Abstract
Improving the effects of human adipose tissue-derived mesenchymal stem cells (ASCs) on the demyelination and neurobehavioral function was investigated in an experimental model of neonatal hypoxic-ischemic encephalopathy (HIE). Seven-day-old male rats were subjected to hypoxia-ischemia-lipopolysaccharide and intracerebroventricularly transplanted with human ASCs (4 × 105 cells/rat) once at postnatal day 10 (PND10) or repeatedly at PND10, 17, 27, and 37. Neurobehavioral abnormalities (at PND20, 30, and 40) and cognitive functions (at PND41–44) were evaluated using multiple test systems. Human ASCs recovered the using ratio of forelimb contralateral to the injured brain, improved locomotor activity, and restored rota-rod performance of HIE animals, in addition to showing a marked improvement of cognitive functions. It was confirmed that transplanted human ASCs migrated to injured areas and differentiated into oligodendrocytes expressing myelin basic protein (MBP). Moreover, transplanted ASCs restored production of growth and neurotrophic factors and expression of decreased inflammatory cytokines, leading to attenuation of host MBP loss. The results indicate that transplanted ASCs restored neurobehavioral functions by producing MBP as well as by preserving host myelins, which might be mediated by ASCs’ anti-inflammatory activity and release of growth and neurotrophic factors.
Keywords: Neonatal hypoxic-ischemic encephalopathy (HIE), Adipose tissue-derived mesenchymal stem cell (ASC), Differentiation, Neurobehavior, Cognition, Anti-inflammation, Growth/neurotrophic factor
INTRODUCTION
Neonatal hypoxia-ischemia encephalopathy (HIE), also called periventricular leukomalacia (PVL) or cerebral palsy, is one of the most devastating neural diseases exhibiting diverse neurobehavioral symptoms resulting from asphyxia during delivery (16). Although asphyxia during delivery is considered an important etiological factor in many cases with PVL, the etiology might be multifactorial including intrauterine infection. Especially, respiratory dysfunction is a predominant factor in preterm infants in which a very high incidence of HIE occurs (3,10,43,44). Motor, perceptual, visual, behavioral, and/or cognitive disorders occur in the majority of cases with HIE (11,24,45). Maturation of oligodendrocytes in the human brain is thought to begin with the oligodendrocyte progenitors, progressing to immature oligodendrocytes that ensheath axons for differentiation into myelin-producing oligodendrocytes (4,35). Late preoligodendrocytes and immature oligodendrocytes were found to be particularly susceptible to free radicals and inflammatory cytokines induced by hypoxia-ischemia (HI) insults (12,22,28). Therefore, demyelination of late preoligodendrocytes and immature oligodendrocytes is a key feature of HIE in premature infants.
The oligodendrocyte progenitors in immature rats are also susceptible to HI insults (3). In previous studies, it was confirmed that ischemia (carotid artery ligation) followed by hypoxia (8% O2 for 2 h) caused demyelination and neurobehavioral abnormalities (29). Thereafter, HI followed by lipopolysaccharide (LPS), a combinational model of carotid artery ligation–exposure to a hypoxic environment–LPS injection (HIL) was demonstrated for the induction of cerebral palsy (CP), displaying severe neurobehavioral deficits following HIE (15,17).
In the treatment of HIE, current therapeutic strategies are very limited and restricted to supportive intensive care. For example, clinical hypothermia using icepacks and experimental administration with erythropoietin or minocycline were found to delay the progress of body dysfunction (6,18,47). Recently, several studies reported that various stem cells such as neural stem cells, multi-potent adult progenitor cells, and mesenchymal stem cells have beneficial effects on HI brain injury (9,26,32,41). Especially, it has also been suggested that murine bone marrow-derived mesenchymal stem cells (BMSCs) not only facilitate axonal sorting, myelination, and functional recovery in paralyzed mice deficient in Schwann cells, which ensheath axons in the peripheral nervous system but also improve the behavioral outcome and induces neuronal and oligodendrocyte regeneration after neonatal HI brain injury (7,40).
Adipose tissue is an accessible and reliable site for the isolation of abundant mesenchymal stem cells (100,000 stem cells/g fat tissue) suitable for tissue engineering and regenerative medicine applications (38). The differential capacity of adipose tissue-derived mesenchymal stem cells (ASCs) is less affected by donor age (20). Another interesting characteristic of mesenchymal stem cells is their ability to mobilize to areas of tissue injury known as lesion tropism. Mesenchymal stem cells intravenously delivered to rats after myocardial infarction localize in the infarct region and improve ventricular function, whereas the cells infused to noninfarcted rats localize to the bone marrow (36). Therefore, the present study was carried out to determine whether transplantation of human ASCs to neonatal HIL brain injury rats improves neurobehavioral and cognitive functions, in addition to neuroprotective effects.
MATERIALS AND METHODS
Animal Model of HIE
Pregnant Sprague–Dawley rats were purchased from Daehan-Biolink (Eumseong, Korea). The animals (n = 7/group) were maintained at a constant temperature (23 ± 2°C), relative humidity of 55 ± 10%, and 12-h light/dark cycle and fed with standard rodent chow and purified water ad libitum. Neonates were obtained from natural delivery, and male pups of postnatal day 7 (PND7) underwent HIL; that is, their left common carotid artery was occluded and placed in an 8% oxygen/92% nitrogen incubator (36°C) for 2 h (29). Finally, the pups were intraperitoneally injected with LPS (1 mg/kg) after 1-h recovery to induce inflammation and returned to their dam. Control animals only underwent the sham operation and vehicle treatment. All experimental procedures were approved and carried out in accordance with the Institutional Animal Care and Use Committee of Laboratory Animal Research Center at Chungbuk National University, Korea.
Preparation and Transplantation of Human ASCs
Human ASCs were prepared under Good Manufacturing Practice conditions (RNL Bio, Seoul, Korea). All human tissues were obtained with the approval of the Korea University Medical Center Institutional Review Board (Seoul, Korea). In brief, abdominal subcutaneous fat tissues were obtained by simple liposuction with the informed consent of a donor (female Korean, 53 years old) for studies on stem cell isolation, culture, and efficacy in animals. Mesenchymal stem cells were isolated by their adherence to plastic from the fat stromal vascular fraction and were culture expanded as previously described in detail (33).
HIL rat pups were intracerebroventricularly transplanted with human ASCs (4 × 105 cells/rat) in 2 µl saline at the following coordinates: anterior/posterior +1 mm, right lateral 2 mm, and ventral 3 mm from bregma. The rats received the cells only once at PND10 in the single-dose group or repeatedly at PND10, 17, 27, and 37 in the repeated-dose group.
Measurement of Neurobehavioral Functions
Cylinder Test. For the evaluation of forelimb use asymmetry by brain damage, the ratio of contralateral (right) forelimb use was analyzed at PND20, 30, and 40. Each animal was individually placed in a glass cylinder (21 cm in diameter, 34 cm in height; Samduk-lab, Guri, Korea) for 3 min. The weight-bearing forepaw to contact the cylinder wall during a full rearing was recorded as left (normal), right (impaired), or both. Paw preference was calculated as [(normal forepaw – impaired forepaw) / (normal forepaw + impaired forepaw + both) × 100%].
Rota-Rod Performance. Motor balance and coordination were evaluated using a rota-rod test system (Panlab Technology, Barcelona, Spain) at PND20, 30, and 40. Rats were placed on a rotating rod at a constant speed of 12 rpm, and the time it took for the rats to fall off the rod was recorded. The average latency was calculated from three consecutive measurements.
Locomotor Activity. Spontaneous activities and exploratory behaviors were evaluated using a video tracking system (Smart v2.5; Panlab Technology), connected to a CCTV (Samsung, Changwon, Korea) at PND20, 30, and 40. Rats were placed in a quiet chamber with a dim light. The types of movement, that is, resting, slow-moving and fast-moving times, were recorded for 5 min, and the ratio was analyzed.
Measurement of Learning and Memory Functions
For the evaluation of memory acquisition during passive avoidance trials, the rats were subjected to the Shuttle box (ENV-010MD; Med Associates Inc., St. Albans, VT, USA) once a day for 4 consecutive days from PND41. The Shuttle box apparatus consists of two light and dark compartments; a light chamber equipped with a lamp and a dark chamber with a steel grid floor for electric shock. On the trials, electric shock (1 mA for 5 s) was delivered when rats entered the dark compartment from the light room through a guillotine door. The latency time in the light room was recorded during 4-day trials. The end-point was set at 300 s, denoting full acquisition of memory.
Double Immunostaining
In order to confirm the distribution and survival of transplanted human ASCs, the rat brain was perfusion-fixed with 10% paraformaldehyde solution (Biopure, Cambridge, MA, USA) and postfixed in the same solution for 48 h, followed by cryoprotection in 30% sucrose (Amresco, Cochran Solon, OH, USA) for 72 h. Coronal cryosections in 30-µm thickness were prepared and processed for double immunostaining for human mitochondria (hMito, for human-derived cells) and Olig2 (for oligodendroglial linage cells), myelin basic protein (MBP, for mature oligodendrocytes), heavy neurofilament (NF-H, for neurons), or glial fibrillary acidic protein (GFAP, for astrocytes). Brain sections were incubated with primary antibodies specific for hMito (1:100; mouse monoclonal, Chemicon, Temecula, CA, USA), Olig2 (1:200; rabbit polyclonal, Chemicon), MBP (1:200; rabbit polyclonal, Chemicon), NF-H (1:200; rabbit polyclonal, Chemicon), or GFAP (1:200; rabbit polyclonal, Chemicon) overnight at 4°C, followed by Alexa Fluor 488- or 594-conjugated anti-rabbit IgG (1:1,000; Molecular Probes, Eugene, OR, USA) for 2 h at room temperature. For determination of the integrity of the host myelin, immunostaining for MBP was also performed using anti-MBP (1:200; rabbit polyclonal, Chemicon) as the primary antibody. All samples were evaluated immediately after staining and photographed with a laser-scanning confocal microscope (LSM710; Zeiss, New York, NY, USA). In order to quantify the immunoreactivity of host MBP, the photographs were analyzed with a Digital Image Analyzer (Image Inside; Focus, Seoul, Korea) for the red intensity and expressed as a percentage of the control (normal) group.
Western Blot Analysis
Whole brains of rats were homogenized in a RIPA buffer (Sigma, St. Louis, MO, USA) with protease inhibitors. Proteins were obtained by centrifugation at 15,000 rpm and 4°C for 15 min and quantified using the BCA Protein Assay kit (Pierce, Rockford, IL, USA). Proteins were denatured by boiling for 5 min at 95°C in a 0.5 M Tris-HCl buffer (pH6.8; Bioshop, Burlington, ON, Canada) containing 10% sodium dodecyl sulfate (SDS; Bioshop) and 10% ammonium persulfate (Sigma-Aldrich), separated by electrophoresis on 12.5% SDS-polyacrylamide gels (SDS-PAGE; Invitrogen, Carlsbad, CA, USA), and transferred to a polyvinylidene difluoride membrane (Millipore, Bedford, MA, USA) in a 25-mM Tris buffer containing 15% methanol (Merck, Darmstadt, Germany), 1% SDS, and 192 mM glycine (Bioshop). After blocking for 2 h with 5% skimmed milk (BD Biosciences, Franklin Lakes, NJ, USA) in Tris-buffered saline-Tween (TBS-T; 20 mM Tris pH 7.6, 137 mM NaCl, and 0.1% Tween 20; Tech&Innovation, Seoul, Korea), the membrane was incubated with antibodies specific for nerve growth factor (NGF) (1:500; rabbit polyclonal, Santa Cruz Biotechnology, Santa Cruz, CA, USA), brain-derived neurotrophic factor (BDNF) (1:500; rabbit polyclonal, Santa Cruz Biotechnology), ciliary neurotrophic factor (CNTF) (1:500; rabbit polyclonal, Santa Cruz Biotechnology), glial cell-derived neurotrophic factor (GDNF) (1:500; rabbit polyclonal, Santa Cruz Biotechnology), vascular endothelial growth factor (VEGF) (1:500; rabbit polyclonal, Santa Cruz Biotechnology), or β-actin (Sigma-Aldrich) overnight at 4°C. After washing with TBS-T, the membrane was incubated with a secondary goat anti-rabbit IgG conjugated with horseradish peroxidase (1:2,000; Santa Cruz Biotechnology) for 2 h at room temperature. The membrane was then developed using an enhanced chemiluminescence solution (Pierce).
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) Analysis
Total RNA was extracted from the brain tissue using TRIzol (Invitrogen). Complimentary DNA templates were obtained from 1 µg of total RNA primed with oligodT primers using 40 U of Moloney Murine Leukemia Virus reverse transcriptase (Promega, Madison, WI, USA) followed by 40 PCR cycles, and RT-PCR products were separated on a 1.2% agarose gel (Bioshop) containing ethidium bromide (Sigma-Aldrich). The primers used for real-time RT-PCR of cytokines are described in Table 1 (Bioneer, Daejeon, Korea).
Table 1.
Primer Sequences for Real-Time RT-PCR
| Gene | Primer | Amino Acid Sequences | Product Size (bp) | Accession No. |
|---|---|---|---|---|
| TNF-α | Forward | 5′-caccacgctcttctgtctactgaac-3′ | 545 | NM_012675 |
| Reverse | 5′-ccggactccgtgatgtctaagtact-3′ | |||
| IL-1β | Forward | 5′-gaagctgtggcagctacctatgtct-3′ | 522 | NM_031512 |
| Reverse | 5′-ctctgcttgagaggtgctgatgtac-3′ | |||
| IL-6 | Forward | 5′-gactgatgttgttgacagccactgc-3′ | 508 | NM_012589 |
| Reverse | 5′-tagccactccttctgtgactctaact-3′ | |||
| GAPDH | Forward | 5′-aacggatttggccgtatcgg-3′ | 299 | NM_017008 |
| Reverse | 5′-agccttctccatggtggtgaagac-3′ |
TNF, tumor necrosis factor; IL, interleukin; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Statistical Analysis
Data are presented as mean ± SEM. The statistical significance between group comparisons for behavioral and cognitive activities and host MBP intensity was determined by one-way analysis of variance, followed by post hoc Tukey’s multiple-comparison test. Values of p < 0.05 were considered to be statistically significant.
RESULTS
In this study, HIL in PND7 rats exhibited various abnormalities of physical and cognitive functions. The characteristics of the HIL model were similar to the development of human HIE (11). Therefore, these diverse neurobehavioral alterations may support that HIL at PND7 could be a good CP model for the evaluation of preventive and/or therapeutic candidates.
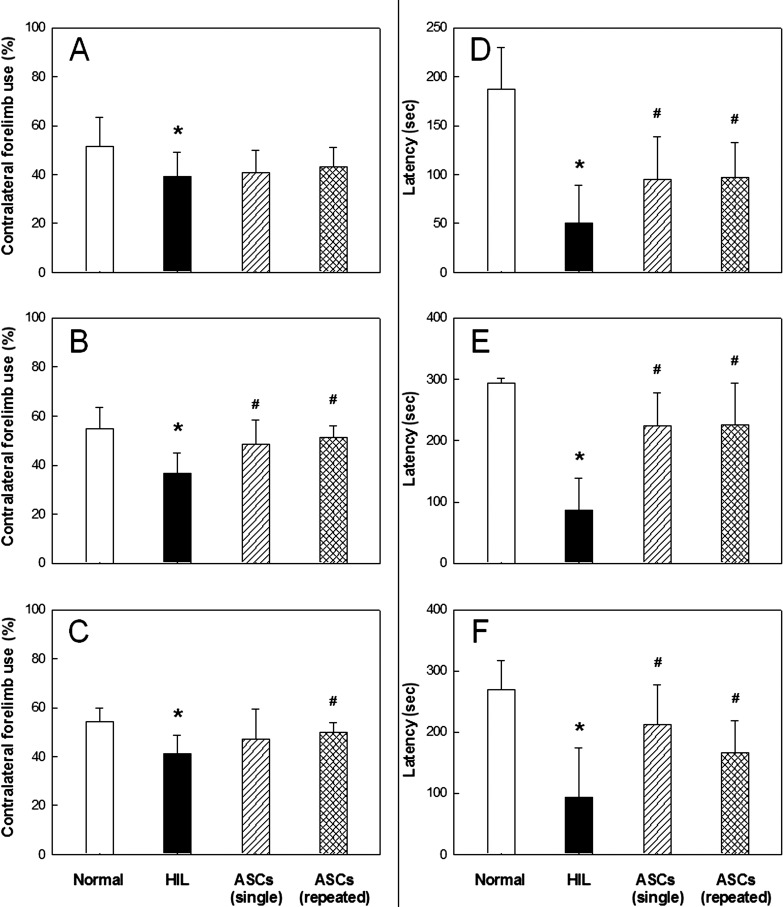
In the cylinder test, normal animals used their left and right forelimbs in similar ratios (50:50%) at PND20, 30, and 40 (Fig. 1A–C). However, rats subjected to HIL at PND7 showed significantly decreased (<40%) use of the contralateral forelimb at PND20, 30, and 40. Such a reduced use of the contralateral forelimb was remarkably recovered at PND30 and 40 by single intracerebroventricular transplantation of human ASCs (4 × 105 cells/rat) at PND10. A similar restoration of physical dysfunction of the contralateral forelimb induced by HIL was achieved with repeated transplantations of human ASCs at PND10, 17, 27, and 37.
Figure 1.
The effects of adipose-derived mesenchymal stem cell (ASC) treatment on behavior after hypoxia-ischemia-lipopolysaccharide (HIL). Cylinder test (A–C) and rota-rod performance (D–F). Rats were subjected to HIL at post-natal day 7 (PND7), and tested at PND20 (A, D), PND30 (B, E), and PND40 (C, F). *Significantly different from the sham control (normal) (p < 0.05). #Significantly different from HIL alone (p < 0.05).
HIL caused impairment of motor coordination in rota-rod performance, leading to a drastic reduction in the latency time by 65–70% at PND20–40 (Fig. 1D–F). Interestingly, however, such decreased rota-rod performances were near-fully restored at all time points tested by the single transplantation of human ASCs at PND10. Also, the functional impairments of HIL rats significantly recovered following repeated transplantations of human ASCs.
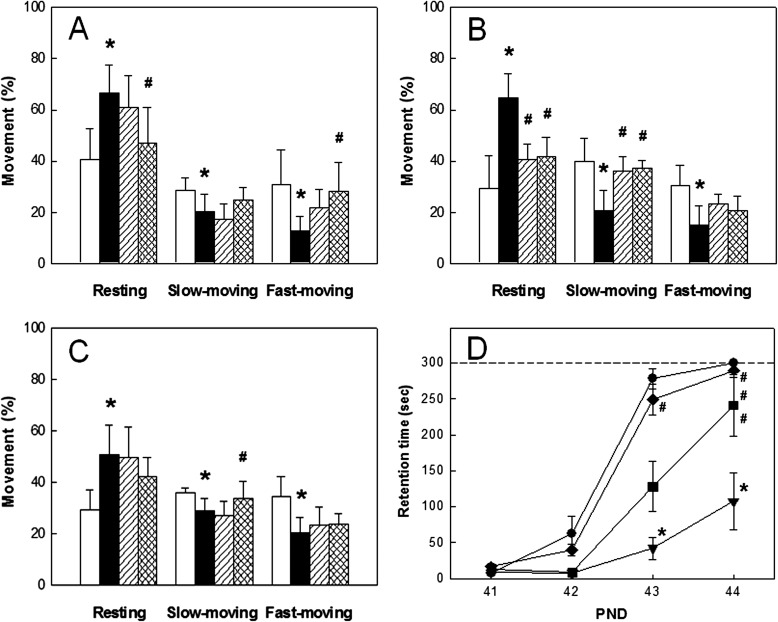
Normal animals exhibited active movement in global activity analysis, in which the sum of slow-moving and fast-moving times was longer than resting time at all PND20–40 (Fig. 2A–C). However, the resting time greatly increased in HIL rats, leading to significant decreases in moving times from PND20 to PND40. By comparison, single transplantation with human ASCs markedly recovered the HIL-induced decrease in locomotor activity. More prominent effects on the restoration of physical activity were obtained by repeated transplantations of human ASCs.
Figure 2.
The effects of ASC treatment on locomotor, passive avoidance, and learning and memory after hypoxia-ischemia-lipopolysaccharide (HIL). Locomotor activity (A–C) and passive avoidance performance (D). Rats were subjected to HIL at PND7 and tested at PND20 (A), PND30 (B), and PND40 (C) for locomotor activity and at PND41–44 (D) for learning and memory function. (A–C) White, sham control (normal); black, HIL alone; shaded, single transplantation; checked, repeated transplantations. (D) ●, sham control (normal); ▼, HIL alone; ■, single transplantation; ◆, repeated transplantations. *Significantly different from the sham control (normal) (p < 0.05). #Significantly different from HIL (p < 0.05).
HIL at PND7 induced severe impairment of cognitive function as assessed by passive avoidance performance at PND41–44. That is, HIL rats displayed much delayed increase in retention time during repeated trials, whereas full memory acquisition was observed on the fourth trial in normal animals (Fig. 2D). By comparison, a single transplantation of human ASCs at PND10 significantly recovered the impaired cognitive function. Notably, repeated transplantations of human ASCs at PND10, 17, 27, and 37 were superior to single treatment, accelerating the passive avoidance performances to a normal level.
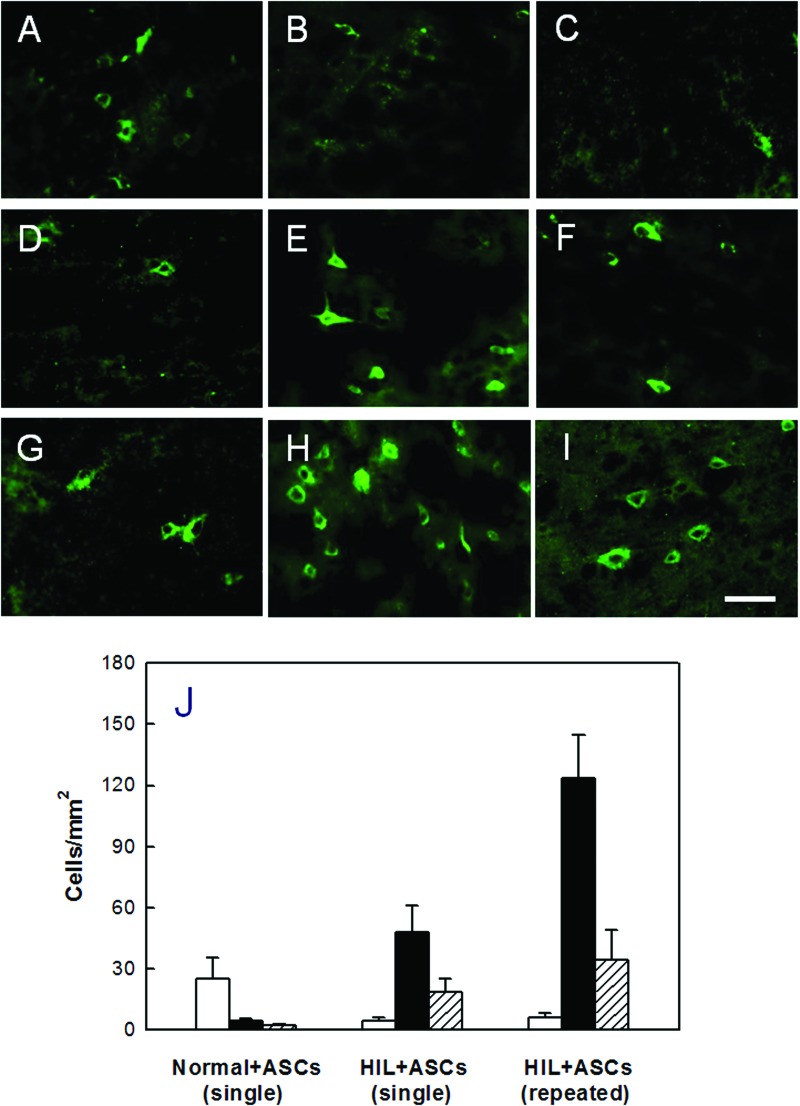
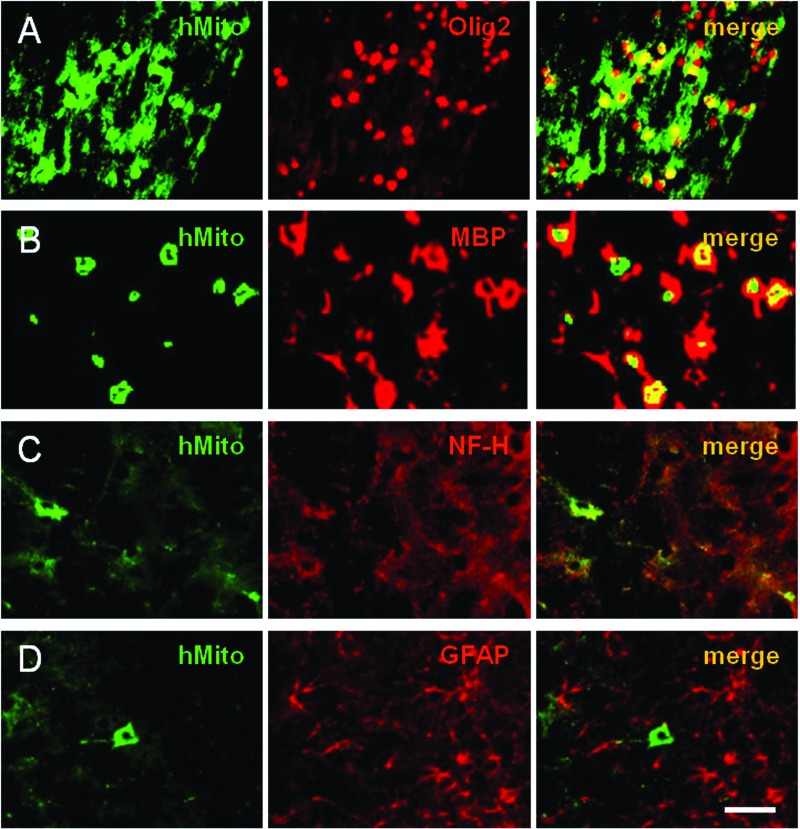
Immunostaining for hMito at PND45 (Fig. 3) revealed that hMito-positive human ASCs (25.1 cells/mm2) were observed predominantly in the hippocampus of normal rats after transplantation at PND10 (Fig. 3A–C, J). By comparison, the transplanted ASCs were distributed in the injured brain regions, corpus callosum and internal capsule, of HIL rats (Fig. 3D–J). In HIL animals, the hMito-positive cells in the corpus callosum were more abundant (2.6-fold) after repeated intracerebroventriculartransplantations (123.5 cells/mm2) than single administration (48.0 cells/mm2). Notably, it was found that the hMito-positive cells also strongly expressed Olig2 in their nucleus (Fig. 4A), confirming that the transplanted cells differentiated into oligodendroglial lineage cells. In addition, transplanted cells were found to mature to oligodendrocytes and produce MBP as confirmed by double immunostaining for cytosolic hMito and surrounding MBP (Fig. 4B). Furthermore, some of the human ASCs differentiated into (NF-H-positive) neurons (Fig. 4C), but not into (GFAP-positive) astrocytes (Fig. 4D).
Figure 3.
Distribution of human ASCs. Transplanted [human mitochondria (hMito)-positive] cells were detected in the hippocampus (A, D, G), corpus callosum (B, E, H), and internal capsule (C, F, I) of normal or hypoxia-ischemia-lipopolysaccharide (HIL)-challenged rats. (A–C) Single transplantation into normal rats. (D–F) Single transplantation into HIL rats. (G–I) Repeated transplantations into HIL rats. (J) Cell numbers at PND45, counted from (A) to (I). White, hippocampus; black, corpus callosum; shaded, internal capsule. Scale bar: 20 µm.
Figure 4.
Differentiation of human ASCs and production of myelin basic proteins (MBPs). Transplanted human ASCs (hMito-positive) were differentiated into oligodendrocyte lineage (A) and matured to oligodendrocytes producing MBP (B), as confirmed by immunoreactivities to Olig2 (a nuclear protein expressed in nuclei) and MBP (a myelin protein surrounding hMito), respectively. A part of the transplanted ASCs were differentiated into neurons [heavy neurofilament (NF-H), C], but not into astrocytes [glial fibrillary acidic protein (GFAP), D]. Scale bar: 20 µm.
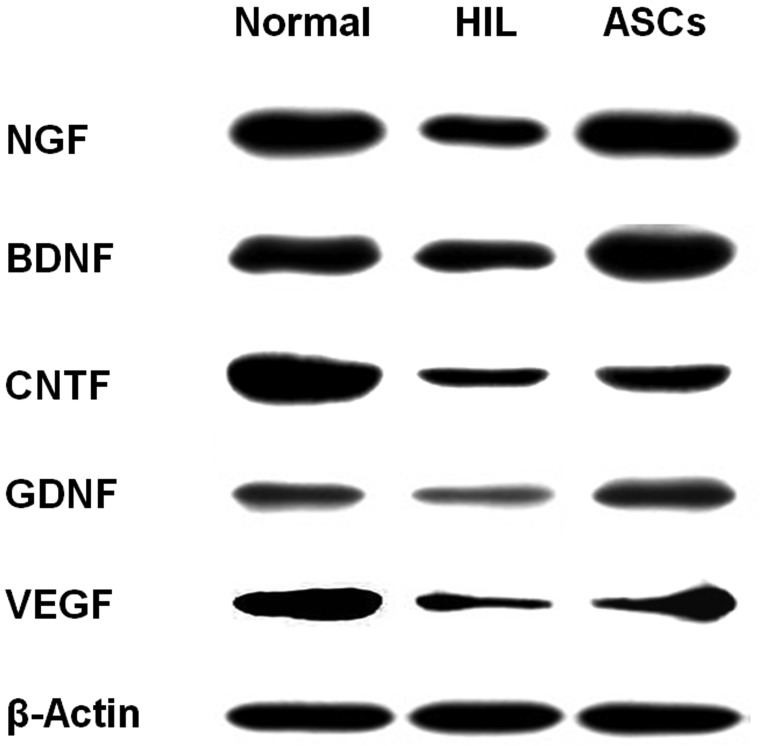
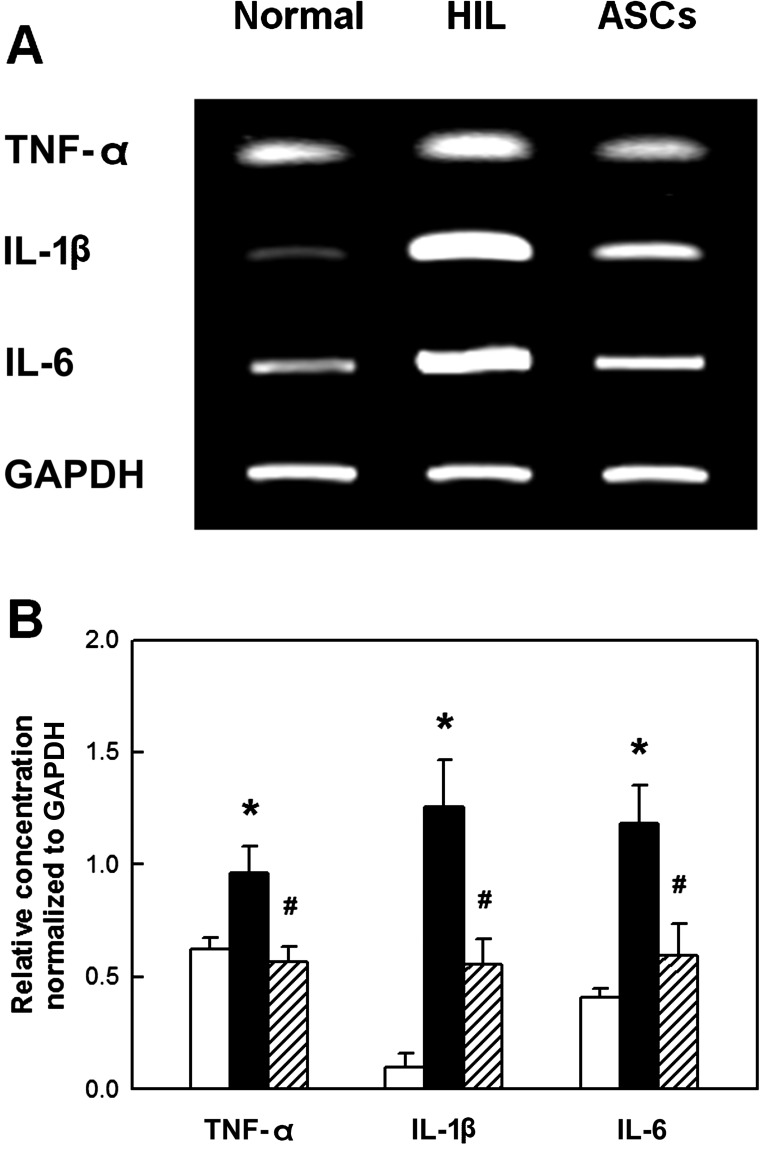
In the brain of HIL rats, the concentrations of growth and neurotrophic factors such as NGF, BDNF, CNTF, GDNF, and VEGF markedly decreased as analyzed at PND45 (Fig. 5). However, the contents of growth/neurotrophic factors were markedly recovered by repeated transplantation of ASCs. The inflammatory cytokines tumor-necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) greatly increased in the HIL-challenged brain (Fig. 6). Notably, the increases in the cytokines were significantly attenuated by ASCs, indicative of the anti-inflammatory activity of ASCs.
Figure 5.
Western blot analysis of brain growth/neurotrophic factors. Growth/neurotrophic factors were analyzed at PND45 following repeated ASC transplantation to hypoxia-ischemia-lipopolysaccharide (HIL)-challenged rats. The decreased production of growth/neurotrophic factors in HIL animals was markedly restored by ASC transplantation. NGF, nerve growth factor; BDNF, brain-derived neurotrophic factor; CNTF, ciliary neurotrophic factor; GDNF, glial cell-derived neurotrophic factor; VEGF, vascular endothelial growth factor.
Figure 6.
Real-time PCR analysis of brain inflammatory cytokines. Expression of the cytokines was analyzed at PND45 following repeated ASCs transplantation to hypoxia-ischemia-lipopolysaccharide (HIL)-challenged rats. (A) Representative bands of mRNA expression. (B) Relative concentration normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The increased expression of major inflammatory cytokines in HIL animals was significantly attenuated by ASC transplantation. TNF-α, tumor-necrosis factor-α; IL-1β, interleukin-1β; IL-6, interleukin-6.
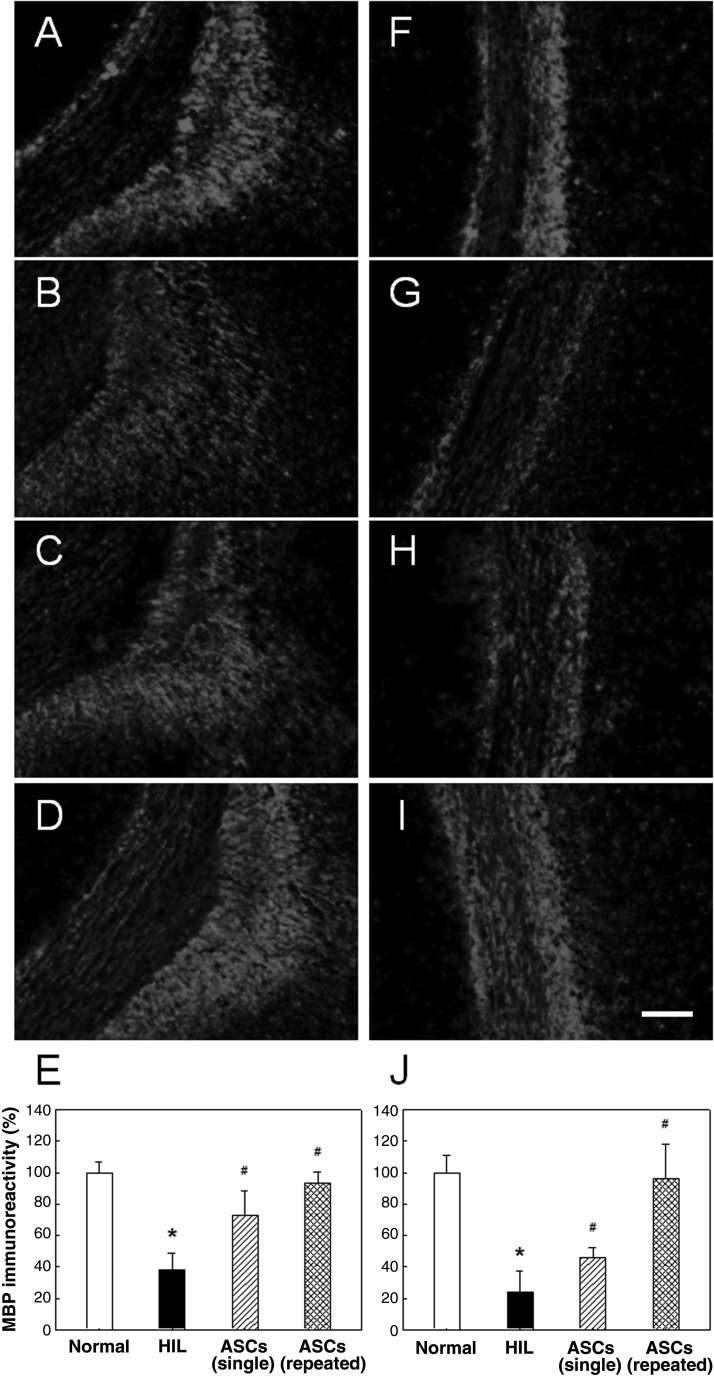
In normal animals, intensive immunoreactivity for MBP of oilgodendrocytic myelin was observed in white matter such as the corpus callosum and internal capsule (Fig. 7). By comparison, the MBP immunoreactivity was markedly reduced to 25–40% of the control level in both the corpus callosum and internal capsule of HIL animals as observed at PND45, indicative of a severe demyelination and loss of host oligodendrocytes (Fig. 7E, J). Notably, however, the HIL-induced loss of host MBP was remarkably attenuated by the single transplantation of human ASCs at PND10. More marked protective effects on MBP depletion were achieved by repeated treatments with human ASCs at PND10, 17, 27, and 37.
Figure 7.
Representative photomicrographs of host myelin basic protein (MBP) immunostaining. (A, F) Sham control (normal). (B, G) Hypoxia-ischemia-lipopolysaccharide (HIL) alone. (C, H) Single ASC transplantation. (D, I) Repeated ASC transplantation. Scale bar: 100 µm. The decreased immunoreactivity of host MBP in both the corpus callosum (A–E) and internal capsule (F–J) in HIL animals was significantly recovered by ASC transplantation.
DISCUSSION
In the present study, intracerebroventricular injection of human ASCs markedly recovered neurobehavioral and cognitive dysfunctions of neonatal HIL rats, along with the remarkable neuroprotective effects preserving MBP reactivity in the corpus callosum and internal capsule. Since the HI animal model leads to relatively mild neurobehavioral deficits (3,26), we injected LPS additionally to HI rats (HIL) for the induction of profound neurobehavioral and cognitive dysfunctions (15,17). It is well known that oxidative and inflammatory injuries of oligodendrocyte progenitors are key factors for the induction of HIE (34,43). Therefore, the primary neuroprotective effect of human ASCs may result from their anti-inflammatory activities through downregulation of cytokines including TNF-α, IL-1β, and IL-6, as also seen in experimental allergic encephalomyelitis (8).
In addition to the immunoregulatory activity, it has been demonstrated that diverse stem cells exert neuroprotective activity by secreting various growth and neurotrophic factors such as NGF, BDNF, CNTF, GDNF, and VEGF (13,37,39,48). Supportively, the host MBP was well preserved after transplantation of ASCs. Microtubule-associated protein 2 was also high following administration of murine BMSCs (40,41) or human ASCs (unpublished results) in the aged and injured brain tissues, implying that the cytoskeleton was preserved and/or regenerated. It was also found that human ASCs produce a large amount of growth/neurotrophic factors including VEGF, NGF, and BDNF (21,27).
Brain injury itself triggers gene expression of growth factors and cytokines as observed in the present study and induce proliferation and mobilization of progenitor cells (31). Interestingly, transplantation of murine BMSCs into the ischemic brain enhanced neurogenesis and oligodendrogenesis (40,41), which might be mediated by the growth/neutrophic factors secreted from the transplanted murine stem cells. Repeated transplantations of murine BMSCs after HI injury increased the expression of diverse growth/neurotrophic factors and cytokines responsible for cellular growth and proliferation (41,42). Therefore, it is believed that human ASCs protected myelins against hypoxic-ischemic and inflammatory (LPS-induced) insults by regulating the growth/neurotrophic factors and cytokines that influence on neuroprotective and neuroregenerative mechanisms together with enhanced remyelination.
Most importantly, transplanted human ASCs substantially ameliorated neurobehavioral and cognitive deficits of HIL rats. In the central nervous system, the increase of NGF and BDNF by transplantation of human ASCs appears to promote the survival and differentiation of cholinergic neurons of the basal forebrain (1,23). A previous study (26) demonstrated physical impairment of HI animals in the cylinder test and recovery of the dysfunction by transplantation of human BMSCs. However, they did not show impairments in rota-rod performance and cognition, which may be due to a relatively mild brain injury compared to our HIL model. In spite of the serious impairments of neurobehavioral and cognitive functions in our model, human ASCs near-fully restored both body functions. Related to cognitive function, neurotrophic factors including BDNF stimulated the cholinergic phenotype by increasing the activity of choline acetyltransferase responsible for acetylcholine synthesis in cultures enriched with embryonic rat motor neurons (46). Several studies demonstrated neuroprotection and improved cognitive function of HI model rats by administering BDNF (2,14). In our recent study, neural stem cells overexpressing NGF restored the cognitive function of ibotenic acid-challenged rats (25). Therefore, it is suggested that human ASCs improved the cognitive deficits of HIL rats by preserving cholinergic neurons via the secretion of neurotrophic factors such as BDNF and NGF (25,27). Such a cognition-recovering effect of ASCs was much higher after repeated treatments compared to single transplantation.
Improved rota-rod performances were achieved as early as 4 days after cell transplantation (data not shown) and lasted longer than 5 weeks. From the full improvement of contralateral forelimb dysfunction, in addition to rota-rod performances, it is assumed that the physical function of the HIL animals was recovered by human ASCs, implying that the integrity of neuromuscular transmission was spared. Moreover, analysis of the locomotor activity of HIL animals revealed that their overall physical functions were protected and restored following human ASC transplantation, leading to enhanced global activity similar to normal rats. In comparison with the superior effectiveness of repeated transplantation to single treatment, there were no remarkable differences in the improvement of behavioral and movement functions between single and repeated transplantations of ASCs. In our previous and follow-up studies, it was confirmed that such physical activity-improving effects of ASCs lasted longer than 7 weeks in HIL (unpublished results) and aged (30) animals.
It was reported that human ASCs expressed growth factor and chemokine receptors, and their migration to injury sites was induced by chemoattractants such as TNF-α (5). In the present study, the number of ASCs observed in the corpus callosum of HIL rats were more (approximately twice) than those in the hippocampus of normal animals after intracerebroventricular injection. ASCs might be attracted by cytokines from injured brain tissue in HIL animals (5). However, specific factors attracting the migration of human ASCs to the hippocampus of normal brain remain to be clarified, although it was reported that murine BMSCs were distributed to all brain regions (26). A previous study indicated that ASCs have self-renewal capacity and multipotency and can be differentiated into functional neurons by growth factors (19). It was also confirmed that human ASCs highly express growth/neurotrophic factors including BDNF, NGF, and VEGF following exposure to hypoxia or after retinoic acid-induced differentiation into neurons (27). In our study, transplanted human ASCs differentiated predominantly into oligodendrocytes, partially into neurons, but not into astrocytes, which was somewhat different from human BMSCs that mainly differentiated into astrocytes and microglia (26).
Since loss and demyelination of oligodendrocyte progenitor cells are key features of PVL in premature infants, it is of interest to note that the transplanted human ASCs differentiate into oligodendrocytes and produce MBP in the injury sites. In comparison with previous studies (40,41) demonstrating a preventive mode of action of the transplanted murine BMSCs via secretion of neurotrophic factors and cytokines, human ASCs showed additional action mechanisms, that is, differentiation into oligodendrocytes and production of MBP as a therapeutic mode, leading to a high efficacy in a severe brain injury model (HIL) showing overall neurobehavioral and cognitive dysfunctions. In our follow-up studies, similar beneficial effects were achieved with delayed transplantation of ASCs from PND20 or PND30 following HIL challenge at PND7 (unpublished results). Therefore, it is suggested that human ASCs could be a promising candidate for cell therapy of HIE including neonatal cerebral palsy.
ACKNOWLEDGMENTS
This work was supported in part by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2011-0021322). The authors declare no conflicts of interest.
REFERENCES
- 1. Alderson R. F.; Alterman A. L.; Barde Y. A.; Lindsay R. M. Brain-derived neurotrophic factor increases survival and differentiated functions of rat septal cholinergic neurons in culture. Neuron 5:297–306; 1990. [DOI] [PubMed] [Google Scholar]
- 2. Almli C. R.; Levy T. J.; Han B. H.; Shah A. R.; Gidday J. M.; Holtzman D. M. BDNF protects against spatial memory deficits following neonatal hypoxia-ischemia. Exp. Neurol. 166:99–114; 2000. [DOI] [PubMed] [Google Scholar]
- 3. Back S. A.; Han B. H.; Luo N. L.; Chricton C. A.; Xanthoudakis S.; Tam J.; Arvin K. L.; Holtzman D. M. Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J. Neurosci. 22:455–463; 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Back S. A.; Riddle A.; McClure M. M. Maturation-dependent vulnerability of perinatal white matter in premature birth. Stroke 38:724–730; 2007. [DOI] [PubMed] [Google Scholar]
- 5. Baek S. J.; Kang S. K.; Ra J. C. In vitro migration capacity of human adipose-derived mesenchymal stem cells and their expression of a distinct set of chemokine and growth factor receptors. Exp. Mol. Med. 43:596–603; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buller K. M.; Carty M. L.; Reinebrant H. E.; Wixey J. A. Minocycline: Aneuroprotective agent for hypoxic-ischemic brain injury in the neonate? J. Neurosci. Res. 87:599–608; 2009. [DOI] [PubMed] [Google Scholar]
- 7. Carlson K. B.; Singh P.; Feaster M. M.; Ramnarain A.; Pavlides C.; Chen Z. L.; Yu W. M.; Feltri M. L.; Strickland S. Mesenchymal stem cells facilitate axon sorting, myelination, and functional recovery in paralyzed mice deficient in Schwann cell-derived laminin. Glia 59: 267–277; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Constantin G.; Marconi S.; Rossi B.; Angiari S.; Calderan L.; Anghileri E.; Gini B.; Bach S. D.; Martinello M.; Bifari F.; Galiè M.; Turano E.; Budui S.; Sbarbati A.; Krampera M.; Bonetti B. Adipose-derived mesenchymal stem cells ameliorate chronic experimental autoimmune encephalomyelitis. Stem Cells 27:2624–2635; 2009. [DOI] [PubMed] [Google Scholar]
- 9. Daadi M. M.; Davis A. S.; Arac A.; Li Z.; Maag A. L.; Bhatnagar R.; Jiang K.; Sun G.; Wu J. C.; Steinberg G. K. Human neural stem cell grafts modify microglial response and enhance axonal sprouting in neonatal hypoxic-ischemic brain injury. Stroke 41:516–523; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Deng W.; Pleasure J.; Pleasure D. Progress in periventricular leukomalacia. Arch. Neurol. 65:1291–1295; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De Vries L. S.; Eken P.; Groenendaal F.; van Haastert I. C.; Meiners L. C. Correlation between the degree of periventricular leukomalacia diagnosed using cranial ultrasound and MRI later in infancy in children with cerebral palsy. Neuropediatrics 24:263–268; 1993. [DOI] [PubMed] [Google Scholar]
- 12. Fern R.; Moller T. Rapid ischemic cell death in immature oligodendrocytes: A fatal glutamate release feedback loop. J. Neurosci. 20:34–42; 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frielingsdorf H.; Simpson D. R.; Thal L. J.; Pizzo D. P. Nerve growth factor promotes survival of new neurons in the adult hippocampus. Neurobiol. Dis. 26:47–55; 2007. [DOI] [PubMed] [Google Scholar]
- 14. Galvin K. A.; Oorschot D. E. Continuous low-dose treatment with brain-derived neurotrophic factor or neurotrophin-3 protects striatal medium spiny neurons from mild neonatal hypoxia/ischemia: A stereological study. Neuroscience 118:1023–1032; 2003. [DOI] [PubMed] [Google Scholar]
- 15. Girard S.; Kadhim H.; Beaudet N.; Sarret P.; Sebire G. Developmental motor deficits induced by combined fetal exposure to lipopolysaccharide and early neonatal hypoxia/ischemia: A novel animal model for cerebral palsy in very premature infants. Neuroscience 158:673–682; 2009. [DOI] [PubMed] [Google Scholar]
- 16. Hagberg H.; Peebles D.; Mallard C. Models of white matter injury: Comparison of infectious, hypoxic-ischemic, and excitotoxic insults. Ment. Retard. Dev. Disabil. Res. Rev. 8:30–38; 2002. [DOI] [PubMed] [Google Scholar]
- 17. Ikeda T.; Mishima K.; Aoo N.; Egashira N.; Iwasaki K.; Fujiwara M.; Ikenoue T. Combination treatment of neonatal rats with hypoxia-ischemia and endotoxin induces long-lasting memory and learning impairment that is associated with extended cerebral damage. Am. J. Obstet. Gynecol. 191:2132–2141; 2004. [DOI] [PubMed] [Google Scholar]
- 18. Jacobs S.; Hunt R.; Tarnow-Mordi W.; Inder T.; Davis P. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst. Rev. 2007(4):CD003311; 2007. [DOI] [PubMed] [Google Scholar]
- 19. Jang S.; Cho H. H.; Cho Y. B.; Park J. S.; Jeong H. S. Functional neural differentiation of human adipose tissue-derived stem cells using bFGF and forskolin. BMC Cell Biol. 11:25; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jo J. Y.; Kang S. K.; Choi I. S.; Ra J. C. Comparison of neural cell differentiation of human adipose mesenchymal stem cells derived from young and old ages. Dev. Reprod. 13:227–237; 2009. [Google Scholar]
- 21. Kim B. H.; Son W. C.; Yim C. O.; Kang S. K.; Ra J. C.; Kim Y. C. Antiwrinkle effects of adipose tissue-derived mesenchymal stem cells in a UV-irradiated hairless mouse model. Tissue Eng. Regen. Med. 7:583–591; 2010. [Google Scholar]
- 22. Kinney H. C.; Back S. A. Human oligodendroglial development: Relationship to periventricular leukomalacia. Semin. Pediatr. Neurol. 5:180–189; 1998. [DOI] [PubMed] [Google Scholar]
- 23. Knüsel B.; Winslow J. W.; Rosenthal A.; Burton L. E.; Seid D. P.; Nikolics K.; Hefti F. Promotion of central cholinergic and dopaminergic neuron differentiation by brain-derived neurotrophic factor but not neurotrophin-3. Proc. Natl. Acad. Sci. USA 88:961–965; 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Krageloh-Mann I.; Petersen D.; Hagberg G.; Vollmer B.; Hagberg B.; Michaelis R. Bilateral spastic cerebral palsy-MRI pathology and origin. Analysis from a representative series of 56 cases. Dev. Med. Child Neurol. 37:379–397; 1995. [DOI] [PubMed] [Google Scholar]
- 25. Lee H. J.; Lim I.; Park S. W.; Kim Y. B.; Ko Y.; Kim S. Human neural stem cells genetically modified to express human nerve growth factor gene restore cognition in ibotenic acid-induced cognitive dysfunction. Cell Transplant. 21:2487–2496; 2012. [DOI] [PubMed] [Google Scholar]
- 26. Lee J. A.; Kim B. I.; Jo C. H.; Choi C. W.; Kim E. K.; Kim H. S.; Yoon K. S.; Choi J. H. Mesenchymal stem-cell transplantation for hypoxic-ischemic brain injury in neonatal rat model. Pediatr. Res. 67:42–46; 2010. [DOI] [PubMed] [Google Scholar]
- 27. Lopatina T.; Kalinina N.; Karagyaur M.; Stambolsky D.; Rubina K.; Revischin A.; Pavlova G.; Parfyonova Y.; Tkachuk V. Adipose-derived stem cells stimulate regeneration of peripheral nerves: BDNF secreted by these cells promotes nerve healing and axon growth de novo. PLoS One 6:e17899; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Oka A.; Belliveau M. J.; Rosenberg P. A.; Volpe J. J. Vulnerability of oligodendroglia to glutamate: Pharmacology, mechanisms, and prevention. J. Neurosci. 13:1441–1453; 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Park D.; Kim T. K.; Choi Y. J.; Lee S. H.; Kang H.; Yang Y. H.; Bae D. K.; Yang G.; Kim Y. B. Experimental models of cerebral palsy in infant rats. Lab. Anim. Res. 26:343–351; 2010. [Google Scholar]
- 30. Park D.; Yang G.; Bae D. K.; Lee S. H.; Yang Y. H.; Kyung J.; Kim D.; Choi E. K.; Choi K. C.; Kim S. U.; Kang S. K.; Ra J. C.; Kim Y. B. Human adipose tissue-derived mesenchymal stem cells improve cognitive and physical activities of ageing mice. J. Neurosci. Res. 91(5):660–670; 2013. [DOI] [PubMed] [Google Scholar]
- 31. Picard-Riera N.; Decker L.; Delarasse C.; Goude K.; Nait-Oumesmar B.; Liblau R.; Pham-Dinh D.; Evercooren A. B. Experimental autoimmune encephalomyelitis mobilizes neural progenitors from the subventricular zone to undergo oligodendrogenesis in adult mice. Proc. Natl. Acad. Sci. USA 99:13211–13216; 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pimentel-Coelho P. M.; Mendez-Otero R. Cell therapy for neonatal hypoxic-ischemic encephalopathy. Stem Cells Dev. 19:299–310; 2010. [DOI] [PubMed] [Google Scholar]
- 33. Ra J. C.; Shin I. S.; Kim S. H.; Kang S. K.; Kang B. C.; Lee H. Y.; Kim Y. J.; Jo J. Y.; Yoon E. J.; Choi H. J.; Kwon E. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev. 20:1297–1308; 2011. [DOI] [PubMed] [Google Scholar]
- 34. Rezaie P.; Dean A. Periventricular leukomalacia, inflammation and white matter lesions within the developing nervous system. Neuropathology 22:106–132; 2002. [DOI] [PubMed] [Google Scholar]
- 35. Rivkin M. J.; Flax J.; Mozell R.; Osathanondh R.; Volpe J. J.; Villa-Komaroff L. Oligodendroglial development in human fetal cerebrum. Ann. Neurol. 38:92–101; 1995. [DOI] [PubMed] [Google Scholar]
- 36. Saito T.; Kuang J.; Bittira B.; Al-Khaldi A.; Chiu R. C. J. Xenotransplant cardiac chimera: Immune tolerance of adult stem cells. Ann. Thorac. Surg. 74:19–24; discussion 24; 2002. [DOI] [PubMed] [Google Scholar]
- 37. Schneider A.; Kruger C.; Steigleder T.; Weber D.; Pitzer C.; Laage R.; Aronowski J.; Maurer M. H.; Gassler N.; Mier W.; Hasselblatt M.; Kollmar R.; Schwab S.; Sommer C.; Bach A.; Kuhn H. G.; Schabitz W. R. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J. Clin. Invest. 115:2083–2098; 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sen A.; Lea-Currie Y. R.; Sujkowska D.; Franklin D. M.; Wilkison W. O.; Halvorsen Y. D.; Gimble J. M. Adipogenic potential of human adipose derived stromal cells from multiple donors is heterogeneous. J. Cell. Biochem. 81:312–319; 2001. [DOI] [PubMed] [Google Scholar]
- 39. Shyu W. C.; Lin S. Z.; Yang H. I.; Tzeng Y. S.; Pang C. Y.; Yen P. S.; Li H. Functional recovery of stroke rats induced by granulocyte colony-stimulating factor-stimulated stem cells. Circulation 110:1847–1854; 2004. [DOI] [PubMed] [Google Scholar]
- 40. van Velthoven C. T.; Kavelaars A.; van Bel F.; Heijnen C. J. Mesenchymal stem cell treatment after neonatal hypoxic-ischemic brain injury improves behavioral outcome and induces neuronal and oligodendrocyte regeneration. Brain Behav. Immun. 24:387–393; 2010. [DOI] [PubMed] [Google Scholar]
- 41. van Velthoven C. T.; Kavelaars A.; van Bel F.; Heijnen C. J. Repeated mesenchymal stem cell treatment after neonatal hypoxia-ischemia has distinct effects on formation and maturation of new neurons and oligodendrocytes leading to restoration of damage, corticospinal motor tract activity, and sensorimotor function. J. Neurosci. 30:9603–9611; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van Velthoven C. T.; Kavelaars A.; van Bel F.; Heijnen C. J. Mesenchymal stem cell transplantation changes the gene expression profile of the neonatal ischemic brain. Brain Behav. Immun. 25:1342–1348; 2011. [DOI] [PubMed] [Google Scholar]
- 43. Volpe J. J. Brain injury in premature infants: A complex amalgam of destructive and developmental disturbances. Lancet Neurol. 8:110–124; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang X.; Rousset C. I.; Hagberg H.; Mallard C. Lipopolysaccharide-induced inflammation and perinatal brain injury. Semin. Fetal Neonatal Med. 11:343–353; 2006. [DOI] [PubMed] [Google Scholar]
- 45. Whitaker A. H.; van Rossem R.; Feldman J. F.; Schonfeld I. S.; Pinto-Martin J. A.; Tore C.; Shaffer D.; Paneth N. Psychiatric outcomes in low-birth-weight children at age 6 years: Relation to neonatal cranial ultrasound abnormalities. Arch. Gen. Psychiatry 54:847–856; 1997. [DOI] [PubMed] [Google Scholar]
- 46. Wong V.; Arriaga R.; Ip N. Y.; Lindsay R. M. The neurotrophins BDNF, NT-3 and NT-4/5, but not NGF, upregulate the cholinergic phenotype of developing motor neurons. Eur. J. Neurosci. 5:466–474; 1993. [DOI] [PubMed] [Google Scholar]
- 47. Xiong T.; Qu Y.; Mu D.; Ferriero D. Erythropoietin for neonatal brain injury: Opportunity and challenge. Int. J. Dev. Neurosci. 29:583–591; 2011. [DOI] [PubMed] [Google Scholar]
- 48. Zhang Z. H.; Wang R. Z.; Wang R. Z.; Li G. L.; Wei J. J.; Li Z. J.; Feng M.; Kang J.; Du W. C.; Ma W. B.; Li Y. N.; Yang Y.; Kong Y. G. Transplantation of neural stem cells modified by human neurotrophin-3 promotes functional recovery after transient focal cerebral ischemia in rats. Neurosci. Lett. 444:227–230; 2008. [DOI] [PubMed] [Google Scholar]