;113(4):612‐22. [DOI] [PubMed] [Google Scholar]
- Rodanant N, Friberg TR, Cheng L, Aurora A, Bartsch D, Toyoguchi M, et al. Predictors of drusen reduction after subthreshold infrared (810 nm) diode laser macular grid photocoagulation for nonexudative age‐related macular degeneration. American Journal of Ophthalmology 2002;134(4):577‐85. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Guymer 2014 {published data only}
- Guymer RH, Brassington KH, Dimitrov P, Makeyeva G, Plunkett M, Xia W, et al. Nanosecond‐laser application in intermediate AMD: 12‐month results of fundus appearance and macular function. Clinical and Experimental Ophthalmology 2014;2(5):466‐79. [DOI] [PubMed] [Google Scholar]
Huang 2011 {published and unpublished data}
- Huang YX, Xiang LN, Wang YL, Li MM, Hu YX. Long‐term effect of prophylactic laser treatment for bilateral soft drusen. Chinese Medical Journal 2011;124(4):541‐5. [PubMed] [Google Scholar]
Sarks 1999 {published data only}
- Sarks SH, Arnold JJ, Killingsworth MC, Sarks JP. Early drusen formation in the normal and aging eye and their relation to age related maculopathy: a clinicopathological study. British Journal of Ophthalmology 1999;83(3):358‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sigelman 1991 {published data only}
- Sigelman J. Foveal drusen resorption one year after perifoveal laser photocoagulation. Ophthalmology 1991;98(9):1379‐83. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Sivagnanavel 2004 {published data only}
- Sivagnanavel V, Chong V. Diffuse laser for drusen reduction in high risk age‐related maculopathy. Investigative Ophthalmology and Visual Science 2004;45:ARVO E‐abstract 3097. [Google Scholar]
References to ongoing studies
Beaumont 2011 {published data only}
- Beaumont P, Kang HK, Gorbatov M, Do H, Matthew R. Prophylactic laser photocoagulation of drusen in early age‐related macular degeneration. Clinical and Experimental Ophthalmology 2011;39(Suppl 1):23‐41. [Google Scholar]
NCT01790802 {unpublished data only}
- NCT01790802. A multi‐centre, randomized trial into the safety and efficacy of nanosecond microsurgical laser intervention in early age‐related macular degeneration. clinicaltrials.gov/show/NCT01790802 (accessed 12 August 2015).
Additional references
Abdelsalam 1999
- Abdelsalam A, Priore L, Zarbin MA. Drusen in age‐related macular degeneration: pathogenesis, natural course, and laser photocoagulation‐induced regression. Survey of Ophthalmology 1999;44(1):1‐29. [DOI] [PubMed] [Google Scholar]
AREDS 2001
- Age‐Related Eye Disease Study Research Group. A randomized, placebo‐controlled clinical trial of high‐dose supplementation with vitamins C and E, beta carotene, and zinc for age‐related macular degeneration and vision loss: AREDS report No. 8. Archives of Ophthalmology 2001;119(10):1417‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bressler 1994
- Bressler NM, Silva JC, Bressler SB, Fine SL, Green WR. Clinicopathologic correlation of drusen and retinal pigment epithelial abnormalities in age‐related macular degeneration. Retina 1994;14(2):130‐42. [PubMed] [Google Scholar]
Bressler 1995
- Bressler NM, Munoz B, Maguire MG, Vitale SE, Schein OD, Taylor HR, et al. Five‐year incidence and disappearance of drusen and retinal pigment epithelial abnormalities. Waterman study. Archives of Ophthalmology 1995;113(3):301‐8. [DOI] [PubMed] [Google Scholar]
Brown 2006
- Brown MM, Brown GC, Sharma S, Stein JD, Roth Z, Campanella J, et al. The burden of age‐related macular degeneration: a value‐based analysis. Current Opinion in Ophthalmology 2006;17(3):257‐66. [DOI] [PubMed] [Google Scholar]
Chakravarthy 2005
- Chakravarthy U, Stevenson M. Self‐reported visual functioning and quality of life in age‐related macular degeneration. Current Opinion in Ophthalmology 2005;16(3):179‐83. [DOI] [PubMed] [Google Scholar]
Chong 2007
- Chong EW, Wong TY, Kreis AJ, Simpson JA, Guymer RH. Dietary antioxidants and primary prevention of age related macular degeneration: systematic review and meta‐analysis. BMJ 2007;335(7623):755. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cleasby 1979
- Cleasby GW, Nakanishi AS, Norris JL. Prophylactic photocoagulation of the fellow eye in exudative senile maculopathy. A preliminary report. Modern Problems in Ophthalmology 1979;20:141‐7. [PubMed] [Google Scholar]
Duvall 1985
- Duvall J, Tso MO. Cellular mechanisms of resolution of drusen after laser coagulation. An experimental study. Archives of Ophthalmology 1985;103(5):694‐703. [DOI] [PubMed] [Google Scholar]
EDPRG 2004
- Friedman DS, O'Colmain BJ, Muñoz B, Tomany SC, McCarty C, Jong PT, et al. Prevalence of age‐related macular degeneration in the United States. Archives of Ophthalmology 2004;122(4):564‐72. [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Evans 2012
- Evans JR, Lawrenson JG. Antioxidant vitamin and mineral supplements for slowing the progression of age‐related macular degeneration. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD000254.pub3] [DOI] [PubMed] [Google Scholar]
Frennesson 1998
- Frennesson C, Nilsson SE. Prophylactic laser treatment in early age related maculopathy reduced the incidence of exudative complications. British Journal of Ophthalmology 1998;82(10):1169‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gass 1973
- Gass JD. Drusen and disciform macular detachment and degeneration. Archives of Ophthalmology 1973;90(3):206‐17. [DOI] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
GRADEpro 2015
- GRADEpro GDT. GRADEpro Guideline Development Tool [Software]. McMasters University, 2015 (developed by Evidence Prime, Inc.). Available from www.gradepro.org.
Gross‐Jendroska 1998
- Gross‐Jendroska M, Owens SL, Flaxel CJ, Guymer RH, Bird AC. Prophylactic laser treatment to fellow eyes of unilateral retinal pigment epithelial tears. American Journal of Ophthalmology 1998;126(1):77‐81. [DOI] [PubMed] [Google Scholar]
Guidry 2002
- Guidry C, Medeiros NE, Curcio CA. Phenotypic variation of retinal pigment epithelium in age‐related macular degeneration. Investigative Ophthalmology and Visual Science 2002;43(1):267‐73. [PubMed] [Google Scholar]
Guymer 2001
- Guymer RH, Hageman GS, Bird AC. Influence of laser photocoagulation on choroidal capillary cytoarchitecture. British Journal of Ophthalmology 2001;85(1):40‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443‐57. [DOI] [PubMed] [Google Scholar]
Hassell 2006
- Hassell JB, Lamoureux EL, Keeffe JE. Impact of age related macular degeneration on quality of life. British Journal of Ophthalmology 2006;90(5):593‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Holz 1994
- Holz FG, Wolfensberger TJ, Piguet B, Gross‐Jendroska M, Wells JA, Minassian DC, et al. Bilateral macular drusen in age‐related macular degeneration. Prognosis and risk factors. Ophthalmology 1994;101(9):1522‐8. [DOI] [PubMed] [Google Scholar]
Klein 2004
- Klein R, Peto T, Bird A, Vannewkirk MR. The epidemiology of age‐related macular degeneration. American Journal of Ophthalmology 2004;137(3):486‐95. [DOI] [PubMed] [Google Scholar]
Langan 2012
- Langan D, Higgins JP, Gregory W, Sutton AJ. Graphical augmentations to the funnel plot assess the impact of additional evidence on a meta‐analysis. Journal of Clinical Epidemiology 2012;65(5):511‐9. [DOI] [PubMed] [Google Scholar]
Lavinsky 2011
- Lavinsky D, Cardillo JA, Melo LA Jr, Dare A, Farah ME, Belfort R Jr. Randomized clinical trial evaluating mETDRS versus normal or high‐density micropulse photocoagulation for diabetic macular edema. Investigative Ophthalmology and Visual Science 2011;52(7):4314‐23. [DOI] [PubMed] [Google Scholar]
Luttrull 2012
- Luttrull JK, Dorin G. Subthreshold diode micropulse laser photocoagulation (SDM) as invisible retinal phototherapy for diabetic macular edema: a review. Current Diabetes Reviews 2012;8(4):274‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maguire 2004
- Maguire M, Complications of Age‐Related Macular Degeneration Prevention Trial Research Group. Baseline characteristics, the 25‐Item National Eye Institute Visual Functioning Questionnaire, and their associations in the Complications of Age‐Related Macular Degeneration Prevention Trial (CAPT). Ophthalmology 2004;111(7):1307‐16. [DOI] [PubMed] [Google Scholar]
Mangione 1999
- Mangione CM, Gutierrez PR, Lowe G, Orav EJ, Seddon JM. Influence of age‐related maculopathy on visual functioning and health‐related quality of life. American Journal of Ophthalmology 1999;128(1):45‐53. [DOI] [PubMed] [Google Scholar]
MPSG 1997
- Macular Photocoagulation Study Group. Risk factors for choroidal neovascularization in the second eye of patients with juxtafoveal or subfoveal choroidal neovascularization secondary to age‐related macular degeneration. Archives of Ophthalmology 1997;115(6):741‐7. [DOI] [PubMed] [Google Scholar]
Owen 2003
- Owen CG, Fletcher AE, Donoghue M, Rudnicka AR. How big is the burden of visual loss caused by age related macular degeneration in the United Kingdom?. British Journal of Ophthalmology 2003;87(3):312‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Owen 2012
- Owen CG, Jarrar Z, Wormald R, Cook DG, Fletcher AE, Rudnicka AR. The estimated prevalence and incidence of late stage age related macular degeneration in the UK. British Journal of Ophthalmology 2012;96(5):752‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parodi 2006
- Parodi MB, Spasse S, Iacono P, Stefano G, Canziani T, Ravalico G. Subthreshold grid laser treatment of macular edema secondary to branch retinal vein occlusion with micropulse infrared (810 nanometer) diode laser. Ophthalmology 2006;113(12):2237‐42. [DOI] [PubMed] [Google Scholar]
Parodi 2008
- Parodi MB, Iacono P, Ravalico G. Intravitreal triamcinolone acetonide combined with subthreshold grid laser treatment for macular oedema in branch retinal vein occlusion: a pilot study. British Journal of Ophthalmology 2008;92(8):1046‐50. [DOI] [PubMed] [Google Scholar]
Pauleikhoff 1990a
- Pauleikhoff D, Harper CA, Marshall J, Bird AC. Aging changes in Bruch's membrane. A histochemical and morphologic study. Ophthalmology 1990;97(2):171‐8. [PubMed] [Google Scholar]
Pauleikhoff 1990b
- Pauleikhoff D, Chen JC, Chisholm IH, Bird AC. Choroidal perfusion abnormality with age‐related Bruch's membrane change. American Journal of Ophthalmology 1990;109(2):211‐7. [DOI] [PubMed] [Google Scholar]
Peters 2008
- Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour‐enhanced meta‐analysis funnel plots help distinguish publication bias from other causes of asymmetry. Journal of Clinical Epidemiology 2008;61(10):991‐6. [DOI] [PubMed] [Google Scholar]
Phipps 2003
- Phipps JA, Guymer RH, Vingrys AJ. Loss of cone function in age‐related maculopathy. Investigative Ophthalmology and Visual Science 2003;44(5):2277‐83. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre. The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre. The Cochrane Collaboration, 2014.
Rudnicka 2015
- Rudnicka AR, Kapetanakis VV, Jarrar Z, Wathern AK, Wormald R, Fletcher AE, et al. Incidence of Late‐Stage Age‐Related Macular Degeneration in American Whites: Systematic Review and Meta‐analysis. American Journal of Ophthalmology 2015;160:85‐93. [DOI] [PubMed] [Google Scholar]
Sarraf 1999
- Sarraf D, Gin T, Yu F, Brannon A, Owens SL, Bird AC. Long‐term drusen study. Retina 1999;19(6):513‐9. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Sivaprasad 2010
- Sivaprasad S, Elagouz M, McHugh D, Shona O, Dorin G. Micropulsed diode laser therapy: evolution and clinical applications. Survey of Ophthalmology 2010;55(6):516‐30. [DOI] [PubMed] [Google Scholar]
Smith 2001
- Smith W, Assink J, Kein R, Mitchell P, Klaver CCW, Klein BEK, et al. Risk factors for age‐related macular degeneration. Pooled findings from three continents. Ophthalmology 2001;108(4):607‐704. [DOI] [PubMed] [Google Scholar]
StataCorp 2013 [Computer program]
- StataCorp. 2013. Stata Statistical Software. Version Release 13. College Station, TX: StataCorp LP.
Vingerling 1996
- Vingerling JR, Hofman A, Grobbee DE, Jong PT. Age‐related macular degeneration and smoking. The Rotterdam Study. Archives of Ophthalmology 1996;114(10):1193‐6. [DOI] [PubMed] [Google Scholar]
Wetzig 1994
- Wetzig PC. Photocoagulation of drusen‐related macular degeneration: a long‐term outcome. Transactions of the American Ophthalmological Society 1994;92:299‐303. [PMC free article] [PubMed] [Google Scholar]
White 2008
- White IR, Higgins JPT, Wood AM. Allowing for uncertainty due to missing data in meta‐analyses. Part 1: two‐stage methods. Statistics in Medicine 2008;27(5):711‐27. [DOI] [PubMed] [Google Scholar]
Young 1987
- Young RW. Pathophysiology of age‐related macular degeneration. Survey of Ophthalmology 1987;31(5):291‐306. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Parodi 2007
- Parodi MB, Evans JR. Laser treatment of drusen to prevent progression to advanced age‐related macular degeneration. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD006537] [DOI] [PubMed] [Google Scholar]
Parodi 2009
- Parodi MB, Virgili G, Evans JR. Laser treatment of drusen to prevent progression to advanced age‐related macular degeneration. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD006537.pub2] [DOI] [PubMed] [Google Scholar]


