Abstract
Our experimental approach toward the development of new islet-based treatment for diabetes mellitus has been the creation of a monolayered islet cell construct (islet cell sheet), followed by its transplantation into a subcutaneous pocket. Previous studies describe rat laminin-5 (chain composition: α3, β3, γ2) as a suitable extracellular matrix (ECM) for surfaces comprised of a coated temperature-responsive polymer, poly(N-isopropylacrylamide) (PIPAAm). To progress toward the clinical application of this approach, the present study attempted to identify an optimal human ECM as a coating material on PIPAAm surfaces, which allowed islet cells to attach on the surfaces and subsequently to be harvested as a monolithic cell sheet. Dispersed rat islet cells were seeded onto PIPAAm dishes coated with various human laminin isotypes: human laminin (HL)-211, HL-332, HL-411, HL-511, and HL-placenta. Plating efficiency at day 1, the confluency at day 3, and glucose-stimulated insulin secretion test at day 3 were performed. The highest value of plating efficiency was found in the HL-332-PIPAAm group (83.1 ± 0.7%). The HL-332-PIPAAm group also showed the highest cellular confluency (98.6 ± 0.5%). Islet cells cultured on the HL-332-PIPAAm surfaces showed a positive response in the glucose-stimulated insulin secretion test. By reducing culture temperature from 37°C to 20°C in the HL-332-PIPAAm group, cells were able to be harvested as a monolithic islet sheet. The present study showed that HL-332 was an optimal human-derived ECM on a PIPAAm coating for preparing islet cell sheets.
Key words: Cell sheet, Pancreatic islets, Laminin, Extracellular matrix (ECM), Dispersed islet cells, Temperature-responsive polymer
INTRODUCTION
Cell-based therapy using human pancreatic islets possesses a great potential to provide a metabolic stability for patients with type 1 diabetes and diabetic patients who received a total pancreatectomy (24,26,29). Currently, treatment procedures have been based on infusing islets into the portal circulation for delivering cells to portal pedicles in the liver. However, treatment with portally infused islets was found to be associated with various complications including an instant blood-mediated inflammatory reaction. In addition, the cells are exposed to cytotoxic immunosuppressive drugs, resulting in a state of poor graft survival (4,11,22,31). For treating type 1 diabetes patients with the increasing burden of medical cost, the development of new approaches designating a bioengineered islet system in vivo is highly anticipated.
Our experimental approach to develop a neo-islet system in vivo has been based on the creation of islet cell sheets (15,16,25,27,28,33). Our group has recently established a reliable technology for creating transplantable monolayer islet cell sheets, which are transplanted to the subcutaneous spot of an animal model utilizing a simple procedure by handling the sheet as a patch (25,28). This procedure was found to be able to create functional neo-islet tissues in the subcutaneous site. The ectopically engineered neo-islet tissue in diabetic mouse successfully allows diabetic status to change to a normal glycemic status, which is observed long term. As an important feature, the cell sheet has a uniformly contiguous layer of islet cells keeping their intrinsic intercellular functional connections (15,25,28). A culture surface, where temperature-responsive polymer, poly(N-isopropylacrylamide) (PIPAAm), has been covalently grafted at a nanometer scale with a subsequent coating of rat laminin-5 (chain composition: α3, β3, γ2), is the principle technology for creating islet cell sheets (17,28).
The successful creations of neo-islet tissue in rodents have encouraged us to move forward for transforming this islet cell sheet-based approach into clinical use. To step ahead toward the clinical application of this approach, the present study attempted to identify optimal human extracellular matrix (ECM) as a coating material on PIPAAm surfaces, which allowed islet cells to attach on the surface and the subsequent cell harvesting as a cell sheet. Optimization was carried out in terms of islet cell attachment, expansion, and cellular functions. In addition, this study also investigated the cellular composition of islet cell sheets harvested from the PIPAAm surfaces coated with the identified optimal isotype of human laminin.
MATERIALS AND METHODS
Preparation of Dispersed Islet Cells
Pancreatic islets were isolated from Lewis rats (8- to 12-week-old males; Charles River, Yokohama, Japan) as previously reported (10,28). Briefly, after being injected with type V collagenase solution (Sigma-Aldrich, St. Louis, MO, USA) through the common bile duct of the rat under general anesthesia, the pancreas was removed from the animal and allowed to be digested for 16∼20 min at 37°C. Islets were then purified by density gradient centrifugation with Histopaque (Sigma-Aldrich) followed by a handpicking maneuver. Purified islets were subsequently cultured with Roswell Park Memorial Institute (RPMI)-1640 medium (Sigma-Aldrich) containing 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Waltham, MA, USA) overnight. On the following day, dispersed single islet cells were obtained by incubating with 0.125% trypsin–ethylenediaminetetraacetic acid (EDTA) (Thermo Fisher Scientific). Cell viability was then determined by trypan blue (Sigma-Aldrich) exclusion test. In this study, experiments were conducted only when dispersed islet cell viability exceeded 90%. All animal experiments were performed with the approval of the Institutional Animal Care and Use Committee of Tokyo Women’s Medical University, Tokyo, Japan.
Preparation of Temperature-Responsive Culture Surfaces
Culture surfaces especially for fabricating islet cell sheets were prepared as described previously with slight modifications (28). In brief, 24-well culture dishes (Thermo Fisher Scientific) were covalently grafted with a temperature-responsive polymer, PIPAAm, with approximately three times PIPAAm amount than that of commercially available PIPAAm dishes (UpCell; CellSeed, Tokyo, Japan) according to the polymer grafting procedure as described previously (17). Culture surfaces were further coated for 2 h with various isotypes of laminins: human recombinant laminin-211 (HL-211; 5 µg/cm2; Bio Lamina, Stockholm, Sweden), human recombinant laminin-411 (HL-411; 5 µg/cm2; Bio Lamina), human recombinant laminin-511 (HL-511; 5 µg/cm2; Bio Lamina), human laminin placenta (2 µg/cm2; Sigma-Aldrich), or human recombinant laminin-332 (HL-332; 0.2 µg/cm2; Oriental Yeast, Tokyo, Japan). As the positive control, the previously optimized culture surfaces coated with rat laminin-5 (0.2 µg cm2; EMD Millipore, Billerica, MA, USA) were used (28). After incubation, dishes were gently washed twice with 1× phosphate-buffered saline (PBS; Sigma-Aldrich). Coating procedures were conducted according to the manufacturer’s instruction.
Culture of Dispersed Islet Cells
Dispersed rat islet cells resuspended in RPMI-1640 medium containing 10% FBS were plated on laminin-coated PIPAAm dishes at a density of 0.5 × 106 cells/cm2 and cultured at 37°C. Twenty-four hours later, the culture dishes were washed to remove nonattached cells and replenished with RPMI-1640 containing 10% FBS. At 48 h, medium was changed with fresh medium, and islet cells were cultured for an additional 24 h at 37°C.
Measuring of Plating Efficiency and Confluency
At day 1 (24 h after cell seeding), attached islet cells were counted in four randomly selected fields per dish at a magnification of 200 (n = 4 per each group). Plating efficiency of islet cells was expressed by the percentage of the number of attached islet cells per the total number of plated islet cells. At day 3, there was an islet cell confluency, which was expressed as the percentage of total surface area of attached cells per culture surface area (n = 4 per each group).
Insulin Secretion Assay
At day 3, insulin secretion assay was conducted as described previously (28). In brief, the culture media was changed with fresh RPMI media containing 3.3 mmol/L glucose, and cells were cultured for 180 min. After this incubation step, cells were then incubated with RPMI media containing 3.3 mmol/L glucose for 60 min and then with RPMI containing 20 mmol/L glucose for 60 min. For the final step, cells were incubated with RPMI media containing 3.3 mmol/L glucose for 60 min. At the end of each time point, the culture medium was collected and kept frozen at −20°C until analysis. The amount of secreted insulin in the culture media (n = 3 per time point) was measured by the Mercodia Ultrasensitive Rat Insulin enzyme-linked immunosorbent assay (ELISA) kit (Mercodia, Uppsala, Sweden). The insulin stimulation index (SI) was calculated by the following equation. SI = [insulin]20/[insulin]3.3, where [insulin]20 and [insulin]3.3 represent insulin concentrations in medium containing 20 mmol/L glucose and that of 3.3 mmol/L glucose at the initial incubation, respectively.
Electron Microscopy Analysis of Harvested Islet Cell Sheets
At day 3, islet cells cultured on PIPAAm surfaces coated with HL-332 were harvested as a cell sheet format by reducing culture temperature from 37°C to 20°C for 30 min. This temperature reduction initiated an automatic alteration of PIPAAm character from hydrophobic to hydrophilic, resulting in the spontaneous detachment of the cell sheet from culture surfaces. A piece of wet supporting membrane (CellShifter; CellSeed) was put on islet cells, which were harvested as a contagious cell sheet with the membrane. Harvested islet cell sheet was then fixed with 2% glutaraldehyde (Sigma-Aldrich) in 0.1 mol/L phosphate buffer and postfixed with 1% osmium tetroxide (Sigma-Aldrich). Fixed specimens were dehydrated by ethanol and embedded with TAAB EPSON 812 (Taab Laboratories Equipment, Berkshire, UK). The ultrathin sections (80 nm in thickness) of sheet samples were observed by a transmission electron microscope (JEM1200EX; JEOL, Tokyo, Japan) at 80 kV.
Immunohistochemical Analyses
Cultured islet cells at day 3 were fixed with 4% paraformaldehyde (Wako, Osaka, Japan), rinsed with PBS, and nonspecific binding sites were blocked with 10% goat serum (Vector, Burlingame, CA, USA) for 30 min. The cells were incubated with guinea pig polyclonal anti-insulin antibody (diluted 1:50; Abcam, Cambridge, UK), mouse monoclonal anti-glucagon antibody (diluted 1:2,000; Abcam), or rabbit polyclonal anti-somatostatin antibody (diluted 1:500; Abcam). For fluorescence immunohistochemistry, Alexa Fluor 488 goat anti-guinea pig IgG and Alexa Fluor 594 goat anti-mouse IgG, and Alexa Fluor 488 goat anti-rabbit IgG (Thermo Fisher Scientific) were used as the secondly antibodies. Nuclear DNA was stained with 300 nM 4′,6-diamidino-2-phenylindole (DAPI; Thermo Fisher Scientific).
Statistical Analyses
All values in the present study were expressed as the mean ± standard deviation (SD). The significance of the differences between groups was tested by one-way ANOVA followed by Scheffe’s least significant difference post hoc analysis using PASW Statistics 18 software (IBM Japan, Tokyo). A probability value of less than 0.05 was considered to be statistically significant.
RESULTS
Examination of Human Laminin Isotypes for Adherent Culture of Dispersed Islet Cells
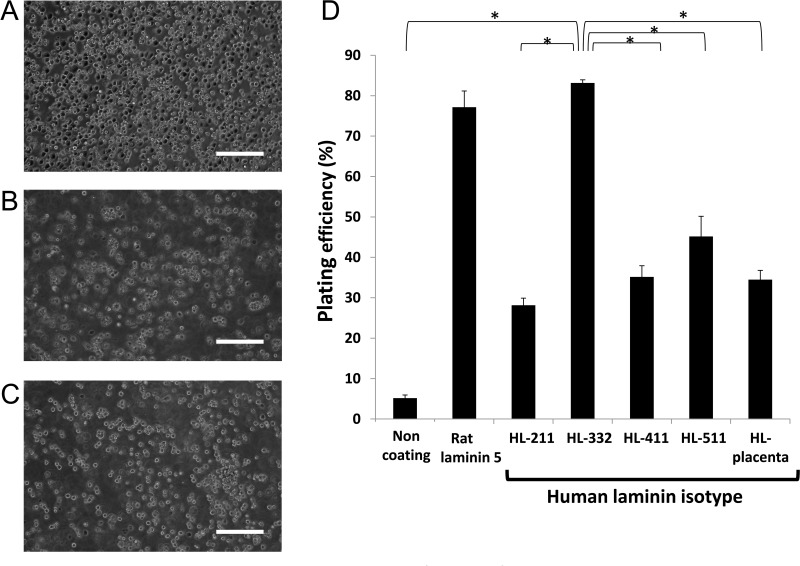
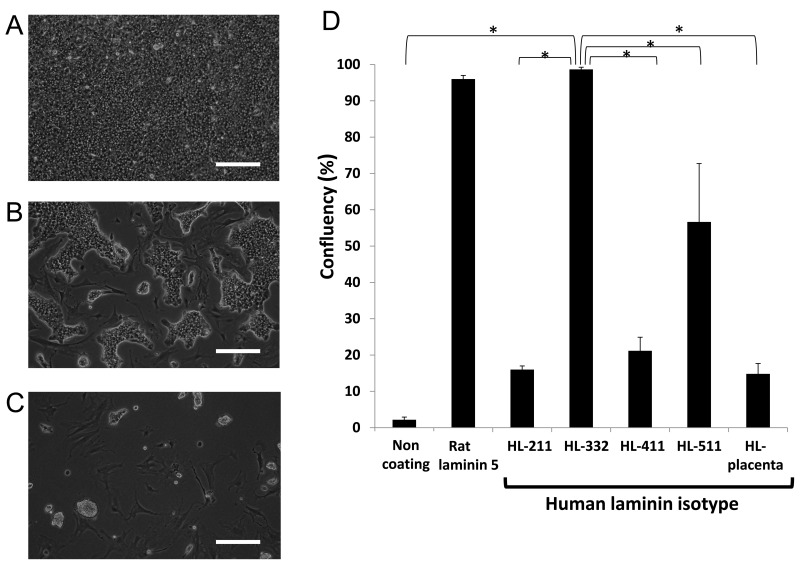
In order to determine a suitable human laminin (HL) that enables efficient adhesion of dispersed islet cells, PIPAAm dishes were coated with various isotypes of HLs, including HL-placenta and recombinant HL-211, HL-332, HL-411, and HL-511. Dispersed rat pancreatic islet cells were seeded onto PIPAAm dishes. Plating efficiency at day 1 showed that PIPAAm with HL-332 surface provided a significantly higher level of cell attachment than those with the other human HL isotypes (Fig. 1). To investigate a possible further cellular adherence and extension on these PIPAAm surfaces, the cell confluency was observed at day 3. As shown in Figure 2, cells on PIPAAm with HL-332 and rat laminin-5 showed nearly full confluent status, while PIPAAm with the other HL isotypes failed to provide such a confluent status.
Figure 1.
Dispersed islet cells adhered on temperature-responsive polymer, poly(N-isopropylacrylamide) (PIPAAm) dishes coated with various human laminins. Dispersed rat islet cells were plated at a density of 0.5 × 106 cells/cm2, and cell attachment status was assessed at day 1. Representative images of plated islet cells on PIPAAm dishes coated with (A) human laminin (HL)-332, (B) HL-511, and (C) HL-411. (D) Plating efficiencies expressed by the percentage of the number of attached islet cells against the total number of plated islet cells (n = 4). Scale bars: 100 μm. *p < 0.05 compared among the groups by one-way ANOVA with Scheffe’s least significant difference post hoc test.
Figure 2.
Morphology and confluent status of islet cells at day 3. Dispersed rat islet cells were plated at a density of 0.5 × 106 cells/cm2, and cell confluency was assessed. Representative images of cultured islet cells on temperature-responsive polymer, poly(N-isopropylacrylamide) (PIPAAm) dishes coated with (A) human laminin (HL)-332, (B) HL-511, and (C) HL-411. (D) Confluency of dispersed islet cells cultured on PIPAAm dishes coated with various human laminins (n = 4). Scale bars: 100 μm. *p < 0.05 compared among the groups by one-way ANOVA with Scheffe’s least significant difference post hoc test.
Functional Assessment of the Adherent Islet Cells on HL-Coated PIPAAm Surfaces
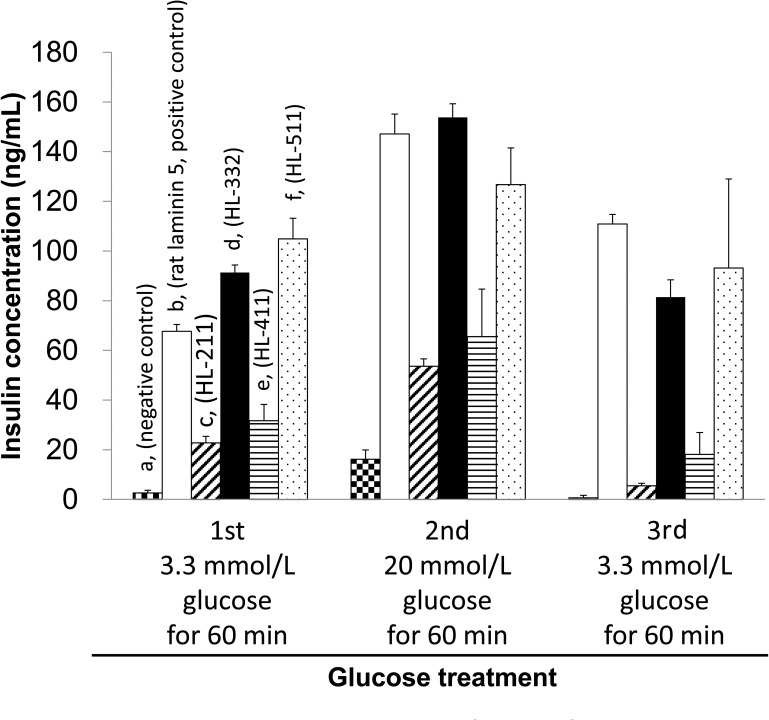
Biological functionality of the cultured islet cells was assessed by their ability to secrete insulin in response to a different level of glucose exposure. At an initial phase of lower basal glucose level (3.3 mmol/L), cells on PIPAAm coated with HL-332, HL-511, and rat laminin-5 secreted the higher amount of insulin than those on the other culture surfaces (Fig. 3). At a higher glucose concentration (20 mmol/L), PIPAAm coated with HL-332 was found to provide the highest insulin secretion among the three culture surfaces. The stimulation index for the cells on PIPAAm with HL-332 was 1.73 ± 0.1 (n = 3). These results indicated that HL-332 was the most adequate human laminin isotype as a coating extracellular matrix on PIPAAm for providing the suitable attachment and subsequent functionality of dispersed islet cells.
Figure 3.
Insulin secretion of cultured islet cells in response to glucose stimulation. At day 3 of culture experiment, cells were incubated with 3.3 mmol/L glucose for 60 min as the first glucose treatment, 20 mmol/L glucose for 60 min as the second treatment, and 3.3 mmol/L glucose for 60 min as the final treatment (n = 4 for each coated surface). The columns (a) in three step treatments represent the amounts of secreted insulin from dispersed islet cells cultured on temperature-responsive polymer, poly (N-isopropylacrylamide) (PIPAAm) dishes (the negative control). The columns (b, c, d, e, and f) represent the amounts of secreted insulin from dispersed islet cells cultured on PIPAAm dishes coated with rat laninin-5 (the positive control), human laminin (HL)-211, HL-332, HL-411, and HL-511, respectively.
Cellular Composition of Islet Cell Sheets on PIPAAm Coated With HL-332
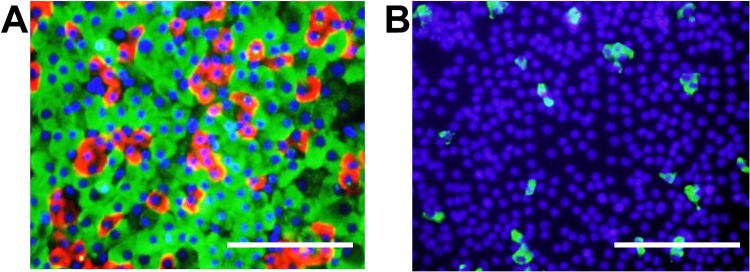
Since the islets are composed of microregions containing at least four types of endocrine cells, the cellular composition of islet cells cultured on PIPAAm coated with HL-332 for 3 days was investigated. The three major cell types were immunohistochemically investigated by immunostaining for insulin (β-cells), glucagon (α-cells), and somatostatin (δ-cells) (Fig. 4). From these images, the ratios of α-, β-, and δ-cells were calculated to be 79.5 ± 5.0%, 18.2 ± 4.6%, and 2.2 ± 0.5% respectively (n = 4).
Figure 4.
Profile of dispersed islet cells cultured on temperature-responsive polymer, poly(N-isopropylacrylamide) (PIPAAm) dishes coated with human laminin-332. To confirm the presence of cytoplasmic insulin, glucagon, and somatostatin of cultured islet cells on PIPAAm culture dishes, cells at day 3 were immunostained by fluorescence method. (A) Insulin-positive cells (β-cells) and glucagon-positive cells (α-cells) were stained green and red, respectively. Nuclei were stained blue. (B) Somatostatin-positive cells (δ-cells) were stained green. Nuclei were stained blue. Scale bars: 100 μm.
Harvesting Islet Cell Sheets and Electron Microscopic Analysis
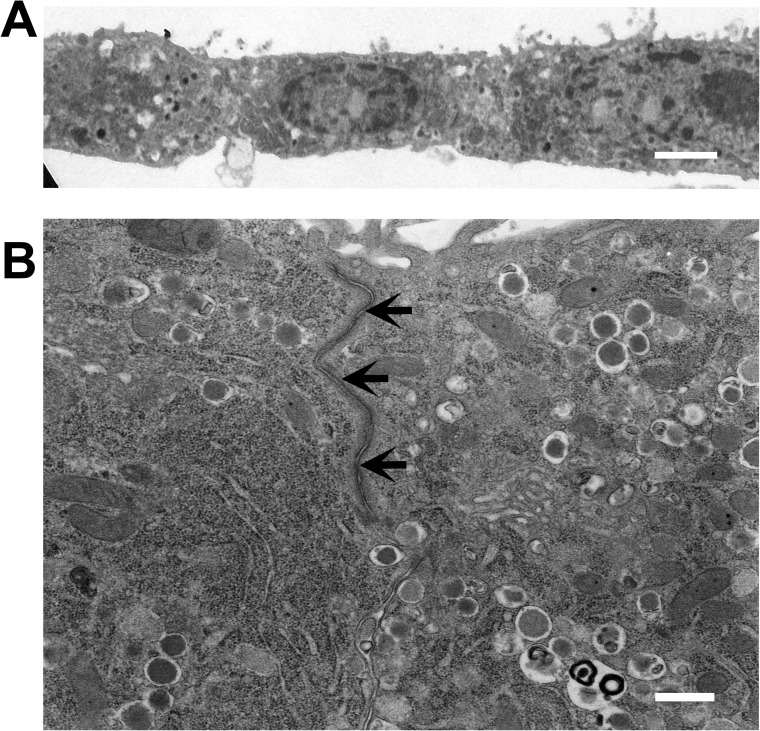
To examine if islet cells cultured on PIPAAm coated with HL-332 were able to be harvested as a cell sheet format, culture temperature was temporarily reduced from 37°C to 20°C for 30 min at day 3. This temperature reduction initiated an islet cell detachment from PIPAAm culture surfaces. With the help of a piece of supporting membrane over the cells, cells were successfully harvested as an intact sheet format. Transmission electron microscopic observation of harvested islet cell sheet showed a monolayered structure containing tight junctions between islet cells (Fig. 5). Furthermore, numerous insulin secretion granules were also observed in the cytoplasm of cells expressing characteristics as a metabolically active islet tissue format (Fig. 5B).
Figure 5.
Transmission electron microscopic images of an islet cell sheet harvested at day 3 from a temperature-responsive polymer, poly(N-isopropylacrylamide) (PIPAAm) dish coated with human laminin-332. Culture temperature was reduced to 20°C for 30 min for inducing natural cell detachment from the dish and harvesting as a cell sheet. (A) Monolayered configuration of the harvested islet cell sheet. (B) Numerous dense-cored insulin secretion granules in the cytoplasm. Tight junctions (arrows) were formed between the islet cells within the cell sheet. Scale bars: 2 μm (A), 500 nm (B).
DISCUSSION
This study demonstrated that HL-332 was the most suitable human laminin isotype, for coating a PIPAAm grafted culture dish, and it allowed the dispersed islet cells to grow to confluence and become a cell monolayer secreting insulin on the culture dish. The cell monolayer was found to contain β-cells as well as α-cells and δ-cells in its structure, and detached from the culture surface as a contiguous cell sheet by reducing the temperature from 37°C to 20°C for 30 min. Electron microscopy found cell-to-cell connections as the tight junctions in the cell sheet and insulin granules in the cytoplasm. On the other hand, dispersed islet cells seeded on a PIPAAm graft culture dish without the HL-332 coating were found to hardly grow. HL-332 was an adequate human laminin isoform for obtaining an islet cell monolayer on PIPAAm grafted culture dish.
Our previous study has found that the most suitable ECM is rat laminin-5 for preparing functional islet sheets on PIPAAm-grafted culture dishes, and the optimal amount of PIPAAm grafted to a culture dish is found to be larger than that of conventional PIPAAm dish for keeping and detaching cells as an islet cell sheet (28). In addition, by transplanting the islet cell sheets in the subcutaneous site of diabetic severe combined immune deficiency (SCID) mouse, newly appeared functional islet tissues are observed and found to allow hyperglycemia to be improved (25). For applying islet cell sheets to the clinical treatment of diabetes mellitus, human islet cell sheets must be prepared. No report describing the culture surface preparation with adequate human ECM coating for producing islet cell sheets on PIPAA graft culture dishes was found. Therefore, this study investigated the most suitable human ECM for coating PIPAAm graft culture dishes for preparing islet cell sheets.
Laminins are cross-shaped heterotrimeric glycoproteins consisting of three polypeptide chains (α-, β-, and γ-chains) (8,30). Fifteen isoforms of laminin isotypes are known with the different combinations of the polypeptide chains (7). The basement membrane (BM) of various cells, including nerve cells and keratinocytes, contains laminins, which play important roles in cellular adhesion, migration, proliferation, differentiation, and apoptosis through the interaction with integrins (1,7). In pancreas, laminin-111 is found in the BM in the epithelium of mouse pancreatic ducts; laminin-511, in the BM of acinar cells; and laminin-411 and 211, in the BM of blood vessels (13). In our previous study, among rat laminin-1 (chain composition: α1, β1, γ1), rat laminin-5 (chain composition: α3, β3, γ2), and type 1 collagen, rat laminin-5 was found to be the most adequate ECM (28). This study attempted to search the most suitable laminin isotype from HL-211, -332, -411, -511, and HL-placenta for the purpose of islet tissue engineering.
Laminin-332, which was found to be the most suitable ECM for culturing a rat islet cell sheets on PIPAAm grafted culture dish, is an extracellular matrix binding with integrin α3β1 that is intensely expressed on the membrane of islet cells (14,19). Integrin β1 contributes to the attachment of islet β-cells to the matrix and their insulin secretion through the interaction with laminin-332 (19). Bosco et al. (5) have reported that the amount of insulin secreted from rat β-cells is increased two times on a culture dish with a high dose of laminin-332 produced by 804G cells. Laminin-332 is known to contribute to the proliferation of cells, as well as the protection of β-cells from apoptosis, which is induced by proinflammatory cytokines such as interleukin-1β, interferon-γ, and tumor necrosis factor-α (2,5,12,19). Since laminin-332 is expressed in rat and human β-cells (19), HL-332 was speculated to be the most suitable human laminin isotype for preparing human islet cell sheets on PIPAAm grafted culture dishes.
Rodent pancreas islets contain at least three different types of endocrine cells with the estimated cell population being 70–80% for β-cells, 10–20% for α-cells, and 2–5% for δ-cells (3,6). In the present study, dispersed islet cells from purified islets by a handpicking maneuver were plated on HL-coated PIPAAm surfaces. Histological analyses revealed that the cellular proportion of these endocrine cells in islet cell sheets made on HL-332-PIPAAm surfaces was nearly identical with those of naive islets. The data indicated that the HL-332-PIPAAm culture surface supported cellular attachment evenly to all three endocrine cell types. The physiological significance of the islet sheets containing β-cells and other cells could be highlighted by following previously reported findings that glucagon provided by α-cells positively affects insulin release from β-cells (20,32), and acetylcholine produced by α-cells interacts with β-cells as a positive regulator of insulin secretion (23). Furthermore, cell-to-cell contacts formed among β-cells and non-β-cells are also important for regulating insulin secretion levels (32). Although these three types of cells were distributed in the islet sheet without regularity, our previous transplantation study demonstrates that neo-islet tissues formed in the subcutaneous site comprised clusters of β-cells surrounded by α-cells, showing a characteristic cellular arrangement of rodent islets (18,25,27). It has been shown that these cellular rearrangements by β-cells and non-β-cells begun as early as day 4 after the islet cell sheet transplantation (27). Future studies are needed to investigate the underlying mechanisms for these intermingled different cell components to rearrange their characteristic formation in vivo.
From our previous experimental findings, neo-islet engineering approaches into the subcutaneous site by islet sheet transplantation may bring several benefits in further developing islet-based therapy (16,25,28). Those benefits include: [1] treatment can be performed under minimally invasive procedures, [2] repetitive procedures could be easily conducted for increasing therapeutic effects, and [3] avoidance of instant blood-mediated inflammatory reaction (IBMIR) and other adverse reactions caused by intravenous cell infusion. As another important benefit, dispersed islet cells would be susceptible for cryopreservation. It has been described that cryopreserved isolated islets are reported to be prone to cellular damage and loss (9,21). In contrast, our group has recently shown that dispersed islet cells can be cryopreserved without causing remarkable loss of viable cells and can be cultured for preparing islet cell sheets (15). A combination of the islet sheet-based tissue engineering approach and a storage/pooling system of islet cells would add a great flexibility to clinical islet-based therapy.
In conclusion, the present study successfully identified a human laminin isotype that favorably supports the monolayered culturing of dispersed islet cells on PIPAAm surfaces. Cultured islet cells were able to be harvested as a contiguous monolayered cell sheet composed of α-, β-, and δ-cells. For translating this technology to humans, further experiments are awaited for creating islet cell sheets made of human dispersed islet cells using HL-332-PIPAAm culture surfaces.
ACKNOWLEDGMENT
The authors would like to thank Ms. Kazuko Kanegae (Tokyo Women’s Medical University) and Mr. Takeshi Masuda (CellSeed) for their technical assistance in islet isolation and culturing, Dr. Norio Ueno (Tokyo Women’s Medical University) for his editorial assistance in the preparation of this manuscript. This work was supported in part by Creation of innovation centers for advanced interdisciplinary research areas Program in the Project for Developing Innovation Systems “Cell Sheet Tissue Engineering Center (CSTEC)” (K.O, T.O) and Grant-in-Aid (Nos. 24300174 and 26670587 to K.O) from the Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. Teruo Okana, Ph.D., is a founder and a member of the board of CellSeed Inc., which has licenses for certain cell sheet-related technologies and patents from Tokyo Women’s Medical University.
REFERENCES
- 1. Adams J. C.; Watt F. M. Regulation of development and differentiation by the extracellular matrix. Development 117(4):1183–1198; 1993. [DOI] [PubMed] [Google Scholar]
- 2. Armanet M.; Wojtusciszyn A.; Morel P.; Parnaud G.; Rousselle P.; Sinigaglia C.; Berney T.; Bosco D. Regulated laminin-332 expression in human islets of Langerhans. FASEB J. 23(12):4046–4055; 2009. [DOI] [PubMed] [Google Scholar]
- 3. Baetens D.; Malaisse-Lagae F.; Perrelet A.; Orci L. Endocrine pancreas: Three-dimensional reconstruction shows two types of islets of Langerhans. Science 206(4424):1323–1325; 1979. [DOI] [PubMed] [Google Scholar]
- 4. Barshes N. R.; Wyllie S.; Goss J. A. Inflammation-mediated dysfunction and apoptosis in pancreatic islet transplantation: Implications for intrahepatic grafts. J. Leukoc. Biol. 77(5):587–597; 2005. [DOI] [PubMed] [Google Scholar]
- 5. Bosco D.; Meda P.; Halban P. A.; Rouiller D. G. Importance of cell-matrix interaction in rat islet β cell secretion in vitro role of α6β1 integrin. Diabetes 49(2):233–243; 2000. [DOI] [PubMed] [Google Scholar]
- 6. Brissova M.; Fowler M. J.; Nicholson W. E.; Chu A.; Hirshberg B.; Harlan D. M.; Powers A. C. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J. Histochem. Cytochem. 53(9):1087–1097; 2005. [DOI] [PubMed] [Google Scholar]
- 7. Colognato H.; Yurchenco P. D. Form and function: The laminin family of heterotrimers. Dev. Dyn. 218(2):213–234; 2000. [DOI] [PubMed] [Google Scholar]
- 8. Cooper A. R.; Kurkinen M.; Taylor A.; Hogan B. L. Studies on the biosynthesis of laminin by murine parietal endoderm cells. Eur. J. Biochem. 119(1):189–197; 1981. [DOI] [PubMed] [Google Scholar]
- 9. Di Carlo A.; Scharp D. W.; Gingerich R. L.; Giannarelli R.; Ansara M.; Olack B. J.; Swanson C. J.; Navalesi R. Insulin and glucagon release from isolated, perifused human islet following low temperature culture and cryopreservation. Transplant. Proc. 26(2):821–822; 1994. [PubMed] [Google Scholar]
- 10. Gotoh M.; Maki T.; Kioizumi T.; Satomi S.; Monako A. P. An improved method for isolation of mouse pancreatic islets. Transplantation 40(4):437–438; 1985. [DOI] [PubMed] [Google Scholar]
- 11. Halban P. A.; German M. S.; Kahn S. E.; Weir G. C. Current status of islet cell replacement and regeneration therapy. J. Clin. Endocrinol. Metab. 95(3):1034–1043; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hammar E.; Parnaud G.; Bosco D.; Perriraz N.; Maedler K.; Donath M.; Rouiller D. G.; Halban P. A. Extracellular matrix protects pancreatic β cells against apoptosis: Role of short-and long-term signaling pathways. Diabetes 53(8):2034–2041; 2004. [DOI] [PubMed] [Google Scholar]
- 13. Jiang F. X.; Naselli G.; Harrison L. C. Distinct distribution of laminin and its integrin receptors in the Pancreas. J. Histochem. Cytochem. 50(12)1625–1632; 2002. [DOI] [PubMed] [Google Scholar]
- 14. Kantengwa S.; Baetens D.; Sadoul K.; Buck C. A.; Halban P. A.; Rouiller D. G. Identification and characterization of α3β1 integrin on primary and transformed rat islet cells. Exp. Cell Res. 237(2):394–402; 1997. [DOI] [PubMed] [Google Scholar]
- 15. Ohashi K.; Mukobata S.; Utoh R.; Yamashita S.; Masuda T.; Sakai H.; Okano T. Production of islet cell sheets using cryopreserved islet cells. Transplant. Proc. 43(9):3188–3191; 2011. [DOI] [PubMed] [Google Scholar]
- 16. Ohashi K.; Okano T. Functional tissue engineering of the liver and islets. Anat. Rec. 297:73–82; 2014. [DOI] [PubMed] [Google Scholar]
- 17. Okano T.; Yamada N.; Sakai H.; Sakurai Y. A novel recovery system for cultured cells using plasma-treated polystyrene dishes grafted with poly(N-isopropylacrylamide). J. Biomed. Mater. Res. 27(10):1243–1251; 1993. [DOI] [PubMed] [Google Scholar]
- 18. Orci L.; Unger R. H. Functional subdivision of islets of Langerhans and possible role of D cells. Lancet 2(7947):1243–1244; 1975. [DOI] [PubMed] [Google Scholar]
- 19. Parnaud G.; Hammar E.; Rouiller D. G.; Armanet M.; Halban P. A.; Bosco D. Blockade of β1 integrin-laminin-5 interaction affects spreading and insulin secretion of rat β cells attached on extracellular matrix. Diabetes 55(5):1413–1420; 2006. [DOI] [PubMed] [Google Scholar]
- 20. Pipeleers D.; in’t Veld P. I.; Maes E.; Van De Winkel M. Glucose-induced insulin release depends on functional cooperation between islet cells. Proc. Natl. Acad. Sci. USA 79(23):7322–7325; 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rich S. J.; Swift S.; Thirdborough S. M.; James R. F.; Bell P. R.; London N. J. Islet cryopreservation: A detailed study of total functional losses. Transplant. Proc. 26(2):823–834; 1994. [PubMed] [Google Scholar]
- 22. Rickels M. R.; Schutta M. H.; Markmann J. F.; Barker C. F.; Naji A.; Teff K. L. β-Cell function following human islet transplantation for type 1 diabetes. Diabetes 54(1):100–106; 2005. [DOI] [PubMed] [Google Scholar]
- 23. Rodrigues-Diaz R.; Dando R.; Jacques-Silvia M. C.; Fachado A.; Molina J.; Abdulreda M. H.; Ricordi C.; Roper S. D.; Berggren P. O.; Caicedo A. Alpha cells secrete acetylcholine as a non-neuronal paracrine signal priming beta cell function in humans. Nat. Med. 17(7):888–892; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ryan E. A.; Lakey J. R.; Paty B. W.; Imes S.; Korbutt G. S.; Kneteman N. M.; Bigam D.; Rajotte R. V.; Shapiro A. M. Successful islet transplantation continued insulin reserve provides long-term glycemic control. Diabetes 51(7):2148–2157; 2002. [DOI] [PubMed] [Google Scholar]
- 25. Saito T.; Ohashi K.; Utoh R.; Shimizu H.; Ise K.; Suzuki H.; Yamato M.; Okano T.; Gotoh M. Reversal of diabetes by the creation of neo-islet tissues into a subcutaneous site using islet cell sheets. Transplantation 92(11):1231–1236; 2011. [DOI] [PubMed] [Google Scholar]
- 26. Shapiro A. M.; Lakey J. R.; Ryan E. A.; Korbutt G. S.; Toth E.; Warnock G. L.; Kneteman N. M.; Rajotte R. V. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 343(4):230–238; 2000. [DOI] [PubMed] [Google Scholar]
- 27. Shimizu H.; Ohashi K.; Saito T.; Utoh R.; Ise K.; Yamato M.; Okano T.; Gotoh M. Topographical arrangement of α- and β-cells within neo-islet tissues engineered by islet cell sheet transplantation in mice. Transplant. Proc. 45(5):1881–1884; 2013. [DOI] [PubMed] [Google Scholar]
- 28. Shimizu H.; Ohashi K.; Utoh R.; Ise K.; Gotoh M.; Yamato M.; Okano T. Bioengineering of functional sheet of islet cells for the treatment of diabetes mellitus. Biomaterials 30(30):5943–5949; 2009. [DOI] [PubMed] [Google Scholar]
- 29. Sutherland D. E.; Gruessner A. C.; Carlson A. M.; Blondet J. J.; Balamurugan A. N.; Reigstad K. F.; Beilman G. J.; Bellin M. D.; Hering B. J. Islet autotransplant outcomes after total pancreatectomy: A contrast to islet allograft outcomes. Transplantation 86(12):1799–1802; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tunggal P.; Smyth N.; Paulsson M.; Ott M. C. Laminins: Structure and genetic regulation. Microsc. Res. Tech. 51(3):214–227; 2000. [DOI] [PubMed] [Google Scholar]
- 31. Van der Windt D. J.; Bottino R.; Casu A.; Campanile N.; Cooper D. K. Rapid loss of intraportally transplanted islets: An overview of pathophysiology and preventive strategies. Xenotransplantation 14(4):288–297; 2007. [DOI] [PubMed] [Google Scholar]
- 32. Wojtusciszyn A.; Armanet M.; Morel P.; Berney T.; Bosco D. Insulin secretion from human beta cells is heterogeneous and dependent on cell-to-cell contacts. Diabetologia 51(10):1843–1852; 2008. [DOI] [PubMed] [Google Scholar]
- 33. Yamashita S.; Ohashi K.; Utoh R.; Kin T.; Shapiro A. M. J.; Yamamoto M.; Gotoh M.; Okano T. Quality of air-transported human islets for preparing single islet cells. Cell Med. 6:33–38; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]