ABSTRACT
Lung injury after influenza infection is characterized by increased permeability of the lung microvasculature, culminating in acute respiratory failure. Platelets interact with activated endothelial cells and have been implicated in the pathogenesis of some forms of acute lung injury. Autopsy studies have revealed pulmonary microthrombi after influenza infection, and epidemiological studies suggest that influenza vaccination is protective against pulmonary thromboembolism; however, the effect of influenza infection on platelet-endothelial interactions is unclear. We demonstrate that endothelial infection with both laboratory and clinical strains of influenza virus increased the adhesion of human platelets to primary human lung microvascular endothelial cells. Platelets adhered to infected cells as well as to neighboring cells, suggesting a paracrine effect. Influenza infection caused the upregulation of von Willebrand factor and ICAM-1, but blocking these receptors did not prevent platelet-endothelial adhesion. Instead, platelet adhesion was inhibited by both RGDS peptide and a blocking antibody to platelet integrin α5β1, implicating endothelial fibronectin. Concordantly, lung histology from infected mice revealed viral dose-dependent colocalization of viral nucleoprotein and the endothelial marker PECAM-1, while platelet adhesion and fibronectin deposition also were observed in the lungs of influenza-infected mice. Inhibition of platelets using acetylsalicylic acid significantly improved survival, a finding confirmed using a second antiplatelet agent. Thus, influenza infection induces platelet-lung endothelial adhesion via fibronectin, contributing to mortality from acute lung injury. The inhibition of platelets may constitute a practical adjunctive strategy to the treatment of severe infections with influenza.
IMPORTANCE There is growing appreciation of the involvement of the lung endothelium in the pathogenesis of severe infections with influenza virus. We have recently shown that the virus can infect human lung endothelial cells, but the functional consequences of this infection are unknown (S. M. Armstrong, C. Wang, J. Tigdi, X. Si, C. Dumpit, S. Charles, A. Gamage, T. J. Moraes, and W. L. Lee, PLoS One 7:e47323, 2012, http://dx.doi.org/10.1371/journal.pone.0047323). Here, we show that this infection causes platelets to adhere to the lung endothelium. Importantly, blocking platelets using two distinct antiplatelet drugs improved survival in a mouse model of severe influenza infection. Thus, platelet inhibition may constitute a novel therapeutic strategy to improve the host response to severe infections with influenza.
INTRODUCTION
Despite annual vaccination programs and widely available antiviral drugs, seasonal influenza alone causes an estimated tens of thousands of deaths in North America annually (1, 2). Most deaths occur due to pulmonary complications, such as primary viral pneumonia (3) or a superimposed bacterial pneumonia (4). In both, respiratory deterioration is marked by acute lung injury (3, 5), a potentially fatal syndrome of pulmonary edema that occurs due to the increased permeability of the lung microvasculature (6, 7). Therapeutic options are limited. While antiviral drugs exist, they only partially reduce mortality (8), they must be administered early to be maximally effective, and their use is complicated by the rapid development of resistance. For instance, almost 100% of H3N2 strains of influenza A are already resistant to amantadine (9). Thus, new therapies for these most severe cases of influenza are urgently needed.
The influenza virus infects the bronchial epithelium, leading to epithelial injury, apoptosis, and desquamation (10). In uncomplicated infections, these changes to the airway epithelium are transient. However, in primary viral pneumonia, the virus also infects the distal lung, particularly type I pneumocytes and ciliated bronchiolar epithelium, leading to alveolar damage and frank alveolar denudement (10). The average thickness of the alveolar capillary membrane is just over 1 μm, which includes a single layer of alveolar epithelium, wisps of interstitial tissue, and a single layer of lung microvascular endothelium (11). In some areas, the membrane is as thin as 200 nm (11). Thus, the death of infected epithelial cells will create gaps in the monolayer and give newly released virions access to the endothelium (10, 12), even in the absence of frank viremia. We have recently reported that human influenza is capable of infecting lung endothelial cells both in vitro (13) and in vivo (7), although very little is known about the functional consequences of such infection. Certainly disruption or injury to either alveolar epithelium or the underlying endothelium or both may contribute to the formation of lung edema (13).
Importantly, whether viral infection affects the relationship of the lung endothelium with other cells in the lung, such as platelets, is unknown. This is an important question, since platelets have been identified to contribute to certain forms of lung injury (14, 15) through their recruitment of leukocytes (16, 17) and induction of an inflammatory response (18). Importantly, the endothelium normally is antithrombogenic and prevents platelet adhesion. An effect of the virus to induce platelet-endothelial adhesion is plausible: numerous reports describe pulmonary thrombi as an important complication of severe infections with influenza (10, 19), while endothelial activation, which would be expected to induce platelet adhesion, was highly correlated with death from influenza in a murine model (20). Furthermore, a study of patients with venous thromboembolism found that vaccination against influenza was associated with a protective effect against pulmonary clots (21). Determining how influenza infection affects platelet-endothelial interactions may have important clinical implications, since numerous platelet antagonists are available already and might constitute useful therapies.
In this study, we elucidated the effect of influenza virus infection on the adhesion of platelets to the lung microvascular endothelium. In addition, we determined whether inhibiting platelets using readily available platelet inhibitors exerted protective effects on the degree of lung injury in a mouse model of severe influenza.
MATERIALS AND METHODS
Cell culture and influenza infection.
Primary human microvascular endothelial cells (HMVECs) derived from lung were obtained from Lonza and were cultured in EBM-2 media with the recommended supplements and used in passages 6 to 8. Influenza A virus X31 (H3N2) was used, since the H3N2 subtype is most commonly associated with complications and death (1, 22); we also used an influenza A virus clinical isolate (H3N2; from Susan Richardson). The effect of a laboratory strain of H1N1 (A/Puerto Rico/8/34, also known as PR8; a gift from Conrad Liles) was tested as well. HMVECs were infected with virus at different multiplicities of infection (MOI; defined as the ratio of PFU to endothelial cells). Virus was added to cells in serum-free media. After 1 h, 0.5% serum was added. All infections were for 24 h. To study any contributions from viral replication, we developed replication-deficient virus by exposing it to UV light for 10 min as previously reported (13). The amount of virus was quantified both by PFU and hemagglutinin units (HAU) using published protocols (23).
Reagents.
To inhibit virus-induced apoptosis, cells were treated with 100 μM ZVAD-FMK (Enzo Life Sciences) for 24 h simultaneously with infection. To evaluate the effect of influenza surface proteins on platelet adhesion, endothelial cells were exposed to recombinant hemagglutinin (HA; 5 to 50 μg/ml; catalogue no. IT-003-00418DTMp; Immune Technology Corp.) and neuraminidase (NA; 250 μg/μl; 6875-NM; Cedarlane) from H3N2 influenza virus for 30 min or 24 h. In other experiments, endothelial cells were exposed to the synthetic viral RNA mimetic poly(I·C) (2 μg/ml; tlr1-pic; Cedarlane) for 24 h prior to the addition of platelets. In one experiment, thrombin (8 U/ml; T6884; Sigma) was used as a positive control to induce platelet adhesion.
Platelet-endothelial adhesion assays.
Gel-filtered platelets were prepared as previously described (24). Briefly, human blood was collected from healthy adult donors by venipuncture and anticoagulated with Na-citrate. Platelet-rich plasma (PRP) was obtained by centrifugation of anticoagulated whole blood at 300 × g for 7 min. Platelets then were isolated from the PRP with the use of a Sepharose 2B column in piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES) buffer (5 mM PIPES, 1.37 mM NaCl, 4 mM KCl, 0.1% glucose, pH 7.0). Following isolation, platelets were diluted to final concentrations required by each experimental condition. Platelets were fluorescently labeled with calcein acetoxymethyl ester (calcein-AM) (1 μg ml−1; Molecular Probes, Eugene, OR). Platelet purity and the lack of activation were verified by flow cytometry for CD41 (25); preparations were 99% single platelets, and >95% were positive for CD41 (not shown).
In the plate-based adhesion assay, endothelial cells were seeded on 96-well plates and grown to confluence (3 to 4 days). Platelets were added to each well at the indicated concentrations and incubated for 1 h. After incubation, cells were washed with phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde (PFA) for 15 min. The adhesion of fluorescently labeled platelets was measured by using a fluorescent plate reader.
In the flow cytometry adhesion assay, endothelial cells were trypsinized and then labeled with LDS 751 (a cell-permeant nucleic acid stain; Life Technologies) before suspending them with calcein-labeled platelets for 15 min. Endothelium-platelet adhesion was detected by flow cytometry using the MACSQuant analyzer (Miltenyi Biotec Inc., San Diego, CA).
Antibodies.
Rabbit anti-von Willebrand factor (vWF) (ab6994), rabbit anti-fibronectin (ab2413), and goat anti-ICAM (EP1442Y) were from Abcam (Cambridge, MA). Mouse anti-P-selectin (MAB2154) was from EMD Millipore, and mouse anti-VE-cadherin (sc-6458), rabbit anti-PECAM-1 (sc-8306), and mouse nuclear protein (NP; sc-80481) were from Santa Cruz Biotechnology. To block integrin-fibronectin binding, platelets were treated with 170 μM Arg-Gly-Asp-Ser (RGDS) peptide (A9041; Sigma-Aldrich) for 30 min before incubation with endothelial cells. Anti-fibrinogen (SC-166968; Santa Cruz) and anti-fibronectin (SC-9068; Santa Cruz) were used in Western blot analyses to detect expression levels of fibrinogen and fibronectin. Antibodies to block cell surface ligands and integrins in adhesion assays were obtained from the following sources: anti-vWF (3.1 g/liter; Dako North America Inc., Carpinteria, CA), anti-ICAM (BBA3; R&D), anti-integrin α5β1 (ab75472; Abcam), and anti-integrin α2β3 (ab3919; Abcam). Cells were treated for 60 min prior to the adhesion assay.
Immunofluorescence.
For VE-cadherin and viral nucleoprotein (NP) immunostains, cells were fixed in 4% PFA for 1 h at room temperature, incubated in 0.15% glycine for 10 min, and then permeabilized in 0.1% Triton X-100 for 20 min. After blocking, cells were treated with anti-VE-cadherin and NP antibodies for 1 h, followed by incubation for 1 h with a fluorescently labeled secondary antibody. Images were acquired by spinning-disc confocal microscopy (Zeiss Axiovert 200M microscope). Microscope settings were kept constant between conditions. All images were randomly chosen and were acquired as z-stack projections (z-stack interval, 0.5 μm).
Apoptosis assays.
Apoptotic cells were detected using an annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit (BioVision, Milpitas, CA) according to the manufacturer's instructions. Annexin V-FITC was detected using a flow cytometer (BD FACSCalibur cytometer; Becton Dickinson, Mississauga, ON, Canada), and the data were analyzed using De Novo Software-FCS Express v 3.0.
Western blotting.
Lysates were prepared with lysis buffer (62.5 mM Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, 10 mM dithiothreitol [DTT]) and separated using 10% polyacrylamide gels. Proteins were transferred to nitrocellulose membranes, blocked for 1 h in 5% milk in Tris-buffered saline (TBS), and probed overnight with primary antibody at 4°C. After washing, blots were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies for 1 h, washed, and then visualized by advanced chemiluminescence (Amersham). Band intensity was quantified using Image J (NIH) and normalized to the loading control after background correction.
Immunohistochemistry.
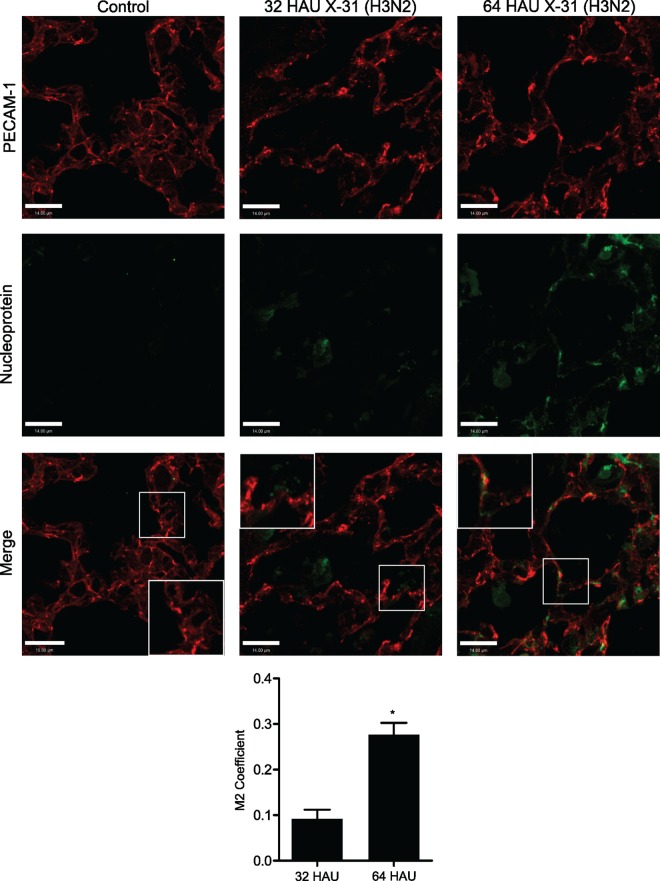
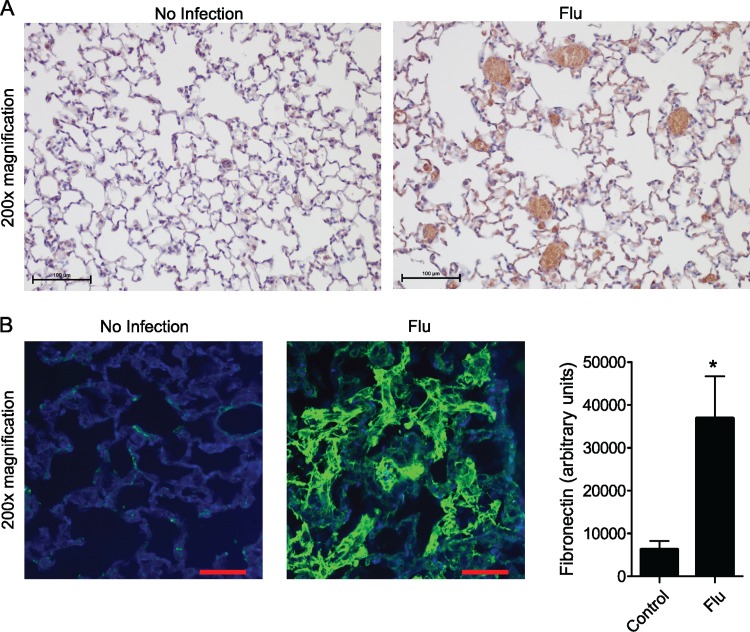
Mice were infected intranasally with 32 or 64 HAU influenza virus and were sacrificed 5 days later. The lungs were collected for histological analysis, fixed in formalin, and embedded in paraffin as previously described (26). For fibronectin, sections were stained with a mouse monoclonal antibody directed against fibronectin (Santa Cruz Biotechnology). Slides were washed and subsequently incubated with Alexa Fluor 488 donkey anti-mouse secondary antibody from Life Technologies Inc. (Burlington, ON, Canada). For platelet deposition, sections were stained in the same manner using anti-CD41 antibody (bs-2636R; Bioss, Woburn, MA) followed by an anti-rabbit secondary antibody and then DAB staining (Dako). Random fields were acquired by spinning-disc confocal microscopy (Zeiss Axiovert 200M microscope) and quantified in a blinded fashion using ImageJ. For the staining of viral nucleoprotein and PECAM-1, antigen retrieval was performed by boiling for 15 min in citric acid buffer, cooling for 45 min, and then blocking and permeabilization. Lung sections were stained with rabbit anti-PECAM-1 (1:500) overnight and with mouse anti-influenza NP (1:75) for 1 h. The colocalization of viral NP and PECAM-1 was quantified using Manders' coefficient (27) as determined using Volocity software.
For experiments with acetylsalicylic acid (ASA; Sigma), mice received 3 mg ASA by intraperitoneal injection at the time of infection and again 24 h later. For adjuvant ASA, mice also received a dose of amantadine by oral gavage twice daily at a concentration of 138 mg/kg of body weight/day starting on the day of infection (28). For platelet inhibition by ticlopidine, mice received a daily dose of 10 mg/kg ticlopidine by intraperitoneal injection (29). Mice were monitored 3 times daily for the duration of the experiment (14 days after infection) and were scored for weight loss, hypothermia, hypoxemia, spontaneous activity (scored from 1 [moribund] to 5 [normal], as described in reference 30), and other clinical features of influenza infection. Mice were euthanized if two or more of the following occurred: weight loss exceeded 30% of initial weight, temperature fell below 31°C, and/or the animal appeared moribund. In one experiment, lung homogenates from ASA-treated and untreated mice were measured for viral PFU 3 days postinfection using published protocols (23).
Pulse oximetry measurements.
Arterial oxygen saturation was measured using the MouseOx Plus device and software (Starr Life Sciences, Oakmont, PA) on conscious (nonanesthetized) mice using a collar clip. Mice were allowed to acclimate to the collar clip for several minutes before the maximal measurement was recorded.
Statistics.
Experiments were repeated a minimum of three times, and data are presented as means and standard errors. Unless otherwise stated, comparisons between greater than 2 groups were performed by one-way analysis of variance (ANOVA) with Bonferroni t tests for post hoc analyses; unpaired student's t tests were performed for comparisons between two groups, and all tests were two-tailed. Statistics were performed using GraphPad Prism software (La Jolla, CA).
Ethics statement.
All mouse experiments were performed in accordance with the regulations of the Canadian Council on Animal Care and were approved by the Animal Care Committee of St. Michael's Hospital (protocol 297). Care was taken to minimize animal discomfort per institutional guidelines. Thus, mice were anesthetized with isoflurane for intranasal instillation and were monitored regularly postinfection.
RESULTS
Influenza induces platelet-endothelial adhesion.
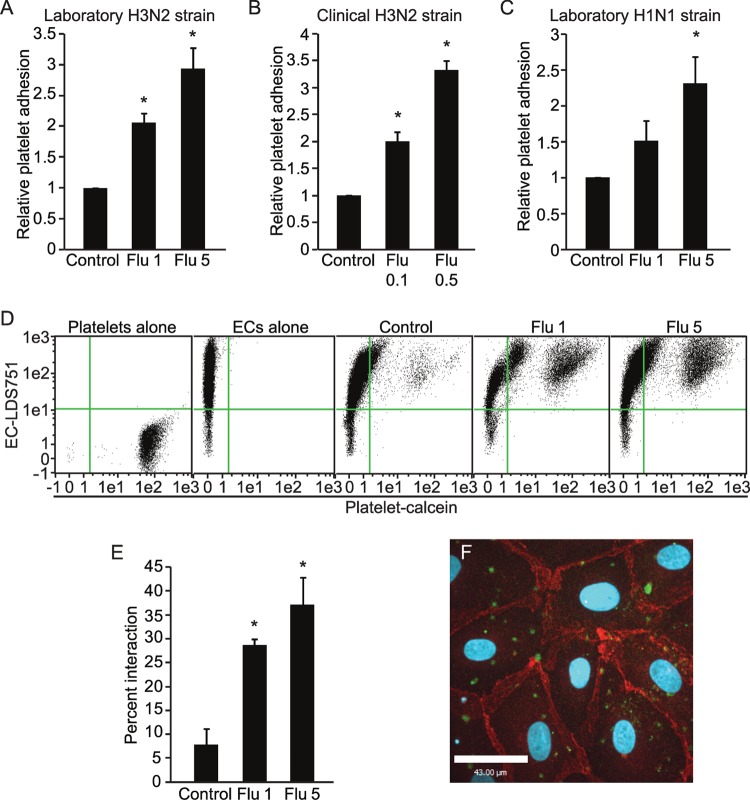
Confluent primary human lung endothelial monolayers were infected with an H3N2 subtype of influenza A virus (X31) for 24 h; we and others have previously reported that human influenza is capable of infecting and replicating in human endothelial cells both in vitro (13, 31, 32) and in vivo (7). This was followed by incubation with purified calcein-labeled human platelets. After washing to remove unbound platelets, the degree of platelet adhesion was measured by a fluorescent plate reader. Viral infection caused a dose-dependent increase in platelet-endothelial adhesion (Fig. 1A); importantly, we observed a similar effect with a clinical isolate (H3N2) of the virus (Fig. 1B) as well as a laboratory H1N1 subtype of influenza A (PR8) (Fig. 1C).
FIG 1.
Influenza A virus induces a dose-dependent increase in platelet-endothelial adhesion. Endothelial monolayers infected with increasing MOIs of influenza (Flu 1, MOI of 1; Flu 5, MOI of 5, etc.) have increased platelet adhesion, whether infected with a laboratory strain of H3N2 (A) (n = 5 to 7), a clinical H3N2 strain (B) (n = 3), or a laboratory strain of H1N1 (C) (n = 3). *, P < 0.05 versus control. (D to E) Influenza virus induces a dose-dependent increase in platelet-endothelial interaction in studies using endothelial cells in suspension. (D) Endothelial cells bound to platelets appear in the upper right quadrant. (E) Quantification of data from panel D. n = 3; *, P < 0.05 versus the control. (F) Influenza virus-induced platelet-endothelial interaction visualized by confocal microscopy. Calcein-AM-labeled platelets are shown in green, while endothelial cell-cell junctions stained with VE-cadherin appear in red. The image is representative of 2 experiments.
We considered the possibility that infection could lead to the retraction of the endothelial cells, exposing the extracellular matrix and favoring the adhesion of platelets to the spaces between cells rather than causing bona fide platelet-endothelial adhesion. To establish that true platelet-endothelial adhesion was occurring, we studied platelet-endothelial interactions in suspension by flow cytometry, thereby eliminating confounding from the extracellular matrix. To do so, we briefly trypsinized control and infected endothelial monolayers before the addition of platelets. Under these conditions, platelet-endothelial adhesion continued to be strongly induced by infection with the virus in a dose-dependent fashion (Fig. 1D and E). To confirm these findings visually, we performed immunofluorescence for the specific endothelial junctional protein VE-cadherin on infected monolayers after the addition of platelets and then imaged the monolayer along the entire z axis. As expected, platelets showed little adherence to uninfected cells (data not shown). In contrast, influenza infection induced the adhesion of platelets to areas of the endothelial monolayer that were both distant and adjacent to cell-cell junctions (Fig. 1F). Thus, influenza infection of endothelial cells induces true platelet-endothelial adhesion.
Influenza-induced platelet adhesion is not due to endothelial apoptosis and occurs with both infected and adjacent cells.
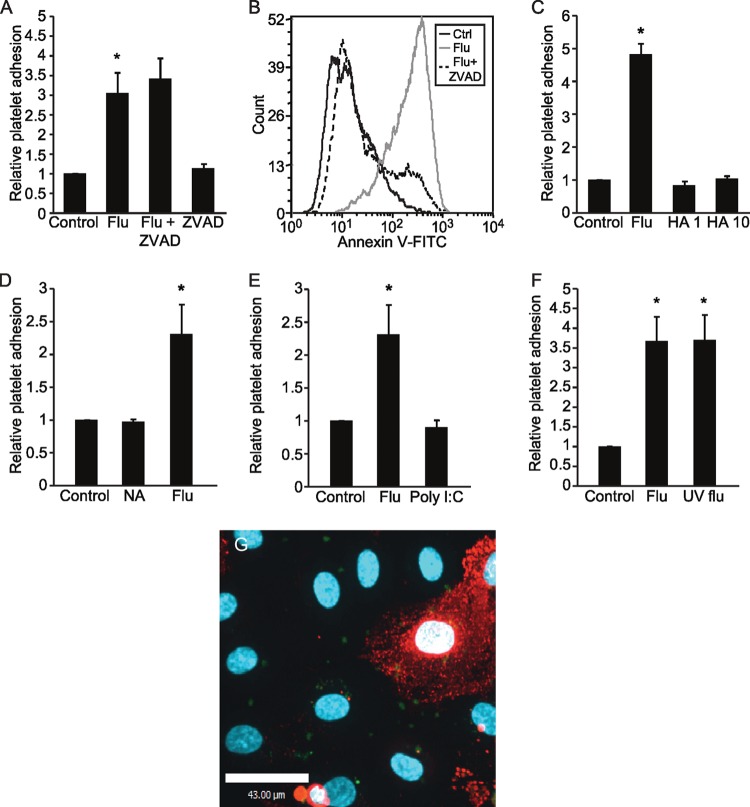
We next considered the possibility that platelet-endothelial adhesion was a consequence of endothelial apoptosis, as viral infection is known to trigger cell death (13), which itself increases adhesiveness to platelets (33). However, treatment with the pan-caspase inhibitor ZVAD had no effect on platelet adhesion (Fig. 2A), even though it did prevent influenza-induced endothelial cell apoptosis (Fig. 2B).
FIG 2.
Exploring the components of the virus responsible for influenza virus-induced endothelium-platelet adhesion. Inhibition of caspases using ZVAD does not affect influenza-induced platelet-endothelial interactions (n = 3; *, P < 0.05 versus the control [Ctrl]) (A), although ZVAD treatment successfully blocks influenza-induced endothelial cell apoptosis (B). The histogram is representative of 3 experiments. (C) Viral binding is not sufficient to induce platelet-endothelial interaction, as the effect was not recapitulated by treatment with the protein hemagglutinin (HA; at 1 or 10 μg/ml). n = 3; *, P < 0.05 versus control and HA groups. (D) Recombinant viral membrane enzyme neuraminidase (NA) cannot recapitulate influenza-induced platelet-endothelial interaction. n = 3; *, P < 0.05 versus control and NA groups. (E) Viral RNA mimetic [poly(I·C)] did not recapitulate influenza-induced platelet-endothelial interaction. n = 3; *, P < 0.05 versus control. (F) Viral replication is not required for influenza-induced platelet-endothelial interaction. Replication-deficient (UV flu) and live virus induced similar degrees of platelet-endothelial adhesion. n = 3; *, P < 0.05 versus control. (G) Influenza-induced platelet-endothelial interaction visualized by confocal microscopy reveals that influenza induces platelet adhesion to endothelial cells in a paracrine fashion. Calcein-AM-labeled platelets are shown in green, while an influenza-infected endothelial cell is labeled using an antibody to viral nucleoprotein and appears red. The image is representative of 2 experiments.
We then performed a series of experiments attempting to recapitulate platelet adhesion using different elements of the influenza virus. The most abundant surface proteins of influenza are hemagglutinin (HA), which mediates viral binding, and neuraminidase (NA), which cleaves sialic acid residues, allowing for the release of newly formed virions. We treated endothelial monolayers with recombinant hemagglutinin or recombinant neuraminidase to determine whether this would be sufficient to induce platelet adhesion. However, at concentrations as high as or higher than those found for the intact virus (34), exposure to recombinant HA and NA for 30 min to 24 h did not induce platelet adhesion (Fig. 2C and D and data not shown). Similarly, we treated monolayers with poly(I·C), a synthetic analogue of double-stranded RNA (35), to evaluate whether the recognition of viral RNA could be responsible for the effect. However, it did not recapitulate platelet adhesion (Fig. 2E). We then generated replication-deficient virus using UV irradiation, which we have shown is still capable of infecting the endothelium and producing viral proteins (13). Under these conditions, platelet-endothelial adhesion still occurred, indicating that viral replication is not required for the effect (Fig. 2F).
The failure of ZVAD to inhibit and of the purified viral components to induce platelet adhesion suggested that endothelium-platelet interactions occur via a paracrine effect rather than being isolated to individual infected cells. Using immunofluorescence for viral nucleoprotein (NP) to denote infected cells, we observed the adherence of platelets to both NP-positive and NP-negative cells, suggesting that adhesion is mediated at least in part by paracrine factors (Fig. 2G).
Endothelial cells are activated after viral infection, and platelet adhesion is mediated by integrins.
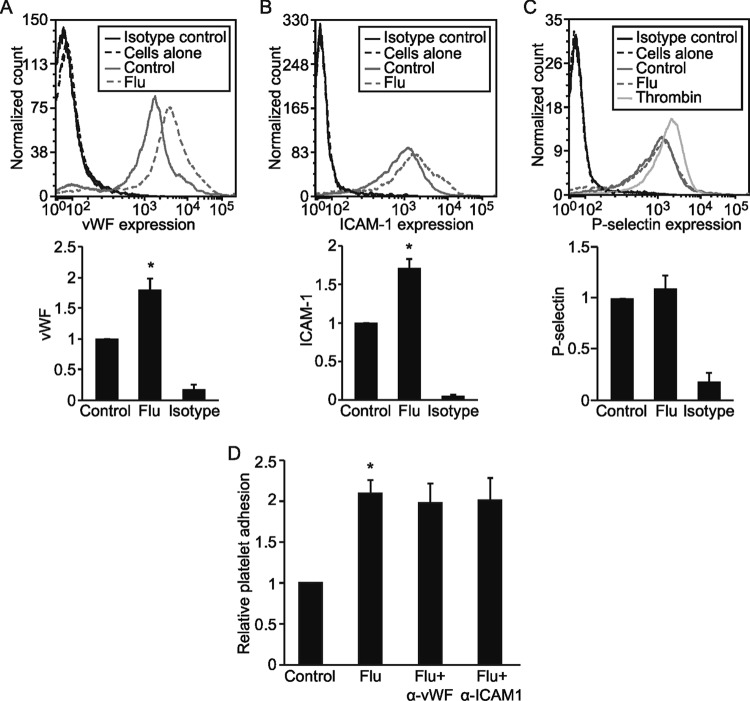
We next sought to determine the cellular mechanism of influenza-induced platelet-endothelial adhesion. We reasoned that viral infection was likely to activate the lung endothelium, leading to increased expression of endothelial ligands capable of engaging platelet receptors. Indeed, infected monolayers demonstrated a significant increase in surface expression of vWF and ICAM-1 but no change in P-selectin levels (Fig. 3A to C). However, treating endothelial cells with blocking antibodies to vWF or ICAM-1 did not prevent platelet adhesion, suggesting that these receptors are not responsible for influenza-induced endothelium-platelet adhesion (Fig. 3D).
FIG 3.
Influenza virus upregulates endothelial vWF and ICAM-1 expression. (A to C) Endothelial expression of vWF (A), ICAM-1 (B), and P-selectin (C) after influenza infection were measured by flow cytometry. Thrombin was used as a positive control in the P-selectin experiment, showing that influenza infection does not affect endothelial P-selectin expression. Histograms and graphs of relative mean fluorescence are representative of 2 to 3 experiments. *, P < 0.05 versus the control; isotype refers to isotype control antibody. (D) ICAM-1 and vWF are not required for flu-induced platelet-endothelial interaction, as the incubation of HMVECs with anti-vWF or anti-ICAM-1 blocking antibodies did not prevent influenza-induced platelet-endothelial adhesion. n = 3; *, P < 0.05 versus the control.
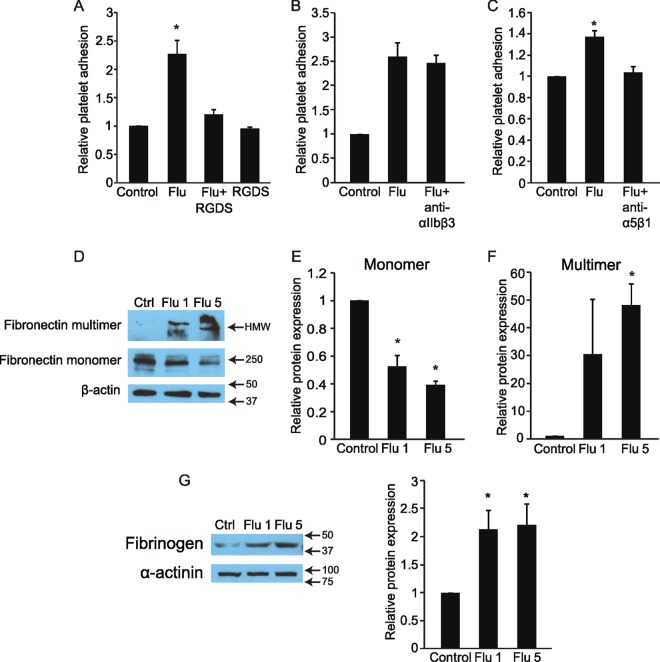
Instead, we considered the possibility that platelet-endothelial adhesion in this context is integrin mediated, as integrins have been reported to be critical in thrombus formation within injured vessels (36). We pretreated platelets with synthetic RGDS peptide, which competitively inhibits integrin binding to fibronectin and related proteins (37). When RGDS-treated platelets were added to infected endothelial monolayers, we observed almost complete abrogation of platelet-endothelial adhesion (Fig. 4A). The inhibitory ability of RGDS peptide suggested a role for endothelial fibronectin or possibly fibrinogen in mediating platelet adhesion. We observed a dose-dependent reduction in monomeric fibronectin in endothelial lysates from infected cells (Fig. 4D and E) coincident with a marked increase in multimeric fibronectin (Fig. 4F) and a significant increase in endothelial fibrinogen (Fig. 4G); however, the latter was not dose dependent. To further establish the involvement of integrins, we pretreated platelets with antibodies blocking the platelet integrin glycoprotein α5β1, which recognizes fibronectin and fibrinogen. Anti-α5β1 antibodies significantly attenuated virus-induced platelet adhesion (Fig. 4C). This was not a nonspecific effect of the antibody, since an antibody to another platelet integrin, αIIbβ3, had no effect (Fig. 4B). While knockdown of endothelial fibronectin using short interfering RNA (siRNA) was achieved, unfortunately exposure to both control (scrambled) and targeted siRNA prevented influenza-induced platelet-endothelial adhesion (not shown), confounding the experiment.
FIG 4.
Integrin binding is required for flu-induced endothelium-platelet binding. (A) Platelets pretreated with RGDS peptide to block platelet integrin binding prior to their addition to influenza-infected and control endothelium show impaired influenza-induced endothelium-platelet adhesion. n = 3; *, P < 0.05 versus control and Flu+RGDS groups. (B) Integrin αIIbβ3 is not required for influenza-induced endothelium-platelet binding, as incubating platelets with an anti-αIIbβ3 antibody prior to their addition to endothelium does not block adhesion. n = 2; *, P < 0.05 versus control. (C) In contrast, blocking antibodies to integrin α5β1 inhibits influenza-induced endothelium-platelet binding. n = 3; *, P < 0.05 versus the control. (D, E, and F) Influenza A virus induces a dose-dependent decrease in endothelial fibronectin monomer expression and increase in fibronectin multimer expression. HMW, high molecular weight. n = 3; *, P < 0.05 versus the control. (G) Influenza induces endothelial fibrinogen expression. n = 3; *, P < 0.05.
Mice infected with influenza demonstrate dose-dependent colocalization of viral nucleoprotein and PECAM-1 as well as platelet and fibronectin deposition in the lung.
To validate our in vitro data, we infected mice intranasally with influenza A virus at a dose that results in 100% mortality by about 7 days after infection; this is in association with lung edema and alveolar neutrophil recruitment (7, 38), akin to severe influenza pneumonia that occurs in humans. This strain of the virus is also capable of infecting mouse lung endothelium in vitro (13); furthermore, we observed colocalization of viral NP and the endothelial membrane protein PECAM-1 (CD31) in the lung sections of infected mice; the degree of colocalization increased significantly in a viral dose-dependent manner (Fig. 5). At a dose of the virus causing 100% mortality within 8 days, over 25% of the viral NP colocalized with the endothelial marker. Examination by immunohistochemistry 5 days after infection revealed marked platelet deposition in the lungs of influenza-infected mice compared to that of uninfected controls (Fig. 6A). The lungs of influenza-infected mice also displayed a marked increase in fibronectin (Fig. 6B), mirroring the in vitro findings.
FIG 5.
Colocalization of viral nucleoprotein and PECAM-1 increases as a function of viral inoculum. Mice were infected with 32 or 64 HAU of influenza A virus (X31), and immunofluorescence on lung sections was performed 5 days later. Sections were probed for viral nucleoprotein and PECAM-1, and colocalization using Manders' coefficient (M2) was quantified using Volocity software; the insets show enlarged areas. *, P < 0.01; n = 3 mice at 32 HAU and 4 mice in each of 64-HAU and control groups; colocalization could not be calculated in control mice due to the absence of viral nucleoprotein. Images were acquired at ×600 magnification.
FIG 6.
Endothelium-platelet interaction in a mouse model of influenza virus-induced lung injury. (A) Lung histology showing that influenza infection is associated with increased platelet deposition in murine lungs. Platelets shown by DAB staining are labeled using an anti-CD41 antibody. Control group, n = 3 animals; flu group, n = 5 animals. (B) Immunostaining for fibronectin reveals increased fibronectin deposition on the lung endothelium of influenza-infected mice compared to that of uninfected controls. Fibronectin is stained green. Control group, n = 3 mice; flu group, n = 5 mice. In all experiments, images are representative of 10 (×200 magnification) fields per lung. *, P < 0.05.
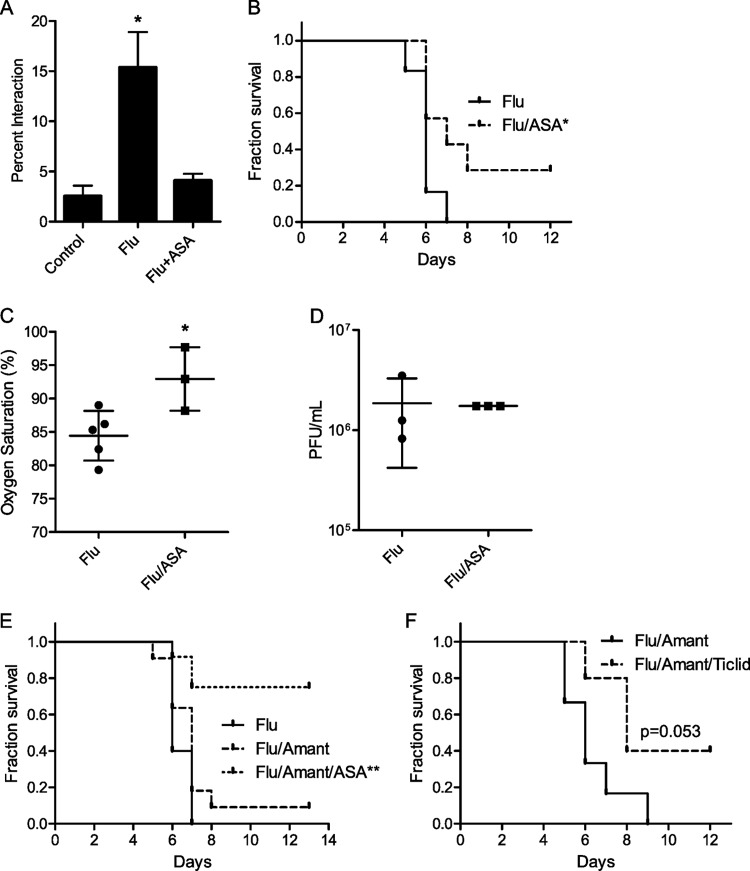
To determine the importance of platelet-endothelial interactions to the pathophysiology of influenza-induced acute lung injury, we administered acetylsalicylic acid (ASA) to mice simultaneously with the onset of infection. ASA inhibits platelet cyclooxygenase, preventing the synthesis of thromboxane A2 and thereby impairing platelet function (39). Treatment of flu-infected endothelial cells with ASA resulted in a significant reduction in platelet-endothelial interaction (Fig. 7A). Under these conditions, ASA significantly improved survival from influenza (Fig. 7B) and decreased arterial hypoxemia (Fig. 7C). Contrary to previous reports, the benefit was not due to a direct or indirect effect on viral replication (40, 41), as viral titers from lung homogenates were unchanged by treatment with ASA (Fig. 7D).
FIG 7.
ASA treatment improves survival following severe influenza virus infection. (A) Influenza-induced platelet-endothelial interaction in vitro is attenuated by treatment with ASA. n = 4 experiments. *, P < 0.01 for the control versus flu groups. (B) Kaplan-Meier survival curves showing that ASA treatment at the time of influenza virus infection improves survival in mice infected with a severe dose of X31 H3N2 influenza virus. Flu group, n = 6; flu/ASA group, n = 7 mice; *, P < 0.05 by Mantel-Cox test. (C) ASA treatment is associated with improved arterial oxygen saturation 5 days after influenza infection. This time point was chosen as mice die shortly after. *, P < 0.05. (D) ASA treatment of influenza-infected mice has no effect on lung viral titer, as measured 3 days after influenza infection. (E) Kaplan-Meier survival curves showing that ASA treatment in combination with the antiviral drug amantadine (Amant) significantly improves survival following severe influenza infection in mice. Flu group, n = 5 mice; flu/Amant group, n = 11 mice; flu/Amant/ASA group, n = 12 mice; **, P < 0.01 by Mantel-Cox test. (F) Platelet inhibition by ticlopidine (Ticlid) in combination with amantadine improves survival. Flu/Amant group, n = 6 mice; flu/Amant/Ticlid group, n = 5 mice.
To more closely mimic the clinical situation, in which a drug like ASA would be given as an adjuvant, we repeated the experiment but administered ASA in conjunction with the antiviral drug amantadine. While amantadine alone exhibited a trend toward modestly improved survival, adjuvant therapy with ASA increased survival from 0 to approximately 80% (Fig. 7E). Finally, to establish the beneficial effect of platelet inhibition by a second method, we tested ticlopidine, a drug that inhibits platelet function via a different pathway (ADP receptor inhibition). As with ASA, ticlopidine administered to mice at the time of infection resulted in a trend toward increased survival (Fig. 7F).
DISCUSSION
Acute lung injury, which manifests clinically as the acute respiratory distress syndrome (ARDS), is a common cause of mortality from severe influenza. Even in combination with optimal supportive care, therapy with antiviral drugs does not completely prevent mortality (8), indicating that new therapeutic approaches are needed. Given reports that microthombi are observed in the lungs of patients with severe influenza (10, 19) and that platelet inhibition ameliorates certain forms of lung injury (14, 15), we hypothesized that the influenza virus induces platelet adhesion to the lung endothelium and that this process contributes to the pathology of severe influenza.
In this study, we have shown using both laboratory and clinical isolates that influenza virus infection of the lung endothelium can induce a dose-dependent adhesion of platelets; this effect was independent of endothelial apoptosis and viral replication and was not recapitulated by purified hemagglutinin or neuraminidase. Our data are most consistent with adhesion being induced by paracrine mediators that are elaborated in response to infection, although the identity of this factor or factors currently is being investigated. Virus-induced platelet adhesion is dependent on platelet integrins and is likely mediated by interaction with endothelial fibronectin and/or fibrinogen. Fibronectin exists in both circulating and cellular forms, and it is the latter that exhibits a tendency to multimerize (42); in turn, multimerized fibronectin is thought to be more thrombogenic (43). It is important to acknowledge that while antibody blockade of vWF and ICAM-1 did not attenuate in vitro platelet adhesion, this does not exclude their potential involvement in vivo; platelet adhesion in vivo is complex and multifactorial, and our in vitro mechanistic approach is necessarily reductionist. A particular issue that is the subject of ongoing work is the role of shear stress, since vWF is known to play a critical role in platelet tethering under conditions of flow.
We and others have previously reported the infection of endothelial cells in vitro by human influenza (13), a phenomenon that also occurs in vivo in the lungs of infected mice and which we now show increases with increasing viral inoculum. However, the functional consequences of endothelial infection by influenza are largely unexplored. Using multiple strains of the virus, our data clearly indicate that infection of endothelial cells is sufficient to induce platelet adhesion. Our model does not preclude a role for other stimuli besides endothelial infection to induce platelet-endothelial adhesion. For instance, influenza infection recently has been reported to induce lung epithelial cytokine production, including that of interleukin-6 (IL-6), IL-8, and granulocyte-macrophage colony-stimulating factor (GM-CSF) (44, 45, 46). Similarly, activated neutrophils and macrophages which are recruited to the lung after influenza infection (17) are themselves major producers of numerous proinflammatory cytokines (47), regulated at least in part by the autonomic nervous system (48, 49). These circulating cytokines likely stimulate platelets (50) and endothelial cells, promoting platelet-endothelial adhesion. Regardless of the specific stimulus, adherent platelets become activated and regulate numerous facets of the inflammatory response, including the release of further proinflammatory mediators and the formation of neutrophil extracellular traps (18, 51) that together contribute to tissue injury. Thus, the relative contribution of endothelial infection to this sequence of events is unknown and technically difficult to assess, as preventing the infection of any one tissue alone in vivo is not currently feasible.
While the manuscript was in preparation, a report was published describing the activation of platelets during influenza virus infection of mice and demonstrating that platelet inhibition improved the outcome (52); the role of the endothelium was not explored. Our findings, which focus on the endothelial sequelae of influenza infection, thereby are complementary and extend the notion that platelets contribute pathologically to influenza-induced acute lung injury. Given the importance of leukocytes and innate immunity in general to the host response to influenza infection (53) and the proposed role of platelets in regulating innate immunity (18, 51), it is at least theoretically possible that platelet inhibition is harmful (54). Instead, we found that treatment with ASA improved survival, increased arterial oxygenation, and did not impair viral clearance in vivo. Furthermore, the synergistic benefit of combined antiplatelet and antiviral therapy strongly supports the notion that targeting the host rather than the pathogen is a useful strategy in the treatment of severe influenza (55).
In conclusion, the infection of lung microvascular endothelial cells by human influenza is capable of inducing platelet adhesion via integrin binding. Using a murine model of severe influenza, the inhibition of platelets significantly improves mortality, and this effect is even greater when combined with an antiviral drug. The wide availability of antiplatelet agents suggests that this approach holds promise for the therapy of severe influenza infections, although further study is required.
ACKNOWLEDGMENTS
We thank Chris Spring and Caterina Di Ciano-Oliveria from the core facilities of the Keenan Research Centre for Biomedical Science for technical help.
This work was supported by an operating grant from the Canadian Institutes of Health Research (CIHR MOP 130564), bridge funds from the Institute of Circulatory and Respiratory Health at the CIHR (OCN 126577), and an Early Researcher Award from the Government of Ontario, all to W.L.L. M.G.S. was supported by a Queen Elizabeth II Graduate Scholarship in Science and Technology and a graduate award from the Ted Rogers Centre for Heart Research. R.Z. is supported by the CREMS Research Scholar program at the University of Toronto.
REFERENCES
- 1.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. 2003. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 2.Schanzer DL, Tam TW, Langley JM, Winchester BT. 2007. Influenza-attributable deaths, Canada 1990-1999. Epidemiol Infect 135:1109–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louria DB, Blumenfeld HL, Ellis JT, Kilbourne ED, Rogers DE. 1959. Studies on influenza in the pandemic of 1957-1958. II. Pulmonary complications of influenza. J Clin Investig 38:213–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morens DM, Taubenberger JK, Fauci AS. 2008. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis 198:962–970. doi: 10.1086/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dominguez-Cherit G, Lapinsky SE, Macias AE, Pinto R, Espinosa-Perez L, de la Torre A, Poblano-Morales M, Baltazar-Torres JA, Bautista E, Martinez A, Martinez MA, Rivero E, Valdez R, Ruiz-Palacios G, Hernandez M, Stewart TE, Fowler RA. 2009. Critically ill patients with 2009 influenza A(H1N1) in Mexico. JAMA 302:1880–1887. doi: 10.1001/jama.2009.1536. [DOI] [PubMed] [Google Scholar]
- 6.Lee WL, Downey GP. 2001. Leukocyte elastase: physiological functions and role in acute lung injury. Am J Respir Crit Care Med 164:896–904. doi: 10.1164/ajrccm.164.5.2103040. [DOI] [PubMed] [Google Scholar]
- 7.Wang C, Armstrong SM, Sugiyama MG, Tabuchi A, Krauszman A, Kuebler WM, Mullen B, Advani S, Advani A, Lee WL. 2015. Influenza primes human lung microvascular endothelium to leak upon exposure to Staphylococcus aureus. Am J Respir Cell Mol Biol 53:459–470. doi: 10.1165/rcmb.2014-0373OC. [DOI] [PubMed] [Google Scholar]
- 8.McGeer A, Green KA, Plevneshi A, Shigayeva A, Siddiqi N, Raboud J, Low DE. 2007. Antiviral therapy and outcomes of influenza requiring hospitalization in Ontario, Canada. Clin Infect Dis 45:1568–1575. doi: 10.1086/523584. [DOI] [PubMed] [Google Scholar]
- 9.van der Vries E, Schutten M, Boucher CA. 2011. The potential for multidrug-resistant influenza. Curr Opin Infect Dis 24:599–604. doi: 10.1097/QCO.0b013e32834cfb43. [DOI] [PubMed] [Google Scholar]
- 10.Kuiken T, Taubenberger JK. 2008. Pathology of human influenza revisited. Vaccine 26(Suppl 4):D59–D66. doi: 10.1016/j.vaccine.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weibel ER, Knight BW. 1964. A morphometric study on the thickness of the pulmonary air-blood barrier. J Cell Biol 21:367–396. doi: 10.1083/jcb.21.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mori I, Komatsu T, Takeuchi K, Nakakuki K, Sudo M, Kimura Y. 1995. In vivo induction of apoptosis by influenza virus. J Gen Virol 76(Part 11):2869–2873. doi: 10.1099/0022-1317-76-11-2869. [DOI] [PubMed] [Google Scholar]
- 13.Armstrong SM, Wang C, Tigdi J, Si X, Dumpit C, Charles S, Gamage A, Moraes TJ, Lee WL. 2012. Influenza infects lung microvascular endothelium leading to microvascular leak: role of apoptosis and claudin-5. PLoS One 7:e47323. doi: 10.1371/journal.pone.0047323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zarbock A, Singbartl K, Ley K. 2006. Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. J Clin Investig 116:3211–3219. doi: 10.1172/JCI29499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Looney MR, Nguyen JX, Hu Y, Van Ziffle JA, Lowell CA, Matthay MA. 2009. Platelet depletion and aspirin treatment protect mice in a two-event model of transfusion-related acute lung injury. J Clin Investig 119:3450–3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caudrillier A, Kessenbrock K, Gilliss BM, Nguyen JX, Marques MB, Monestier M, Toy P, Werb Z, Looney MR. 2012. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Investig 122:2661–2671. doi: 10.1172/JCI61303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Narasaraju T, Yang E, Samy RP, Ng HH, Poh WP, Liew AA, Phoon MC, van Rooijen N, Chow VT. 2011. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am J Pathol 179:199–210. doi: 10.1016/j.ajpath.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rondina MT, Weyrich AS, Zimmerman GA. 2013. Platelets as cellular effectors of inflammation in vascular diseases. Circ Res 112:1506–1519. doi: 10.1161/CIRCRESAHA.113.300512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agarwal PP, Cinti S, Kazerooni EA. 2009. Chest radiographic and CT findings in novel swine-origin influenza A (H1N1) virus (S-OIV) infection. Am J Roentgenol 193:1488–1493. doi: 10.2214/AJR.09.3599. [DOI] [PubMed] [Google Scholar]
- 20.Brandes M, Klauschen F, Kuchen S, Germain RN. 2013. A systems analysis identifies a feedforward inflammatory circuit leading to lethal influenza infection. Cell 154:197–212. doi: 10.1016/j.cell.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu T, Carcaillon L, Martinez I, Cambou JP, Kyndt X, Guillot K, Vergnes MC, Scarabin PY, Emmerich J. 2009. Association of influenza vaccination with reduced risk of venous thromboembolism. Thromb Haemost 102:1259–1264. [DOI] [PubMed] [Google Scholar]
- 22.Peltola VT, McCullers JA. 2004. Respiratory viruses predisposing to bacterial infections: role of neuraminidase. Pediatr Infect Dis J 23:S87–S97. doi: 10.1097/01.inf.0000108197.81270.35. [DOI] [PubMed] [Google Scholar]
- 23.Szretter KJ, Balish AL, Katz JM. 2006. Influenza: propagation, quantification, and storage. Curr Protoc Microbiol Chapter 15:Unit 15G.11. [DOI] [PubMed] [Google Scholar]
- 24.Fine KM, Ashbrook PC, Brigden LP, Maldonado JE, Didishelm P. 1976. Gel-filtered human platelets. Ultrastructure, function, and role of proteins in inhibition of aggregation by aspirin. Am J Pathol 84:11–24. [PMC free article] [PubMed] [Google Scholar]
- 25.Bagamery K, Kvell K, Landau R, Graham J. 2005. Flow cytometric analysis of CD41− labeled platelets isolated by the rapid, one-step OptiPrep method from human blood. Cytometry A 65:84–87. [DOI] [PubMed] [Google Scholar]
- 26.Advani A, Kelly DJ, Cox AJ, White KE, Advani SL, Thai K, Connelly KA, Yuen D, Trogadis J, Herzenberg AM, Kuliszewski MA, Leong-Poi H, Gilbert RE. 2009. The (Pro)renin receptor: site-specific and functional linkage to the vacuolar H+-ATPase in the kidney. Hypertension 54:261–269. doi: 10.1161/HYPERTENSIONAHA.109.128645. [DOI] [PubMed] [Google Scholar]
- 27.Azizi PM, Zyla RE, Guan S, Wang C, Liu J, Bolz SS, Heit B, Klip A, Lee WL. 2015. Clathrin-dependent entry and vesicle-mediated exocytosis define insulin transcytosis across microvascular endothelial cells. Mol Biol Cell 26:740–750. doi: 10.1091/mbc.E14-08-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen JT, Smee DF, Barnard DL, Julander JG, Gross M, de Jong MD, Went GT. 2012. Efficacy of combined therapy with amantadine, oseltamivir, and ribavirin in vivo against susceptible and amantadine-resistant influenza A viruses. PLoS One 7:e31006. doi: 10.1371/journal.pone.0031006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuka H, Fujiwara H, Tanaka M, Yoshifusa H, Nakamura Y, Shibata Y. 1995. Antithrombotic effect of ticlopidine on occlusive thrombi of small coronary arteries in (NZWxBXSB)F1 male mice with myocardial infarction and systemic lupus erythematosus. J Cardiovasc Pharmacol 25:9–13. doi: 10.1097/00005344-199501000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Grauslund M, Thougaard AV, Fuchtbauer A, Hofland KF, Hjorth PH, Jensen PB, Sehested M, Fuchtbauer EM, Jensen LH. 2007. A mouse model for studying the interaction of bisdioxopiperazines with topoisomerase IIalpha in vivo. Mol Pharmacol 72:1003–1014. doi: 10.1124/mol.107.036970. [DOI] [PubMed] [Google Scholar]
- 31.Chan MC, Chan RW, Yu WC, Ho CC, Chui WH, Lo CK, Yuen KM, Guan YI, Nicholls JM, Peiris JS. 2009. Influenza H5N1 virus infection of polarized human alveolar epithelial cells and lung microvascular endothelial cells. Respir Res 10:102. doi: 10.1186/1465-9921-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Visseren FL, Verkerk MS, Bouter KP, Diepersloot RJ, Erkelens DW. 1999. Interleukin-6 production by endothelial cells after infection with influenza virus and cytomegalovirus. J Lab Clin Med 134:623–630. doi: 10.1016/S0022-2143(99)90103-8. [DOI] [PubMed] [Google Scholar]
- 33.Bombeli T, Schwartz BR, Harlan JM. 1999. Endothelial cells undergoing apoptosis become proadhesive for nonactivated platelets. Blood 93:3831–3838. [PubMed] [Google Scholar]
- 34.Getie-Kebtie M, Sultana I, Eichelberger M, Alterman M. 2013. Label-free mass spectrometry-based quantification of hemagglutinin and neuraminidase in influenza virus preparations and vaccines. Influenza Other Respir Viruses 7:521–530. doi: 10.1111/irv.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fortier ME, Kent S, Ashdown H, Poole S, Boksa P, Luheshi GN. 2004. The viral mimic, polyinosinic:polycytidylic acid, induces fever in rats via an interleukin-1-dependent mechanism. Am J Physiol Regul Integr Comp Physiol 287:R759–R766. doi: 10.1152/ajpregu.00293.2004. [DOI] [PubMed] [Google Scholar]
- 36.Ruggeri ZM, Mendolicchio GL. 2007. Adhesion mechanisms in platelet function. Circ Res 100:1673–1685. doi: 10.1161/01.RES.0000267878.97021.ab. [DOI] [PubMed] [Google Scholar]
- 37.Pierschbacher MD, Ruoslahti E. 1984. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature 309:30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- 38.Sugiyama MG, Armstrong SM, Wang C, Hwang D, Leong-Poi H, Advani A, Advani S, Zhang H, Szaszi K, Tabuchi A, Kuebler WM, Van Slyke P, Dumont D, Lee WL. 2015. The Tie2-agonist vasculotide rescues mice from influenza virus infection. Sci Rep 5:11030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toivanen J, Ylikorkala O, Viinikka L. 1985. Differential inhibition of platelet thromboxane and lung prostacyclin production by sulphinpyrazone, acetylsalicylic acid and indomethacin by human tissues in vitro. Thromb Res 37:493–502. doi: 10.1016/0049-3848(85)90095-7. [DOI] [PubMed] [Google Scholar]
- 40.Mazur I, Wurzer WJ, Ehrhardt C, Pleschka S, Puthavathana P, Silberzahn T, Wolff T, Planz O, Ludwig S. 2007. Acetylsalicylic acid (ASA) blocks influenza virus propagation via its NF-kappaB-inhibiting activity. Cell Microbiol 9:1683–1694. doi: 10.1111/j.1462-5822.2007.00902.x. [DOI] [PubMed] [Google Scholar]
- 41.Coulombe F, Jaworska J, Verway M, Tzelepis F, Massoud A, Gillard J, Wong G, Kobinger G, Xing Z, Couture C, Joubert P, Fritz JH, Powell WS, Divangahi M. 2014. Targeted prostaglandin E2 inhibition enhances antiviral immunity through induction of type I interferon and apoptosis in macrophages. Immunity 40:554–568. doi: 10.1016/j.immuni.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 42.Chen R, Gao B, Huang C, Olsen B, Rotundo RF, Blumenstock F, Saba TM. 2000. Transglutaminase-mediated fibronectin multimerization in lung endothelial matrix in response to TNF-alpha. Am J Physiol Lung Cell Mol Physiol 279:L161–L174. [DOI] [PubMed] [Google Scholar]
- 43.Cho J, Mosher DF. 2006. Role of fibronectin assembly in platelet thrombus formation. J Thromb Haemost 4:1461–1469. doi: 10.1111/j.1538-7836.2006.01943.x. [DOI] [PubMed] [Google Scholar]
- 44.Ito Y, Correll K, Zemans RL, Leslie CC, Murphy RC, Mason RJ. 2015. Influenza induces IL-8 and GM-CSF secretion by human alveolar epithelial cells through HGF/c-Met and TGF-alpha/EGFR signaling. Am J Physiol Lung Cell Mol Physiol 308:L1178–L1188. doi: 10.1152/ajplung.00290.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pan H, Zhang Y, Luo Z, Li P, Liu L, Wang C, Wang H, Li H, Ma Y. 2014. Autophagy mediates avian influenza H5N1 pseudotyped particle-induced lung inflammation through NF-kappaB and p38 MAPK signaling pathways. Am J Physiol Lung Cell Mol Physiol 306:L183–L195. doi: 10.1152/ajplung.00147.2013. [DOI] [PubMed] [Google Scholar]
- 46.Adachi M, Matsukura S, Tokunaga H, Kokubu F. 1997. Expression of cytokines on human bronchial epithelial cells induced by influenza virus A. Int Arch Allergy Immunol 113:307–311. doi: 10.1159/000237584. [DOI] [PubMed] [Google Scholar]
- 47.Mantovani A, Cassatella MA, Costantini C, Jaillon S. 2011. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol 11:519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 48.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ. 2003. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 49.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. 2000. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 50.Lumadue JA, Lanzkron SM, Kennedy SD, Kuhl DT, Kickler TS. 1996. Cytokine induction of platelet activation. Am J Clin Pathol 106:795–798. [DOI] [PubMed] [Google Scholar]
- 51.Sreeramkumar V, Adrover JM, Ballesteros I, Cuartero MI, Rossaint J, Bilbao I, Nacher M, Pitaval C, Radovanovic I, Fukui Y, McEver RP, Filippi MD, Lizasoain I, Ruiz-Cabello J, Zarbock A, Moro MA, Hidalgo A. 2014. Neutrophils scan for activated platelets to initiate inflammation. Science 346:1234–1238. doi: 10.1126/science.1256478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Le VB, Schneider JG, Boergeling Y, Berri F, Ducatez M, Guerin JL, Adrian I, Errazuriz-Cerda E, Frasquilho S, Antunes L, Lina B, Bordet JC, Jandrot-Perrus M, Ludwig S, Riteau B. 2015. Platelet activation and aggregation promote lung inflammation and influenza virus pathogenesis. Am J Respir Crit Care Med 191:804–819. doi: 10.1164/rccm.201406-1031OC. [DOI] [PubMed] [Google Scholar]
- 53.Tate MD, Ioannidis LJ, Croker B, Brown LE, Brooks AG, Reading PC. 2011. The role of neutrophils during mild and severe influenza virus infections of mice. PLoS One 6:e17618. doi: 10.1371/journal.pone.0017618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van den Boogaard FE, Schouten M, de Stoppelaar SF, Roelofs JJ, Brands X, Schultz MJ, Van't Veer C, van der Poll T. 2015. Thrombocytopenia impairs host defense during murine Streptococcus pneumoniae pneumonia. Crit Care Med 43:e75–83. doi: 10.1097/CCM.0000000000000853. [DOI] [PubMed] [Google Scholar]
- 55.Armstrong SM, Mubareka S, Lee WL. 2013. The lung microvascular endothelium as a therapeutic target in severe influenza. Antiviral Res 99:113–118. doi: 10.1016/j.antiviral.2013.05.003. [DOI] [PubMed] [Google Scholar]