Abstract
Interleukin 6 (IL-6) is considered a proliferation and survival factor for B cells. To assess the role of IL-6 in Kaposi sarcoma-associated herpesvirus (KSHV) latency, KSHV latency locus-transgenic mice (referred to as latency mice) lacking IL-6 were evaluated. IL-6−/− latency mice had the same phenotypes as the latency mice, i.e., increased frequency of marginal zone B cells, hyperplasia, and hyperglobulinemia, indicating that the KSHV latency locus, which includes all viral microRNAs (miRNAs), can compensate for lack of IL-6 in premalignant B cell activation.
TEXT
Aberrant interleukin 6 (IL-6) signaling is associated with tumorigenesis in preclinical and clinical models of lymphoma. Mice overexpressing IL-6 develop IgG1 plasmacytoma (1, 2), while IL-6 knockout (IL-6−/−) mice exhibit a lower incidence of chemically induced liver cancer and resistance to pristane-induced plasmacytoma (3, 4). Anti-IL-6 (siltuximab) and anti-IL-6 receptor (tocilizumab) antibodies have clinical efficacy against multicentric Castleman's disease (MCD) (5–8). MCD is a preneoplastic hyperplasia of B cells, the plasmablastic variant of which is associated with Kaposi sarcoma-associated herpesvirus (KSHV). KSHV is also the etiologic agent of Kaposi sarcoma, primary effusion lymphoma (PEL) (9), and an IL-6-associated disorder called KSHV inflammatory cytokine syndrome (KICS) (10, 11). PELs produce IL-6 (12, 13), and an anti-IL-6 antibody inhibited growth of PELs both in vitro and in vivo (14, 15); however, some PEL cell lines, such as BCBL-1, do not express or depend on IL-6 (15, 16). KSHV encodes a viral IL-6 homolog which is expressed at various levels in PEL (17, 18). To understand the role of endogenous IL-6 in premalignant KSHV pathogenesis, we investigated KSHV transgenic mice without IL-6.
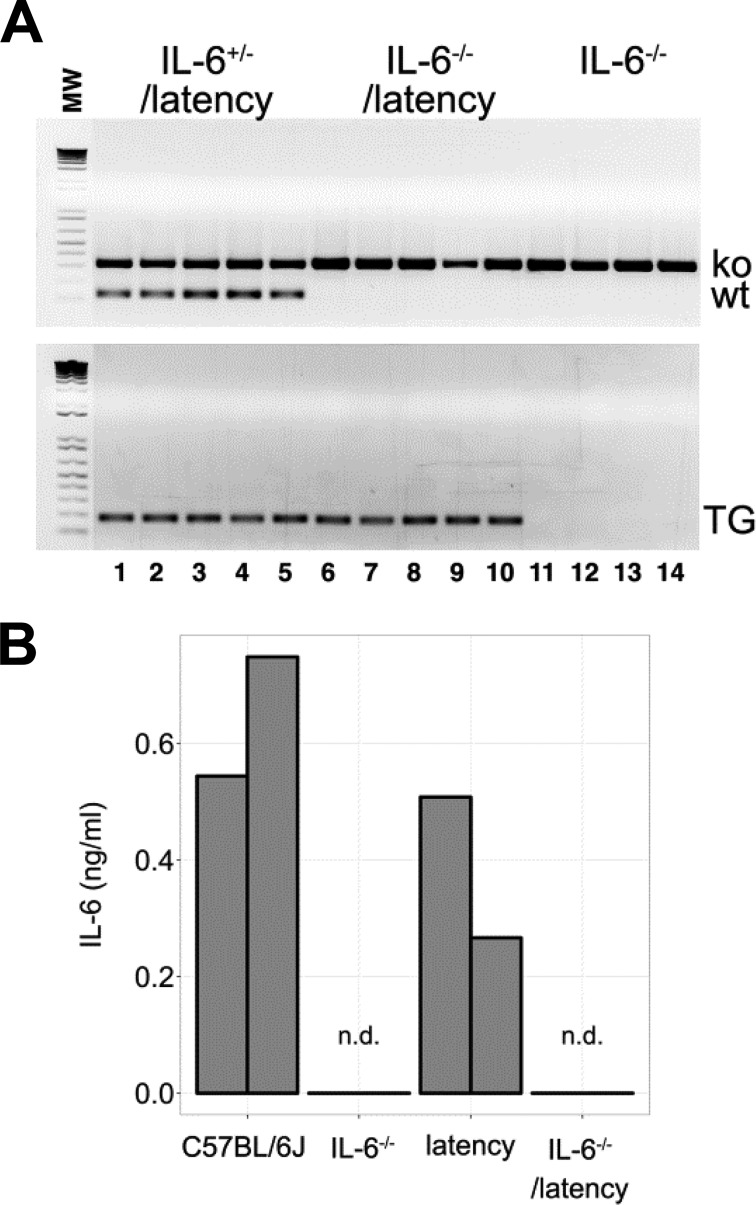
KSHV latency-associated nuclear antigen (LANA)-transgenic mice develop B cell hyperplasia, which is dependent on CD19 (19, 20). C57BL/6J KSHV latency locus-transgenic mice (referred to as latency mice), which in addition to LANA express all viral microRNAs (miRNAs), exhibit consistent expansion of the marginal zone (MZ) and plasma cells (PCs), as well as hypergammaglobulinemia (21). These mice were crossed to isogenic IL-6−/− knockout mice (B6;129S2-Il6tm1Kopf/J). Genotyping was performed according to the supplier's protocol and as published elsewhere (Fig. 1A) (20). Splenocytes from 7- to 8-week-old mice were cultured with 100 ng/ml lipopolysaccharides (LPS) for 48 h, and IL-6 levels were measured by enzyme-linked immunosorbent assay (ELISA) (eBioscience). In response, cells from C57BL/6J and the latency mice secreted IL-6, while cells from IL-6−/− and IL-6−/− latency mice did not (Fig. 1B).
FIG 1.
IL-6−/− latency transgenic mice. (A) Agarose gel of PCR products obtained with primers specific for IL-6−/− and a latency gene. (B) Level of IL-6 in supernatant from splenocytes which were cultured with 100 ng/ml LPS (from Escherichia coli 0111:B4; Invivogen). n.d., not detected.
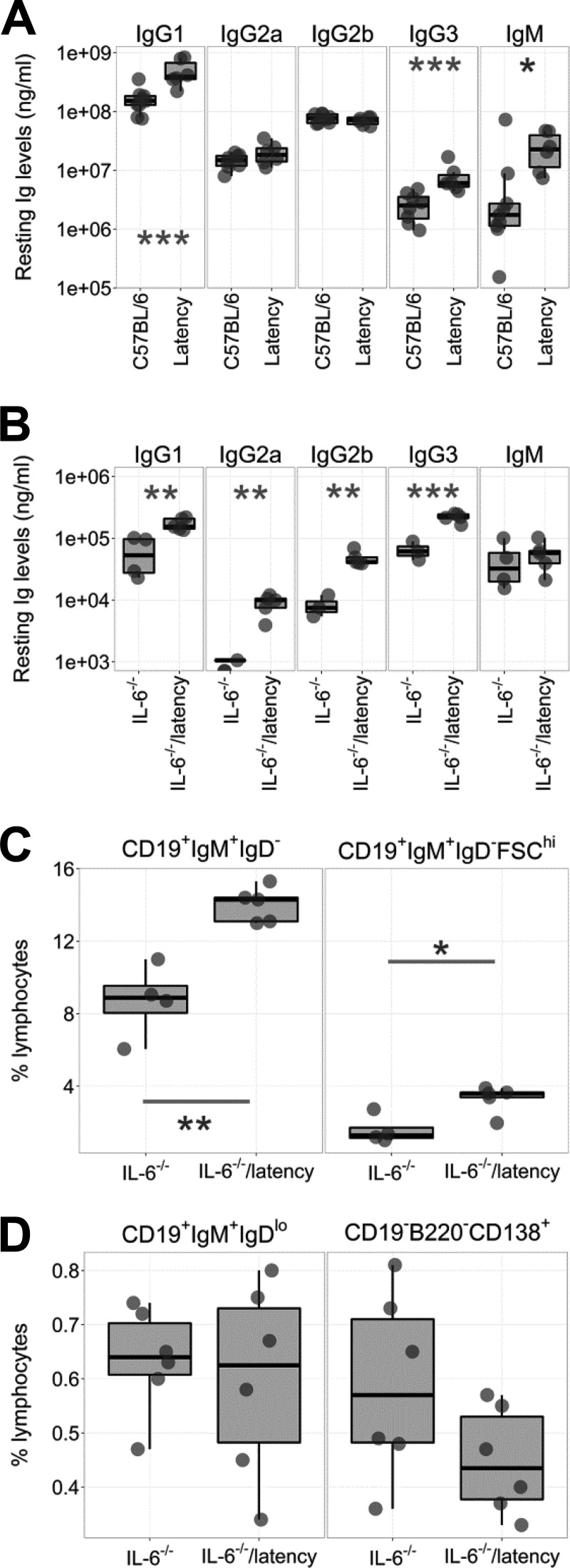
IL-6 plays important roles in immunoglobulin (Ig) secretion by sustaining long-lived PCs (reviewed in reference 22). IgG production is impaired in IL-6−/− mice (23–25), whereas IgG hyperglobulinemia is a consistent phenotype of the latency mice (Fig. 2A). To examine the genetic interaction between KSHV latency genes and lack of IL-6, we examined serum Ig levels by ELISA as described previously (21). Total IgG1, IgG2a, IgG2b, and IgG3 levels were higher in IL-6−/− latency than IL-6−/− mice (Fig. 2B). This demonstrates that KSHV latent genes (and miRNAs) in B cells can compensate for the absence of IL-6 in B cell maturation.
FIG 2.
Phenotypes of IL-6−/− latency mice. Box plots show the 1st and 3rd quartiles, with the median indicated by the band. Whiskers extend to 1.5× the interquartile range. (A) Peripheral Ig levels were plotted from 9 C57BL/6J and 6 latency mice as determined by ELISA. This represents a meta-analysis. Some of the data points were previously reported (21). (B) Peripheral Ig levels were plotted from IL-6−/− and IL-6−/− latency mice as determined by ELISA (n = 5). (C) Splenic marginal zone B cells (CD19+ IgM+ IgD−) and activated marginal zone B cells (CD19+ IgM+ IgD− FSChi) (n = 5). (D) Immature B cells (CD19+ IgM+ IgDlo) and plasma cells (CD19− B220− CD138+) in BM were plotted (n = 6). Data are frequencies, shown as a percentage of total lymphocytes. *, **, and ***, P ≤ 0.05, P ≤ 0.005, and P ≤ 0.0005, respectively, by ANOVA.
To assess the effect of IL-6 on B cell development, cells were isolated from the spleen or bone marrow (BM) of 7- to 11-week-old IL-6−/− and IL-6−/− latency mice and analyzed by flow cytometry. IL-6−/− latency mice displayed the same phenotypes as the latency mice, specifically, increased frequencies of MZ cells (CD19+ IgM+ IgD−) and activated MZ B cells (CD19+ IgM+ IgD− FSChi) in spleen (Fig. 2C; Table 1). Frequencies of mature B cells and plasma cells (PC) were not significantly different. This held true for spleen (data not shown) and BM (Fig. 2D). This suggests that the KSHV latency-associated hyperplasia of naive, pre-GC B cells was not dependent on IL-6.
TABLE 1.
MZ frequency of the IL-6−/−, IL-6−/− latency, latency, and C57BL/6 micea
| Class | Marker | IL-6−/− |
IL-6−/− latencyb |
Latencyb |
C57BL/6 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | SD | n | % | SD | n | P (vs IL-6−/−) | % | SD | n | P (vs IL-6−/− latency) | % | SD | n | P (vs IL-6−/−) | P (vs latency) | ||
| MZ | CD19+ IgM+ IgD− | 8.7 | 2.0 | 4 | 14.0 | 1.0 | 5 | 0.001 | 15.8 | 2.2 | 9 | NS | 11.8 | 3.1 | 8 | NS | 0.01 |
| Activated MZ | CD19+ IgM+ IgD− FSChi | 1.6 | 0.8 | 4 | 3.3 | 0.8 | 5 | 0.01 | 4.5 | 1.5 | 9 | NS | 2.6 | 1.1 | 8 | NS | 0.01 |
The background of the latency, IL-6−/−, and IL-6−/− latency mouse was C57BL/6. Splenic cells were analyzed using flow cytometry. Values are the percentages in total lymphocytes. MZ, marginal zone B cells; SD, standard deviation; n, number of animal analyzed; NS, not significant.
This represents a meta-analysis. Frequencies of MZ and activated MZ from the latency and C57BL/6 mice were previously reported (21).
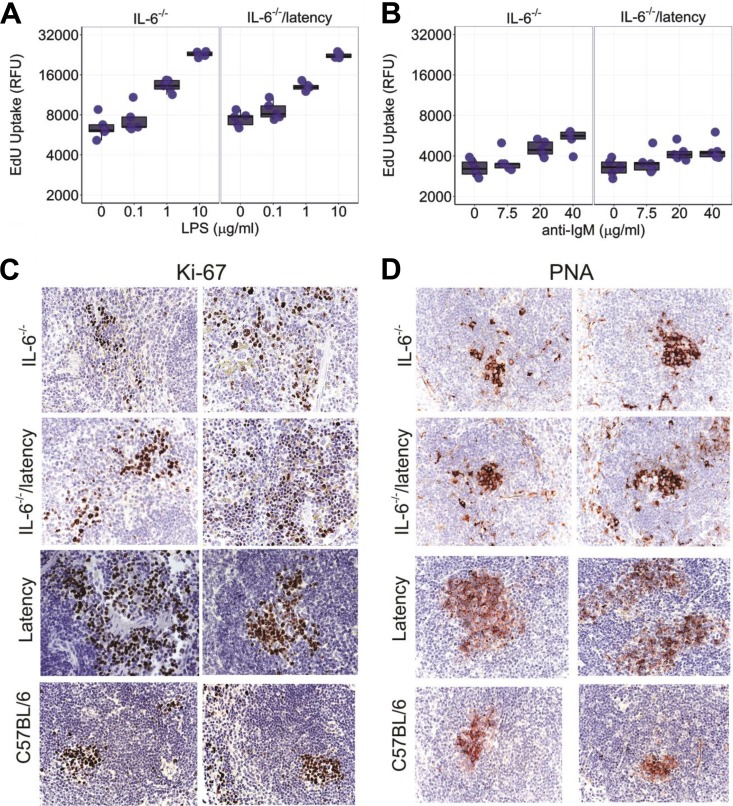
Ex vivo hyperresponsiveness to B cell stimuli is a distinct phenotype of KSHV latency mice (21). To test the hypothesis that this phenotype was dependent on IL-6, splenic B cells from 5- to 6-week-old IL-6−/− and IL-6−/− latency mice were purified by negative selection and cultured with LPS or anti-IgM antibody. Proliferation was measured using a Click-iT EdU assay (Invitrogen). The Toll-like receptor (TLR)-driven responsiveness to LPS persisted in the absence of IL-6 (Fig. 3A; Table 2); however, the B cell receptor (BCR)-driven responsiveness was damped in the absence of IL-6 (Fig. 3B; Table 2). This is consistent with a mechanism whereby BCR-induced B cell proliferation was aided by an IL-6 feedback loop but TLR-induced proliferation was not.
FIG 3.
The degree of ex vivo proliferation of B cells was plotted in response to LPS (A) or anti-IgM antibody (B). The box plots (n = 5) show the 1st and 3rd quartiles, with the median indicated by the band. Whiskers extend to 1.5× the interquartile range. Significance levels were determined by ANOVA. Immunostaining of spleen sections (5 IL-6−/− or IL−/− latency mice; 3 C57BL/6 or latency mice) with Ki-67 (C) and PNA (D). Representative images are shown. Magnification, ×400.
TABLE 2.
Ex vivo proliferation of splenic B cells from IL-6−/−, IL-6−/− latency, latency, and C57BL/6 micea
| Treatment | IL-6−/− |
IL-6−/− latency |
Latencyb |
C57BL/6b |
||||
|---|---|---|---|---|---|---|---|---|
| Slope | Slope | P (vs IL-6−/−) | Slope | P (vs IL-6−/− latency) | Slope | P (vs IL-6−/−) | P (vs IL-6−/− latency) | |
| LPS | 1,474.0 | 1,348.9 | NS | 1,629.5 | NS | 910.4 | NS | NS |
| Anti-IgM | 53.8 | 29.4 | NS | 229.3 | 0.001 | 158.1 | 0.04 | 0.007 |
The background of the latency, IL-6−/−, and IL-6−/− latency mice was C57BL/6. The degree of ex vivo proliferation of splenic B cells was analyzed by ANOVA. Five mice per genotype were analyzed. The regression line slope and its 95% confidence interval were calculated based on t distribution for each genotype. NS, not significant.
This represents a meta-analysis. The ex vivo response to LPS and anti-IgM of the latency and C57BL/6 mice was previously reported (21).
To document the in vivo phenotype of IL-6−/− latency mice, formalin-fixed, paraffin-embedded spleen sections were prepared and evaluated by immunohistochemistry for two established proliferation markers, Ki-67 and peanut agglutinin (PNA). We could not find a difference in staining degree or intensity between IL-6−/− and IL-6−/− latency mice; however, the staining was stronger in tissues from latency mice than in either C57BL/6 or IL-6−/− latency mice (Fig. 3C and D).
The rates of lymphoma and splenic lymphoid hyperplasia as ascertained by hematoxylin and eosin (H&E) stain showed no difference (Table 3). Mesenteric lymph nodes (MLNs) are chronically stimulated by gut microbiota. MiR-155 knockout mice exhibit a lower frequency of germinal center (GC) B cells in MLNs (26). KSHV encodes miR-K12-11, which is an ortholog of miR-155 and rescued the miR-155 deficiency-associated phenotype in MLNs (21, 27). IL-6−/− latency mice, which express miR-K12-11, had the same rate of lymphoid hyperplasia as the latency mice (Table 3). Another phenotype of KSHV latency mice is severe extramedullary hematopoiesis (EMH). The rates of EMH in spleen and liver were not dependent on the presence of IL-6 (Table 3).
TABLE 3.
In vivo phenotypesa
| Tissue | Phenotype | IL-6−/− |
IL-6−/− latency |
Latencyb |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of mice | Rate (%) | No. of mice | Rate (%) | P (vs IL-6−/−) | No. of mice | Rate (%) | P (vs IL-6−/−) | P (vs IL-6−/− latency) | ||
| Spleen | Lymphoma | 0 | 0.0 | 1 | 3.0 | 1 | 8 | 16.0 | 0.093 | 0.076 |
| Lymphoid hyperplasia | 6 | 31.6 | 9 | 27.3 | 1 | 13 | 26.0 | 1 | 1 | |
| Normal | 13 | 68.4 | 23 | 69.7 | 29 | 58.0 | ||||
| Severe EMH | 2 | 10.5 | 3 | 9.1 | 1 | 11 | 22.0 | 0.491 | 0.147 | |
| Liver | Severe EMH | 5 | 26.3 | 2 | 6.7 | 0.085 | NA | |||
| Total no. of mice | 19 | 33 | 50 | |||||||
| MLN | Lymphoma | 2 | 14.3 | 0 | 0.0 | 0.333 | NA | |||
| Lymphoid hyperplasia | 12 | 85.7 | 20 | 95.2 | 1 | NA | ||||
| Normal | 0 | 0.0 | 1 | 4.8 | NA | |||||
| Total no. of mice | 14 | 21 | NA | |||||||
Data were analyzed using ANOVA. A P value of ≤0.05 was regarded as significant. NA, data not available.
This represents a meta-analysis. The phenotype of the latency mice was previously reported (21).
B cell hyperplasia in spleen and proliferation in lymph nodes, as scored here, are complex and progressive phenotypes that develop over months and are subject to a multitude of compensatory and counterbalancing mechanisms in the animal. Clearly, IL-6 is needed for maximal B cell function and sustained proliferation during normal development and in preneoplastic scenarios, such as MCD. However, many mechanisms are known to relieve the dependence on IL-6 in disease. Augmented NF-κB signaling was found in an IL-6-independent variant of multiple myeloma (28). Activation of STAT3 (signal transducers and activators of transcription 3), an important intermediate of IL-6 signaling, was observed in IL-6-independent plasmacytomas (29). The genetic experiment presented here suggests that KSHV latent genes, too, can compensate for IL-6 in the early stages of B cell activation and development. The miRNAs are known for their profound effects on cell lineage development and differentiation. The KSHV miRNAs, most likely, evolved to foster initial infection and latent persistence in naive B cells and eventual, preferential expansion of infected cells. One mechanism to facilitate this “goal” would be to compensate for limiting host activators, such as IL-6.
ACKNOWLEDGMENTS
We thank Blossom Damania for critical reading and helpful discussion.
This study was supported by a faculty development award to S.-H.S. under UNC CFAR P30 AI50410, and public health service grants CA109232 and CA019014 (D.P.D). The UNC LCCC Animal Histopathology Core is supported in part by an NCI Center Core Support Grant (CA16086) to the UNC LCCC. The UNC Flow Cytometry Core Facility is supported in part by an NCI Center Core Support Grant (P30CA016086) to the UNC LCCC.
REFERENCES
- 1.Kovalchuk AL, Kim JS, Park SS, Coleman AE, Ward JM, Morse HC, Kishimoto T, Potter M, Janz S. 2002. IL-6 transgenic mouse model for extraosseous plasmacytoma. Proc Natl Acad Sci U S A 99:1509–1514. doi: 10.1073/pnas.022643999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suematsu S, Matsuda T, Aozasa K, Akira S, Nakano N, Ohno S, Miyazaki J, Yamamura K, Hirano T, Kishimoto T. 1989. IgG1 plasmacytosis in interleukin 6 transgenic mice. Proc Natl Acad Sci U S A 86:7547–7551. doi: 10.1073/pnas.86.19.7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. 2007. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science 317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 4.Lattanzio G, Libert C, Aquilina M, Cappelletti M, Ciliberto G, Musiani P, Poli V. 1997. Defective development of pristane-oil-induced plasmacytomas in interleukin-6-deficient BALB/c mice. Am J Pathol 151:689–696. [PMC free article] [PubMed] [Google Scholar]
- 5.Beck J, Hsu S, Wijdenes J, Bataille R, Klein B, Vesole D, Hayden K, Jagannath S, Barlogie B. 1994. Brief report: alleviation of systemic manifestations of Castleman's disease by monoclonal anti-interleukin-6 antibody. N Engl J Med 330:602–605. doi: 10.1056/NEJM199403033300904. [DOI] [PubMed] [Google Scholar]
- 6.Brandt SJ, Bodine DM, Dunbar CE, Nienhuis AW. 1990. Dysregulated interleukin 6 expression produces a syndrome resembling Castleman's disease in mice. J Clin Invest 86:592–599. doi: 10.1172/JCI114749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshizaki K, Matsuda T, Nishimoto N, Kuritani T, Taeho L, Aozasa K, Nakahata T, Kawai H, Tagoh H, Komori T. 1989. Pathogenic significance of interleukin-6 (IL-6/BSF-2) in Castleman's disease. Blood 74:1360–1367. [PubMed] [Google Scholar]
- 8.Burger R, Wendler J, Antoni K, Helm G, Kalden JR, Gramatzki M. 1994. Interleukin-6 production in B-cell neoplasias and Castleman's disease: evidence for an additional paracrine loop. Ann Hematol 69:25–31. doi: 10.1007/BF01757344. [DOI] [PubMed] [Google Scholar]
- 9.Carbone A, Vaccher E, Gloghini A, Pantanowitz L, Abayomi A, de Paoli P, Franceschi S. 2014. Diagnosis and management of lymphomas and other cancers in HIV-infected patients. Nat Rev Clin Oncol 11:223–238. doi: 10.1038/nrclinonc.2014.31. [DOI] [PubMed] [Google Scholar]
- 10.Tamburro KM, Yang D, Poisson J, Fedoriw Y, Roy D, Lucas A, Sin S-H, Malouf N, Moylan V, Damania B, Moll S, van der Horst C, Dittmer DP. 2012. Vironome of Kaposi sarcoma associated herpesvirus-inflammatory cytokine syndrome in an AIDS patient reveals co-infection of human herpesvirus 8 and human herpesvirus 6A. Virology 433:220–225. doi: 10.1016/j.virol.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uldrick TS, Wang V, O'Mahony D, Aleman K, Wyvill KM, Marshall V, Steinberg SM, Pittaluga S, Maric I, Whitby D, Tosato G, Little RF, Yarchoan R. 2010. An interleukin-6-related systemic inflammatory syndrome in patients co-infected with Kaposi sarcoma-associated herpesvirus and HIV but without multicentric Castleman disease. Clin Infect Dis 51:350–358. doi: 10.1086/654798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asou H, Said JW, Yang R, Munker R, Park DJ, Kamada N, Koeffler HP. 1998. Mechanisms of growth control of Kaposi's sarcoma-associated herpes virus-associated primary effusion lymphoma cells. Blood 91:2475–2481. [PubMed] [Google Scholar]
- 13.Miles SA, Rezai AR, Salazar-González JF, Vander Meyden M, Stevens RH, Logan DM, Mitsuyasu RT, Taga T, Hirano T, Kishimoto T. 1990. AIDS Kaposi sarcoma-derived cells produce and respond to interleukin 6. Proc Natl Acad Sci U S A 87:4068–4072. doi: 10.1073/pnas.87.11.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foussat A, Wijdenes J, Bouchet L, Gaidano G, Neipel F, Balabanian K, Galanaud P, Couderc J, Emilie D. 1999. Human interleukin-6 is in vivo an autocrine growth factor for human herpesvirus-8-infected malignant B lymphocytes. Eur Cytokine Netw 10:501–508. [PubMed] [Google Scholar]
- 15.Jones KD, Aoki Y, Chang Y, Moore PS, Yarchoan R, Tosato G. 1999. Involvement of interleukin-10 (IL-10) and viral IL-6 in the spontaneous growth of Kaposi's sarcoma herpesvirus-associated infected primary effusion lymphoma cells. Blood 94:2871–2879. [PubMed] [Google Scholar]
- 16.Sin S-H, Roy D, Wang L, Staudt MR, Fakhari FD, Patel DD, Henry D, Harrington WJ, Damania BA, Dittmer DP. 2007. Rapamycin is efficacious against primary effusion lymphoma (PEL) cell lines in vivo by inhibiting autocrine signaling. Blood 109:2165–2173. doi: 10.1182/blood-2006-06-028092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neipel F, Albrecht J, Ensser A, Huang Y, Li J, Friedman-Kien A, Fleckenstein B. 1997. Human herpesvirus 8 encodes a homolog of interleukin-6. J Virol 71:839–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore PS, Boshoff C, Weiss RA, Chang Y. 1996. Molecular mimicry of human cytokine and cytokine response pathway genes by KSHV. Science 274:1739–1744. doi: 10.1126/science.274.5293.1739. [DOI] [PubMed] [Google Scholar]
- 19.Fakhari FD, Jeong JH, Kanan Y, Dittmer DP. 2006. The latency-associated nuclear antigen of Kaposi sarcoma-associated herpesvirus induces B cell hyperplasia and lymphoma. J Clin Invest 116:735–742. doi: 10.1172/JCI26190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sin S-H, Fakhari FD, Dittmer DP. 2010. The viral latency-associated nuclear antigen augments the B-cell response to antigen in vivo. J Virol 84:10653–10660. doi: 10.1128/JVI.00848-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sin S-H, Dittmer DP. 2013. Viral latency locus augments B-cell response in vivo to induce chronic marginal zone enlargement, plasma cell hyperplasia, and lymphoma. Blood 121:2952–2963. doi: 10.1182/blood-2012-03-415620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tangye SG. 2011. Staying alive: regulation of plasma cell survival. Trends Immunol 32:595–602. doi: 10.1016/j.it.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Jourdan M, Cren M, Robert N, Bollore K, Fest T, Duperray C, Guilloton F, Hose D, Tarte K, Klein B. 2014. IL-6 supports the generation of human long-lived plasma cells in combination with either APRIL or stromal cell-soluble factors. Leukemia 28:1647–1656. doi: 10.1038/leu.2014.61. [DOI] [PubMed] [Google Scholar]
- 24.Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, Zinkernagel R, Bluethmann H, Kohler G. 1994. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature 368:339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- 25.Yoshizaki K, Nakagawa T, Fukunaga K, Tseng LT, Yamamura Y, Kishimoto T. 1984. Isolation and characterization of B cell differentiation factor (BCDF) secreted from a human B lymphoblastoid cell line. J Immunol 132:2948–2954. [PubMed] [Google Scholar]
- 26.Thai T-H, Calado DP, Casola S, Ansel KM, Xiao C, Xue Y, Murphy A, Frendewey D, Valenzuela D, Kutok JL, Schmidt-Supprian M, Rajewsky N, Yancopoulos G, Rao A, Rajewsky K. 2007. Regulation of the germinal center response by microRNA-155. Science 316:604–608. doi: 10.1126/science.1141229. [DOI] [PubMed] [Google Scholar]
- 27.Sin S-H, Kim YB, Dittmer DP. 2013. Latency locus complements microRNA 155 deficiency in vivo. J Virol 87:11908–11911. doi: 10.1128/JVI.01620-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verdelli D, Nobili L, Todoerti K, Mosca L, Fabris S, D'Anca M, Pellegrino E, Piva R, Inghirami G, Capelli C, Introna M, Baldini L, Chiaramonte R, Lombardi L, Neri A. 2014. Molecular events underlying interleukin-6 independence in a subclone of the CMA-03 multiple myeloma cell line. Genes Chromosomes Cancer 53:154–167. doi: 10.1002/gcc.22127. [DOI] [PubMed] [Google Scholar]
- 29.Rawat R, Rainey GJ, Thompson CD, Frazier-Jessen MR, Brown RT, Nordan RP. 2000. Constitutive activation of STAT3 is associated with the acquisition of an interleukin 6-independent phenotype by murine plasmacytomas and hybridomas. Blood 96:3514–3521. [PubMed] [Google Scholar]