ABSTRACT
Interferon beta (IFN-β) is a key component of cellular innate immunity in mammals, and it constitutes the first line of defense during viral infection. Studies with cultured cells previously showed that almost all nucleated cells are able to produce IFN-β to various extents, but information about the in vivo sources of IFN-β remains incomplete. By applying immunohistochemistry and employing conditional-reporter mice that express firefly luciferase under the control of the IFN-β promoter in either all or only distinct cell types, we found that astrocytes are the main producers of IFN-β after infection of the brain with diverse neurotropic viruses, including rabies virus, Theiler's murine encephalomyelitis virus, and vesicular stomatitis virus. Analysis of a panel of knockout mouse strains revealed that sensing of viral components via both RIG-I-like helicases and Toll-like receptors contributes to IFN induction in the infected brain. A genetic approach to permanently mark rabies virus-infected cells in the brain showed that a substantial number of astrocytes became labeled and, therefore, must have been infected by the virus at least transiently. Thus, our results strongly indicate that abortive viral infection of astrocytes can trigger pattern recognition receptor signaling events which result in secretion of IFN-β that confers antiviral protection.
IMPORTANCE Previous work indicated that astrocytes are the main producers of IFN after viral infection of the central nervous system (CNS), but it remained unclear how astrocytes might sense those viruses which preferentially replicate in neurons. We have now shown that virus sensing by both RIG-I-like helicases and Toll-like receptors is involved. Our results further demonstrate that astrocytes get infected in a nonproductive manner under these conditions, indicating that abortive infection of astrocytes plays a previously unappreciated role in the innate antiviral defenses of the CNS.
INTRODUCTION
Interferons (IFN) play a crucial role in antiviral defense. They are produced within hours after viral infection of cells and act by binding to specific cell surface receptors in an autocrine or paracrine fashion, thereby inducing a signaling cascade that leads to the induction of a large number of IFN-stimulated genes (ISGs). ISGs can serve diverse functions and establish an antiviral state (1).
Invading pathogens are detected by pattern recognition receptors (PRRs). PRRs differ in their cellular localization and ligand specificity. The two major receptor families, the cytoplasmic RIG-I-like receptors (RLR) and membrane-bound Toll-like receptors (TLR), control the induction of IFN upon RNA virus infection. RLRs and the TLRs associated with the endosomal compartment are mainly responsible for the detection of intracellular pathogens such as viruses. Upon viral infection, PRRs are triggered by pathogen-associated molecular patterns (PAMPs), such as double-stranded or tri-phosphorylated RNA. Downstream of RLRs, the adaptor protein MAVS (mitochondrial antiviral-signaling protein) leads to the activation of various transcription factors that confer enhanced expression of IFN genes. TLR3 signals through the adaptor protein TRIF (TIR-domain-containing adapter-inducing interferon-β), whereas all other TLRs share the common adaptor protein MyD88 (myeloid differentiation primary response gene 88).
The brain is a delicate organ, as the regeneration potential of its cells is very limited. The brain is protected from invading infectious agents by the blood-brain barrier. Nevertheless, it still encounters viral infections via different routes (2). IFN-based mechanisms can restrict viral replication in the brain (3). Using mice lacking functional IFN receptors specifically on neuroectodermal cells, it could be shown that a local IFN response in the brain is required to block the spread of vesicular stomatitis virus (VSV) (4). Interestingly, however, dedicated IFN producer cells such as plasmacytoid dendritic cells are absent from the brain parenchyma (5). Thus, brain-resident cells must produce substantial amounts of IFN after virus infection. It appears that all brain cells are capable of producing IFN, although to greatly differing extents (6, 7). Most experimental attempts to identify IFN-producing cells in the brain have been in vitro approaches, and only a few of those studies have given insights into the in vivo situation. Due to quick secretion of IFN by the producer cells, it is intrinsically difficult to identify the cellular sources of IFN in vivo. Thus, a clear picture of the identity of IFN-producing cells in the virus-infected brain is still missing.
In a previous study (8) in which we employed reporter mice harboring firefly luciferase under the transcriptional control of the IFN-β promoter (9), it became clear that astrocytes are prominent IFN-β producers during infection with La Crosse virus. This finding was surprising because La Crosse virus preferentially infects neurons, raising the issue of how astrocytes may sense the infection. It further remained unclear whether massive production of IFN by astrocytes was a unique response of the brain to infection with La Crosse virus or whether astrocytes should be regarded as professional IFN-producing cells of the virus-infected brain.
By applying immunohistochemistry and employing conditional-reporter mice that express firefly luciferase under the control of the IFN-β promoter in either all or only distinct cell types, we found that astrocytes are the main producers of IFN-β after infection of the brain with diverse neurotropic viruses, including rabies virus (RABV), Theiler's murine encephalomyelitis virus (TMEV), and vesicular stomatitis virus (VSV). We addressed the issues of whether detection of viral components by PRRs is necessary to induce IFN-β in the brain and whether a nonproductive infection of astrocytes could be responsible for IFN production. The analysis of a panel of knockout mouse strains revealed that sensing of viral components via both RIG-I-like helicases and Toll-like receptors contributes to IFN induction in the virus-infected brain. Using an in vivo approach to permanently mark infected cells, we observed that a substantial number of astrocytes must have transiently encountered virus during an early stage of the infection. Taken together, our data strongly indicate that abortive viral infection of astrocytes triggers pattern recognition receptor signaling, which results in the secretion of IFN-β.
MATERIALS AND METHODS
Mice.
All mice were handled in accordance with local animal welfare regulation. The majority of mice were bred in the animal facility of the Institute of Virology, University Medical Center Freiburg. Mavs-, Trif-, and Myd88-deficient mice were bred in the animal facilities of the Helmholtz Centre for Infection Research, Braunschweig, Germany, or TWINCORE, Centre for Experimental and Clinical Infection Research, Hanover, Germany. All mutant mice used were of the C57BL/6 genetic background or were backcrossed onto the C57BL/6 background for at least 10 generations. Reporter mice expressing firefly luciferase under the control of the IFN-β promoter in all cell types (Δβ-luc) (9) were used as heterozygotes; i.e., they all carried one functional allele of the IFN-β gene. Tissue-specific reporter mice were described in a report of previous studies by our group (8). To generate conditional-reporter mice, IFN-βflox-luc/flox-luc (10) mice were crossed to the following tissue-specific Cre deleter strains: syn1-cre (11), addressing neurons; thy1-cre (12), addressing astrocytes and neurons; and lysM-cre (10, 13), addressing microglia and macrophages. Tomatoflox mice harboring a floxed STOP cassette upstream of a red fluorescent protein (tdTomato) gene (14) were obtained from the Jackson Laboratory (stock no. 007914).
Viruses and infections.
Mice were anesthetized and infected intracranially by applying 10 μl of phosphate-buffered saline (PBS) containing TMEV GDVII (15), RABV SAD L16 (16), or RABV SAD-Cre using a Gilson precision pipette. RABV SAD-Cre was generated by introducing a gene cassette encoding Cre recombinase between the G and L genes of SAD L16. The cassette comprised a copy of the N/P gene transcription stop/restart signal followed by the coding sequence of the recombinase. The cassette was introduced into the HindIII restriction site located in the G gene 3′ untranscribed region (UTR) (position 5337 of SAD L16). The Cre recombinase (GenBank accession no. X03453) was engineered to include a 5′ terminal nuclear localization signal (NLS) (MVPKKKRKV) to promote genomic recombination (NLS-Cre cDNA was kindly provided by U. Koszinowski, Munich, Germany). Recombinant virus was recovered employing standard reverse genetics technology (17). The infection doses are specified in the respective figure legends. Intranasal infections of RABV SAD carrying the G protein of the more neurotropic CVS strain (SAD-GCVS) and of VSV were done by administrating 5-μl samples of diluted virus stock into each nostril under anesthesia. Mice were euthanized at various time points after infection with or without perfusion before brains were harvested.
Luciferase activity.
Whole brains or brain parts were homogenized in PBS using 0.25-in.-diameter ceramic spheres and a FastPrep-24 instrument (MP Biomedicals). Homogenates were mixed 1:5 with 5× passive lysis buffer (Promega) and incubated for at least 30 min on ice. To determine the enzymatic activity of firefly luciferase, 10-μl samples of tissue homogenate were added to 50 μl of luciferase substrate (luciferase assay system; Promega) and light emission was measured in a luminometer (Sirius; Berthold Technologies).
Virus titration.
To determine TMEV GDVII titers, plaque assays were performed using BHK-21 cells. Confluent cells in a 6-well plate were infected with serial dilutions of brain homogenates and incubated for 1 h. Next, the inoculum was replaced by Dulbecco's modified Eagle's medium (Gibco, Life Technologies) containing 3% Avicel cellulose (FMC BioPolymer) and 0.1% bovine serum albumin. Cells were incubated for 72 h at 37°C. Supernatant was removed, and the cells were fixed by applying 4% paraformaldehyde (PFA) for 15 min. Plaques in the cell monolayer were visualized by staining with crystal violet.
Immunofluorescence staining.
Mice were sacrificed by intraperitoneal injection of a lethal dose of Ketamin/Xylazin/Vetranquil. Whole-animal perfusion was done via the left ventricle with 0.9% NaCl supplemented with 10 U/ml of heparin followed by 4% PFA. Brains were removed and placed in 4% PFA for another 6 h at 4°C. For luciferase staining, 50-μm-thick horizontal free-floating sections of brain tissue were prepared using a Vibratom (Leica). Sections were treated with Peroxo-Block peroxidase inhibitor (Life Technologies) for 1 min followed by three PBS washing steps. Blocking and permeabilization were done with PBS containing 5% normal donkey serum and 0.1% Triton X-100 for 30 min. Antibody diluent comprised of PBS and 3% donkey normal serum was used for all dilution steps. Brain slices were stained overnight at 4°C with a rabbit anti-luciferase antibody (70C-CR2020RAP; Fitzgerald) and simultaneously with mouse anti-glial fibrillary acidic protein (GFAP) (G3893; Sigma-Aldrich) specific for astrocytes. To detect luciferase signals, an amplification step was performed using a Biotin-SP-conjugated donkey anti-rabbit antibody (Jackson ImmunoResearch Laboratories) followed by a tyramide signal amplification fluorescein system (PerkinElmer) according to the manufacturer's manual. For detection of GFAP, a donkey anti-mouse DyLight549 secondary antibody (Jackson ImmunoResearch Laboratories) was used.
After perfusion and postfixation, brains of Tomatoflox mice were saturated successively with 15% and 30% sucrose overnight, embedded in a cryomold with Tissue-Tek O.C.T. compound (Sakura Finetek), and frozen with the help of a liquid nitrogen-cooled aluminum block. Tissue was cut into 8-to-10-μm-thick sections using a Cryocut instrument (Leica) and dried overnight at 37°C. After rehydration with PBS, sections were treated with blocking reagent and permeabilized as described above. Slides were stained overnight with rabbit serum against RABV-N (gift of Stefan Finke, FLI, Germany), mouse anti-GFAP, or chicken anti-MAP2 (ab5392; abcam) followed by washing steps and incubation with the respective Alexa Fluor 488 (Life Technologies) and Alexa Fluor 647 (Jackson ImmunoResearch Laboratories) secondary antibodies. All slides were mounted with DAPI (4′,6-diamidino-2-phenylindole) staining ImmunoSelect (IS) mounting medium (Dianova). Images were acquired with a Zeiss AxioPlan 2 microscope (including ApoTome) using AxioVision 4 software.
Graphical and statistical analysis.
Graphs and statistics were generated using GrapPad Prism software version 5.02.
RESULTS
Astrocytes are the main IFN-β producers during intracranial infection of the brain with TMEV and RABV.
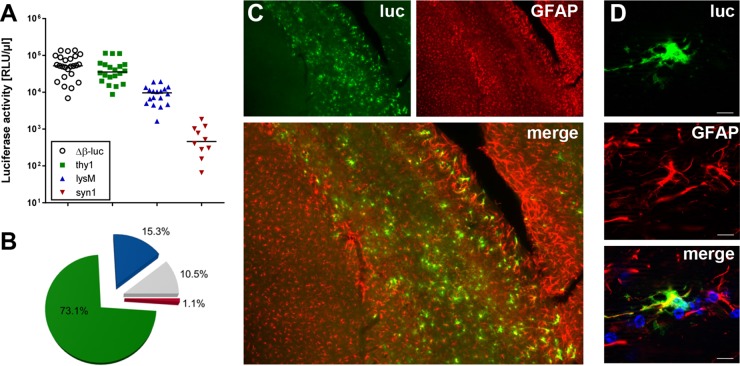
To assess the contributions of different cell types to IFN-β production after virus infection of the brain, we used previously described reporter mice that express firefly luciferase under the control of the IFN-β promoter either in all cell types (Δβ-luc) or in only neurons (syn1), astrocytes and neurons (thy1), or microglia and macrophages (lysM) (8, 9). We infected these reporter mice intracranially with neurotropic TMEV strain GDVII, and, 3.5 days later, we determined luciferase activity in the brain homogenates. TMEV-infected Δβ-luc mice showed high luciferase activity (Fig. 1A). Interestingly, thy1 reporter mice showed only marginally reduced luciferase activity, whereas neuron-specific syn1 mice had luciferase activities only slightly above the background level seen with uninfected mice (100 relative light units [RLU]/μl), suggesting that astrocytes are responsible for about 73% of IFN-β production in the TMEV-infected brain (Fig. 1B). The remaining luciferase activity was due to microglia/macrophages (15.3%), neurons (1.1%), and undefined other cell types (10.5%).
FIG 1.
Astrocytes are the main source of IFN-β during intracranial infection with TMEV. (A) Reporter mice in which the luciferase gene can be induced in all cell types (Δβ-luc), in astrocytes and neurons (thy1), in neurons only (syn1), or in microglia/macrophages (lysM) were infected with 104 PFU of TMEV GDVII, and luciferase activities in samples of brain homogenate were measured at day 3.5 postinfection. Background levels for uninfected mice were usually around 100 relative light units (RLU) per μl. (B) The average contributions to luciferase activity of different cell types are shown as pie charts. The mean activity of global Δβ-luc reporter mice was set to 100%. Green = astrocytes, blue = microglia/macrophages, red = neurons, gray = contribution of unidentified cells. (C) Luciferase-producing cells in the corpus callosum/hippocampus region were visualized by immunostaining. Most of the luciferase (luc)-producing cells were identified as astrocytes by costaining for GFAP. (D) A single luciferase-positive cell is shown at higher magnification to better visualize colocalization with GFAP. Bar = 10 μm.
To visualize luciferase production by astrocytes, we performed immunostaining experiments with a luciferase-specific antibody and an antibody against glial fibrillary acidic protein (GFAP), an astrocyte marker. The majority of luciferase-positive cells were observed in the corpus callosum and the hippocampus of infected brains (Fig. 1C). Colocalization of GFAP and luciferase was observed in most cells, supporting our conclusion described above that the majority of luciferase-positive cells are astrocytes (Fig. 1D). As reported earlier (8), immunostaining experiments cannot provide truly quantitative data for several technical reasons. First, GFAP does not mark all astrocytes of the brain. Second, our staining protocol has limited sensitivity and may fail to detect cells which express luciferase at low levels. Third, unlike GFAP, which is associated with distinct subcellular structures, luciferase accumulates diffusely in the cytoplasm, which renders colocalization studies notoriously difficult. Nevertheless, by measuring luciferase activity in brains of Cre recombinase-expressing mice and by immunostaining for luciferase, we obtained similar data, strongly indicating that astrocytes are the main producers of luciferase in TMEV-infected reporter mice.
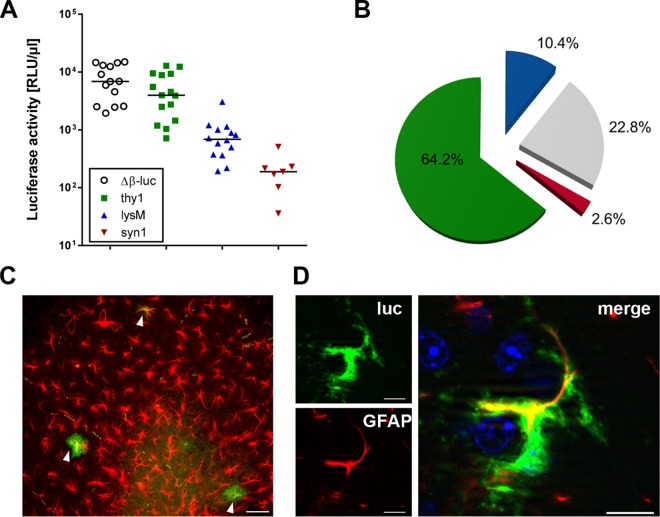
A similar picture was observed during infection of mice with RABV strain SAD L16 (Fig. 2A). About 65% of the luciferase signal in RABV-infected brains could be attributed to astrocytes, whereas the microglia/macrophage compartment and neurons played only minor roles, with contributions to the overall luciferase signal of 10.4% and 2.6%, respectively (Fig. 2B). Costaining experiments confirmed that luciferase is mainly produced by GFAP-positive astrocytes also in the case of RABV (Fig. 2C). However, the overall abundance of luciferase-positive cells was much lower than in TMEV-infected brains.
FIG 2.
Astrocytes produce IFN-β in response to intracranial infection with RABV. (A) Reporter mice in which the luciferase gene can be induced in all cell types (Δβ-luc), in astrocytes and neurons (thy1), in neurons only (syn1), or in microglia/macrophages (lysM) were infected with 105 PFU of RABV strain SAD L16, and levels of luciferase activity in samples of brain homogenate were measured at day 3.5 postinfection. (B) The average contributions of different cell types to luciferase activity in brains of mice infected with RABV strain SAD L16 are shown as pie charts as explained in the legend to Fig. 1. (C) Luciferase-producing cells in the hippocampus region were visualized by immunostaining and identified as astrocytes by costaining with GFAP (arrowheads). Bar = 50 μm. (D) A single luciferase-positive cell is shown at higher magnification to better visualize colocalization with GFAP. Bar = 10 μm.
Astrocytes are the major source of IFN-β in the olfactory bulb after intranasal infection with RABV or VSV.
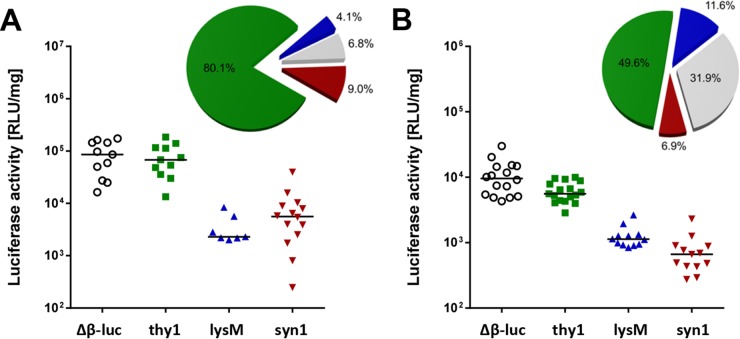
To evaluate the influence of the infection route on IFN-β production in the central nervous system (CNS), we performed intranasal infections with a recombinant RABV carrying the G protein of the more neurotropic CVS strain (SAD-GCVS) and vesicular stomatitis virus (VSV) strain Indiana, both of which can infect mice via the intranasal route (4, 18). Rabies virus induced the luciferase reporter gene in all brain parts of Δβ-luc reporter mice, whereas reporter gene expression in VSV-infected mice was restricted to the olfactory bulb (data not shown). After rabies virus infection of cell type-specific reporter mice, we observed that about 80% of the luciferase activity in the olfactory bulb originated from astrocytes and less than 10% from either neurons or microglia/macrophages (Fig. 3A). Similarly, in the olfactory bulb of VSV-infected mice, astrocytes were the major IFN-β producers (Fig. 3B). Taken together, these data clearly demonstrated that astrocytes are the predominant source of IFN-β in the virus-infected brain, irrespective of the nature of the infecting virus and the route of infection.
FIG 3.
Astrocytes are the main IFN-β producers in the olfactory bulb after intranasal infection with (A) RABV or (B) VSV. Global-reporter mice (Δβ-luc), as well as reporter mice specific for astrocytes and neurons (thy1), for neurons only (syn1), or for microglia/macrophages (lysM), were infected by the intranasal route with (A) 106 PFU of RABV strain SAD-GCVS or (B) 103 PFU of VSV strain Indiana, and the luciferase activity of homogenates from the olfactory bulb was determined at day 7 (RABV) or day 4 (VSV) postinfection. The average contributions of different cell types are shown as pie charts (see legend to Fig. 1).
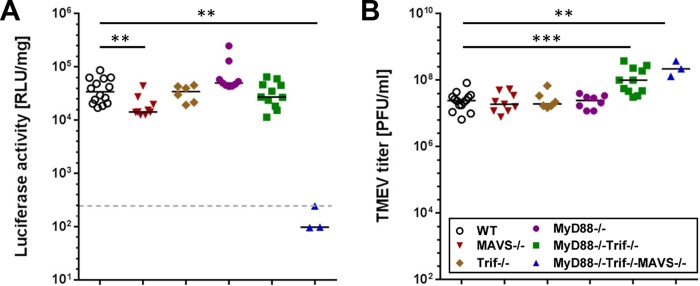
TLR and RLR signaling contribute to TMEV-induced IFN-β production in the brain.
To investigate which pathogen-sensing pathways contribute to IFN-β production in the brain, infection experiments with TMEV GDVII were performed in IFN-β reporter mice lacking downstream components of either TLRs or RLRs or both (Fig. 4). Δβ-luc/MAVS−/− mice, deficient in signaling via RLRs, showed slightly but significantly reduced luciferase signals in brain homogenates compared to wild-type mice (Fig. 4A). In TLR signaling-deficient Δβ-luc/MyD88−/−/Trif−/− mice (Fig. 4A) as well as in the respective single-knockout mice (Δβ-luc/MyD88−/− and Δβ-luc/Trif−/− mice), no significant decreases in luciferase activity could be detected. However, viral titers (Fig. 4B) in brains of Δβ-luc/MyD88−/−/Trif−/− mice were slightly higher than in wild-type controls, suggesting a moderate impairment of the antiviral response in these mice. Reporter activity was at the background level only in brain homogenates of Δβ-luc/MyD88−/−/Trif−/−/MAVS−/− triply deficient mice, which lack functional RLR and TLR systems (Fig. 4A). These data clearly indicated that both RLR-mediated signaling and TLR-mediated signaling contribute to IFN-β production in the TMEV-infected brain.
FIG 4.
Contribution of TLR and RLR signaling to TMEV-induced IFN-β production in the brain. Global-reporter mice lacking the indicated components of the TLR or RLR signaling pathways were infected by the intracerebral route with 104 PFU of TMEV strain GDVII. At day 3 postinfection, brains were analyzed for (A) luciferase activity and (B) virus titers. The dotted line shows average basal luciferase activity in brains of noninfected mice. Statistical analyses were performed using the Mann-Whitney U test (**, P < 0.01; **, P < 0.001). WT, wild type.
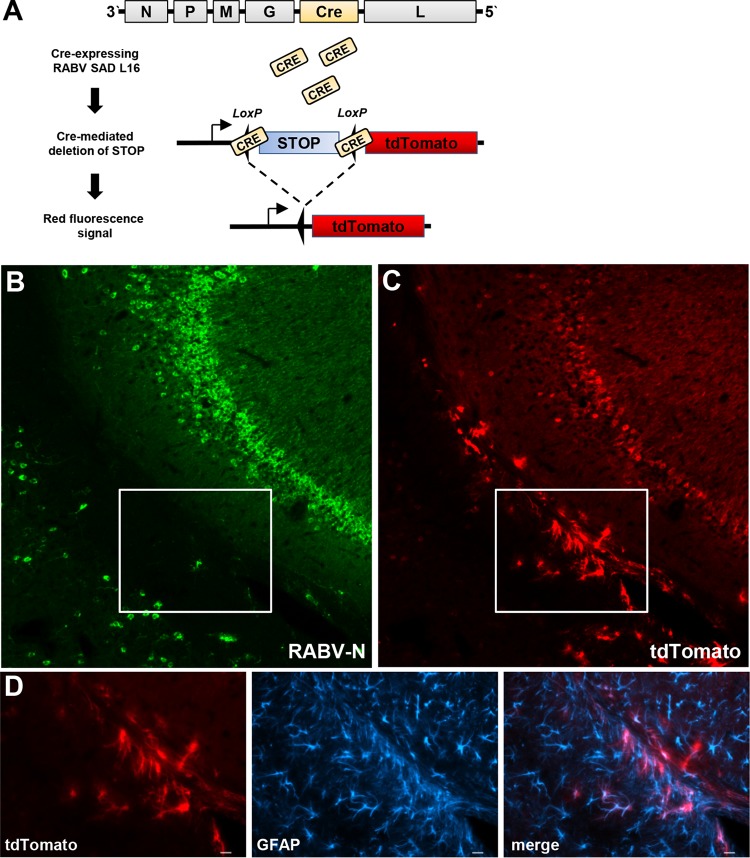
Astrocytes transiently encounter virus during brain infection with RABV.
TMEV, VSV, and RABV were previously reported to predominantly infect neurons in mice (19–21). Nevertheless, our results presented above clearly indicated that astrocytes rather than neurons are the main source of IFN-β in the brain after infection with these viruses. We therefore hypothesized that abortive infection of astrocytes might play a crucial role during virus recognition by the innate immune system. To permanently mark infected cells in the brain even in cases in which infection was transient only, we used a Cre recombinase-expressing rabies virus (RABV-Cre) to infect tdTomatoflox reporter mice. In these mice, a floxed STOP cassette prevents transcription of a downstream red fluorescent protein variant (tdTomato) (14). After intracerebral infection with this recombinant RABV, Cre recombinase was expressed in infected brain cells. As a consequence, the STOP cassette was deleted, which resulted in permanent expression of the tdTomato marker gene (Fig. 5A). Six days postinfection, a large number of tdTomato-positive brain cells were observed (Fig. 5B), most of which were also positive for RABV antigen (Fig. 5C). As expected, the vast majority of these doubly positive cells expressed the neuron marker MAP2 (data not shown). Importantly, a substantial number of cells in RABV-infected brains were tdTomato positive and RABV antigen negative (see boxes in Fig. 5B and C), as would be expected if some nonproductive virus infections had occurred. The antibody used to detect RABV-N exhibited both high specificity and high sensitivity in control experiments (data not shown), excluding the possibility that the absence of a virus signal in these cells was simply due to technical limitations. Costaining for the astrocyte marker GFAP (Fig. 5D) revealed that the abortively infected cells were astrocytes. Interestingly, 2 days earlier (at 4 days postinfection with RABV-Cre), no tdTomato-positive cells had been observed, although RABV antigen was abundantly present at that time point (data not shown), indicating that Cre-mediated recombination is slower than the expression of viral N protein in our infection model. We noted that tdTomato-positive astrocytes were predominantly found in the white matter and most frequently in or close to the corpus callosum and the hippocampus (Fig. 5B), suggesting that these particular astrocytes are more susceptible to virus infection than astrocytes located in other brain regions.
FIG 5.
Abortive RABV infection of astrocytes. (A) Cartoon showing Cre recombinase-expressing RABV and predicted Cre-mediated deletion of a STOP cassette in Tomatoflox mice. (B and C) tdTomatoflox mice were infected with 105 PFU of Cre recombinase-encoding RABV. At day 6 postinfection, brain slices were analyzed for expression of tdTomato (B) and RABV-N antigen (C). A section of the hippocampus is shown. (D) Double staining showed that dtTomato-positive cells in the boxed areas of panels B and C represent GFAP-positive astrocytes. Bar = 20 μm.
Attempts to directly verify the hypothesis that the tdTomato-positive astrocytes produce IFN-β were not successful (data not shown). This was most likely due to differences in the kinetics of virus-induced synthesis of IFN, which is a fast but usually transient process, and of Cre-mediated recombination and subsequent expression of the tdTomato reporter gene, which is a slow but permanent process. Therefore, it is likely that virus-infected astrocytes had stopped producing IFN-β by the time the tdTomato reporter gene was expressed at detectable levels. Nevertheless, these results clearly demonstrated that a substantial number of abortively infected astrocytes are present in rabies virus-infected mouse brains. Based on the fact that the majority of abortively infected astrocytes were located in a region of the hippocampus in which luciferase-positive cells were usually detected (compare 5B with Fig. 1C and 2C), it further seems likely that abortively infected cells represent the main source of IFN-β in the virus-infected mouse brains.
DISCUSSION
Previous work in our laboratories indicated that astrocytes are the main producers of IFN after infection of the CNS with La Crosse virus (8) or VSV (22), but how astrocytes might sense those viruses which preferentially replicate in neurons remained unclear. In the current study, we extended this observation by demonstrating that TMEV and RABV, two other virus species that also selectively replicate in neurons, showed the same behavior and preferentially triggered IFN-β synthesis in astrocytes of infected mouse brains. Thus, RNA viruses of different taxonomic families and with largely different replication strategies that all preferentially replicate in neurons are sensed by astrocytes, indicating that these cells play a previously unappreciated role in innate antiviral defenses of the CNS.
The brain possesses functional RLR and TLR systems (3, 23), and constitutive expression as well as upregulation of both systems in response to virus infection was previously described for astrocytes (24, 25). Using mice with defects in the known innate signaling pathways, we analyzed which sensors might determine IFN-β synthesis in the virus-infected brain. Although TMEV GDVII was shown to induce IFN via signaling through MDA5 in earlier studies (26), we noted that both the TLR and the RLR pathways played important roles. Mice possessing only one of these sensor systems responded surprisingly well. In contrast, only mice lacking both signaling pathways were unable to produce IFN-β in response to challenge with TMEV. A similar observation was made in a recent study with VSV in which both TLRs and RLRs were found to be necessary to mount a protective IFN response (27).
From the results of our earlier work with La Crosse virus, we had postulated that abortive infection of astrocytes might trigger IFN-β synthesis in the brain. This speculation was based on the finding that the magnitude of the IFN response in astrocytes, but not microglia, was influenced by the viral antagonistic factor NSs (8). Since NSs of La Crosse virus inhibits the host IFN response from within the infected cells by reducing the activity of cellular RNA polymerase II (28), bona fide infection of astrocytes rather than a simple contact of astrocytes with viral material seemed to have occurred. However, since replication of La Crosse virus in neurons is fulminant, it was technically impossible to distinguish low-grade viral infection of astrocytes from nonspecific staining of these cells due to uptake of viral antigens from infected neurons.
The recent generation of transgenic mice carrying inactive reporter genes that may be rendered transcriptionally active by Cre-mediated recombination offers new experimental strategies to permanently introduce genetic marks into cell populations which are susceptible to infection by a Cre recombinase-encoding virus. Here we used Tomatoflox reporter mice and a rabies virus expressing Cre recombinase to permanently mark infected cells. A substantial number of astrocytes of infected mouse brains could be labeled under such experimental conditions. Importantly, the vast majority of labeled astrocytes were negative for viral antigen by classical immunostaining, strongly suggesting that rabies virus infection of these cells was abortive. Thus, residual activity of the viral polymerase during abortive infection appears to produce sufficiently high levels of Cre recombinase for successful excision of the STOP cassette.
We assume that transiently infected astrocytes are largely responsible for the synthesis of IFN-β in the infected brain. Indirect evidence that the abortively infected astrocytes identified by the tdTomato marker are responsible for IFN-β synthesis at early times postinfection comes from their localization in the brain. Tomato-positive astrocytes were mainly found in distinct patches in the white matter of the brain, a location where the majority of luciferase-positive cells are usually detected (see, for example, Fig. 1C). However, we were unable to directly prove this assumption by visualizing enhanced luciferase levels in tdTomato-positive astrocytes. This was not unexpected given the fact that virus-induced expression of the IFN-β gene is a transient process (at least in abortively infected cells) that peaks at about day 3 postinfection, whereas Cre recombinase-mediated excision of inhibitory DNA and subsequent expression of the Tomato cassette take more than 4 days to become detectable in our system. On the one hand, this unfavorable constellation is due to the relatively short in vivo half-life of luciferase, which is only about 2 to 3 h (29, 30). On the other hand, this also appears to be due to the intrinsically slow Cre-mediated recombination in rabies virus-infected brain cells. Slow recombination of a green fluorescent protein (GFP) reporter gene was also observed in neurons infected with rabies virus expressing Cre recombinase. In this case, GFP became visible only at day 15 postinfection, although high levels of viral RNA were already observed at day 7 (31). It is conceivable that various cellular processes, including nuclear import of host cell proteins, get altered in rabies virus-infected cells, which might slow down Cre-mediated recombination. Indeed, strongly dysregulated gene expression was observed in neurons derived from rabies virus-infected mouse brains (31).
Considering all our data, we propose that at least two different PRR pathways must contribute to triggering IFN synthesis in astrocytes. First, IFN production in astrocytes seems to be due to cytoplasmic detection of viral RNA via RLRs. These pathways were previously shown to contribute to recognition of rabies virus and TMEV strain GDVII (25, 26). Second, IFN production via MyD88/Trif-dependent TLR signaling seems to play a role. In particular, TLR3-mediated recognition was observed in the case of the neurovirulent TMEV strains DA and BeNa (32, 33). Further, TLR2 and TLR4 may get activated after infection with some viruses, including VSV (34–36). Viral PAMPs triggering these latter pathways are most likely not nucleic acids but rather viral proteins, such as the VSV glycoprotein (36). Third, IFN production by astrocytes might result from recognition of damage-associated molecular patterns or viral nucleic acids released from dying cells that accumulate in the extracellular space. Extracellular RNA of cellular or viral origin can be taken up by endocytosis and may result in activation of TLRs, especially TLR3 but also RLRs (37).
In summary, our data clearly show that astrocytes represent the major source of IFN-β in the virus-infected brain. Abortive infection of these cells most likely triggers PRR signaling, leading to the secretion of IFN-β. Due to their specific location in the infected brain, we provide hints that the tdTomato-positive astrocytes might exhibit very high susceptibility to virus infection and, at the same time, restrict viral replication particularly well. Such special features appear to favor strong innate immune responses which promote antiviral defense. We currently do not know whether the subset of astrocytes identified here, which seem to play a key role in pathogen defense, might possess some unique markers which could be used to address them more easily in future studies.
ACKNOWLEDGMENTS
We thank Stefan Finke (FLI, Riems, Germany) for providing antibodies for RABV-N and Kerstin Schlegel for technical assistance.
TWINCORE is a joint venture between the Helmholtz Centre for Infection Research, Braunschweig, Germany, and the Hanover Medical School, Hanover, Germany.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Schoggins JW, Wilson SJ, Panis M, Murphy MY, Jones CT, Bieniasz P, Rice CM. 2011. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature 472:481–485. doi: 10.1038/nature09907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koyuncu OO, Hogue IB, Enquist LW. 2013. Virus infections in the nervous system. Cell Host Microbe 13:379–393. doi: 10.1016/j.chom.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carty M, Reinert L, Paludan SR, Bowie AG. 2014. Innate antiviral signalling in the central nervous system. Trends Immunol 35:79–87. doi: 10.1016/j.it.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 4.Detje CN, Meyer T, Schmidt H, Kreuz D, Rose JK, Bechmann I, Prinz M, Kalinke U. 2009. Local type I IFN receptor signaling protects against virus spread within the central nervous system. J Immunol 182:2297–2304. doi: 10.4049/jimmunol.0800596. [DOI] [PubMed] [Google Scholar]
- 5.Steinman RM. 1991. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol 9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 6.Sorgeloos F, Kreit M, Hermant P, Lardinois C, Michiels T. 2013. Antiviral type I and type III interferon responses in the central nervous system. Viruses 5:834–857. doi: 10.3390/v5030834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Owens T, Khorooshi R, Wlodarczyk A, Asgari N. 2014. Interferons in the central nervous system: a few instruments play many tunes. Glia 62:339–355. doi: 10.1002/glia.22608. [DOI] [PubMed] [Google Scholar]
- 8.Kallfass C, Ackerman A, Lienenklaus S, Weiss S, Heimrich B, Staeheli P. 2012. Visualizing production of beta interferon by astrocytes and microglia in brain of La Crosse virus-infected mice. J Virol 86:11223–11230. doi: 10.1128/JVI.01093-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lienenklaus S, Cornitescu M, Zietara N, Lyszkiewicz M, Gekara N, Jablonska J, Edenhofer F, Rajewsky K, Bruder D, Hafner M, Staeheli P, Weiss S. 2009. Novel reporter mouse reveals constitutive and inflammatory expression of IFN-beta in vivo. J Immunol 183:3229–3236. doi: 10.4049/jimmunol.0804277. [DOI] [PubMed] [Google Scholar]
- 10.Solodova E, Jablonska J, Weiss S, Lienenklaus S. 2011. Production of IFN-beta during Listeria monocytogenes infection is restricted to monocyte/macrophage lineage. PLoS One 6:e18543. doi: 10.1371/journal.pone.0018543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu Y, Romero MI, Ghosh P, Ye Z, Charnay P, Rushing EJ, Marth JD, Parada LF. 2001. Ablation of NF1 function in neurons induces abnormal development of cerebral cortex and reactive gliosis in the brain. Genes Dev 15:859–876. doi: 10.1101/gad.862101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dewachter I, Reverse D, Caluwaerts N, Ris L, Kuiperi C, Van den Haute C, Spittaels K, Umans L, Serneels L, Thiry E, Moechars D, Mercken M, Godaux E, Van Leuven F. 2002. Neuronal deficiency of presenilin 1 inhibits amyloid plaque formation and corrects hippocampal long-term potentiation but not a cognitive defect of amyloid precursor protein [V717I] transgenic mice. J Neurosci 22:3445–3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. 1999. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res 8:265–277. doi: 10.1023/A:1008942828960. [DOI] [PubMed] [Google Scholar]
- 14.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. 2010. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tangy F, McAllister A, Brahic M. 1989. Molecular cloning of the complete genome of strain GDVII of Theiler's virus and production of infectious transcripts. J Virol 63:1101–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schnell MJ, Mebatsion T, Conzelmann KK. 1994. Infectious rabies viruses from cloned cDNA. EMBO J 13:4195–4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghanem A, Kern A, Conzelmann KK. 2012. Significantly improved rescue of rabies virus from cDNA plasmids. Eur J Cell Biol 91:10–16. doi: 10.1016/j.ejcb.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Finke S, Conzelmann KK. 2005. Replication strategies of rabies virus. Virus Res 111:120–131. doi: 10.1016/j.virusres.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Brahic M, Bureau JF, Michiels T. 2005. The genetics of the persistent infection and demyelinating disease caused by Theiler's virus. Annu Rev Microbiol 59:279–298. doi: 10.1146/annurev.micro.59.030804.121242. [DOI] [PubMed] [Google Scholar]
- 20.Trottier MD Jr, Palian BM, Reiss CS. 2005. VSV replication in neurons is inhibited by type I IFN at multiple stages of infection. Virology 333:215–225. doi: 10.1016/j.virol.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 21.Baloul L, Lafon M. 2003. Apoptosis and rabies virus neuroinvasion. Biochimie 85:777–788. doi: 10.1016/S0300-9084(03)00137-8. [DOI] [PubMed] [Google Scholar]
- 22.Detje CN, Lienenklaus S, Chhatbar C, Spanier J, Prajeeth CK, Soldner C, Tovey MG, Schluter D, Weiss S, Stangel M, Kalinke U. 24 December 2014. Upon intranasal vesicular stomatitis virus infection, astrocytes in the olfactory bulb are important interferon Beta producers that protect from lethal encephalitis. J Virol doi: 10.1128/JVI.02044-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paul S, Ricour C, Sommereyns C, Sorgeloos F, Michiels T. 2007. Type I interferon response in the central nervous system. Biochimie 89:770–778. doi: 10.1016/j.biochi.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Furr SR, Chauhan VS, Sterka D Jr, Grdzelishvili V, Marriott I. 2008. Characterization of retinoic acid-inducible gene-I expression in primary murine glia following exposure to vesicular stomatitis virus. J Neurovirol 14:503–513. doi: 10.1080/13550280802337217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faul EJ, Wanjalla CN, Suthar MS, Gale M, Wirblich C, Schnell MJ. 2010. Rabies virus infection induces type I interferon production in an IPS-1 dependent manner while dendritic cell activation relies on IFNAR signaling. PLoS Pathog 6:e1001016. doi: 10.1371/journal.ppat.1001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin YH, Kim SJ, So EY, Meng L, Colonna M, Kim BS. 2012. Melanoma differentiation-associated gene 5 is critical for protection against Theiler's virus-induced demyelinating disease. J Virol 86:1531–1543. doi: 10.1128/JVI.06457-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spanier J, Lienenklaus S, Paijo J, Kessler A, Borst K, Heindorf S, Baker DP, Kroger A, Weiss S, Detje CN, Staeheli P, Kalinke U. 2014. Concomitant TLR/RLH signaling of radioresistant and radiosensitive cells is essential for protection against vesicular stomatitis virus infection. J Immunol 193:3045–3054. doi: 10.4049/jimmunol.1400959. [DOI] [PubMed] [Google Scholar]
- 28.Verbruggen P, Ruf M, Blakqori G, Overby AK, Heidemann M, Eick D, Weber F. 2011. Interferon antagonist NSs of La Crosse virus triggers a DNA damage response-like degradation of transcribing RNA polymerase II. J Biol Chem 286:3681–3692. doi: 10.1074/jbc.M110.154799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ignowski JM, Schaffer DV. 2004. Kinetic analysis and modeling of firefly luciferase as a quantitative reporter gene in live mammalian cells. Biotechnol Bioeng 86:827–834. doi: 10.1002/bit.20059. [DOI] [PubMed] [Google Scholar]
- 30.Thompson JF, Hayes LS, Lloyd DB. 1991. Modulation of firefly luciferase stability and impact on studies of gene regulation. Gene 103:171–177. doi: 10.1016/0378-1119(91)90270-L. [DOI] [PubMed] [Google Scholar]
- 31.Gomme EA, Wirblich C, Addya S, Rall GF, Schnell MJ. 2012. Immune clearance of attenuated rabies virus results in neuronal survival with altered gene expression. PLoS Pathog 8:e1002971. doi: 10.1371/journal.ppat.1002971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Salleeh F, Petro TM. 2007. TLR3 and TLR7 are involved in expression of IL-23 subunits while TLR3 but not TLR7 is involved in expression of IFN-beta by Theiler's virus-infected RAW264.7 cells. Microbes Infect 9:1384–1392. doi: 10.1016/j.micinf.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 33.So EY, Kang MH, Kim BS. 2006. Induction of chemokine and cytokine genes in astrocytes following infection with Theiler's murine encephalomyelitis virus is mediated by the Toll-like receptor 3. Glia 53:858–867. doi: 10.1002/glia.20346. [DOI] [PubMed] [Google Scholar]
- 34.Barbalat R, Lau L, Locksley RM, Barton GM. 2009. Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands. Nat Immunol 10:1200–1207. doi: 10.1038/ni.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michaud F, Coulombe F, Gaudreault E, Kriz J, Gosselin J. 2010. Involvement of TLR2 in recognition of acute gammaherpesvirus-68 infection. PLoS One 5:e13742. doi: 10.1371/journal.pone.0013742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Georgel P, Jiang Z, Kunz S, Janssen E, Mols J, Hoebe K, Bahram S, Oldstone MB, Beutler B. 2007. Vesicular stomatitis virus glycoprotein G activates a specific antiviral Toll-like receptor 4-dependent pathway. Virology 362:304–313. doi: 10.1016/j.virol.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 37.Nellimarla S, Mossman KL. 2014. Extracellular dsRNA: its function and mechanism of cellular uptake. J Interferon Cytokine Res 34:419–426. doi: 10.1089/jir.2014.0002. [DOI] [PubMed] [Google Scholar]