Abstract
A sample of 398 African American youth, residing in rural counties with high poverty and unemployment, were followed from age 11 to age 19. Protective parenting was associated with better health whereas elevated SES-risk was associated with poorer health at age 19. Genomewide epigenetic variation assessed in young adulthood (age 19), was associated with both SES-risk and protective parenting. Three categories of genes were identified whose methylation was associated with parenting, SES-risk, and young adult health. Methylation was a significant mediator of the impact of parenting and SES-risk on young adult health. Variation in mononuclear white blood cell types was also examined and controlled, showing that it did not account for observed effects of parenting and SES-risk on health.
Keywords: epigenetic, methylation, African American, parenting, SES
Because of widespread economic disadvantage in the Southern coastal plain, African American youth living in the Southeast are exposed to a range of socio-economic status (SES) risk at developmentally sensitive stages. Exposure to stressful, recurrent, and uncontrollable difficulties throughout childhood potentially sets the stage for increased endocrine and autonomic responses (Miller & Cole, 2012), and ultimately epigenetic change that may forecast changes in transcriptional response and differential risk for adverse health outcomes (Cole et al., 2011). Sustained economic adversity during childhood has been found to predict a range of negative physiological consequences (Evans, Chen, Miller, & Seeman, 2012; Maholmes & King, 2012), including decreased life expectancy (Braveman, Cubbin, Egerter, Williams, & Pamuk, 2010) and poorer health manifested in childhood as well as in adulthood (Pollit, Kaufman, Rose, et al., 2008; Starfield, Robertson, & Riley, 2002; Yoshikawa, Aber, & Beardslee, 2012).
Poverty and economic hardship also have been shown to influence protective family and social processes that have the potential to influence later health and well-being (Conger, Wallace, Sun, Simons, McLoyd, & Brody, 2002; Maholmes & King, 2012), making it important to clarify whether such family processes are best conceptualized as an aspect of SES-risk and related hardships, or can be viewed as contributing independently to long-term health outcomes. Several lines of research converge to support the hypothesis that parenting practices in particular may help counter the impact of SES-risk exposure on long-term health outcomes (Miller, Chen, & Parker, 2011). Recent evidence, for instance, suggests that parental emotional support buffers children’s and adolescents’ physiological stress reactions, potentially ameliorating physiological risk factors that develop following exposure to childhood adversity (Brody, Yu, Beach, Kogan, Windle, & Philibert, 2014; Chen, Miller, Kobor, & Cole, 2011). Harsh or abusive parenting has been found to have a direct negative influence on health outcomes (Heim, Newport, Mletzko, Miller, & Nemeroff, 2008) and contribute to vulnerability to chronic diseases later in life (Dube et al., 2009; Miller & Chen, 2010; Shonkoff, Boyce, & McEwen, 2009; Repetti, Taylor, & Seeman, 2002). These results support the proposition that parenting comprised of the presence of positive parenting behaviors and absence of negative parenting behaviors during late childhood and early adolescence can be considered “protective parenting,” and may have beneficial effects on stress-regulatory systems and, ultimately, young adult health.
Although the ways in which childhood and adolescent stressful life experiences are turned into long-term biological changes with health consequences (cf. Hertzman, 1999) is not yet fully understood, one possible mechanism is epigenetic change in organized categories of genes. To the extent that SES-risk and protective parenting are associated with shared patterns of epigenetic change, they may jointly influence biological processes via altered gene expression and produce changes in cellular functioning with long-term health implications (see Philibert & Beach, 2015 for additional information about epigenetics). Due to the relative ease of measurement of DNA methylation, and because it is believed to provide a stable and comprehensive record of epigenetic influence (Hochberg et al., 2011), measurement of DNA methylation has emerged as a powerful tool for the examination of the ways in which non-genomic influences may result in stable changes gene activity and later phenotypes (Kramer, 2005). Because the immune system continues to develop during childhood and adolescence (West, 2002), and because opportunities for SES-risk and parenting to influence methylation potentially related to this system may continue over this same period (Curley, Jensen, Mashoodh, Champagne, 2011), it is useful to explore SES-risk and protective parenting effects on methylation during childhood and early adolescence (Masten, 2001).
Recent theorizing within the life history tradition (Belsky, Steinberg, & Draper, 1991; Charnov, 1993;) suggests that early adversity may be manifested in changes in the distribution of particular cell types in blood (Irwin & Cole, 2011) as well as in the epigenetic programming of such cells (Miller, Chen, Parker, 2011). The two different types of effects suggest alternative mechanisms resulting in the early programming of adaptive responses (Gluckman, Hanson, & Spencer, 2005; Rickard & Lummaa, 2007), and suggest the need to examine two alternative indirect pathways to health outcomes. Previous research indicates that mononuclear white blood cell types may be reliably detected using information from the epigenome (Accomando, Weinke, Houseman, Nelson, Kelsey, 2014; Houseman et al, 2012), allowing effects of social circumstances on cell type variation to be estimated from the same data base used to assess effects on DNA methylation. This allows a joint examination of the extent to which salient facets of childhood experience combine to influence the composition as well as the activity of mononuclear white blood cells, and ultimately young adult health.
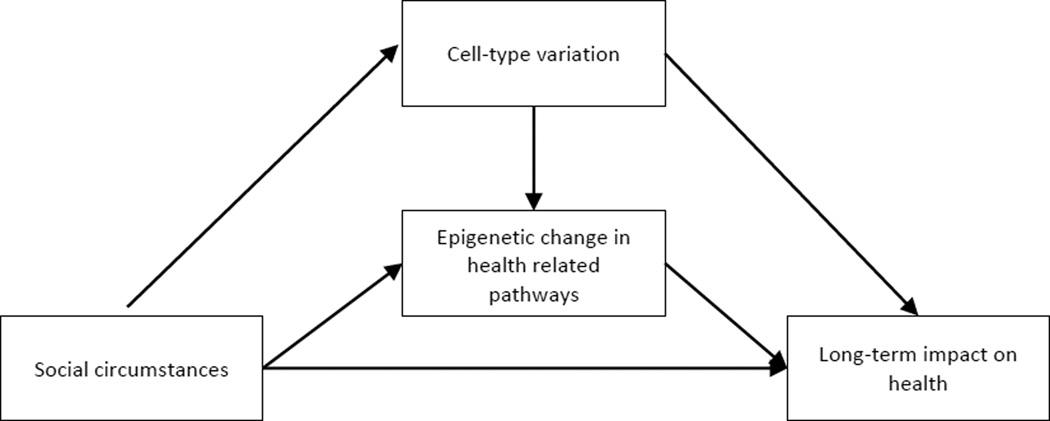
A general model emerging from the foregoing considerations portrays SES-risk and protective parenting as having direct effects on health as well as having indirect effects via methylation of health-related genes and changes in the proportions of different mononuclear white blood cell types. To the extent that indirect effects are found, they focus theoretical attention on shared biological pathways conferring long-lasting effects of cumulative SES risk and protective parenting on health. A portrayal of the resulting general conceptual model can be seen in Figure 1. Protective social circumstances in childhood and adolescence, such as protective parenting, and adverse social circumstances, such as SES-risk, are hypothesized to exert opposing effects on health as well on the epigenetic patterns and cell-type variation associated with later health. To the extent that epigenetic mechanisms or cell-type variation explain the impact of SES-risk or parenting effects on health, it should be possible to demonstrate mediation.
Figure 1.
The conceptual model showing two potential shared, biological, indirect pathways from early SES-risk and Parenting to young adult health.
In the current investigation, we examine the hypothesis that more protective parenting will be associated with differences in DNA methylation genomewide as well as with self-reported health in young adulthood. Of particular interest is the possibility that protective parenting may counteract some changes associated with exposure to SES-risk producing effects, in part, via shared epigenetic mechanisms. We examine six main hypotheses with the first and fourth comprised of two sub-hypotheses:
-
H1
Protective parenting (H1a) and SES-risk (H1b) will each be associated with methylation genomewide to a degree greater than expected by chance and each will overlap with loci associated with young-adult health.
-
H2
Parenting and SES-risk will be associated with individual differences in proportions of different types of mononuclear white blood cells that, in turn, will be significantly associated with young adult health.
-
H3
Categories of genes identified by GoMiner™ (Zeeberg, et al., 2003) as linking SES-risk and health will overlap with categories identified as linking parenting and health, allowing construction of a methylation index that captures shared categories of epigenetic effects and allowing direct comparison of the effect of SES-risk and protective parenting on shared epigenetic mechanisms.
-
H4
Protective parenting and SES-risk will each account for unique variance and opposite effects in (a) the methylation index identified in step 3, and (b) young adult health.
-
H5
Both methylation and cell-type variation will mediate the effect of protective parenting and SES-risk on youth reported health.
-
H6
Parenting and SES-risk will interact to predict self-reported health, methylation, and cell-type variation, and in each case interaction terms will account for significant variance beyond the main effects of parenting and SES-risk.
Method
Participants
Participants were 398 primary caregivers and target youth residing in rural Georgia who provided data yearly between youth ages of 11 and 19. Participants were selected randomly from a larger sample of youth participating in an ongoing longitudinal study being conducted by the Center for Family Research. Participants resided in nine rural counties in Georgia. In these counties, families live in communities in which poverty rates are among the highest in the U.S. and unemployment rates are above the national average (Dalaker, 2001). Family economic circumstances and parenting interactions were provided by African American primary caregivers. Youth provided blood for epigenetic assessments as well as reports of the caregiver’s parenting and their own physical health. Target youth mean age was 11.7 years at the first assessment and 19.2 years at the time of epigenetic assessment based on a blood draw. At the first assessment, primary caregivers in the sample worked an average of 39.9 hour per week, and 42.3% lived below federal poverty standards. Of the youth in the sample, 55% were female.
Procedure
All data were collected in participants’ residences. A standardized assessment protocol lasting two hours, on average, was used at each wave of data collection. Two African American field researchers met with each family to allow separate and simultaneous data collection from the primary caregiver and the target youth. Interviews were conducted with no other family members present or able to overhear the conversation. Primary caregivers consented to their own and the youths’ participation in the study, and the youths under 18 assented to their own participation and then consented when they participated as adults.
Measures
Preadolescent cumulative SES risk
Preadolescent cumulative SES risk was assessed across six indicators and three waves of data collected from primary caregivers when the target youths were 11, 12, and 13 years of age. Each indicator was scored dichotomously (0 if absent, 1 if present. The six risk indicators were (a) family poverty, defined as being below the poverty level, taking into account both family income and number of family members; (b) primary caregiver non-completion of high school or an equivalent; (c) primary caregiver unemployment; (d) single-parent family structure; (e) family receipt of Temporary Assistance for Needy Families; and (f) income rated by the primary caregiver as not adequate to meet all needs.. Cumulative SES risk was defined as the average number of risk factors across the three assessments, yielding an index with a theoretical range of 0 to 6 (M = 2.33, SD = 1.35).
Parenting
The protective parenting processes of support, communication, and monitoring and the adverse practice of harsh parenting were assessed via target youth reports as well as parent reports across five scales. Youths and primary caregivers responded to three scales in common with wording changes as appropriate: the Interaction Behavior Questionnaire (IBQ; Prinz, Foster, Kent, & O'Leary, 1979), Nurturant-Involved Parenting Scale (Conger, Ge, Elder, Lorenz, & Simons, 1994), and the Harsh-Inconsistent Parenting Scale (Brody et al., 2001). Youths also completed a revised version of the four-item Social Support for Emotional Reasons subscale from the COPE scale to assess levels of parental support (Carver, Scheier, & Weintraub, 1989). Primary caregivers also completed the Destructive Arguing Inventory to assess styles of conflict and conflict resolution within parent-child relationship (Kurdek et al., 1994). For caregivers, Cronbach’s alphas ranged from .73 to .84 for the three measures other than harsh parenting across the three waves. For youth, Cronbach’s alphas for completed measures ranged from .76 to .85 across the three measures other than harsh parenting and across the three waves. In each case, harsh parenting displayed lower alphas that other measures (.54 to .60 for caregivers; .53 to .59 for youth).
Each of the parenting scales was standardized and then averaged across the first three waves of assessment (i.e., ages 11–13). We reversed negatively valenced parenting measures to insure that for all measures higher scores indicated more protective parenting and fewer negative practices. All the parenting measures were summed to form the overall measure of protective parenting.
Young adult health
Youths reported their general health twice in young adulthood shortly before the blood draw to assess methylation. The General Health Perceptions subscale from the RAND 36-Item Short-Form Health Survey (Hays, Sherbourne, & Mazel, 1993) was used. This five-item subscale includes a single-item rating of overall health ranging from 1 (excellent) to 5 (poor) and four items assessing youths' ratings of their current health status ranging from 1 (definitely false) to 5 (definitely true); e.g., "I am as healthy as anybody I know"; "I seem to get sick a little easier than other people". In keeping with standard scoring, responses 1 through 5 were recoded to values of 100, 75, 50, 25, 0. Items were reversed scored when necessary so that higher scores on the subscale indicated more health problems and poorer general health. After reverse scoring, all items were averaged to yield a General Health Problems score with a range of 0 to 100 (α = .72 at age 18 and α = .76 at age 19). The correlation between self-reported health at 18 and 19 was .555, p=.000, and so the two assessments were averaged to create a single index of young adult health.
Methylation
A certified phlebotomist drew four tubes of blood (30 ml) from each participant and shipped it the same day to a laboratory for preparation. Procedures for preparation of blood, quality control, and standardization of methylation values, along with our analytic plan, are described in detail in supplement S1. Briefly, mononuclear cell pellets were separated from the diluted blood specimen by centrifugation. The Illumina (San Diego, CA) HumanMethylation450 Beadchip was used to assess genome-wide DNA methylation. After quality control analyses, quantile normalization methods (Pidsley et al., 2013) were used to derive the methylation indices used for the current investigation. Cell type variation was quantified using methods developed by Reinus, et al., 2012.
Results
Results are presented in six stages, corresponding to the six specific hypotheses outlined above. As can be seen in Table 1, primary study variables were correlated at a zero-order level. Protective parenting and SES-risk were significantly negatively associated with each other (r = −.137, p = .006), and both were significantly associated with youth reports of health in young adulthood (r = −.157, p = .002; and r = .212, p = .000, respectively).
Table 1.
Correlation Matrix for the Major Study Variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Parenting | —— | |||||||||
| 2. SES-risk | −.137** | —— | ||||||||
| 3. Methylation index | .144** | −.221** | —— | |||||||
| 4. Self-reported health | −.157** | .212** | −.225** | —— | ||||||
| 5. Male | .007 | −.037 | .045 | −.175** | —— | |||||
| 6. Age | .000 | .084† | .007 | −.069 | −.026 | —— | ||||
| 7. Factor 1 | .075 | −.094† | .557** | −.055 | −.149** | .032 | —— | |||
| 8. Factor 2 | .035 | −.108* | .646** | −.111* | .128* | −.009 | .003 | —— | ||
| 9. Factor 3 | −.166** | .015 | −.093† | .102* | −.218** | −.026 | −.005 | −.011 | —— | |
| 10. Factor 4 | .049 | −.085† | .090† | .036 | −.082 | .027 | −.002 | .008 | .004 | —— |
| Mean | .000 | .000 | .000 | 25.792 | .452 | 19.123 | .084 | .017 | .031 | −.002 |
| SD | 1.000 | 1.000 | .475 | 16.323 | .498 | .607 | 30.618 | 14.263 | 6.499 | 4.805 |
p ≤ .01;
p ≤.05;
p<.10 (two-tailed tests).
The methylation index is the average of CpG values connecting health to both parenting and SES-risk. Factors 1 to 4 are the four principle components reflecting cell-type variation in the current data.
(N = 398)
H1. Examination of Parenting, SES-risk, and Health Associations with Methylation Genomewide
To examine H1, we ran three separate genomewide analyses using the cleaned and normalized methylation data; parent, SES-risk, and youth health were each regressed on methylation genomewide, controlling for sex and age effects and excluding x and y chromosomes. For H1a, results indicated substantial association of parenting with methylation genomewide. Using the 473,111 available loci in the cleaned data set, there were 23,982 loci associated with parenting at p < .01, and 15 of these were significant genomewide at false discovery rate (FDR) < .05 (Benjamini & Hochberg, 1995). This suggests a substantial reliable association of parenting during preadolescence with genomewide methylation. For H1b examining SES-risk and methylation genomewide, 28,640 loci were associated at the p < .01 level of significance, with 2,032 loci associated at FDR < .05. Thus, both parenting and SES-risk were associated with a larger number of CpG sites genomewide than would be anticipated by chance (i.e. 4731 at p < .01).
Youth reported health was regressed on methylation genomwide, controlling for sex and age effects, identifying 12,431 CpG sites that were associated at p < .01, with no sites significant at FDR < .05.Among loci significantly associated with protective parenting at p < .01, 471 CpGs also were associated with health at p < .01. Among loci significantly associated with SES-risk at p < .01, 2734 loci were also associated with health at p < .01.
H2. Parenting, SES-risk, and Health Associations with Cell-type variation
Cell type variation was indexed using the first four principle components found in the 99 most informative loci (for additional detail see S1). After deriving factor scores based on the principle components analysis, we correlated factor scores with parenting, SES-risk, the methylation index, and youth reported health. As can be seen in Table 1, the factor scores indicative of individual differences in cell type variation were associated with predictors, with the hypothesized mediator, and with youth health. However, factor 3 was the only factor related significantly to parenting (r = −.166, p = .001). Factor 2 was the only factor associated significantly with SES-risk (r = −.108, p = .031). Factors 1 and 2 were both robustly associated with the hypothesized mediator (r = .557, p = .000; and r = .646, p = .000, respectively), and factors 2 and 3 were significantly associated with self-reported health (r = −.111, p = .026; and r = .102, p = .041, respectively). The impact of cell-type variation on the mediator and outcome variables were controlled in analyses examining the impact of parenting and SES-risk on methylation and youth health. Plausible alternative mediating biological pathways from protective parenting to cell-type factor 3, and from SES-risk to cell-type factor 2 were also examined in the final model.
H3. Identification of CpG sites on gene categories linking protective parenting and SES-risk to youth health
Significant Gominer™ categories linking youth reported health to parenting were identified as were categories linking youth reported health to SES-risk. Ten categories of genes were identified as being significant at FDR < .05 for the loci common to protective parenting and health. For SES-risk and health, 144 categories of genes were identified as significant at FDR < .05. The three categories identified by Gominer™ in both analyses were GO:0035466, regulation of signaling pathway; GO:0023033, signaling pathway, GO:0023052, signaling. These three categories indicated a potential shared biological mechanism of effect for parenting and SES-risk. To create a methylation index that would capture this potentially shared mechanism we used all CpG sites associated with either protective parenting or SES-risk that were on the genes in the three common pathways. Among the 471 CpGs associated p < .01 with both protective parenting and health, 78 CpGs were on one or more of the three overlapping categories. Among the 2,734 CpGs associated p < .01 with both SES-risk and health, 363 CpGs were on one or more of the three overlapping categories. Because there were 21 CpGs that overlapped between the two sets, we ultimately identified 420 loci (i.e., 441 – 21) on genes associated with the three significant Gominer™ categories identified. Loci negatively associated with parenting or positively associated with SES-risk were reverse scored to insure that all reflected a common direction of effect, then the 420 values were standardized and averaged. The resulting total methylation index reflects level of methylation across the 420 loci linking youth reported health to parenting and SES-risk. (Individual locus level detail provided in S2)
H4. Opposite effects for Protective Parenting and SES-risk
As can be seen in Table 1, the correlation for protective parenting and the methylation index (r = .144, p = .004) was in the opposite direction to that for SES-risk (r = −.221, p = .000), supporting hypothesis 4a. Likewise, the correlation for protective parenting and youth reported health (r = −.157, p = .002) was opposite in direction to the correlation between SES-risk and youth-reported health (r = 212, p = .000) supporting hypothesis 4b. In regressions controlling for age, gender, and plate there were also significant effects of parenting and SES-risk on the mediator (methylation) and the outcome (Health). Parenting accounted for significant unique variance in the methylation index and in youth-reported health (β = .076, p =.000; β = −.147, p =.003) respectively as did SES-risk (β = −.044, p =.003; β = .197, p =.000) respectively. As was the case for the univariate analyses, the effect of parenting and SES-risk were significant in opposite directions.
H5. Methylation and cell-type variation mediate effects of Parenting and SES-risk on Health
Using the total methylation index (H3) and the factor scores representing cell-type variation (H2), we examined whether the effects of protective parenting and SES-risk on self-reported health were mediated by methylation, cell type variation, or both. Because loci for the methylation index were selected to be linked to health, we allowed for correlated measurement errors between the methylation index and youth-reported health. We used the function (MODEL INDIRECT) in Mplus version 7.1 (Muthén & Muthén, 2012) and obtained bootstrap confidence intervals for the effect of the independent variables (parenting and SES risk exposure) on the outcome variable (young adult self-reported health) through the mediator (methylation index) using 1000 replicates to assess the bias-corrected 95% confidence intervals for the indirect effects (Hayes, 2009). This approach estimates direct and indirect effects simultaneously, does not assume a standard normal distribution when calculating the p-value for the indirect effect, and repeatedly samples the data to estimate the indirect effect.
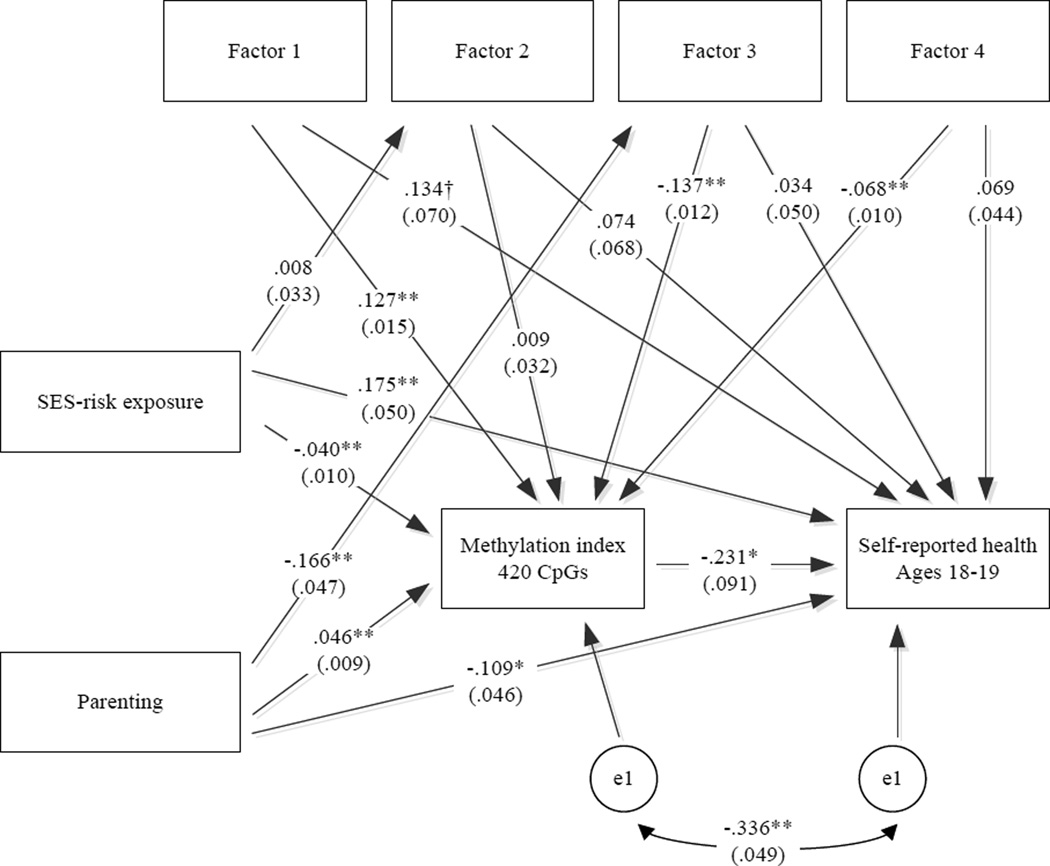
Supporting H5, we found that both parenting and SES risk exposure were associated with the methylation index score as well as with self-reported health in young adulthood in a multivariate context (See Figure 2). In addition, the impact of both protective parenting and SES-risk exposure on self-reported health in young adulthood was partially, but not fully, mediated by impact on the methylation index. As can be seen in Table 2, the methylation index accounted for 8.12% of the variance in young adult poor health attributable to parenting, with a significant indirect effect (IE) of parenting on health, IE = −.173, 95% CI = [−.353, −.032] and a significant unstandardized direct effect of B = −1.78, 95% CI = [−3.26, −.221]. The methylation index also accounted for 5.02% of the variance in young adult poor health attributable to SES risk exposure, with a significant indirect effect of SES risk on health of IE = .151, 95% CI = [.030, .323] and a significant unstandardized direct effect of B = 4.20, 95% CI = [1.27, 4.42].
Figure 2.
Model of direct and indirect effects of SES-risk and Parenting on Self-reported health using the total methylation index (420 CpG sites) as the mediator.
Note: χ2 = 16.372, df = 19, p = .632; CFI = 1.000; RMSEA = .000. Batch effect, males, and age are controlled. Values presented are standardized parameter estimates with robust standard errors in parentheses. Factor scores reflect cell-type variation.
**p ≤ .01; *p ≤ .05, †p < .10 (two-tailed tests), n = 398.
Table 2.
Indirect and Direct Effects From SES-risk and Parenting to Health in Figure 2 Showing Significant Indirect Effects Through Methylation From SES-risk to Health and From Parenting to Health, but no Indirect Effect Through Cell-type Variation.
| Predictor | Mediators | Outcome | Indirect effect [95% CI] |
The portion of the total variance explained by mediators |
|---|---|---|---|---|
| SES-risk exposure | Methylation index | Self-reported health | .151 [.030, .323] |
5.020% |
| Parenting | Methylation index | Self-reported health | −.173 [−.353, −.032] |
8.118% |
| SES-risk exposure | Factor 2 | Self-reported health | .009 [−.108, .125] |
---- |
| Parenting | Factor 3 | Self-reported health | −.091 [−.385, .187] |
---- |
| Parenting | Factor 3 | Methylation index, | −.086 [−.196, −.013] |
4.036% |
Contrary to hypotheses, there was no significant mediation of parenting or SES-risk effects on health through individual differences in cell-type. However, cell type variation was significantly associated with both the hypothesized mediator and with youth reported health, indicating its importance as a control variable. In addition, there was a significant indirect effect from parenting to the methylation index via cell-type variation (−.091, 95% CI = [−.196, −.013]), indicating that parenting influenced the methylation mediator indirectly through variation in the cell-type variation captured by factor 3 as well as through its direct association. Factor 3 captures the relative dominance of monocytes to B cells, and so can be taken as reflecting the relative dominance of activity of the innate relative to the acquired immune system, a negative pattern that is potentially important for long-term inflammation related disease.
In the final model, shown in Figure 2, which drops nine non-significant pathways from control variables, parenting and SES-risk continue to be associated with both youth reported health and with the methylation mediator in opposing directions, indicating that their apparent competing effects are observable at multiple levels of analysis and with controls for potential confounds.
Hypothesis 6. Interaction between parenting and SES-risk
To examine whether Parenting and SES-risk interacted to predict youth-reported health, the methylation index, or any of the four factors reflecting cell-type variation, we conducted a series of moderated regression analyses. In each case, the interaction term was entered along with sex, age, and the main effects of parenting and SES-risk. In no case was the interaction term significant. For youth-reported health, β = .022, t = .463, p = .644, NS. For the methylation index, β = −.056, t = −1.147, p = .252, NS. For the four factor scores, β’s for the interaction term ranged from −.010 to .057, all p’s > .15. Accordingly, the interaction term between parenting and SES-risk was not included in the multivariate model, leaving figure 2 as the final model.
Discussion
The examination of the way that early social environments influence health and remodel biological systems through epigenetic change is just beginning. There is promising preliminary work on multiple fronts suggesting the potential for productive exploration of the way in which epigenetic mechanisms may be related to, and help account for, long-term health effects stemming from childhood experiences such as exposure to elevated SES-risk or experience of protective parenting. In the current study, we used a longitudinal research design to test hypotheses regarding the opposing effects of parenting and SES-risk across preadolescence on shared epigenetic mechanisms linked to health. As predicted, both SES-risk and protective parenting were associated with methylation at more loci than would be expected by chance, and it was possible to identify three shared Gominer™ categories linking both parenting and SES-risk to young adult health. Greater protective parenting was linked to patterns of methylation in these three gene categories and ultimately linked to positive health whereas SES-risk was linked to patterns in the same categories of genes in the opposite direction and ultimately linked to poorer health. Finally, as predicted, the methylation index partially mediated the prospective association between protective parenting and health 6 years later as well as the effect of cumulative SES risk on young adult health six years later. Accordingly, it appears that protective parenting may play a role in offsetting the adverse impact of early cumulative SES risk on young adult health, in part, by countering epigenetic effects of SES risk exposure.
Consistent with study hypotheses, we found that supportive family environments, assessed longitudinally, can exert a long-term effect on epigenetic profile, with parenting at ages 11–13 associated with epigenetic patterns assessed at age 19. In addition, these effects were not spurious associations due to overlap with SES-risk, nor was it possible to eliminate the pattern by controlling for individual differences in cell type. Findings were also consistent with the proposition that poor health and health disparities during young adulthood may be tied to growing up in environments characterized by high SES risk (Shonkoff et al., 2009). Moreover, findings suggest that some of the biological impact arising from exposure to elevated levels of SES risk in pre-adolescence may be offset by protective parenting and that this influence may occur, in part, through impact on shared biological mechanisms. Importantly, however, because there was no evidence of a significant interaction effect, the data do not conform to a buffering hypothesis. Although identification of the specific signaling pathways affected by SES-risk and protective parenting is beyond the scope of the current investigation, it is encouraging to find that differential methylation of broad categories of signaling genes are common to both.
SES-risk and protective parenting demonstrated opposite effects on alteration in mononuclear white blood cell signaling processes that, in turn, were linked to young adult health. This finding indicates that in pre and early adolescence, youths’ physiological internalization of stress may be a function of messages from parents and changes in parenting behavior as well as direct observation and experience of ongoing SES-related stressors. Thus, shared effects on epigenetic pathways leading to health would be anticipated and changes in signaling processes, net of effects on individual differences in cell type composition, may be central to understanding the common biological mechanism of influence shared by SES-risk and parenting on young adult health. Previous studies have focused on the effect of early childhood life stress on subsequent biological and genomic functioning (e.g., Essex et al. 2013; Miller, Lachman, Chen et al., 2011), whereas our results suggest that risk and protective factors measured later, i.e., during pre and early adolescence, may also be associated with health in young adults. Exploratory Gominer™ analyses identified 144 gene categories significant at FDR < .05 related to the genes included on the methylation index that mediated the impact of protective parenting and SES-risk on health, suggesting a broad range of potentially affected biological activities. Together, these results encourage additional investigation of physiological weathering effects and genomic alterations induced by SES risk exposure and protective factors that occur during pre-adolescence or later and that may be mediated by epigenetic changes.
More broadly, however, the findings suggest that the dual pathway model outlined in Figure 1 does not account for the majority of health related effects of SES-risk and protective parenting during pre and early adolescence. Importantly, there was no evidence of effects through shared impact on proliferation of specific cell-types. Although parenting in pre and early adolescence was related to a shift in cell-types, in the full model SES-risk was not. Even at the zero-order level, where significant associations were observed for both SES-risk and protective parenting, significant associations were found with different patterns of cell-type variation. Likewise, examination of potential buffering effects failed to identify significant interactions of parenting and SES-risk, providing no support for models of amplification or buffering. Finally, the amount of mediation through common mechanisms that was observed was partial, accounting for only 5.02% and 8.11% of the total variance in health associated with SES-risk and protective parenting respectively. This suggests that for each, the impact of unshared pathways on health may be more numerous or more potent than the shared pathways.
Limitations of the present study also should be noted. First, future replications with younger rural African American children would be useful to better capture the interplay of very early SES risk exposure and parenting on gene methylation. It may be that work with younger samples would demonstrate a stronger effect of SES risk exposure, or might show greater effects on individual differences in cell-type. In addition, because we did not measure gene expression, or protein synthesis for the genes affected by differential methylation, the current results await replication and extension using methods that can clarify whether the observed effects are associated with other markers of altered cellular functioning and result in either up or down regulation of affected sets of genes.
Despite the limitations and need for future replication, the current results provide a useful demonstration of the impact of protective parenting and SES-risk during pre-adolescence on methylation and youths’ long-term health outcomes. SES-risk and protective parenting influence both shared and non-shared biological pathways that partially account for their association with young adult health. Jointly, the results suggest the value of continued investigation of epigenetic change related to parenting and its potential for impact many years later. Because the epigenetic pathways identified as common to parenting and SES-risk focus on signaling, there is potential for more focused future investigation to identify additional factors influencing these mechanisms, potentially elaborating the current model. At the same time, it should be noted that much of the effect of SES-risk and parenting appears to be through non-shared pathways, with some of the pathways likely reflecting non-shared social mechanisms whereas others reflect non-shared biological mechanisms. This suggests that, at a practical level, there may be many approaches to enhance the impact of family-based approaches on young adult health outcomes.
Supplementary Material
Acknowledgments
This research was supported by Award 5R01HD030588-16A1 from the National Institute of Child Health and Human Development and Award 1P30DA027827 from the National Institute on Drug Abuse. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Child Health and Human Development, the National Institute on Drug Abuse, or the National Institutes of Health.
Contributor Information
Steven R. H. Beach, University of Georgia
Man Kit Lei, University of Georgia.
Gene H. Brody, University of Georgia
Sangjin Kim, University of Georgia.
Allen W. Barton, University of Georgia
Meesha V. Dogan, University of Iowa
Robert A. Philibert, University of Iowa
References
- Accomando WP, Wiencke JK, Houseman EA, Nelson HN, Kelsey KT. Quantitative reconstruction of leukocyte subsets using DNA methylation. Genome Biology. 2014;15:R50. doi: 10.1186/gb-2014-15-3-r50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Steinberg L, Draper P. Childhood experience, interpersonal development, and reproductive strategy: and evolutionary theory of socialization. Child Development. 1991;62:647–670. doi: 10.1111/j.1467-8624.1991.tb01558.x. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B. 1995;57(1):289–300. MR 1325392. [Google Scholar]
- Braveman PA, Cubbin C, Egerter S, Williams DR, Pamuk E. Socioeconomic disparities in health in the United States: What the patterns tell us. American Journal of Public Health. 2010;100(S1):S186–S196. doi: 10.2105/AJPH.2009.166082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Ge X, Conger RD, Gibbons FX, Murry VM, Gerrard M, Simons RL. The influence of neighborhood disadvantage, collective socialization, and parenting on African American children's affiliation with deviant peers. Child Development. 2001;72:1231–1246. doi: 10.1111/1467-8624.00344. [DOI] [PubMed] [Google Scholar]
- Brody GH, Yu T, Beach SRH, Kogan SM, Windle M, Philibert RA. Harsh parenting and adolescent health: A longitudinal analysis with genetic moderation. Health Psychology. 2014;33:401–409. doi: 10.1037/a0032686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, Scheier MF, Weintraub JK. Assessing coping strategies: A theoretically based approach. Journal of Personality and Social Psychology. 1989;56:267–283. doi: 10.1037//0022-3514.56.2.267. [DOI] [PubMed] [Google Scholar]
- Charnov EL. Life History Invariants. Oxford, UK: Oxford University Press; 1993. [Google Scholar]
- Chen E, Miller GE, Kobor MS, Cole SW. Maternal warmth buffers the effects of low early-life socioeconomic status on pro-inflammatory signaling in adulthood. Molecular Psychiatry. 2011;16:729–737. doi: 10.1038/mp.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conger RD, Ge X, Elder GH, Lorenz FO, Simons RL. Economic stress, coercive family process, and developmental problems of adolescents. Child Development. 1994;65(2):541–561. [PubMed] [Google Scholar]
- Cole SW, Hawkley LC, Arevalo JMG, Cacioppo JT. Transcript origin analysis identifies antigen-presenting cells as primary targets of socially regulated gene expression in leukocytes. Proceedings of the National Academy of Sciences. 2011;108(7):3080–3085. doi: 10.1073/pnas.1014218108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conger RD, Wallace LE, Sun Y, Simons RL, McLoyd VC, Brody GH. Economic pressure in African American families: A replication and extension of the family stress model. Developmental Psychology. 2002;38:179–193. [PubMed] [Google Scholar]
- Curley JP, Jensen CL, Mashoodh R, Champagne FA. Social influences on neurobiology and behavior: Epigenetic effects during development. Psychoneuroendocrinology. 2011;36:352–371. doi: 10.1016/j.psyneuen.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalaker J. Poverty in the United States, 2000. Washington, DC: U. S. Government Printing Office; 2001. Sep, (U. S. Census Bureau Current Population Reports Series P60–214) [Google Scholar]
- Dube SR, Fairweather D, Pearson WS, Felitti VJ, Anda RF, Croft JB. Cumulative childhood stress and autoimmune diseases in adults. Psychosomatic Medicine. 2009;71:243–250. doi: 10.1097/PSY.0b013e3181907888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essex MJ, Thomas Boyce W, Hertzman C, Lam LL, Armstrong JM, Neumann S, Kobor MS. Epigenetic vestiges of early developmental adversity: childhood stress exposure and DNA methylation in adolescence. Child Development. 2013;84(1):58–75. doi: 10.1111/j.1467-8624.2011.01641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW, Chen E, Miller GE, Seeman TE. How poverty gets under the skin: A life course perspective. In: Maholmes V, King R, editors. The Oxford Handbook of Poverty and Child Development. New York, Y: Oxford University Press; 2012. pp. 13–36. [Google Scholar]
- Gluckman PD, Hanson MA, Spencer HG. Predictive Adaptive Responses and Human Evolution. Trends in Ecology & Evolution. 2005;20(10):527–533. doi: 10.1016/j.tree.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Hayes AF. Beyond Baron and Kenny: Statistical mediation analysis in the new millennium. Communication Monographs. 2009;76(4):408–420. [Google Scholar]
- Hays RD, Sherbourne CD, Mazel RM. The RAND 36-item health survey 1.0. Health Economics. 1993;2:217–227. doi: 10.1002/hec.4730020305. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33(6):693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Hertzman C. The biological embedding of early experience and its effects on health in adulthood. Annals of the New York Academy of Sciences. 1999;896:85–95. doi: 10.1111/j.1749-6632.1999.tb08107.x. [DOI] [PubMed] [Google Scholar]
- Hochberg Z, Feil R, Constancia M, Fraga M, Junien C, Carel JC, Boileau P, Le Bouc Y, Deal CL, Lillycrop K, Scharfmann R, Sheppard A, Skinner M, Szyf M, Waterland RA, Waxman DJ, Whitelaw E, Ong K, Albertsson-Wikland K. Child health, developmental plasticity, and epigenetic programming. Endocrine Review. 2011;32(2):159–224. doi: 10.1210/er.2009-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houseman EA, Accomando WP, Koestler DC, Christensen BC, Marsit CJ, Nelson HH, Wiencke JK, Kelsey KT. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinformatics. 2012;13:86–101. doi: 10.1186/1471-2105-13-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Cole SW. Reciprocal Regulation of the Neural and Innate Immune Systems. Nature Reviews Immunology. 2011;11(9):625–632. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer DA. Commentary: Gene–environment interplay in the context of genetics, epigenetics, and gene expression. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44:19–27. doi: 10.1097/01.chi.0000145804.30112.6b. [DOI] [PubMed] [Google Scholar]
- Kurdek LA. Areas of conflict for gay, lesbian, and heterosexual couples: What couples argue about influences relationship satisfaction. Journal of Marriage and the Family. 1994;56:923–934. [Google Scholar]
- Maholmes V, King R. The Oxford Handbook of Poverty and Child Development. New York, NY: Oxford University Press; 2012. [Google Scholar]
- Masten AS. Ordinary Magic: Resilience Processes in Development. American Psychologist. 2001;56(3):227–238. doi: 10.1037//0003-066x.56.3.227. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E. Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychological Science. 2010;21(6):848–856. doi: 10.1177/0956797610370161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychological Bulletin. 2011;137:959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Cole SW. Clustering of depression and inflammation in adolescents previously exposed to childhood adversity. Biological Psychiatry. 2012;72(1):34–40. doi: 10.1016/j.biopsych.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Lachman ME, Chen E, Gruenewald TL, Karlamangla AS, Seeman TE. Pathways to resilience: Maternal nurturance as a buffer against the effects of childhood poverty on metabolic syndrome at midlife. Psychological Science. 2011;22:1591–1599. doi: 10.1177/0956797611419170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus user's guide. 7th ed. Los Angeles, CA: Muthén & Muthén; 1998–2012. [Google Scholar]
- Philibert RA, Beach SRH. The Genetic and Epigenetic Essentials of Modern Humans. In: Pluess M, editor. Genetics of Psychological Well-Being. Oxford University Press; 2015. [Google Scholar]
- Pidsley R, Wong CCY, Volta M, Lunnon K, Mill J, Schalkwyk LC. A data-driven approach to preprocessing Illumina 450K methylation array data. BMC Genomics. 2013;14:293–302. doi: 10.1186/1471-2164-14-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollitt RA, Kaufman JS, Rose KM, Diez-Roux AV, Zeng D, Heiss G. Cumulative life course and adult socioeconomic status and markers of inflammation in adulthood. Journal of Epidemiology and Community Health. 2008;62:484–491. doi: 10.1136/jech.2006.054106. [DOI] [PubMed] [Google Scholar]
- Prinz RJ, Foster SL, Kent RN, O'Leary KD. Multivariate assessment of conflict in distressed and nondistressed mother–adolescent dyads. Journal of Applied Behavior Analysis. 1979;12:691–700. doi: 10.1901/jaba.1979.12-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinius LE, Acevedo N, Joerink M, Pershagen G, Dahlén SE, Greco D, Söderhäll C, Scheynius A, Kere J. Differential DNA methylation in purified human blood cells: implications for cell lineage and studies on disease susceptibility. PLoS One. 2012;7:e41361. doi: 10.1371/journal.pone.0041361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psychological Bulletin. 2002;128(2):330–336. [PubMed] [Google Scholar]
- Rickard IJ, Lummaa V. The Predictive Adaptive Response and Metabolic Syndrome: Challenges for the Hypothesis. Trends in Endocrinology and Metabolism. 2007;18(3):94–99. doi: 10.1016/j.tem.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: Building a new framework for health promotion and disease prevention. Journal of the American Medical Association. 2009;301:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- West LJ. Defining critical windows in the development of the human immune system. Human & Experimental Toxicology. 2002;21(9–10):499–505. doi: 10.1191/0960327102ht288oa. [DOI] [PubMed] [Google Scholar]
- Yoshikawa H, Aber JL, Beardslee WR. The effects of poverty on the mental, emotional, and behavioral health of children and youth: Implications for prevention. American Psychologist. 2012;67(4):272. doi: 10.1037/a0028015. [DOI] [PubMed] [Google Scholar]
- Zeeberg BR, Feng W, Wang G, Wang MD, Fojo AT, Sunshine M, Weinstein JN. GoMiner: a resource for biological interpretation of genomic and proteomic data. Genome Biology. 2003;4(4):1–8. doi: 10.1186/gb-2003-4-4-r28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.