ABSTRACT
Lifelong antiretroviral therapy (ART) for HIV-1 does not diminish the established latent reservoir. A possible cure approach is to reactivate the quiescent genome from latency and utilize immune responses to eliminate cells harboring reactivated HIV-1. It is not known whether antibodies within HIV-1-infected individuals can recognize and eliminate cells reactivated from latency through antibody-dependent cellular cytotoxicity (ADCC). We found that reactivation of HIV-1 expression in the latently infected ACH-2 cell line elicited antibody-mediated NK cell activation but did not result in antibody-mediated killing. The lack of CD4 expression on these HIV-1 envelope (Env)-expressing cells likely resulted in poor recognition of CD4-induced antibody epitopes on Env. To examine this further, cultured primary CD4+ T cells from HIV-1+ subjects were used as targets for ADCC. These ex vivo-expanded primary cells were modestly susceptible to ADCC mediated by autologous or heterologous HIV-1+ serum antibodies. Importantly, ADCC mediated against these primary cells could be enhanced following incubation with a CD4-mimetic compound (JP-III-48) that exposes CD4-induced antibody epitopes on Env. Our studies suggest that with sufficient reactivation and expression of appropriate Env epitopes, primary HIV-1-infected cells can be targets for ADCC mediated by autologous serum antibodies and innate effector cells. The results of this study suggest that further investigation into the potential of ADCC to eliminate reactivated latently infected cells is warranted.
IMPORTANCE An HIV-1 cure remains elusive due to the persistence of long-lived latently infected cells. An HIV-1 cure strategy, termed “shock and kill,” aims to reactivate HIV-1 expression in latently infected cells and subsequently eliminate the reactivated cells through immune-mediated killing. While recent research efforts have focused on reversing HIV-1 latency, it remains unclear whether preexisting immune responses within HIV-1+ individuals can efficiently eliminate the reactivated cells. HIV-1-specific antibodies can potentially eliminate cells reactivated from latency via Fc effector functions by recruiting innate immune cells. Our study highlights the potential role that antibody-dependent cellular cytotoxicity might play in antilatency cure approaches.
INTRODUCTION
A major barrier to curing HIV-1 infection is the persistence of quiescent integrated replication-competent viral genomes in long-lived latently infected cells (1). The “shock and kill” cure approach proposes reactivation of latent HIV-1 to render cells harboring latent proviruses susceptible to immune-mediated clearance (2). Several latency reversal agents (LRAs) have been examined both in vitro and in vivo for their ability to “shock” latent HIV-1 and induce viral protein expression (3–7). Although some LRAs have shown potent HIV-1 reactivation in vitro and ex vivo, in vivo studies have been less promising. While panobinostat and romidepsin have induced low-level plasma viremia in human trials (5, 8), these LRAs failed to reduce total integrated HIV-1 DNA or, in the case of panobinostat, failed to prevent recrudescence of viremia after analytical antiretroviral therapy (ART) interruption. These observations imply that latency reversal in the context of preexisting immune responses, at least with these LRAs, is insufficient to clear cells harboring latent proviruses. Supportive of this notion are ex vivo data showing that unadulterated autologous cytotoxic T lymphocytes (CTLs) from ART-treated patients do not kill cells reactivated with vorinostat (9). If the infected cells are not efficiently killed following reactivation, these cells may revert to a latent state and reconstitute the latent reservoir. As such, more-potent immune responses may need to be utilized to ensure efficient clearance of reactivated latently infected cells.
Cytolysis of reactivated cells harboring HIV-1 provirus could theoretically be achieved via antibody-dependent cellular cytotoxicity (ADCC) (10). Anti-HIV-1 antibodies trigger ADCC upon binding cell surface viral proteins and the IgG constant region receptor, FcγRIIIa or CD16, of effector cells such as natural killer (NK) cells and monocytes (11–13). Evidence of the antiviral efficacy of anti-HIV-1 ADCC is provided through the association of this immune response with slower disease progression (14–16) as well as vaccine efficacy (17–19). Recent studies, however, demonstrate that HIV-1 evades ADCC by concealing important ADCC epitopes on the envelope (Env) glycoprotein trimer and by reducing the amount of Env on the surface of infected cells (20, 21). Downregulation of CD4 by HIV-1 Vpu and Nef reduces the likelihood of Env entering a CD4-bound conformation, resulting in the concealment of many CD4-induced (CD4i) antibody epitopes (22, 23). This could be a barrier for ADCC antibody recognition since a high proportion of ADCC antibodies in HIV-1-infected sera recognize CD4i epitopes (23). Additionally, inhibition of tetherin by Vpu prevents accumulation of nascent HIV-1 virions at the surface of the infected cell, thereby reducing the amount of surface Env available for antibody binding (22, 24, 25). These evasion mechanisms might prevent ADCC from killing reactivated cells following administration of LRAs. To overcome CD4 downregulation on the surface of infected cells, CD4-mimetic compounds (CD4mc) have been rationally designed to bind to Env and induce the CD4-bound conformation (26, 27). Importantly, these CD4mc are able to improve binding of ADCC-mediating antibodies to Env and sensitize HIV-1-infected cells to ADCC (28).
In this study, we examined if antibodies from HIV-1-infected subjects could activate primary NK cells or eliminate a reactivated latently infected cell line. We also studied the effect of ADCC on ex vivo-expanded primary cells from HIV-1+ subjects following in vitro reactivation and culture. Although NK effector cells exhibited some antibody-dependent activation when cultured with reactivated cell lines, we found that the cell lines were not susceptible to antibody-mediated killing. In contrast, ex vivo-expanded primary CD4+ T cells were susceptible to ADCC, and this could be improved by increasing exposure of CD4i epitopes on Env using a CD4mc.
MATERIALS AND METHODS
Participants.
Whole blood was obtained from 14 HIV-1-uninfected donors. As a source of effector cells for antibody-dependent NK cell activation and ADCC assays, peripheral blood mononuclear cells (PBMCs) were isolated via Ficoll-Paque density gradient centrifugation (GE Healthcare). NK cells were enriched from PBMCs using the EasySep Human NK Cell Enrichment kit (Stemcell Technologies). Plasma from an HIV-1-infected subject known to have anti-HIV-1 antibodies capable of activating NK cells was obtained from the Melbourne Sexual Health Centre (29). For analysis of ADCC against primary HIV-1-infected target cells, HIV-1+ blood and serum samples from a total of 7 viremic subjects were obtained from the Montreal Primary HIV Infection Cohort and the Canadian Cohort of HIV-infected Slow Progressors (Table 1). Informed consent was obtained prior to collection of blood samples, and the described studies were approved by the relevant human ethics committees.
TABLE 1.
Demographic, virological, and immunological characterization of HIV-1-infected individuals used to expand endogenously infected CD4+ T cells
| Donor ID | Time after infection (mo) | Age (yr) | Gender | Race | CD4 count (cells/mm3) | Viral load (RNA copies/ml) |
|---|---|---|---|---|---|---|
| 1 | 23 | 43 | Male | Caucasian | 356 | 193,437 |
| 2 | 4 | 38 | Male | Caucasian | 320 | 132,886 |
| 3 | 31 | 22 | Male | African | 329 | 19,067 |
| 4 | 88 | 49 | Male | Caucasian | 281 | 44,848 |
| 5a | 132 | 42 | Male | Caucasian | 453 | 68 |
| 6 | 18 | 25 | Male | Hispanic | 371 | 9,871 |
| 7 | 131 | 38 | Male | Hispanic | 138 | 14,614 |
Donor currently on ART.
Antibodies and cell lines.
Polyclonal HIVIG, the anti-gp120 monoclonal antibody 2G12, the CEM.NKr-CCR5 cell line and the latently infected ACH-2 T cell line were all obtained from the NIH AIDS Reagent Program (Division of AIDS, NIAID, NIH).
Reactivation of latent HIV-1.
The latently infected ACH-2 cells were reactivated with 10 μg/ml phytohemagglutinin (PHA; Thermo Fisher) and 10 nM phorbol 12-myristate 13-acetate (PMA; Sigma) for 24 h prior to incubation with effector cells (30, 31). Levels of HIV-1 reactivation were measured via intracellular staining for p24 (clone KC57-RD1; Beckman Coulter) and surface staining for Env using 2G12 (5 μg/ml) with an Alexa Fluor 488-conjugated secondary anti-human IgG1 antibody (Invitrogen).
To obtain primary infected cells, CD4+ T cells were purified from HIV-1+ individuals (Table 1) and reactivated with PHA (10 μg/ml) for 36 h, followed by incubation with recombinant interleukin-2 (IL-2; 100 U/ml; NIH AIDS Reagent Program) for 4 to 8 days. HIV-1 reactivation was measured via intracellular staining for p24. The binding of HIV-1+ sera to reactivated cells was assessed with an AF647-conjugated secondary anti-human IgG1 antibody (Invitrogen).
Antibody-dependent NK cell activation assay.
The antibody-dependent NK cell activation assay was performed as previously described, measuring intracellular NK cell gamma interferon (IFN-γ) and CD107a expression (32). Briefly, freshly isolated effector cells (healthy donor PBMCs or enriched NK cells) were incubated with target ACH-2 cells in the presence of HIV-1-infected plasma (1:1,000 dilution), HIV-1-uninfected plasma (1:1,000 dilution), or HIV Ig (HIVIG; 50 μg/ml). The effector and target cells were incubated together for 5 h at 37°C with anti-CD107a antibody (APC conjugate, clone H4A3; BD), brefeldin A (5 μg/ml; Sigma), and monensin (6 μg/ml; BD). For certain experimental conditions, the effector and target cells were separated by a semipermeable transwell membrane with 0.4-μm pores to prevent effector-to-target cell contact (Sigma). After the 5-h incubation, cells were stained for surface expression of CD3 (PerCP conjugate, clone SK7; BD) and CD56 (PE-Cy7 conjugate, clone NCAM16; BD). Cells were fixed with 1% formaldehyde and permeabilized with fluorescence-activated cell sorter (FACS) permeabilization buffer (BD) prior to intracellular staining for IFN-γ (AF700 conjugate, clone B27; BD). The samples were fixed with 1% formaldehyde prior to being acquired on the BD LSRFortessa flow cytometer (BD). Flow cytometry data were analyzed using FlowJo version 10 (TreeStar).
LDH release cytotoxicity assay.
The CytoTox96 nonradioactive cytotoxicity assay kit (Promega) was used to measure HIV-1-specific ADCC through release of cytosolic lactate dehydrogenase (LDH) from target cells, as previously described (32). Effector PBMCs were incubated with the target ACH-2 cells at 25:1 and 10:1 effector-to-target ratios in the presence or absence of HIVIG (25 μg/ml) for 4 h. Controls conducted for the calculation of percent cytotoxicity (%cytotoxicity) were performed according to the manufacturer's instructions. Absorbance values measured at 492 nm were used to calculate %cytotoxicity with the following formula: (experimental − effector minimum − target minimum)/(target maximum − target minimum) × 100.
Flow cytometry-based infected cell elimination assay.
ADCC against reactivated ACH-2 cells and primary HIV-1-infected cells mediated by HIV-1+ sera was measured using a flow cytometry-based infected cell elimination assay as previously described (28, 33). Reactivated primary HIV-1-infected cells that were cultured for 4 to 8 days were incubated with autologous PBMCs in the presence or absence of autologous or heterologous HIV-1+ sera (1:1,000 dilution) for 5 h. As shown previously, the optimal HIV-1+ serum dilution to use for ADCC is a dilution of 1:1,000 (33). Furthermore, the 1:1,000 dilution was chosen, as previously published ADCC studies have described a prozone phenomenon where ADCC is suboptimal at higher antibody concentrations (low serum dilutions of 1:10 to 1:100) (34, 35). The CD4mc JP-III-48 (50 μM) was also added in some experimental conditions to assess its effect on HIV-1+ serum binding and ADCC. The target cells were stained for intracellular p24 expression, and the percent killing (%killing) was calculated with the following formula: [(%p24+ cells in targets) − (%p24+ cells in targets + effectors ± HIV-1+ serum)]/(%p24+ cells in targets) × 100.
Statistics.
Statistical analyses were performed with GraphPad Prism version 6. Comparisons between matched pairs were analyzed using the nonparametric Wilcoxon signed-rank test. Statistical tests were considered significant when P values were less than 0.05. Statistics given in Results are presented in the following format: (median [interquartile range] versus median [interquartile range], P value of statistical test).
RESULTS
Reactivation of latently infected ACH-2 cells.
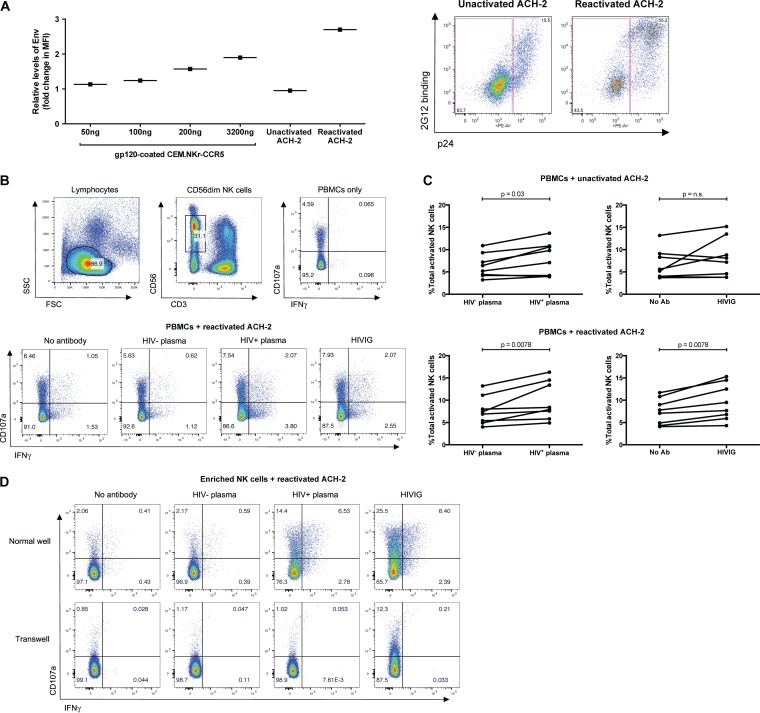
We initially utilized the latently infected ACH-2 T cell line as a model of HIV-1 latency. For ADCC antibodies to readily target infected cells, HIV-1 Env antigens need to be expressed on the cell surface. To determine the level of Env expression on reactivated ACH-2 cells, we compared the relative binding of a conformational-independent anti-Env Ab, 2G12, to reactivated ACH-2 cells and CEM.NKr-CCR5 cells coated with a series of dilutions of recombinant gp120 protein (22). Unactivated ACH-2 cells expressed relatively low levels of gp120, similar to those expressed by CEM.NKr-CCR5 cells coated with 50 ng/ml of gp120. Conversely, reactivated ACH-2 cells expressed high levels of gp120, higher than that observed for CEM.NKr-CCR5 cells coated with 3.2 μg/ml of gp120 (Fig. 1A, left panel). The majority of Env-expressing ACH-2 cells also expressed p24 (Fig. 1A, right panel).
FIG 1.
Assessment of anti-HIV-1 antibody-dependent NK cell activation against ACH-2 cells. (A) (Left) To determine the relative levels of Env on the surface of reactivated ACH-2 cells, uninfected CEM.NKr-CCR5 cells were first pulsed with increasing amounts of recombinant gp120 (50 to 3,200 ng/ml). Next, unactivated ACH-2 cells, reactivated ACH-2 cells, and gp120-pulsed CEM.NKr-CCR5 cells were surface stained with 2G12 (5 μg/ml) using an Alexa Fluor 488-conjugated secondary anti-human IgG1 antibody. The y axis denotes fold change in median fluorescence intensity (MFI) over background secondary antibody binding. (Right) Unactivated and reactivated ACH-2 cells were stained simultaneously for intracellular p24 and surface Env expression (2G12 binding). (B) PBMCs or enriched NK cells were used as effector cells and incubated with target ACH-2 cells at a 10:1 or 1:1 effector-to-target ratio, respectively. The top panel depicts the gating strategy utilized to analyze flow cytometry data for the NK cell activation assay. Gating was on the lymphocyte population, followed by CD3− CD56dim NK cells, and lastly, NK cell activation was evaluated via surface expression of the CD107a degranulation marker and intracellular production of IFN-γ. The bottom panels are four representative plots of NK cell activation against reactivated ACH-2 cells in the presence of no-antibody, HIV-1− plasma (1:1,000 dilution), HIV-1+ plasma (1:1,000 dilution), or HIVIG (50 μg/ml). (C) Graphs depict total NK cell activation against unactivated (top) and reactivated ACH-2 cells (bottom) in the presence of no-antibody, HIV-1− plasma, HIV-1+ plasma, or HIVIG. The percent total activated NK cells was obtained by summing up the values for the CD107a+ quadrant, IFN-γ+ quadrant, and CD107a+ IFN-γ+ quadrant. The data were analyzed with the nonparametric Wilcoxon signed-rank test. (D) Incubations were performed in normal plates (top) or transwell plates (bottom) in which effector and target cells were separated by a semipermeable membrane, which allowed transfer of small molecules but prevented cell-cell contact. Instead of PBMCs, enriched NK cells were used as effector cells to exclude possible antibody-dependent NK cell activation mediated by bystander virion-coated CD4+ T cells. Incubations in transwell plates were performed to exclude possible antibody-dependent NK cell activation mediated by soluble immune complexes instead of cell-cell contact between effector and target cells.
Antibody-dependent NK cell activation against reactivated ACH-2 cells.
Given that a large proportion of reactivated ACH-2 cells were expressing high levels of gp120 following 24 h of stimulation with mitogens, we next determined if reactivated ACH-2 cells served as targets for HIV-1-specific antibody-dependent NK cell activation. Previously, we have demonstrated robust NK cell activation by gp120-pulsed CEM.NKr-CCR5 cells and primary T cells in the presence of anti-HIV-1 antibodies (32). The gating strategy to analyze antibody-dependent NK cell activation by ACH-2 cells is shown in Fig. 1B. For reactivated ACH-2 cells (Fig. 1C, bottom panel), HIV-1+ plasma and HIVIG mediated a small but statistically significant increase in total NK cell activation (CD107a and/or IFN-γ expression) compared to HIV-1− plasma and the no-antibody controls (8.2% [6.3 to 14.2%] versus 7.2% [5.0 to 10.3%]), P = 0.0078, for HIV-1+ plasma versus HIV-1− plasma, and 8.6% [6.1 to 14.0%] versus 7.5% [4.5 to 10.4%]), P = 0.0078, for HIVIG versus no-antibody controls). Healthy PBMC donors also varied substantially in the capacity of their NK cells to become activated in the presence of both HIV-1-specific antibodies and reactivated ACH-2 cells. Unactivated ACH-2 cells elicited a slight but significant increase in NK cell activation when cultured with HIV-1+ plasma compared to HIV-1− plasma (8.5% [4.1 to 10.8%] versus 5.8% [4.3 to 8.8%], P = 0.03), but not with HIVIG compared to the no-antibody control (7.7% [4.0 to 12.3%] versus 5.4% [3.9 to 8.9%], not significant [n.s.]) (Fig. 1C, top panel). It is, however, important to note that there was no significant difference between antibody-dependent NK cell activation (with background subtracted) against reactivated ACH-2 cells and against unactivated ACH-2 cells.
Since PBMCs were used as effector cells, we reasoned that virions produced by reactivated ACH-2 cells could bind to CD4+ T cells within PBMCs, thereby making virion-coated CD4+ T cells bystander targets capable of activating NK cells in an antibody-dependent manner. Furthermore, HIV-1-specific antibodies could possibly bind to free virions in solution to form immune complexes and activate NK cells in a cell contact-independent manner. To exclude these two bystander mechanisms, we performed the NK cell activation assay in transwell plates using enriched NK cells. For these experiments, NK effectors and ACH-2 targets were separated by a semipermeable membrane that prevented cell-cell contact but allowed the transfer of soluble immune complexes. As depicted in Fig. 1D, both HIV-1+ plasma and HIVIG mediated activation of enriched NK cells against reactivated ACH2 cells when the effector and target cells were cultured together in normal wells (23.7% total NK cell activation for HIV-1+ plasma; 34.3% total NK cell activation for HIVIG). When separated in a transwell, however, HIV-1+ plasma did not mediate NK activation above background levels. Transwell incubations in the presence of HIVIG resulted in 12.5% total NK cell activation. This NK activation mediated by HIVIG through the transwell membrane possibly occurred through the formation of soluble immune complexes with virions produced from ACH-2 cells. Thus, the majority of both CD107a and IFN-γ expression was dependent upon cell-cell contact between the effector NK cells and reactivated ACH-2 cells.
ADCC against reactivated ACH-2 cells.
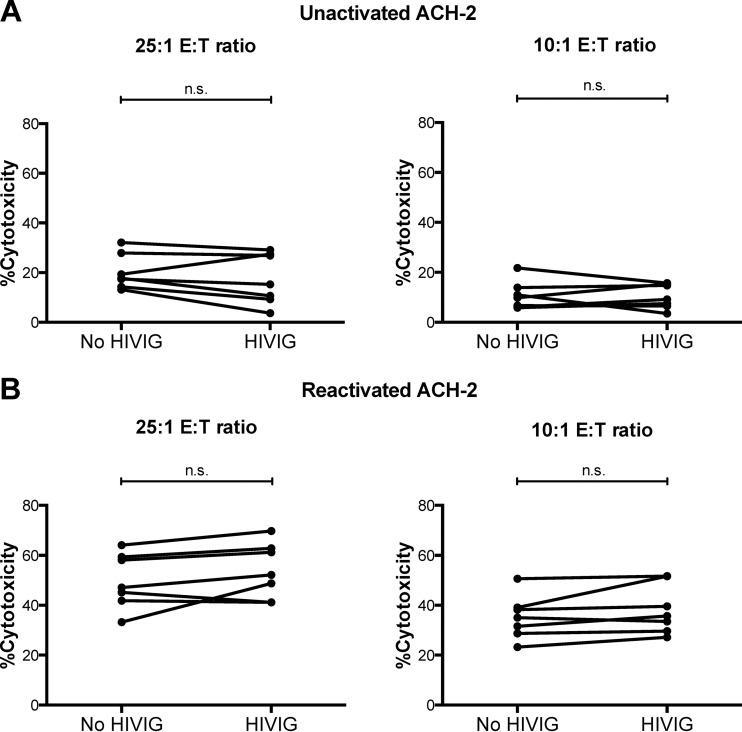
The data presented thus far demonstrate that reactivated ACH-2 cells can elicit some NK cell activation in an HIV-1-specific antibody-dependent manner and that the level of NK activation is donor dependent. We next assessed whether the ADCC activity in polyclonal HIVIG could kill reactivated ACH-2 cells using an LDH release cytotoxicity assay. No significant difference in cytotoxicity was observed for unactivated ACH-2 cells between samples incubated with or without HIVIG for both 25:1 and 10:1 effector-to-target ratios (15.3% [9.3 to 27.4%]) versus 17.8% [14.3 to 27.9%], n.s. for the 25:1 ratio and 9.2% [6.5 to 15.4%] versus 9.8% [6.1 to 13.9%], n.s. for the 10:1 ratio) (Fig. 2A). A similar pattern was observed for reactivated ACH-2 cells, although background levels of natural killing increased. Again, no significant difference in cytotoxicity was observed between the samples incubated with or without HIVIG under either 25:1 or 10:1 effector-to-target cell ratio conditions (52.1% [41.2 to 62.9%] versus 47.1% [41.8 to 59.3%], n.s. for the 25:1 ratio and 35.7% [29.7 to 51.6%] versus 35.0% [28.7 to 39.1%], n.s. for the 10:1 ratio) (Fig. 2B). Thus, even though there was a slight but significant increase in antibody-dependent NK cell activation (degranulation and cytokine secretion) against reactivated ACH-2 cells, this was not capable of killing the virus-expressing reactivated ACH-2 cells.
FIG 2.
Anti-HIV-1 ADCC against ACH-2 cells. PBMC effector cells isolated from 7 healthy donors were incubated with unactivated (A) or reactivated (B) ACH-2 cells at 25:1 and 10:1 effector-to-target ratios in the presence or absence of HIVIG (25 μg/ml). Anti-HIV-1 ADCC was assessed using the LDH release cytotoxicity assay. The data were analyzed with the nonparametric Wilcoxon signed-rank test.
A CD4mc enhances binding of HIV-1+ sera to reactivated ACH-2 cells but does not enhance killing of the ACH-2 cells.
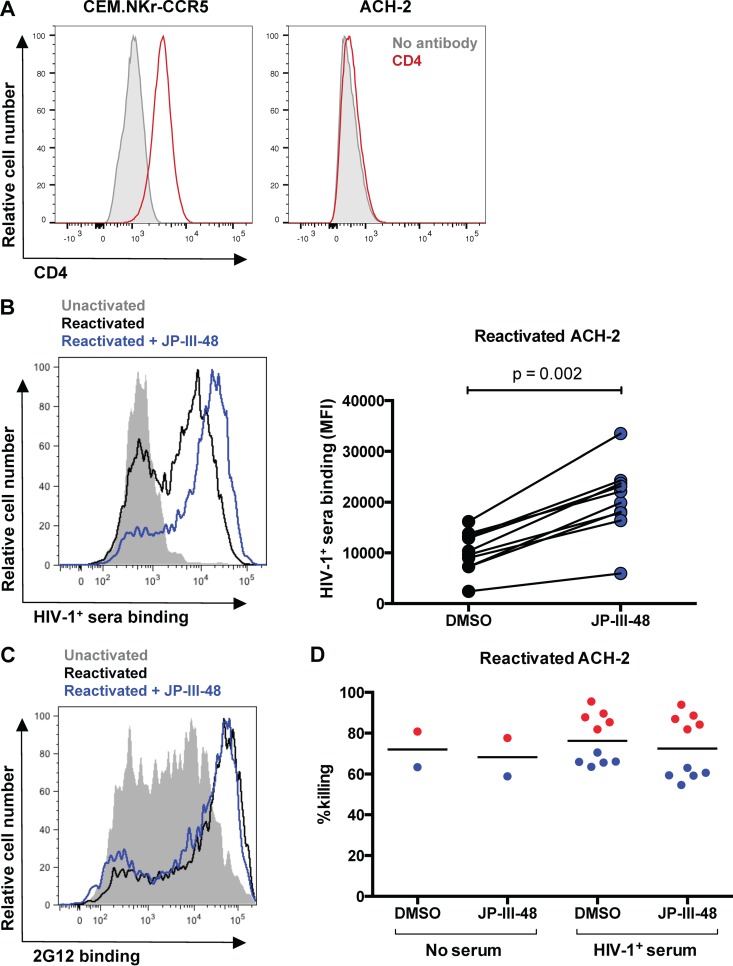
Recent studies have shown that ADCC antibodies from HIV-1+ individuals predominantly bind to CD4i epitopes on Env, which may be concealed as a result of Vpu- and Nef-mediated CD4 downregulation (22, 23). We hypothesized that the poor ADCC activity against reactivated ACH-2 cells could be due to insufficient binding of HIVIG to Env trimers, as a consequence of occluded CD4i epitopes. Indeed, CD4 is not present on the surface of ACH-2 cells (Fig. 3A). To induce the CD4-bound conformation of Env and expose CD4i epitopes, we incubated reactivated ACH-2 cells with the CD4mc JP-III-48. As shown in Fig. 3B, incubation with JP-III-48 significantly increased binding of HIV-1+ sera from all 10 donors tested to reactivated ACH-2 cells compared to the negative dimethyl sulfoxide (DMSO) control (P = 0.002). To assess if increased binding of HIV-1+ sera to reactivated ACH-2 cells was due to the exposure of CD4i epitopes, we assessed the effect of incubation with CD4mc on 2G12 binding. Since 2G12 binds to a glycan-based outer domain epitope on gp120 that is CD4 independent (36), the presence of JP-III-48 did not affect binding of 2G12 to reactivated ACH-2 cells (Fig. 3C). Following this, we assessed whether the increased binding of HIV-1+ serum antibodies in the presence of JP-III-48 also increased killing of reactivated ACH-2 cells by using a flow cytometry-based infected cell elimination assay. Cytolysis of reactivated ACH-2 cells in the presence of HIV-1+ sera (1:1,000 dilution) from 10 donors was minimally above the high natural killing (no serum) background observed with this cell line in the absence of CD4mc (DMSO control) and was not increased following incubation with JP-III-48 (Fig. 3D).
FIG 3.
The CD4mc JP-III-48 enhances binding of HIV-1+ sera to reactivated ACH-2 cells but does not enhance ADCC. (A) Surface staining for CD4 expression on CEM.NKr-CCR5 cells and ACH-2 cells. (B) The effect of CD4mc JP-III-48 on the binding of HIV-1+ sera from 10 donors to ACH-2 cells was assessed via flow cytometry. On the left is a representative plot of HIV-1+ sera binding to unactivated ACH-2 cells (shaded gray), reactivated ACH-2 cells (black line), and reactivated ACH-2 cells in the presence of JP-III-48 (blue line). The graph on the right depicts the level of HIV-1+ sera from 10 donors binding to reactivated ACH-2 cells in the presence of JP-III-48 or the vehicle (DMSO). (C) To determine if CD4mc affects binding of antibodies to non-CD4i epitopes, we assessed binding of 2G12 to unactivated ACH-2 cells (shaded gray), reactivated ACH-2 cells (black line), and reactivated ACH-2 cells in the presence or absence of JP-III-48 (blue line). (D) The effect of CD4mc on ADCC against reactivated ACH-2 cells was assessed using a flow cytometry-based infected cell elimination assay (28, 33). Effector PBMCs from 2 healthy donors (depicted in red and blue) were incubated with reactivated ACH-2 cells in the presence or absence of HIV-1+ sera (1:1,000 dilution) from 10 donors (5 for each PBMC donor). Incubations were performed in the presence of JP-III-48 or the vehicle (DMSO). The data in this figure were analyzed using the Wilcoxon signed-rank test, and the black lines represent the medians of each group.
HIV-1+ serum binding and killing of primary ex vivo-expanded HIV-1-infected CD4+ T cells.
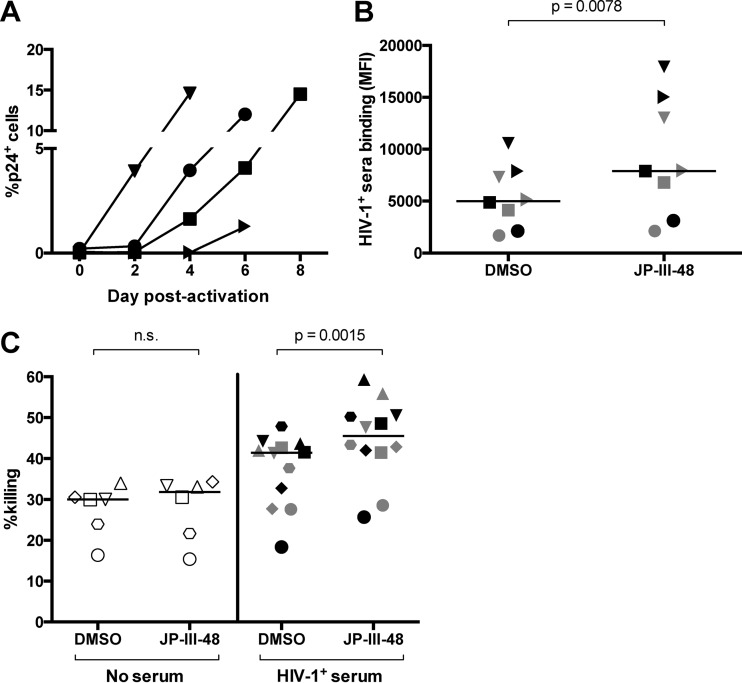
Although cell lines can serve as useful in vitro models of HIV-1 latency for preliminary studies, their surface molecules and high levels of baseline virus expression poorly mimic the quiescent, resting phenotypes of most latently infected cells in vivo (37). Moreover, their high susceptibility to direct NK cell-mediated lysis might render it difficult to assess ADCC activity above background natural killing. However, latently infected primary CD4+ T cells are rare in the peripheral blood of HIV-1+ subjects on ART (approximately 1 replication-competent virus per million resting CD4+ T cells [38]) and are difficult to purify in sufficient numbers to serve directly as ex vivo targets for ADCC assays. Thus, we isolated CD4+ T cells from viremic HIV-1-infected individuals (described in Table 1) and cultured them under activating conditions to obtain sufficient numbers of HIV-1-expressing cells. Virus expression was achieved by activating the cells with PHA for 36 h followed by incubation with recombinant IL-2 for 4 to 8 days, as reported previously (28). Note that this method does not study the effect of ADCC on the very low numbers of cells initially expressing HIV-1 at the start of the culture but rather the effect on cells infected during the 4- to 8-day culture period. The culture was successful in inducing production of p24 in CD4+ T cells over the culture period, as shown in Fig. 4A. The percentage of p24-expressing cells for all 4 donors was below detectable levels at the start of the culture and increased to a range of 1.3 to 14.6% by 4 to 8 days. Next, we examined whether autologous or heterologous HIV-1+ serum antibodies (from one heterologous donor) could bind to the cultured primary cells and whether addition of the CD4mc JP-III-48 affected this binding. As shown in Fig. 4B, autologous (black) and heterologous (gray) HIV-1+ sera bound to the infected cells and incubation with JP-III-48 enhanced this binding significantly compared to the negative DMSO control (P = 0.0078). We next incubated the cultured CD4+ T cells with autologous effector PBMCs in the absence of sera (white) or in the presence of autologous (black) or heterologous (gray) HIV-1+ sera (1:1,000 dilution) and examined ADCC responses using the flow cytometry-based infected-cell elimination assay (Fig. 4C). The proportion of infected cells killed in the presence of both autologous and heterologous HIV-1+ sera was higher than that of the no-serum controls (41.4% [29 to 43.4%] versus 30.0% [22.0 to 31.4%]). There was no apparent difference in the ability of either autologous or heterologous HIV-1+ sera to mediate killing of the infected cells (the black and gray symbols in Fig. 4C mostly overlap). In the presence of JP-III-48 and HIV-1+ sera, 45.5% (41.6 to 50.5%) of infected cells were eliminated versus 31.8% (20.1 to 33.6%) in the presence of JP-III-48 without sera. Importantly, killing of infected primary cells in the presence of HIV-1+ sera was enhanced following incubation with JP-III-48 compared to the negative DMSO control (45.5% [41.6 to 50.5%] versus 41.4% [29.0 to 43.4%], P = 0.0015).
FIG 4.
The CD4mc JP-III-48 enhances binding of HIV-1+ sera to primary infected cells and enhances ADCC. (A) To obtain primary infected cells, CD4+ T cells were purified from HIV-1+ individuals and activated with PHA for 36 h, followed by incubation with recombinant IL-2 for 4 to 8 days. HIV-1 expression was assessed via intracellular staining for p24 and was measured 2 to 8 days postactivation with PHA. (B) The effect of CD4mc or the vehicle (DMSO) on the binding of autologous (black) and one other heterologous (gray) HIV-1+ sera to primary infected cells was assessed using flow cytometry. The different shapes depict different primary cell donors. (C) Anti-HIV-1 ADCC against the cultured primary infected cells was assessed using a flow cytometry-based infected cell elimination assay. Following ex vivo expansion, primary CD4+ T cells were incubated with autologous effector PBMCs in the absence of serum (white) or in the presence of autologous sera (black) or heterologous HIV-1+ sera (gray) (1:1,000 dilution). Incubations were performed in the presence of JP-III-48 or the vehicle (DMSO). Data for this figure were analyzed using the Wilcoxon signed-rank test, and the black lines represent the medians of each group.
DISCUSSION
Recent research efforts have focused on reactivating the HIV-1 latent reservoir as a strategy to unveil latently infected cells to the immune system. Immune-mediated clearance may be needed to eliminate these reactivated cells before they revert to a latent undetectable state. In this study, we examined whether polyclonal HIV-1-specific antibodies from HIV-1-infected subjects could mediate ADCC against a latently infected cell line following HIV-1 reactivation and ex vivo-expanded CD4+ T cells from HIV-1-infected subjects. We first showed that reactivated ACH-2 cells activate NK cells to express IFN-γ and CD107a in the presence of HIV-1-specific antibodies. Antibody-mediated activation, however, did not result in antibody-mediated killing of the reactivated ACH-2 cells. Antibodies in the sera of HIV-1-infected individuals that mediate ADCC have been shown to predominantly bind to CD4i epitopes on Env (23), which are likely to be concealed on the CD4− ACH-2 cells. Thus, we incubated the reactivated cells with a CD4mc (JP-III-48) to induce the CD4-bound conformation of Env. Despite detecting an increase in binding of HIV-1+ serum antibodies to reactivated ACH-2 cells, this did not result in increased ADCC. This raises important caveats about the utility of ADCC in targeting reactivated latently infected cells, at least in this model.
One consideration with interpreting the killing of ACH-2 cells is that the reactivation of HIV-1 expression using PHA or PMA resulted in the ACH-2 cells becoming better targets for NK cell-mediated natural cytotoxicity, as seen by the high %killing in the absence of HIV-1-specific antibodies (median of 17.8% natural killing for unactivated ACH-2 versus 47.1% natural killing for reactivated ACH-2, 25:1 effector-to-target ratio). Although this may reduce the sensitivity of the cytotoxicity assay to detect antibody-mediated killing above the high background natural killing, we have observed that even with high natural cytotoxicity of a major histocompatibility complex class I (MHC-I)-devoid B cell line (721.221 cells), we can still detect significant antibody-mediated killing above background with the anti-CD20 monoclonal antibody rituximab (K. J. Selva, S. J. Kent, and M. S. Parsons, unpublished data). Thus, at least in the setting of optimal antigen expression and optimal antibody potency, we would have expected to detect HIV-1-specific ADCC activity against the reactivated ACH-2 cells.
While several studies have utilized latently infected cell lines to examine reactivation of the latent provirus (39, 40) and even elimination of reactivated cells (41), transformed cell lines may not be ideal models of HIV-1 latency and may not be reflective of primary CD4+ T cells as targets for ADCC. Latently infected CD4+ T cells are rare in HIV-1+ subjects on ART (approximately 1 replication-competent virus per million resting CD4+ T cells [38]), and to obtain sufficient HIV-1-infected CD4+ T cell targets, it was necessary to activate the primary cells with mitogens and culture them for several days. Although the latent reservoirs from HIV-1+ individuals were not enriched in this current study, we show that cultures of primary infected CD4+ T cells from HIV-1+ individuals can be eliminated by ADCC triggered by both autologous and heterologous HIV-1+ serum antibodies and autologous effector PBMCs. Immune-mediated clearance of reactivated cells should ideally occur much earlier than in this experimental system and with less potent LRAs. Stimulation of the CD4+ T cell targets with mitogens will likely increase expression of certain activating NK cell receptor ligands that synergize with CD16 to augment ADCC responses (42, 43). It is not known whether this enhancement will occur with the non-globally activating LRAs currently being examined in clinical trials. Many studies are now assessing potential synergistic combinations of different LRAs to achieve more robust latency reactivation, of which some have shown promising results (44, 45).
An essential question that remains to be addressed is whether current latency-reversing strategies that have shown promise in vitro can achieve sufficiently high reactivation in vivo for the immune system to detect and eliminate reactivated cells. More importantly, even if high levels of latency reversal were achieved in vivo, would immune cells within HIV-1+ subjects on treatment be capable of eliminating the reactivated cells? Improving the functionality of effector cells might be required for patients who exhibit higher levels of immune exhaustion. For NK cells in particular, it has previously been shown that inhibition of certain matrix metalloproteinases can prevent downmodulation of the Fc receptor CD16, thereby improving the functionality of chronically activated NK cells (46–48).
The capacity of ADCC antibodies to recognize HIV-1 antigens expressed on the surface of reactivated cells is likely to be an important limitation for antilatency ADCC responses. ADCC antibodies within HIV-1+ sera primarily recognize CD4-induced Env epitopes concealed as a result of Vpu- and Nef-mediated CD4-downregulation (23). The inhibition of Nef and Vpu might be a possible strategy to increase recognition of Env-expressing cells (49). On a similar note, we show here that CD4mc have potential therapeutic utility because they can efficiently induce the CD4-bound conformation and increase binding of HIV-1+ serum antibodies to ex vivo-expanded primary CD4+ T cells. If the levels of HIV-1-specific ADCC antibodies within patients on long-term ART have declined due to a lack of antigenic stimulation (50), boosting ADCC antibody levels through passive transfer could be a possible therapeutic approach. Indeed, a study performed in HIV-1-infected humanized mice found that the passive transfer of three broadly neutralizing antibodies (BnAbs) following administration of three distinct LRAs led to a significant decrease in viral rebound in the absence of ART (51). BnAbs that have been shown to suppress viremia (52–54), in part through Fc-mediated effector functions (55), are attractive in that regard as they bind to a wide range of HIV-1 strains and recognize native Env epitopes that are not CD4 dependent. That being said, we have found that monoclonal antibodies directed against anti-cluster A CD4i epitopes on Env are much better mediators of ADCC in vitro than BnAbs that are not CD4 dependent (56).
In summary, we show that primary HIV-1-infected cells can be targets for ADCC mediated by autologous serum antibodies and immune effector cells. ADCC against these reactivated cells was enhanced with the administration of the CD4mc JP-III-48 that increased binding of HIV-1+ serum antibodies. Future studies will need to examine whether the non-globally activating LRAs can achieve sufficient reactivation for infected cells to become efficient targets for antibody-mediated clearance and whether sufficient depletion of the latent reservoir can be achieved to prevent viral rebound off-ART.
ACKNOWLEDGMENTS
We thank Hao Kim Lu for discussing theoretical aspects of the presented work, Nathalie Brassard for technical assistance, Jean-Pierre Routy for clinical samples, Mario Legault for cohort coordination and the CRCHUM Flow Cytometry Platform. We thank all subjects for their participation
We declare that we have no conflicts of interest.
Funding Statement
This work was supported by Australia NHMRC awards 1052979 and 10471832, an Australia-India Strategic Research Fund award, a Canada Foundation for Innovation Program Leader grant, Canadian Institutes for Health Research (CIHR) operating grants 119334 and 134117, Establishment of Young Scientist grant 26702 to A.F., the FRQS AIDS and Infectious Diseases Network, NIH grants AI100645, AI100663, and GM56550, and Center for HIV/AIDS Vaccine Immunology and Immunogen Design (CHAVI-ID) and National Institutes of Health grant RO1 HL-092565. A.F. is a recipient of a Canada Research Chair on Retroviral Entry. J.R. and M.S.P. are recipients of CIHR Fellowship Awards. D.E.K. is supported by a Research Scholar Career Award from the Quebec Health Research Fund (FQRS). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.Siliciano JD, Kajdas J, Finzi D, Quinn TC, Chadwick K, Margolick JB, Kovacs C, Gange SJ, Siliciano RF. 2003. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med 9:727–728. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- 2.Deeks SG. 2012. HIV: shock and kill. Nature 487:439–440. doi: 10.1038/487439a. [DOI] [PubMed] [Google Scholar]
- 3.Archin NM, Bateson R, Tripathy MK, Crooks AM, Yang KH, Dahl NP, Kearney MF, Anderson EM, Coffin JM, Strain MC, Richman DD, Robertson KR, Kashuba AD, Bosch RJ, Hazuda DJ, Kuruc JD, Eron JJ, Margolis DM. 2014. HIV-1 expression within resting CD4+ T cells after multiple doses of vorinostat. J Infect Dis 210:728–735. doi: 10.1093/infdis/jiu155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elliott JH, Wightman F, Solomon A, Ghneim K, Ahlers J, Cameron MJ, Smith MZ, Spelman T, McMahon J, Velayudham P, Brown G, Roney J, Watson J, Prince MH, Hoy JF, Chomont N, Fromentin R, Procopio FA, Zeidan J, Palmer S, Odevall L, Johnstone RW, Martin BP, Sinclair E, Deeks SG, Hazuda DJ, Cameron PU, Sekaly RP, Lewin SR. 2014. Activation of HIV transcription with short-course vorinostat in HIV-infected patients on suppressive antiretroviral therapy. PLoS Pathog 10:e1004473. doi: 10.1371/journal.ppat.1004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rasmussen TA, Tolstrup M, Brinkmann CR, Olesen R, Erikstrup C, Solomon A, Winckelmann A, Palmer S, Dinarello C, Buzon M, Lichterfeld M, Lewin SR, Østergaard L, Søgaard OS. 2014. Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: a phase 1/2, single group, clinical trial. Lancet HIV 1:e13–e21. doi: 10.1016/S2352-3018(14)70014-1. [DOI] [PubMed] [Google Scholar]
- 6.DeChristopher BA, Loy BA, Marsden MD, Schrier AJ, Zack JA, Wender PA. 2012. Designed, synthetically accessible bryostatin analogues potently induce activation of latent HIV reservoirs in vitro. Nat Chem 4:705–710. doi: 10.1038/nchem.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beans EJ, Fournogerakis D, Gauntlett C, Heumann LV, Kramer R, Marsden MD, Murray D, Chun TW, Zack JA, Wender PA. 2013. Highly potent, synthetically accessible prostratin analogs induce latent HIV expression in vitro and ex vivo. Proc Natl Acad Sci U S A 110:11698–11703. doi: 10.1073/pnas.1302634110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sogaard OS, Graversen ME, Leth S, Olesen R, Brinkmann CR, Nissen SK, Kjaer AS, Schleimann MH, Denton PW, Hey-Cunningham WJ, Koelsch KK, Pantaleo G, Krogsgaard K, Sommerfelt M, Fromentin R, Chomont N, Rasmussen TA, Ostergaard L, Tolstrup M. 2015. The depsipeptide romidepsin reverses HIV-1 latency in vivo. PLoS Pathog 11:e1005142. doi: 10.1371/journal.ppat.1005142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shan L, Deng K, Shroff NS, Durand CM, Rabi SA, Yang HC, Zhang H, Margolick JB, Blankson JN, Siliciano RF. 2012. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity 36:491–501. doi: 10.1016/j.immuni.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee WS, Parsons MS, Kent SJ, Lichtfuss M. 2015. Can HIV-1-specific ADCC assist the clearance of reactivated latently infected cells? Front Immunol 6:265. doi: 10.3389/fimmu.2015.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kramski M, Parsons MS, Stratov I, Kent SJ. 2013. HIV-specific antibody immunity mediated through NK cells and monocytes. Curr HIV Res 11:388–406. doi: 10.2174/1570162X113116660061. [DOI] [PubMed] [Google Scholar]
- 12.Daeron M. 1997. Fc receptor biology. Annu Rev Immunol 15:203–234. doi: 10.1146/annurev.immunol.15.1.203. [DOI] [PubMed] [Google Scholar]
- 13.Smalls-Mantey A, Connors M, Sattentau QJ. 2013. Comparative efficiency of HIV-1-infected T cell killing by NK cells, monocytes and neutrophils. PLoS One 8:e74858. doi: 10.1371/journal.pone.0074858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baum LL, Cassutt KJ, Knigge K, Khattri R, Margolick J, Rinaldo C, Kleeberger CA, Nishanian P, Henrard DR, Phair J. 1996. HIV-1 gp120-specific antibody-dependent cell-mediated cytotoxicity correlates with rate of disease progression. J Immunol 157:2168–2173. [PubMed] [Google Scholar]
- 15.Chung AW, Navis M, Isitman G, Wren L, Silvers J, Amin J, Kent SJ, Stratov I. 2011. Activation of NK cells by ADCC antibodies and HIV disease progression. J Acquir Immune Defic Syndr 58:127–131. doi: 10.1097/QAI.0b013e31822c62b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wren LH, Chung AW, Isitman G, Kelleher AD, Parsons MS, Amin J, Cooper DA, ADCC study collaboration investigators, Stratov I, Navis M, Kent SJ. 2013. Specific antibody-dependent cellular cytotoxicity responses associated with slow progression of HIV infection. Immunology 138:116–123. doi: 10.1111/imm.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomaras GD, Ferrari G, Shen X, Alam SM, Liao HX, Pollara J, Bonsignori M, Moody MA, Fong Y, Chen X, Poling B, Nicholson CO, Zhang R, Lu X, Parks R, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Gilbert PB, Kim JH, Michael NL, Montefiori DC, Haynes BF. 2013. Vaccine-induced plasma IgA specific for the C1 region of the HIV-1 envelope blocks binding and effector function of IgG. Proc Natl Acad Sci U S A 110:9019–9024. doi: 10.1073/pnas.1301456110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonsignori M, Pollara J, Moody MA, Alpert MD, Chen X, Hwang KK, Gilbert PB, Huang Y, Gurley TC, Kozink DM, Marshall DJ, Whitesides JF, Tsao CY, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Kim JH, Michael NL, Tomaras GD, Montefiori DC, Lewis GK, DeVico A, Evans DT, Ferrari G, Liao HX, Haynes BF. 2012. Antibody-dependent cellular cytotoxicity-mediating antibodies from an HIV-1 vaccine efficacy trial target multiple epitopes and preferentially use the VH1 gene family. J Virol 86:11521–11532. doi: 10.1128/JVI.01023-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kramski M, Stratov I, Kent SJ. 2015. The role of HIV-specific antibody-dependent cellular cytotoxicity in HIV prevention and the influence of the HIV-1 Vpu protein. AIDS 29:137–144. doi: 10.1097/QAD.0000000000000523. [DOI] [PubMed] [Google Scholar]
- 21.Veillette M, Richard J, Pazgier M, Lewis G, Parsons MS, Finzi A. 2016. Role of HIV-1 envelope glycoproteins conformation and accessory proteins on ADCC responses. Curr HIV Res 14:9–23. [DOI] [PubMed] [Google Scholar]
- 22.Veillette M, Desormeaux A, Medjahed H, Gharsallah NE, Coutu M, Baalwa J, Guan Y, Lewis G, Ferrari G, Hahn BH, Haynes BF, Robinson JE, Kaufmann DE, Bonsignori M, Sodroski J, Finzi A. 2014. Interaction with cellular CD4 exposes HIV-1 envelope epitopes targeted by antibody-dependent cell-mediated cytotoxicity. J Virol 88:2633–2644. doi: 10.1128/JVI.03230-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veillette M, Coutu M, Richard J, Batraville LA, Dagher O, Bernard N, Tremblay C, Kaufmann DE, Roger M, Finzi A. 2015. The HIV-1 gp120 CD4-bound conformation is preferentially targeted by antibody-dependent cellular cytotoxicity-mediating antibodies in sera from HIV-1-infected individuals. J Virol 89:545–551. doi: 10.1128/JVI.02868-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arias JF, Heyer LN, von Bredow B, Weisgrau KL, Moldt B, Burton DR, Rakasz EG, Evans DT. 2014. Tetherin antagonism by Vpu protects HIV-infected cells from antibody-dependent cell-mediated cytotoxicity. Proc Natl Acad Sci U S A 111:6425–6430. doi: 10.1073/pnas.1321507111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alvarez RA, Hamlin RE, Monroe A, Moldt B, Hotta MT, Rodriguez Caprio G, Fierer DS, Simon V, Chen BK. 2014. HIV-1 Vpu antagonism of tetherin inhibits antibody-dependent cellular cytotoxic responses by natural killer cells. J Virol 88:6031–6046. doi: 10.1128/JVI.00449-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lalonde JM, Le-Khac M, Jones DM, Courter JR, Park J, Schon A, Princiotto AM, Wu X, Mascola JR, Freire E, Sodroski J, Madani N, Hendrickson WA, Smith AB III. 2013. Structure-based design and synthesis of an HIV-1 entry inhibitor exploiting X-ray and thermodynamic characterization. ACS Med Chem Lett 4:338–343. doi: 10.1021/ml300407y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schon A, Madani N, Klein JC, Hubicki A, Ng D, Yang X, Smith AB III, Sodroski J, Freire E. 2006. Thermodynamics of binding of a low-molecular-weight CD4 mimetic to HIV-1 gp120. Biochemistry 45:10973–10980. doi: 10.1021/bi061193r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richard J, Veillette M, Brassard N, Iyer SS, Roger M, Martin L, Pazgier M, Schon A, Freire E, Routy JP, Smith AB III, Park J, Jones DM, Courter JR, Melillo BN, Kaufmann DE, Hahn BH, Permar SR, Haynes BF, Madani N, Sodroski JG, Finzi A. 2015. CD4 mimetics sensitize HIV-1-infected cells to ADCC. Proc Natl Acad Sci U S A 112:E2687–E2694. doi: 10.1073/pnas.1506755112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stratov I, Chung A, Kent SJ. 2008. Robust NK cell-mediated human immunodeficiency virus (HIV)-specific antibody-dependent responses in HIV-infected subjects. J Virol 82:5450–5459. doi: 10.1128/JVI.01952-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clouse KA, Powell D, Washington I, Poli G, Strebel K, Farrar W, Barstad P, Kovacs J, Fauci AS, Folks TM. 1989. Monokine regulation of human immunodeficiency virus-1 expression in a chronically infected human T cell clone. J Immunol 142:431–438. [PubMed] [Google Scholar]
- 31.Pomerantz RJ, Trono D, Feinberg MB, Baltimore D. 1990. Cells nonproductively infected with HIV-1 exhibit an aberrant pattern of viral RNA expression: a molecular model for latency. Cell 61:1271–1276. doi: 10.1016/0092-8674(90)90691-7. [DOI] [PubMed] [Google Scholar]
- 32.Gooneratne SL, Richard J, Lee WS, Finzi A, Kent SJ, Parsons MS. 2015. Slaying the Trojan horse: natural killer cells exhibit robust anti-HIV-1 antibody-dependent activation and cytolysis against allogeneic T-cells. J Virol 89:97–109. doi: 10.1128/JVI.02461-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richard J, Veillette M, Batraville LA, Coutu M, Chapleau JP, Bonsignori M, Bernard N, Tremblay C, Roger M, Kaufmann DE, Finzi A. 2014. Flow cytometry-based assay to study HIV-1 gp120 specific antibody-dependent cellular cytotoxicity responses. J Virol Methods 208:107–114. doi: 10.1016/j.jviromet.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 34.Cox JH. 1999. HIV-1-specific antibody-dependent cellular cytotoxicity (ADCC). Methods Mol Med 17:373–381. [DOI] [PubMed] [Google Scholar]
- 35.Pollara J, Hart L, Brewer F, Pickeral J, Packard BZ, Hoxie JA, Komoriya A, Ochsenbauer C, Kappes JC, Roederer M, Huang Y, Weinhold KJ, Tomaras GD, Haynes BF, Montefiori DC, Ferrari G. 2011. High-throughput quantitative analysis of HIV-1 and SIV-specific ADCC-mediating antibody responses. Cytometry A 79:603–612. doi: 10.1002/cyto.a.21084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trkola A, Purtscher M, Muster T, Ballaun C, Buchacher A, Sullivan N, Srinivasan K, Sodroski J, Moore JP, Katinger H. 1996. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J Virol 70:1100–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim M, Hosmane NN, Bullen CK, Capoferri A, Yang HC, Siliciano JD, Siliciano RF. 2014. A primary CD4+ T cell model of HIV-1 latency established after activation through the T cell receptor and subsequent return to quiescence. Nat Protoc 9:2755–2770. doi: 10.1038/nprot.2014.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bruner KM, Hosmane NN, Siliciano RF. 2015. Towards an HIV-1 cure: measuring the latent reservoir. Trends Microbiol 23:192–203. doi: 10.1016/j.tim.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Savarino A, Mai A, Norelli S, El Daker S, Valente S, Rotili D, Altucci L, Palamara AT, Garaci E. 2009. “Shock and kill” effects of class I-selective histone deacetylase inhibitors in combination with the glutathione synthesis inhibitor buthionine sulfoximine in cell line models for HIV-1 quiescence. Retrovirology 6:52. doi: 10.1186/1742-4690-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rasmussen TA, Schmeltz Sogaard O, Brinkmann C, Wightman F, Lewin SR, Melchjorsen J, Dinarello C, Ostergaard L, Tolstrup M. 2013. Comparison of HDAC inhibitors in clinical development: effect on HIV production in latently infected cells and T-cell activation. Hum Vaccin Immunother 9:993–1001. doi: 10.4161/hv.23800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lan J, Yang K, Byrd D, Hu N, Amet T, Shepherd N, Desai M, Gao J, Gupta S, Sun Y, Yu Q. 2014. Provirus activation plus CD59 blockage triggers antibody-dependent complement-mediated lysis of latently HIV-1-infected cells. J Immunol 193:3577–3589. doi: 10.4049/jimmunol.1303030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bryceson YT, March ME, Ljunggren HG, Long EO. 2006. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood 107:159–166. doi: 10.1182/blood-2005-04-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cerboni C, Zingoni A, Cippitelli M, Piccoli M, Frati L, Santoni A. 2007. Antigen-activated human T lymphocytes express cell-surface NKG2D ligands via an ATM/ATR-dependent mechanism and become susceptible to autologous NK-cell lysis. Blood 110:606–615. doi: 10.1182/blood-2006-10-052720. [DOI] [PubMed] [Google Scholar]
- 44.Laird GM, Bullen CK, Rosenbloom DI, Martin AR, Hill AL, Durand CM, Siliciano JD, Siliciano RF. 2015. Ex vivo analysis identifies effective HIV-1 latency-reversing drug combinations. J Clin Invest 125:1901–1912. doi: 10.1172/JCI80142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang G, Mendes EA, Kaiser P, Wong DP, Tang Y, Cai I, Fenton A, Melcher GP, Hildreth JE, Thompson GR, Wong JK, Dandekar S. 2015. Synergistic reactivation of latent HIV expression by ingenol-3-angelate, PEP005, targeted NF-kB signaling in combination with JQ1 induced p-TEFb activation. PLoS Pathog 11:e1005066. doi: 10.1371/journal.ppat.1005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang CC, Isitman G, Bruneau J, Tremblay C, Bernard NF, Kent SJ, Parsons MS. 2015. Phenotypic and functional profiles of NK cells exhibiting MMP-mediated CD16 cleavage after anti-HIV antibody-dependent activation. Clin Exp Immunol 181:275–285. doi: 10.1111/cei.12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parsons MS, Tang CC, Jegaskanda S, Center RJ, Brooks AG, Stratov I, Kent SJ. 2014. Anti-HIV antibody-dependent activation of NK cells impairs NKp46 expression. J Immunol 192:308–315. doi: 10.4049/jimmunol.1301247. [DOI] [PubMed] [Google Scholar]
- 48.Liu Q, Sun Y, Rihn S, Nolting A, Tsoukas PN, Jost S, Cohen K, Walker B, Alter G. 2009. Matrix metalloprotease inhibitors restore impaired NK cell-mediated antibody-dependent cellular cytotoxicity in human immunodeficiency virus type 1 infection. J Virol 83:8705–8712. doi: 10.1128/JVI.02666-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bouchet J, Basmaciogullari SE, Chrobak P, Stolp B, Bouchard N, Fackler OT, Chames P, Jolicoeur P, Benichou S, Baty D. 2011. Inhibition of the Nef regulatory protein of HIV-1 by a single-domain antibody. Blood 117:3559–3568. doi: 10.1182/blood-2010-07-296749. [DOI] [PubMed] [Google Scholar]
- 50.Madhavi V, Ana-Sosa-Batiz FE, Jegaskanda S, Center RJ, Winnall WR, Parsons MS, Ananworanich J, Cooper DA, Kelleher AD, Hsu D, Pett S, Stratov I, Kramski M, Kent SJ. 2015. Antibody-dependent effector functions against HIV decline in subjects on antiretroviral therapy. J Infect Dis 211:529–538. doi: 10.1093/infdis/jiu486. [DOI] [PubMed] [Google Scholar]
- 51.Halper-Stromberg A, Lu CL, Klein F, Horwitz JA, Bournazos S, Nogueira L, Eisenreich TR, Liu C, Gazumyan A, Schaefer U, Furze RC, Seaman MS, Prinjha R, Tarakhovsky A, Ravetch JV, Nussenzweig MC. 2014. Broadly neutralizing antibodies and viral inducers decrease rebound from HIV-1 latent reservoirs in humanized mice. Cell 158:989–999. doi: 10.1016/j.cell.2014.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shingai M, Nishimura Y, Klein F, Mouquet H, Donau OK, Plishka R, Buckler-White A, Seaman M, Piatak M Jr, Lifson JD, Dimitrov DS, Nussenzweig MC, Martin MA. 2013. Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia. Nature 503:277–280. doi: 10.1038/nature12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barouch DH, Whitney JB, Moldt B, Klein F, Oliveira TY, Liu J, Stephenson KE, Chang HW, Shekhar K, Gupta S, Nkolola JP, Seaman MS, Smith KM, Borducchi EN, Cabral C, Smith JY, Blackmore S, Sanisetty S, Perry JR, Beck M, Lewis MG, Rinaldi W, Chakraborty AK, Poignard P, Nussenzweig MC, Burton DR. 2013. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature 503:224–228. doi: 10.1038/nature12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Caskey M, Klein F, Lorenzi JC, Seaman MS, West AP Jr, Buckley N, Kremer G, Nogueira L, Braunschweig M, Scheid JF, Horwitz JA, Shimeliovich I, Ben-Avraham S, Witmer-Pack M, Platten M, Lehmann C, Burke LA, Hawthorne T, Gorelick RJ, Walker BD, Keler T, Gulick RM, Fatkenheuer G, Schlesinger SJ, Nussenzweig MC. 2015. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature 522:487–491. doi: 10.1038/nature14411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bournazos S, Klein F, Pietzsch J, Seaman MS, Nussenzweig MC, Ravetch JV. 2014. Broadly neutralizing anti-HIV-1 antibodies require Fc effector functions for in vivo activity. Cell 158:1243–1253. doi: 10.1016/j.cell.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ding S, Veillette M, Coutu M, Prévost J, Scharf L, Bjorkman PJ, Ferrari G, Robinson JE, Stürzel C, Hahn BH, Sauter D, Kirchhoff F, Lewis GK, Pazgier M, Finzi A. 4 December 2015. A highly-conserved residue of the HIV-1-gp120 inner domain is important for ADCC responses mediated by anti-cluster A antibodies. J Virol doi: 10.1128/JVI.02779-15. [DOI] [PMC free article] [PubMed] [Google Scholar]