Abstract
Aluminum is known to exert neurotoxic effects associated with various neurodegenerative disorders, including Alzheimer's disease (AD). Ibuprofen is a well-known non-steroidal anti-inflammatory drug, which has demonstrated potential efficacy in the treatment of numerous inflammatory and neurodegenerative disorders, including AD. The present study aimed to investigate the protective effects of ibuprofen on cognitive function, and the expression levels of neuronal pentraxins (NPs) and interleukin (IL)-1β in an aluminum chloride (AlCl3)-induced mouse model of neurotoxicity. The effects of ibuprofen (100 mg/kg/day for 12 days) on learning and memory were evaluated in the AlCl3-induced neurotoxic mice using a Morris water maze and open field tests. In addition, ibuprofen was assessed for its effects on the expression levels of NPs and IL-1β in the hippocampus, cortex and amygdala of the brain. Treatment of the AlCl3-treated mice with ibuprofen decreased anxiety levels (6.90±0.34 min) compared with the AlCl3-treated group (1.80±0.29 min), as indicated by the time spent in the central area in an open field test. Furthermore, the expression levels of NP1 (1.32±0.47) and IL-1β (0.99±0.21) were significantly decreased in the hippocampus of mice following ibuprofen treatment, as compared with the AlCl3-treated mice (8.62±1.54 and 7.47±0.53, respectively). In the present study, ibuprofen was able to target novel structures in order to attenuate the inflammation associated with an AlCl3-induced mouse model of neurotoxicity; thus suggesting that ibuprofen may be considered a potential therapeutic option for the treatment of neurodegenerative diseases, including AD.
Keywords: pentraxins, ibuprofen, memory, interleukin-1β, neurodegeneration
Introduction
Ibuprofen is an over-the-counter non-steroidal anti-inflammatory drug (NSAID) (1), which is predominantly used as an analgesic, anti-inflammatory and antipyretic compound (2). Ibuprofen has previously been demonstrated to limit the production of pro-inflammatory cytokines and to exert neuroprotective effects (1). Furthermore, ibuprofen has demonstrated effectiveness in the treatment of Parkinson's disease (PD) (3), neuroinflammation associated with β-amyloid (Aβ) deposition (4), and motor deficits in hepatic encephalopathy (5), due to its anti-inflammatory and antioxidant properties. In addition, it may reduce the risk of developing Alzheimer's disease (AD), as increased expression levels of inflammatory cytokines have previously been associated with AD (6).
The pathogenesis of various neurodegenerative disorders is attributed to neurotoxicity, which is associated with various factors, including aluminum (Al) (7). Previous studies have demonstrated a role for Al in dementia (8), PD (9) and AD (10). Furthermore, an aluminum chloride (AlCl3)-induced model has previously been established for the investigation of neurotoxicity (11), AD (12), dementia (13), and neuroinflammation (14).
Neuronal pentraxins (NPs), which have key roles in neuroinflammation (15), belong to a family of proteins that are homologous to immune system proteins, including C-reactive and acute phase proteins, and are believed to be involved in synaptic functions (16). The NPs, including NP1, NP2 and NP receptor (NPR) (17), are proteins that were initially identified due to their ability to bind to the snake poison taipoxin (18) and mediate its internalization, leading to cell death. As taipoxin blocks neuromuscular transmission at the pre-synaptic junction (19), it has been suggested that NPs may have a role in the neuronal uptake process (18). NP2 is a secreted glycoprotein that has been demonstrated to enhance the growth of neuronal dendrites (20), and does not bind to taipoxin; thus suggesting that it has a distinct function from NP1 (20).
NP1 is expressed in the neurons of the cerebellum, hippocampus and cerebral cortex (21), whereas NP2 is widely expressed in the testis, liver, pancreas, skeletal muscles, heart and brain (20). Furthermore, the expression of NP2 has been demonstrated to be upregulated in response to neuronal activity, upon which it may mediate clustering of 2-amino-3-(3-hydroxy-5-methyl-isoxazol-4-yl) propanoic acid receptors (22). NP1, NP2 and NPR have 50% identity with each other and their C-terminal halves have 20–30% identity with immune system pentraxins, including the serum amyloid protein (23), C-reactive protein (24) and long pentraxin 3 (25).
Previous studies have suggested that NPs may contribute to the pathogenesis of neurodegenerative disorders, including AD (26) and PD (16). A predominant characteristic of the AD brain is accumulated Aβ protein, which interferes with synaptic functions and induces neuronal cell death (27). Aβ toxicity has been demonstrated to initiate the overexpression of NP1, which is an apoptotic protein (28), resulting in the activation of programmed cell death due to loss of neuronal functions (26). In addition, NP2 has been demonstrated to be markedly upregulated in PD (16); thus suggesting an important role for NPs in neuroinflammatory pathways. The present study aimed to analyze the effects of ibuprofen on the expression levels of NPs, and inflammation in an AlCl3-induced mouse model of neurotoxicity.
Materials and methods
Agents
Aluminum chloride hexahydrate (AL0770) was obtained from Sigma-Aldrich (St. Louis, MO, USA). Sodium chloride (SO0225) was purchased from Scharlab, S.L. (Barcelona, Spain). Ibuprofen tablets (600 mg; RN: 007850) were purchased from Abbot Laboratories, Ltd. (Karachi, Pakistan). Chloroform (24216) was obtained from Riedel-de Haën (Seelze, Germany). RNA was extracted using TRIzol® reagent obtained from Invitrogen Life Technologies (Carlsbad, CA, USA), and reverse transcription-polymerase chain reaction (RT-PCR) was performed using Taq polymerase, dNTPs and reverse transcriptase, all obtained from Thermo Fisher Scientific, Inc. (Pittsburgh, PA, USA).
Mice
Male BALB/c mice (age, 3 months; weight, 35–40 g; n=10 per group) were purchased from the National Institute of Health (Islamabad, Pakistan). All experiments performed complied with the guidelines outlined by the Institute of Laboratory Animal Research, Division on Earth and Life Sciences, National Institute of Health, USA (Guide for the Care and Use of Laboratory Animals: Eighth Edition, 2011). The protocol for the present study was approved by the Ethical Committee for Research on Animals Internal Review Board at the Atta-ur-Rahman School of Applied Biosciences, National University of Sciences and Technology (Islamabad, Pakistan). The mice were maintained in a controlled environment (22–25°C) within an animal house, with a natural day and night cycle. The animals were administered tap water and fed a standard diet. Mice were divided in three groups (n=10 per group), as follows: Control, treated with normal saline intraperitoneally (i.p.); AlCl3-treated, which received 150 mg/kg/day AlCl3 (i.p.); and ibuprofen-treated, which received 100 mg/kg/day (i.p.).
Administration of agents
Ibuprofen (100 mg/kg/day) and AlCl3 (150 mg/kg/day) were administered for 12 consecutive days. AlCl3 was dissolved in distilled water and injected (i.p.) into the AlCl3-treated group mice (29). Normal saline (0.9%) was administered to the control group according to body weight. Ibuprofen was administered orally via feed-stuff to the ibuprofen-treated group mice.
Morris water maze test
The procedure used was identical to the method outlined by Ahmed and Gilani (30). The experimental apparatus consisted of a circular tub containing an invisible platform submerged 1.5 cm below the surface of water. The temperature of the water was maintained at 20–23°C. External cues, including pictures and objects, were present and the position of the cues remained constant throughout the study.
The Morris water maze test was conducted for 7 consecutive days. Each mouse received two trials per day between 10:00 AM and 5:00 PM. The mice were randomly placed in one of four quadrants of the tub, facing towards the wall of the tub, and were allowed to swim until they reached the platform. The time taken to reach the platform was recorded, and once a mouse reached the platform it was allowed to rest for 15 sec. If a mouse failed to locate the platform within 90 sec, it was placed on the platform and allowed to rest for 15 sec.
Open field test
The open field test procedure was based on the method outlined by Choleris et al (31), with minor alterations. Briefly, the mice were placed into a square chamber and their activity was recorded using a SteadyShot DSC-W610 camera (Sony Corporation, Tokyo, Japan) fixed in a tripod stand at the top of the chamber. The box was divided into central and peripheral areas. A minor alteration of this method compared with Choleris et al was that the peripheral area was ≤2 cm from the walls of the chamber. The test duration was 30 min and the time spent by the animal in the central and peripheral areas was recorded.
Gene expression analysis
The gene expression analysis was conducted according to the procedure outlined by Ahmed et al (32), with minor alterations. The mice were sacrificed under diethyl ether anesthesia (676845; Sigma-Aldrich), after which the brains were removed in order to extract the cortex, hippocampus and amygdala. RNA was extracted using TRIzol® reagent. The quantity and quality of the RNA was determined using agarose gel electrophoresis. RNA (1 µg in 40 µl reaction volume) was reverse transcribed into cDNA, which was used as a template for PCR with specific primers (Eurofins Scientific, Luxembourg), which are listed in Table I. PCR cycling was performed using a 2720 Thermal Cycler (Applied Biosystems, Inc., Waltham, MA, USA). cDNA (3 µl) was used for the PCR reactions in the following 25-µl reaction mixture: 10 µM each primer, 25 µM MgCl2, 10 µM dNTPs final concentration and 0.625 U Taq Polymerase (Thermo Fisher Scientific, Waltham, MA, USA). PCR cycling was conducted as follows: Initial denaturation at 95°C for 5 min, followed by denaturation at 94°C for 30 sec, annealing (at temperatures given in Table I) for 30 sec and extension at 72°C for 30 sec for the number of cycles given for each gene in Table I. This was followed by a final extension step at 72°C for 10 min. The PCR products were separated by 2% agarose gel electrophoresis, and visualized with ethidium bromide. The intensity of each PCR product band was quantified using ImageJ software (National Institutes of Health, Bethesda, MA, USA), and β-actin was used as an internal control.
Table I.
List of primers and PCR conditions
| Primer no. | Gene | Primer sequence (5′-3′) | Annealing temperatures (°C) | Number of cycles |
|---|---|---|---|---|
| 1 | Actin | F=GCCTTCCTTCTTGGGTATGG | 55 | 32 |
| R=CAGCTCAGTAACAGTCCGC | ||||
| 2 | NP1 | F=CAGGACACTCTAGGTGGAGG | 55 | 32 |
| R=GAGGGGAGAAGAGAGACGAT | ||||
| 3 | NP2 | F=CATCAACGACAAGGTCGCAC | 55 | 32 |
| R=CTCTTCACAGGTCTCCACAGG | ||||
| 4 | NPR | F=CAAGAGCAGGATACCTTGGG | 55 | 32 |
| R=GAAGTGGGAGGTATAGCCAG | ||||
| 5 | IL-1β | F=TGAAGAAGAGCCCATCCTCTG | 55 | 32 |
| R=GGGTGTGCCGTCTTTCATTA |
NP1, neuronal pentraxin 1; NP2, neuronal pentraxin 2; NPR, neuronal pentraxin receptor; IL-1β, interleukin-1β; F, forward; R, reverse.
Statistical analysis
The data are presented as the mean ± standard error of the mean. The results were analyzed by two way analysis of variance and the Bonferroni post hoc test using GraphPad Prism® software (GraphPad, Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Effects of ibuprofen on learning and memory in an AlCl3-induced neurotoxicity mouse model
In order to analyze the pharmacological effects of ibuprofen on the learning and memory abilities of AlCl3-treated mice, ibuprofen (100 mg/kg/day) and AlCl3 (150 mg/kg/day) were administered to the appropriate groups for 12 days, after which the mice were subjected to memory testing using the Morris water maze test for the final 7 days of treatment, prior to sacrifice. On day two, the control group demonstrated a significant decrease in escape latency time (32.94±10.18), as compared with the AlCl3-treated group (58.88±11.70; P<0.05; Fig. 1).
Figure 1.
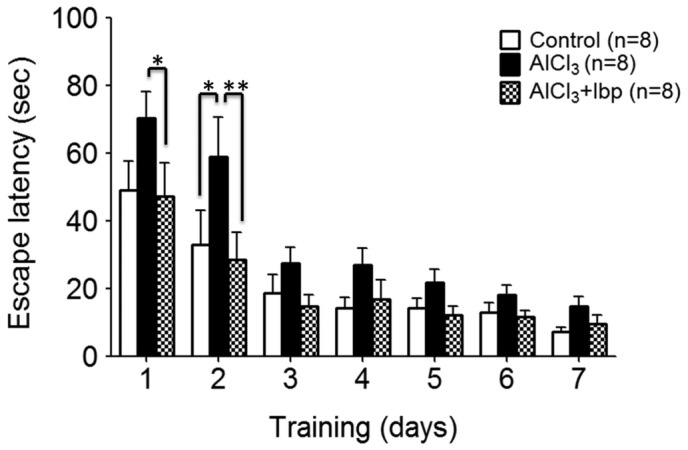
Morris water maze test was used to assess the effects of ibuprofen treatment on learning and memory in an AlCl3-induced mouse model of neurotoxicity. On days 1 and 2, ibuprofen treatment significantly improved the memory of mice in the ibuprofen-treated group, as compared with the AlCl3-treated group. Data are presented as the mean ± standard error of the mean (n=8/group). *P<0.05 vs. the AlCl3-treated group; **P<0.01 vs. the AlCl3-treated group. Ibp, ibuprofen; AlCl3, aluminum chloride.
Treatment with ibuprofen significantly improved the spatial reference memory of mice in the treated group (47.25±9.93) on day one, as compared with the AlCl3-treated group (70.38±7.75; P<0.05). On the second day, the ibuprofen-treated group exhibited significantly improved learning and memory abilities (28.63±7.94), as compared with the AlCl3-treated group on the same day (58.88±11.7; P<0.01; Fig. 1). However, on all other days, no significant differences between the three groups were observed (P>0.05).
Effects of ibuprofen on anxiety and locomotion in AlCl3-induced neurotoxicity model
In order to investigate the therapeutic effects of ibuprofen on anxiety and locomotion, open field tests were conducted on day 12 of treatment. The control group spent less time (22.20±0.41 min) in the peripheral area of the chamber, as compared with the AlCl3-treated group (28.2±0.29 min; Fig. 2), whereas the time spent by the control group mice in the center (7.80±0.41 min) was greater than the AlCl3-treated group (1.80±0.29 min); thus suggesting that mice in the AlCl3-treated group had elevated levels of anxiety (Fig. 2).
Figure 2.
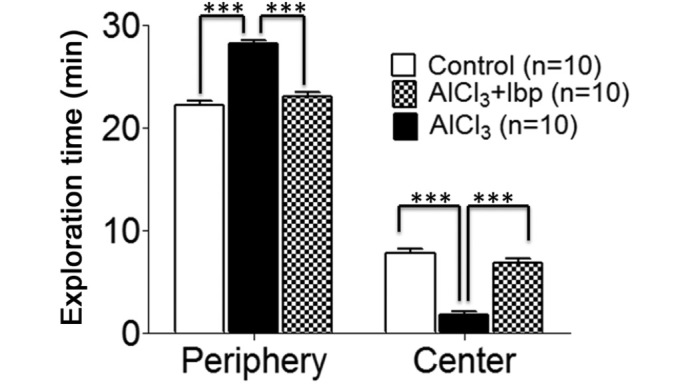
Open field test was used to investigate the effects of ibuprofen on anxiety and locomotion in an AlCl3-induced mouse model of neurotoxicity. The control and ibuprofen-treated groups spent significantly less time in the periphery and more time in the central area, as compared with the AlCl3-treated group. Data are presented as the mean ± standard error of the mean (n=10/group). The test was performed on day 12 following the start of treatment with the drugs. The time spent in the periphery and center of the chamber was calculated. ***P<0.001 vs. the AlCl3-treated group. Ibp, ibuprofen; AlCl3, aluminum chloride.
Following treatment with ibuprofen, a decrease in the time spent in the periphery (23.10±0.34 min) was observed in the ibuprofen-treated group, as compared with the AlCl3-treated group (28.20±0.29 min; Fig. 2), whereas, the time spent in the center (6.90±0.34 min) was significantly increased in the ibuprofen-treated group, as compared with the AlCl3-treated group (1.80±0.29 min; P<0.001; Fig. 2).
Effects of ibuprofen on the expression levels of NPs in the hippocampus, cortex and amygdala
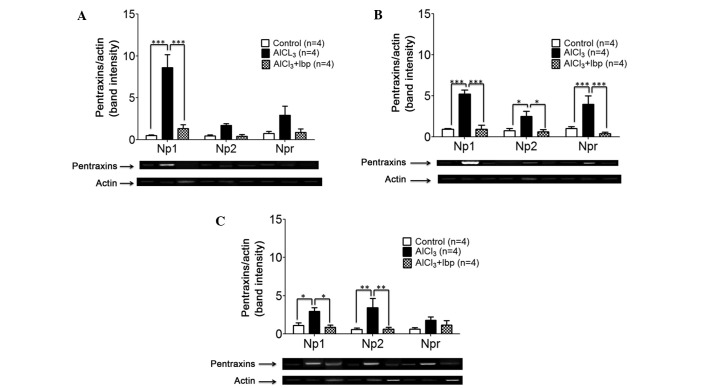
In order to evaluate the effects of ibuprofen on the expression levels of NPs, the mice were sacrificed at the end of treatment and RNA was extracted for analysis. Briefly, RNA was reverse transcribed into cDNA, which was used as a template for PCR. Ibuprofen treatment significantly decreased the expression levels of NP1 (1.32±0.47) in the hippocampus of ibuprofen-treated mice, as compared with the AlCl3-treated group (8.62±1.54; P<0.001; Fig. 3A). No significant decreases in the expression levels of NP2 and NPR were observed among the three groups; however, a trend of decreasing expression was detected in the ibuprofen-treated group, as compared with the AlCl3-treated group (Fig. 3A).
Figure 3.
Effects of ibuprofen treatment on the expression levels of neuronal pentraxins in the various brain structures of an AlCl3-induced mouse model of neurotoxicity. (A) Ibuprofen treatment significantly decreased the expression levels of NP1 in the hippocampus, as compared with the AlCl3-treated group. (B) Ibuprofen significantly decreased the expression levels of NP1, NP2 and NPR in the cortex, as compared with the AlCl3-treated group. (C) Ibuprofen significantly decreased the expression levels of NP1 and NP2 in the amygdala, as compared with the AlCl3-treated group. Reverse transcription-polymerase chain reaction and agarose gel electrophoresis were used to analyze the expression levels of NP1, NP2 and NPR in the various brain compartments. Data are presented as the mean ± standard error of the mean (n=4). *P<0.05 vs. the AlCl3-treated group; **P<0.01 vs. the AlCl3-treated group; ***P<0.001 vs. the AlCl3-treated group. NP1, neuronal pentraxin-1; NP2, neuronal pentraxin-2; NPR, neuronal pentraxin receptor; Ibp, ibuprofen; AlCl3, aluminum chloride.
In the cortex, ibuprofen treatment was associated with a significant decrease in the expression levels of NP1 (0.94±0.48; P<0.001), NP2 (0.62±0.28; P<0.05) and NPR (0.43±0.14; P<0.001), as compared with the expression levels of NP1 (5.21±0.51), NP2 (2.50±0.65) and NPR (4.00±1.01) in the AlCl3-treated group (Fig. 3B).
In the amygdala, ibuprofen treatment was associated with a significant decrease in the expression levels of NP1 (0.90±0.26; P<0.05) and NP2 (0.62±0.26; P<0.01), as compared with the expression levels of NP1 (2.95±0.47) and NP2 (3.43±1.22) in the AlCl3-treated group; however, no significant difference in the expression levels of NPR were detected among the groups (P>0.05; Fig. 3C).
Effects of ibuprofen on the expression levels of IL-1β in the hippocampus, cortex and amygdala
In order to analyze the effects of ibuprofen on inflammation in the AlCl3-treated group, IL-1β expression levels were analyzed. The control group exhibited significantly reduced levels of IL-1β expression in the hippocampus (0.69±0.10), cortex (0.98±0.33) and amygdala (0.67±0.18), as compared with the levels observed in the hippocampus (7.47±0.53), cortex (7.75±1.13) and amygdala (7.45±0.17) of the AlCl3-treated group (P<0.001; Fig. 4). Furthermore, ibuprofen treatment significantly decreased the expression levels of IL-1β in the hippocampus (0.99±0.21), cortex (0.86±0.09) and amygdala (0.49±0.13), as compared with the hippocampus, cortex and amygdala of AlCl3-treated mice (P<0.001; Fig. 4).
Figure 4.
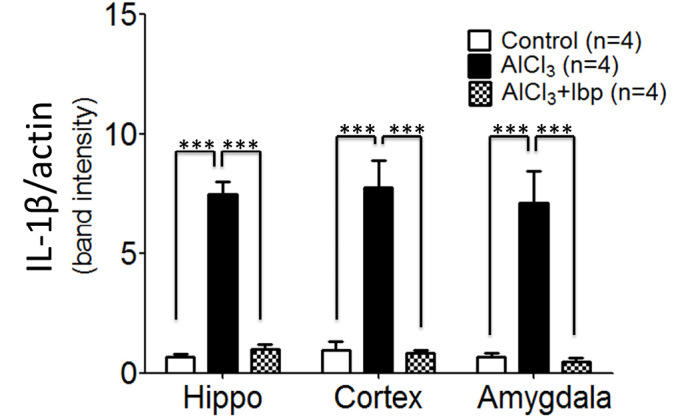
Effects of ibuprofen on the expression levels of IL-1β in the various brain compartments of an AlCl3-induced mouse model of neurotoxicity. IL-1β expression levels were analyzed using reverse transcription-polymerase chain reaction and agarose gel electrophoresis. Data are presented as the mean ± standard error of the mean. ***P<0.001 vs. the AlCl3-treated group. IL-1β, interleukin-1β; AlCl3, aluminum chloride; Ibp, ibuprofen; Hippo, hippocampus.
Discussion
It has previously been suggested that NSAIDs exert neuroprotective effects, in addition to their established anti-inflammatory functions (33). Ibuprofen is an NSAID that has been demonstrated to readily cross the blood-brain barrier (34) and exert memory-enhancing effects (29). The present study investigated the neuroprotective effects of ibuprofen (100 mg/kg/day) in an AlCl3-induced mouse model of neurotoxicity. In order to assess hippocampus-dependent spatial and reference memory (35), a Morris water maze test was conducted. The results of the present study demonstrated that learning and memory were impaired in AlCl3-treated mice, and that treatment with ibuprofen was able to reverse these effects; thus suggesting that ibuprofen is able to exert memory-enhancing effects. However, the effects were not significant in the later training days.
In order to investigate the effects of ibuprofen on the levels of anxiety, locomotion, and exploratory behavior in the AlCl3-treated mice, an open field test was conducted in the present study (36). Blanchard et al (37) previously suggested that the reluctance of an animal to move from one place to another, or into the central area in the test, is indicative of elevated levels of anxiety in the mice. The results of the present study demonstrated an increase in the anxiety levels of AlCl3-treated mice, as compared with the control group. Treatment with ibuprofen reversed the effects of AlCl3-treatment and was associated with a decrease in the levels of anxiety in ibuprofen-treated mice. Anxiety has been reported to be a predominant clinical symptom of neurodegenerative disorders (38); therefore, ibuprofen may have an additional therapeutic effect in the treatment of neurodegenerative disorder-associated anxiety.
In order to investigate the underlying mechanisms of ibuprofen, the effects of ibuprofen on the expression levels of NPs and IL-1β in the AlCl3-treated mice were analyzed. Previous studies have detected an association between the accumulation of Aβ and the upregulation of NP1, which is an apoptotic protein that promotes the death of damaged neurons (26). The results of the present study indicated that treatment with AlCl3 led to a significant increase in the expression levels of NP1 in the hippocampus; thus suggesting a possible role of NP1 in AlCl3-induced neurotoxicity, which is consistent with a previous report (26). The increase in NP2 and NPR expression levels in the hippocampus was not significant; however minor upregulation was observed in the AlCl3-treated group, as compared with the control group. Increased expression levels of NP1, NP2 and NPR were observed in the cortex, whereas NP1 and NP2 expression levels were increased in the amygdala, indicating that NPs have a role in AlCl3-induced neurodegeneration, dementia and learning and memory. Notably, treatment with ibuprofen was able to attenuate memory impairment. Inflammation is a hallmark of AD pathogenesis (39,40) and IL-1β, a pro-inflammatory cytokine, has a role in inflammation (41). It has previously been suggested that IL-1β overexpression is associated with the formation of plaques (42). The results of the present study demonstrated a significant increase in IL-1β expression levels in the hippocampus, cortex, and amygdala of AlCl3-treated mice, supporting previous data that IL-1β is elevated in a degenerating brain (43). Furthermore, treatment with ibuprofen was able to significantly decrease the expression levels of IL-1β in the various brain compartments, and the memory improvement observed may be due to this, as it has previously been demonstrated that IL-1β has a prominent role in memory impairment and aging (44). In addition, significant decreases in the expression levels of NPs following treatment with ibuprofen illustrated that ibuprofen may have a role in the expression of NPs, as well as interleukins; thus suggesting that there may be a possible link between NPs and neuroinflammation. NPs may promote the upregulation of interleukins; however, the underlying mechanism by which NPs are associated with inflammation remains unknown. The present study identified novel ibuprofen targets and demonstrated that treatment with ibuprofen may reduce the risk of developing neurodegeneration. Therefore, ibuprofen may be considered a potential therapeutic option for the treatment of patients with neurodegenerative disorders, including AD.
In conclusion, the present study analyzed the therapeutic effects of ibuprofen on the performance of mice in various behavioral tests, and on the expression levels of NPs. Ibuprofen increased learning and memory, and decreased anxiety in mice. Furthermore, ibuprofen was able to reduce inflammation, which is a hallmark of neurodegeneration and AD pathogenesis. Ibuprofen also decreased the expression levels of NPs, which are associated with neuropathology. Future studies should endeavor to elucidate the mechanisms by which NPs are involved in inflammation and the expression of IL-1β.
Acknowledgements
The present study was supported by The Atta-ur-Rahman School of Applied Biosciences, National University of Sciences and Technology, Islamabad, Pakistan.
References
- 1.Dokmeci D. Ibuprofen andA lzheimer's disease. Folia Med (Plovdiv) 2004;46:5–10. [PubMed] [Google Scholar]
- 2.Bushra R, Aslam N. An overview of clinical pharmacology of Ibuprofen. Oman Med J. 2010;25:155–1661. doi: 10.5001/omj.2010.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaminelli T, Gradowski RW, Bassani TB, Barbiero JK, Santiago RM, Maria-Ferreira D, Baggio CH, Vital MA. Antidepressant and antioxidative effect of Ibuprofen in the rotenone model of Parkinson's disease. Neurotox Res. 2014;26:351–362. doi: 10.1007/s12640-014-9467-y. [DOI] [PubMed] [Google Scholar]
- 4.Zurita MP, Muñoz G, Sepúlveda FJ, Gómez P, Castillo C, Burgos CF, Fuentealba J, Opazo C, Aguayo LG. Ibuprofen inhibits the synaptic failure induced by the amyloid-β peptide in hippocampal neurons. J Alzheimers Dis. 2013;35:463–473. doi: 10.3233/JAD-122314. [DOI] [PubMed] [Google Scholar]
- 5.Cauli O, Rodrigo R, Piedrafita B, Llansola M, Mansouri MT, Felipo V. Neuroinflammation contributes to hypokinesia in rats with hepatic encephalopathy, Ibuprofen restores its motor activity. J Neurosci Res. 2009;87:1369–1374. doi: 10.1002/jnr.21947. [DOI] [PubMed] [Google Scholar]
- 6.Lim GP, Yang F, Chu T, Chen P, Beech W, Teter B, Tran T, Ubeda O, Ashe KH, Frautschy SA, Cole GM. Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer's disease. J Neurosci. 2000;20:5709–5714. doi: 10.1523/JNEUROSCI.20-15-05709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zawilla NH, Taha FM, Kishk NA, Farahat SA, Farghaly M, Hussein M. Occupational exposure to aluminum and its amyloidogenic link with cognitive functions. J Inorg Biochem. 2014;139:57–64. doi: 10.1016/j.jinorgbio.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Khan KA, Kumar N, Nayak PG, Nampoothiri M, Shenoy RR, Krishnadas N, Rao CM, Mudgal J. Impact of caffeic acid on aluminium chloride-induced dementia in rats. J Pharm Pharmacol. 2013;65:1745–1752. doi: 10.1111/jphp.12126. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed SS, Santosh W. Metallomic profiling and linkage map analysis of early Parkinson's disease: A new insight to aluminum marker for the possible diagnosis. PLoS One. 2010;5:e11252. doi: 10.1371/journal.pone.0011252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawahara M, Kato-Negishi M. Link between aluminum and the pathogenesis of Alzheimer's disease: The integration of the aluminum and amyloid cascade hypotheses. Int J Alzheimers Dis. 2011;2011:276393. doi: 10.4061/2011/276393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He BP, Strong MJ. Motor neuronal death in sporadic amyotrophic lateral sclerosis (ALS) is not apoptotic. Histopathology. Neuropathol Appl Neurobiol. 2000;26:150–160. doi: 10.1046/j.1365-2990.2000.026002150.x. [DOI] [PubMed] [Google Scholar]
- 12.Pan R, Qiu S, Lu DX, Dong J. Curcumin improves learning and memory ability and its neuroprotective mechanism in mice. Chin Med J (Engl) 2008;121:832–839. [PubMed] [Google Scholar]
- 13.Shen LX, Jin LQ, Zhang DS, Xue GP. Effect of osthol on memory impairment of mice in AlCl3-induced acute senile model. Yao Xue Xue Bao. 2002;37:178–180. [PubMed] [Google Scholar]
- 14.Zaky A, Mohammad B, Moftah M, Kandeel KM, Bassiouny AR. Apurinic/apyrimidinic endonuclease 1 is a key modulator of aluminum-induced neuroinflammation. BMC Neurosci. 2013;14:26. doi: 10.1186/1471-2202-14-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veerhuis R. Histological and direct evidence for the role of complement in the neuroinflammation of AD. Curr Alzheimer Res. 2011;8:34–58. doi: 10.2174/156720511794604589. [DOI] [PubMed] [Google Scholar]
- 16.Moran LB, Hickey L, Michael GJ, Derkacs M, Christian LM, Kalaitzakis ME, Pearce RK, Graeber MB. Neuronal pentraxin II is highly upregulated in Parkinson's disease and a novel component of Lewy bodies. Acta Neuropathol. 2008;115:471–478. doi: 10.1007/s00401-007-0309-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bjartmar L, Huberman AD, Ullian EM, Rentería RC, Liu X, Xu W, Prezioso J, Susman MW, Stellwagen D, Stokes CC, et al. Neuronal pentraxins mediate synaptic refinement in the developing visual system. J Neurosci. 2006;26:6269–6281. doi: 10.1523/JNEUROSCI.4212-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schlimgen AK, Helms JA, Vogel H, Perin MS. Neuronal pentraxin, a secreted protein with homology to acute phase proteins of the immune system. Neuron. 1995;14:519–526. doi: 10.1016/0896-6273(95)90308-9. [DOI] [PubMed] [Google Scholar]
- 19.Goodman AR, Cardozo T, Abagyan R, Altmeyer A, Wisniewski HG, Vilcek J. Long pentraxins, An emerging group of proteins with diverse functions. Cytokine Growth Factor Rev. 1996;7:191–202. doi: 10.1016/1359-6101(96)00019-6. [DOI] [PubMed] [Google Scholar]
- 20.Tsui CC, Copeland NG, Gilbert DJ, Jenkins NA, Barnes C, Worley PF. Narp, a novel member of the pentraxin family, promotes neurite outgrowth and is dynamically regulated by neuronal activity. J Neurosci. 1996;16:2463–2478. doi: 10.1523/JNEUROSCI.16-08-02463.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamenskaya MA, Thesleff S. The neuromuscular blocking action of an isolated toxin from the elapid (Oxyuranus scutellactus) Acta Physiol Scand. 1974;90:716–724. doi: 10.1111/j.1748-1716.1974.tb05639.x. [DOI] [PubMed] [Google Scholar]
- 22.Xu D, Hopf C, Reddy R, Cho RW, Guo L, Lanahan A, Petralia RS, Wenthold RJ, O'Brien RJ, Worley P. Narp and NP1 form heterocomplexes that function in developmental and activity-dependent synaptic plasticity. Neuron. 2003;39:513–528. doi: 10.1016/S0896-6273(03)00463-X. [DOI] [PubMed] [Google Scholar]
- 23.Dowton SB, McGrew SD. Rat serum amyloid P component. Histopathology. Biochem J. 1990;270:553–556. doi: 10.1042/bj2700553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whitehead AS, Zahedi K, Rits M, Mortensen RF, Lelias JM. Mouse C-reactive protein. Histopathology. Biochem J. 1990;266:283–290. doi: 10.1042/bj2660283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breviario F, d'Aniello EM, Golay J, Peri G, Bottazzi B, Bairoch A, Saccone S, Marzella R, Predazzi V, Rocchi M, et al. Interleukin-1-inducible genes in endothelial cells. Cloning of a new gene related to C-reactive protein and serum amyloid P component. J Biol Chem. 1992;267:22190–22197. [PubMed] [Google Scholar]
- 26.Abad MA, Enguita M, DeGregorio-Rocasolano N, Ferrer I, Trullas R. Neuronal pentraxin 1 contributes to the neuronal damage evoked by amyloid-beta and is overexpressed in dystrophic neurites in Alzheimer's brain. J Neurosci. 2006;26:12735–12747. doi: 10.1523/JNEUROSCI.0575-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walsh DM, Selkoe DJ. Deciphering the molecular basis of memory failure in Alzheimer's disease. Neuron. 2004;44:181–193. doi: 10.1016/j.neuron.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 28.DeGregorio-Rocasolano N, Gasull T, Trullas R. Overexpression of neuronal pentraxin 1 is involved in neuronal death evoked by low K(+) in cerebellar granule cells. J Biol Chem. 2001;276:796–803. doi: 10.1074/jbc.M007967200. [DOI] [PubMed] [Google Scholar]
- 29.Hashmi AN, Yaqinuddin A, Ahmed T. Pharmacological effects of Ibuprofen on learning and memory, muscarinic receptors gene expression and APP isoforms level in pre-frontal cortex of AlCl3-induced toxicity mouse model. Int J Neurosci. 2015;125:277–287. doi: 10.3109/00207454.2014.922972. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed T, Gilani AH. Inhibitory effect of curcuminoids on acetylcholinesterase activity and attenuation of scopolamine-induced amnesia may explain medicinal use of turmeric in Alzheimer's disease. Pharmacol Biochem Behav. 2009;91:554–559. doi: 10.1016/j.pbb.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 31.Choleris E, Thomas AW, Kavaliers M, Prato FS. A detailed ethological analysis of the mouse open field test, Effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field. Neurosci Biobehav Rev. 2001;25:235–260. doi: 10.1016/S0149-7634(01)00011-2. [DOI] [PubMed] [Google Scholar]
- 32.Ahmed T, Enam SA, Gilani AH. Curcuminoids enhance memory in an amyloid-infused rat model of Alzheimer's disease. Neuroscience. 2010;169:1296–1306. doi: 10.1016/j.neuroscience.2010.05.078. [DOI] [PubMed] [Google Scholar]
- 33.Asanuma M, Miyazaki I, Ogawa N. Neuroprotective effects of nonsteroidal anti-inflammatory drugs on neurodegenerative diseases. Curr Pharm Des. 2004;10:695–700. doi: 10.2174/1381612043453072. [DOI] [PubMed] [Google Scholar]
- 34.Parepally JM, Mandula H, Smith QR. Brain uptake of nonsteroidal anti-inflammatory drugs, Ibuprofen, flurbiprofen, and indomethacin. Pharm Res. 2006;23:873–881. doi: 10.1007/s11095-006-9905-5. [DOI] [PubMed] [Google Scholar]
- 35.Bromley-Brits K, Deng Y, Song W. Morris water maze test for learning and memory deficits in Alzheimer's disease model mice. J Vis Exp. 2011;2920(53) doi: 10.3791/2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walsh RN, Cummins RA. The Open-Field Test: A critical review. Psychol Bull. 1976;83:482–504. doi: 10.1037/0033-2909.83.3.482. [DOI] [PubMed] [Google Scholar]
- 37.Blanchard DC, Griebel G, Blanchard RJ. Mouse defensive behaviors, Pharmacological and behavioral assays for anxiety and panic. Neurosci Biobehav Rev. 2001;25:205–218. doi: 10.1016/S0149-7634(01)00009-4. [DOI] [PubMed] [Google Scholar]
- 38.Ferretti L, McCurry SM, Logsdon R, Gibbons L, Teri L. Anxiety and Alzheimer's disease. J Geriatr Psychiatry Neurol. 2001;14:52–58. doi: 10.1177/089198870101400111. [DOI] [PubMed] [Google Scholar]
- 39.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, et al. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/S0197-4580(00)00124-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tuppo EE, Arias HR. The role of inflammation in Alzheimer's disease. Int J Biochem Cell Biol. 2005;37:289–305. doi: 10.1016/j.biocel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 41.Ren K, Torres R. Role of interleukin-1beta during pain and inflammation. Brain Res Rev. 2009;60:57–64. doi: 10.1016/j.brainresrev.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mrak RE, Griffin WS. Interleukin-1, neuroinflammation and Alzheimer's disease. Neurobiol Aging. 2001;22:903–908. doi: 10.1016/S0197-4580(01)00287-1. [DOI] [PubMed] [Google Scholar]
- 43.Cacabelos R, Alvarez XA, Fernández-Novoa L, Franco A, Mangues R, Pellicer A, Nishimura T. Brain interleukin-1 beta in Alzheimer's disease and vascular dementia. Methods Find Exp Clin Pharmacol. 1994;16:141–151. [PubMed] [Google Scholar]
- 44.Ahmed T, Gilani AH. A comparative study of curcuminoids to measure their effect on inflammatory and apoptotic gene expression in an Aβ plus ibotenic acid-infused rat model of Alzheimer's disease. Brain Res. 2011;1400:1–18. doi: 10.1016/j.brainres.2011.05.022. [DOI] [PubMed] [Google Scholar]