Abstract
Patients with mediastinal lymph node (LN) downstaging following neoadjuvant chemotherapy exhibit improved outcomes compared with patients with persistent N2 disease. The aim of this study was to compare clinicopathological characteristics and survival between patients with unexpected and expected persistent N2 disease following surgery for non-small-cell lung cancer (NSCLC). This retrospective analysis included 348 patients with NSCLC who underwent surgery following chemotherapy at the Shanghai Pulmonary Hospital, Tongji University School of Medicine, between 1995 and 2012. According to the results of the imaging examinations and postoperative pathology, the patients were divided into three groups, namely groups I (nodal downstaging, pN0-1), II (expected persistent N2 disease) and III (unexpected persistent N2 disease). The rates of overall survival (OS) and disease-free survival (DFS) were estimated by the Kaplan-Meier method. Univariate and multivariate analyses were performed to identify the independent risk factors for OS and DFS. The mortality rate was 1.1% during the postoperative period. Perioperative complications occurred in 45 patients (12.9%). The 5-year OS rate was 32.2, 6.3 and 25.9% in groups I, II and III, respectively (group I vs. III, P=0.023; and group III vs. II, P<0.001). The 5-year DFS rate was 30.1, 5.1 and 22.4% in groups I, II and III, respectively (group I vs. III, P=0.012; and group III vs. II, P<0.001). Grouping, predicted forced expiratory volume in 1 sec, N downstaging and skip N2 metastasis were identified as independent predictive factors associated with OS, whereas the independent risk factors associated with DFS were grouping and N downstaging. Patients with unexpected persistent N2 disease exhibited better survival compared with those with expected persistent N2 disease. Surgery following chemotherapy remains the optimal approach for a proportion of patients with persistent N2 disease.
Keywords: neoadjuvant chemotherapy, unexpected persistent N2 disease, surgery, non-small-cell lung cancer
Introduction
Mediastinal lymph node (LN) metastasis is one of the most significant prognostic factors in patients with non-small-cell lung cancer (NSCLC) and accurate N staging is a crucial step prior to decision making regarding the treatment of NSCLC patients (1). However, the optimal treatment approach for NSCLC patients with operable N2 disease remains controversial. According to a previous study, direct surgery for patients with clinically apparent N2 disease achieved a long-term survival rate of <10% of (2). However, certain phase II trials and a limited number of phase III trials suggested improved long-term survival for NSCLC patients with N2 disease treated with neoadjuvant therapy followed by surgical resection, compared with those undergoing surgery alone (3–7). Additionally, Pataer et al reported that the histopathological response to neoadjuvant chemotherapy was significantly associated with long-term overall survival (OS) in NSCLC patients with N2 disease (8). In a prospective phase II study evaluating neoadjuvant cisplatin and docetaxel, the 3-year survival rate was 61% in patients with pathologically negative mediastinal LNs, compared with 11% in those with persistent N2 disease, following chemotherapy (9). Despite meticulous efforts to assess the nodal status, unexpected persistent N2 disease following induction (neoadjuvant) chemotherapy may be identified following surgical treatment for NSCLC, as false-negative results are intrinsic to the preoperative staging workup. The fact that persistent N2 disease was unexpectedly detected in surgical specimens of patients with clinical N0-1 disease following neoadjuvant chemotherapy suggests that the pathological extent of N2 disease may be microscopic rather than macroscopic in such cases (10). However, the optimal treatment selection for this subgroup of patients remains debated upon and the role of surgery following induction therapy has not yet been clearly determined.
We reviewed our experience with patients treated with neoadjuvant chemotherapy for N2 NSCLC followed by surgical resection. The objective of this study was to compare the clinicopathological characteristics and survival outcomes between patients with unexpected persistent N2 disease (clinical N0-1, pathological N2) and those with expected persistent N2 disease (clinical N2, pathological N2) undergoing surgery following neoadjuvant chemotherapy for NSCLC.
Patients and methods
Patient population
The medical records of 392 consecutive patients with a pathological diagnosis of NSCLC who received neoadjuvant chemotherapy at the Department of General Thoracic Surgery, Shanghai Pulmonary Hospital (Shanghai, China) between January, 1995 and December, 2012 were reviewed. All the patients underwent contrast-enhanced thoracic computed tomography (CT) or 18F-fluorodeoxyglucose (FDG)-positron emission tomography (PET)/CT prior to surgery. Additional routine pretreatment evaluations included chest radiography, abdominal ultrasonography or CT scan, brain magnetic resonance imaging or CT scan, cardiopulmonary function tests and whole-body radionuclide bone scanning. Mediastinoscopy or endobronchial ultrasonography-guided transbronchial needle aspiration biopsy were routinely performed on patients with bulky mediastinal LNs on CT (short-axis diameter >1.5 cm). The patients were staged according to the seventh edition of the TNM classification (11).
The inclusion criteria were patients with pathological N2 disease prior to treatment and administration of neoadjuvant chemotherapy. The chemotherapeutic regimens included platinum in combination with gemcitabine, vinorelbine, paclitaxel or docetaxel. The exclusion criteria were patients with superior sulcus tumors, lack of indications for surgery, treatment with induction radiotherapy and palliative surgery. Finally, a total of 348 patients were enrolled in this study.
Response to neoadjuvant chemotherapy
After two cycles of neoadjuvant chemotherapy, the response to treatment was classified according to the World Health Organization criteria by CT scan and bronchoscopy (12) as follows: Complete response, disappearance of all disease on radiographic and bronchoscopic (when performed) examination; partial response, >50% reduction in the volume of all measurable lesions; stable disease, no detectable change in tumor volume of all lesions or change in the size of all measured lesions between a 50% reduction and a 25% increase, with no additional disease detected; progressive disease, increase of >25% of all measured lesions or appearance of new lesions. Complete and partial response are both classified as objective response. Only patients with objective response or stable disease were considered eligible for surgery.
Definitions and grouping
A resection was considered as complete (R0) when there was no residual tumor at the bronchial or vascular margins and no residual LN disease. Nodal downstaging was defined as resected mediastinal LNs free of gross and microscopic disease. Clinical nodal downstaging was defined as a long-axis diameter of mediastinal LNs <1 cm on chest CT or negative PET/CT findings; pathological nodal downstaging was defined as absence of microscopic disease in the resected mediastinal nodes.
According to the definition of clinical or pathological nodal downstaging, all the enrolled cases were assigned into three study groups as follows: Group I, nodal downstaging (pN0-1); group II, no nodal downstaging (expected persistent N2 disease); and group III, clinical nodal downstaging without pathological nodal downstaging (unexpected persistent N2 disease).
Surgical procedures
All the patients received R0 resection. The surgical procedures included lobectomy, bilobectomy, sleeve resection and pneumonectomy. Systemic lymphadenectomy was defined as removal of at least three mediastinal stations and >10 nodes, additional to the resection of hilar nodes (13). Systemic lymphadenectomy was mandatory for all the patients in this study. Mediastinal LN dissection consisted of en bloc resection of all nodes at stations 2R, 4R, 7, 8, 9 and 10R for right-sided tumors and nodes at stations 4L, 5, 6, 7, 8, 9 and 10L for left-sided tumors. Bronchial stumps underwent routine frozen sections to ensure microscopically tumor-free margins in all the cases.
Postoperative treatment and follow-up
Adjuvant chemotherapy was administered to the patients included in this study and mediastinal regional radiotherapy was performed in patients with persistent N2 disease, provided they were able to tolerate additional treatments. The patients were regularly evaluated by CT every 6 months for the first 2 years after surgery and every 12 months thereafter.
All the patients were followed up by trained staff every 6 months, by phone or e-mail. All patient information regarding survival, cancer recurrence or metastasis and cause of death were recorded. In the present study, the endpoint of follow-up was December, 2012, with a mean follow-up of 55 months.
Statistical analysis
Numerical data are expressed in terms of frequency, mean and standard deviation and categorical variables as percentages. The Chi-square test or Fisher's exact test were used to compare proportional data. One-way analysis of variance or the Kruskal-Wallis test, depending on the normality of distribution, were used to compare continuous variables among the three groups. Survival curves were constructed using the Kaplan-Meier method and were compared univariately using the log-rank test. The Cox-regression test was used for multivariate analysis of survival. All the statistical tests were two-sided, with a significance level set at 0.05.
Results
Patient characteristics
The patient characteristics are summarized in Table I. The study included 273 men and 75 women, with a mean age of 55 years (range, 27–78 years). The most common comorbidities were chronic obstructive pulmonary disease in 79 (22.7%) and cardiovascular diseases in 41 (11.8%) patients. A total of 264 (75.9%) patients underwent invasive staging of the mediastinum by mediastinoscopy or endobronchial ultrasonography-guided transbronchial needle aspiration biopsy, whereas 84 (24.1%) were staged by percutaneous lung puncture biopsy and CT or PET/CT scan. The tumors were considered fully resectable in all the patients. More patients in group I underwent lobectomy compared with groups II and III (P<0.001) and fewer patients in group II achieved an objective response compared with groups I and III (P<0.001). As regards histological subtype, the percentage of adenocarcinoma was 28.9, 20.3 and 39.7% in groups I, II and III, respectively (P=0.002). In addition, a lower proportion of group II patients exhibited a higher clinical T stage compared with the other two groups (P=0.002) (Table II).
Table I.
Clinical characteristics of the patients (n=348).
| Variables | Patient no. (%) |
|---|---|
| Groups | |
| I | 211 (60.6) |
| II | 79 (22.7) |
| III | 58 (16.7) |
| Gender | |
| Male | 273 (78.4) |
| Female | 75 (21.6) |
| Age (years) | |
| ≤60 | 189 (54.3) |
| >60 | 159 (45.7) |
| Smoking history | |
| Yes | 249 (71.6) |
| No | 99 (28.4) |
| Predicted FEV1 | |
| ≤60% | 40 (11.5) |
| >60% | 308 (88.5) |
| Clinical T stage | |
| T1 | 34 (9.8) |
| T2 | 196 (56.3) |
| T3 | 92 (26.4) |
| T4 | 26 (7.5) |
| Comorbidities | |
| Yes | 124 (35.6) |
| No | 224 (64.4) |
| Clinical N2 levels | |
| Single | 298 (85.6) |
| Multiple | 50 (14.4) |
| Clinical stage | |
| IIIA | 323 (92.8) |
| IIIB | 25 (7.2) |
| Imaging response | |
| Objective response | 264 (75.9) |
| Stable disease | 84 (24.1) |
| Type of operation | |
| Lobectomy | 181 (52.0) |
| Bilobectomy | 55 (15.8) |
| Pneumonectomy | 112 (32.2) |
| Histology | |
| Adenocarcinoma | 100 (28.7) |
| Squamous cell carcinoma | 195 (56.0) |
| Adenosquamous carcinoma | 43 (12.4) |
| Others | 10 (2.9) |
| N downstaging | |
| Yes | 211 (60.6) |
| No | 137 (39.4) |
| Skip N2 metastasis | |
| Yes | 60 (17.2) |
| No | 288 (82.8) |
FEV1; forced expiratory volume in 1 sec; CT; computed tomography.
Table II.
Comparison of main clinicopathological characteristics among the three groups.
| Patient no. (%) | ||||
|---|---|---|---|---|
| Variables | Group I (n=211) | Group II (n=79) | Group III (n=58) | P-value |
| Gender | 0.343 | |||
| Male | 171 (81.0) | 59 (74.7) | 43 (74.1) | |
| Female | 40 (19.0) | 20 (25.3) | 15 (25.9) | |
| Age (years) | 0.707 | |||
| ≤60 | 113 (53.6) | 46 (58.2) | 30 (51.7) | |
| >60 | 98 (46.4) | 33 (41.8) | 28 (48.3) | |
| Smoking history | 0.738 | |||
| Yes | 148 (70.1) | 59 (74.7) | 42 (72.4) | |
| No | 63 (29.9) | 20 (25.3) | 16 (27.6) | |
| Predicted FEV1 | 0.106 | |||
| ≤60% | 27 (12.8) | 4 (5.1) | 9 (15.5) | |
| >60% | 184 (87.2) | 75 (94.9) | 49 (84.5) | |
| Clinical T stage | 0.002 | |||
| T1 | 19 (9.0) | 11 (13.9) | 4 (6.9) | |
| T2 | 131 (62.1) | 32 (40.5) | 33 (56.9) | |
| T3 | 41 (19.4) | 33 (41.8) | 18 (31.0) | |
| T4 | 20 (9.5) | 3 (3.8) | 3 (5.2) | |
| Comorbidities | 0.481 | |||
| Yes | 80 (37.9) | 24 (30.4) | 20 (34.5) | |
| No | 131 (62.1) | 55 (69.6) | 38 (65.5) | |
| Clinical N2 levels | 0.087 | |||
| Single | 187 (88.6) | 62 (78.5) | 49 (84.5) | |
| Multiple | 24 (11.4) | 17 (21.5) | 9 (15.5) | |
| Clinical stage | 0.252 | |||
| IIIA | 192 (91.0) | 76 (96.2) | 55 (94.8) | |
| IIIB | 19 (9.0) | 3 (3.8) | 3 (5.2) | |
| Imaging response | <0.001 | |||
| Objective response | 199 (94.3) | 7 (8.9) | 58 (100.0) | |
| Stable disease | 12 (5.7) | 72 (91.1) | 0 (0.0) | |
| Type of operation | <0.001 | |||
| Lobectomy | 132 (62.6) | 27 (34.2) | 22 (37.9) | |
| Bilobectomy | 37 (17.5) | 11 (13.9) | 7 (12.1) | |
| Pneumonectomy | 42 (19.9) | 41 (51.9) | 29 (50.0) | |
| Histology | 0.002 | |||
| Adenocarcinoma | 61 (28.9) | 16 (20.3) | 23 (39.7) | |
| Squamous cell carcinoma | 127 (60.2) | 40 (50.6) | 28 (48.3) | |
| Adenosquamous carcinoma | 17 (8.1) | 20 (25.3) | 6 (10.3) | |
| Others | 6 (2.8) | 3 (3.8) | 1 (1.7) | |
| N downstaging | <0.001 | |||
| Yes | 211 (100.0) | 0 (0.0) | 0 (0.0) | |
| No | 0 (0.0) | 79 (100.0) | 58 (100.0) | |
| Skip N2 metastasis | <0.001 | |||
| Yes | 0 (0.0) | 32 (40.5) | 28 (48.3) | |
| No | 211 (100.0) | 47 (59.5) | 30 (51.7) | |
FEV1; forced expiratory volume in 1 sec; CT, computed tomography.
Outcomes of neoadjuvant chemotherapy
All the chemotherapeutic regimens contained platinum in combination with gemcitabine in 135 cases (38.8%), vinorelbine in 58 (16.7%), paclitaxel in 31 (8.9%) and docetaxel in 124 (35.6%) cases (data not shown). Following completion of the neoadjuvant chemotherapy, imaging was repeated in all the patients (CT in 82% and PET/CT in 18% of the patients). In total, 264 patients (75.9%) exhibited a radiographic objective response to chemotherapy (31 complete and 233 partial responses), whereas 84 cases exhibited stable disease.
Survival
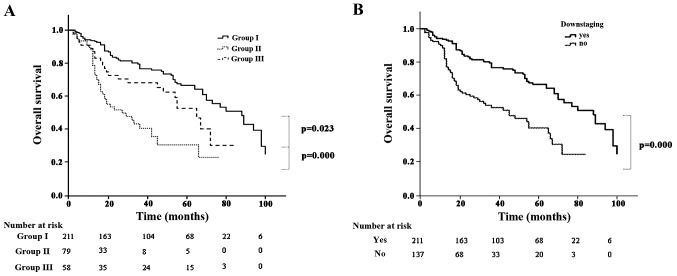
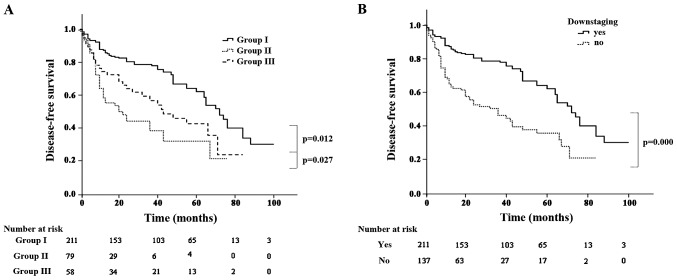
The mortality rate was 1.1% during the postoperative period (n=4). The causes of death included pneumonia (n=1), cardiovascular disease (n=2) and acute respiratory distress syndrome (n=1). Perioperative complications occurred in 45 patients (12.9%). The median duration of follow-up for the entire cohort was 55.2 months (range, 1–100 months). A total of 18 patients were lost to follow-up. At the end of the follow-up period, 137 patients (39.4%) had died (17 from tumor-unrelated causes), 165 patients (47.4%) remained alive and free of disease and 46 (13.2%) remained alive with recurrence. The 5-year OS rate was 32.2, 6.3 and 25.9% in groups I, II and III, respectively (group I vs. III, P=0.023; and group III vs. II, P<0.001) (Fig. 1A). Patients with persistent N2 disease had a lower 5-year OS compared with that of patients whose disease was downstaged (14.6 vs. 32.2%, respectively; P<0.001) (Fig. 1B). Recurrence occurred in 166 patients (47.7%). The pattern of recurrence was locoregional in 69 patients, distant in 71 and both in 26. The 5-year disease-free survival (DFS) rate was 30.1, 5.1 and 22.4% in groups I, II and III, respectively (group I vs. III, P=0.012; and group III vs. II, P=0.027) (Fig. 2A). Patients with persistent N2 disease had a lower 5-year DFS compared with that of patients whose disease was downstaged (12.5. vs. 30.7%, respectively; P<0.001) (Fig. 2B).
Figure 1.
(A) Overall survival according to subgroup; (B) overall survival according to N downstaging.
Figure 2.
(A) Disease-free survival according to subgroup; (B) disease-free survival according to N downstaging.
Multivariate analysis of OS and DFS
All the variables were included in the multivariate analysis. The results are summarized in Tables III and IV. Grouping (P=0.016), predicted forced expiratory volume in 1 sec (P=0.035), N downstaging (P=0.013) and skip N2 metastasis (P=0.003) were identified as independent predictive factors associated with OS, whereas grouping (P=0.037) and N downstaging (P=0.032) were found to be independent risk factors associated with DFS.
Table III.
Multivariate analysis of overall survival.
| Variables | Standard error | Hazard ratio | 95% CI | P-value |
|---|---|---|---|---|
| Grouping | 0.359 | 1.419 | 0.207–0.848 | 0.016 |
| Predicted FEV1 | 0.247 | 1.680 | 1.036–2.724 | 0.035 |
| N downstaging | 0.675 | 2.305 | 1.423–5.074 | 0.013 |
| Skip N2 metastasis | 0.253 | 2.104 | 1.281–3.466 | 0.003 |
FEV1; forced expiratory volume in 1 sec; CI, confidence interval.
Table IV.
Multivariate analysis of disease-free survival.
| Variables | Standard error | Hazard ratio | 95% CI | P-value |
|---|---|---|---|---|
| Grouping | 0.351 | 1.481 | 0.242–0.958 | 0.037 |
| N downstaging | 0.253 | 2.145 | 1.125–3.523 | 0.032 |
CI, confidence interval.
Discussion
The optimal treatment for resectable N2 NSCLC remains controversial. Neoadjuvant chemotherapy was shown to improve survival compared with surgery alone in certain randomized trials (7,14,15). However, these results were not confirmed by other randomized studies (16,17). Additionally, a potential role for radiation therapy in the treatment of N2 disease has been considered. A study by van Meerbeeck et al reported no differences in survival between surgery and radiation therapy following induction chemotherapy (18). However, the different selection criteria determining the optimal approach may be difficult to evaluate, due to the significant heterogeneity among N2 patients.
Previous studies demonstrated that a mediastinal pathological complete response was associated with improved outcomes following resection compared with persistent N2 disease, with long-term survival rates of patients with persistent N2 disease of <10–15% (6,9). In our study, the 5-year survival of patients with persistent N2 disease was 14.6%, which was significantly lower compared with that in patients with N downstaging (32.2%, P<0.001). This was also consistent with previous data. Therefore, certain studies concluded that appropriate adjuvant therapies may be associated with better outcomes in patients with persistent N2 disease compared with surgery alone (2,6,7).
CT or PET/CT imaging may yield false-positive or false-negative results, as they are based on the morphology or FDG uptake of LNs. Silvestri et al reported the sensitivities of CT and PET/CT for accurate detection of LN metastasis to be 51 and 74%, respectively, with respective specificities of 86 and 85% (19). Consequently, false-negative results may be obtained with imaging examinations, i.e., unexpected persistent N2 disease following surgery, even when preoperative imaging suggests N downstaging. Several authors have reported on the outcomes of unexpected N2 disease (20,21); however, the number of studies investigating unexpected persistent N2 disease is limited. In our study, a total of 348 patients with resectable N2 NSCLC received neoadjuvant chemotherapy. Among these patients, 58 with persistent N2 disease had been considered to be N-downstaged on either CT or PET/CT prior to surgery (16.7%).
In the present study, we compared the survival outcomes among patients with N downstaging (pN0-1, group I), those with positive imaging findings and pathological N2 disease (expected persistent N2, group II) and those with negative imaging findings but positive pathological N2 disease (unexpected persistent N2, group III). The 5-year OS rate was 32.2 and 25.9% in groups I and III, respectively (P=0.023), whereas the 5-year DFS rate was 30.1 and 22.4% in groups I and III, respectively (P=0.012). OS and DFS in group I were significantly higher compared with those in group III. This may be attributed to patients in group III undergoing more extensive resection compared with those in group I (P<0.001), although the main reason was the disease stage of the patients in group III. Of note, in our study, there was a significant difference in OS and DFS between groups II and III. Alhough both these groups had persistent N2 disease, different clinical characteristics were associated with different survival. Furthermore, the 5-year OS rate in group III was significantly higher compared with that of patients with persistent N2 disease (22.4 vs. 14.6%, P=0.033), suggesting that surgery was beneficial for certain patients with persistent N2 disease.
The rate of objective response to chemotherapy was 75.9% (n=264). It was previously reported that objective response was observed in >50% of cases (4,5,7,22) and certain studies reported that response to chemotherapy was a strong prognostic factor (4,7,21). Accordingly, in our study, grouping and N downstaging were found to be independent predictive factors associated with OS and DFS, reflecting response to chemotherapy. Mediastinal node downstaging on the resected specimen was a powerful and independent prognostic factor, reflecting the radical resection of systemic disease and effectiveness of chemotherapy. However, this factor was based on postoperative specimen examination and patient selection may be difficult. However, in our study, we demonstrated that patients with unexpected persistent N2 disease exhibited satisfactory survival rates. Port reported that certain patients may be cured by surgery, particularly in case of previous response to chemotherapy (23).
In patients with persistent N2 disease, mediastinal LN-related variables, such as the number of N2 levels, may play a role (3,6). In our series, we did not find N2-related variables to be associated with survival. All the patients in our study received neoadjuvant chemotherapy and the variability of protocols and regimens may be a significant prognostic factor. In fact, there was no difference regarding clinical response or pathological nodal status and we did not identify an association between regimen of chemotherapy and survival. In our study, mediastinal regional radiotherapy was performed in patients with persistent N2 disease, provided they were able to tolerate additional treatments.
Our study had certain limitations. Due to the retrospective nature of the study, some potentially predictive clinical variables were not included; therefore, this study had certain intrinsic drawbacks. Our study was limited by the small patient sample and important information regarding the optimal local treatment modality for patients with persistent N2 disease could not be obtained. Our results suggested that surgery provided a significant survival benefit for patients with unexpected N2 disease and should be first considered in this population. As regards the optimal treatment for patients with expected N2 disease, our experience suggests that a definitive course of radiotherapy (≥60 Gy) is generally recommended, combined with chemotherapy.
In conclusion, we retrospectively analyzed the clinical and pathological characteristics of NSCLC patients with N2 disease who underwent surgery following neoadjuvant chemotherapy. The survival outcomes were compared according to whether persistent N2 disease was preoperatively expected on the basis of CT or PET/CT following chemotherapy. Patients with unexpected persistent N2 disease exhibited better survival outcomes compared with those with expected persistent N2 disease. These findings may be used to select the optimal therapeutic approach to persistent N2 disease.
References
- 1.Groome PA, Bolejack V, Crowley JJ, et al. The IASLC Lung Cancer Staging Project: validation of the proposals for the revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:694–705. doi: 10.1097/JTO.0b013e31812d05d5. [DOI] [PubMed] [Google Scholar]
- 2.Martini N, Flehinger BJ. The role of surgery in N2 lung cancer. Surg Clin North Am. 1987;67:1037–1049. doi: 10.1016/s0039-6109(16)44341-0. [DOI] [PubMed] [Google Scholar]
- 3.Andre F, Grunenwald D, Pignon JP, Dujon A, Pujol JL, Brichon PY, Brouchet L, Quoix E, Westeel V, Le Chevalier T. Survival of patients with resected N2 non-small-cell lung cancer. Evidence for a subclassification and implications. J Clin Oncol. 2000;18:2981–2989. doi: 10.1200/JCO.2000.18.16.2981. [DOI] [PubMed] [Google Scholar]
- 4.Albain KS, Rusch VW, Crowley JJ, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non-small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol. 1995;13:1880–1892. doi: 10.1200/JCO.1995.13.8.1880. [DOI] [PubMed] [Google Scholar]
- 5.Martini N, Kris MG, Flehinger BJ, et al. Preoperative chemotherapy for stage IIIa (N2) lung cancer: The Sloan-Kettering experience with 136 patients. Ann Thorac Surg. 1993;55:1365–1373. doi: 10.1016/0003-4975(93)91072-U. discussion 1373–1374. [DOI] [PubMed] [Google Scholar]
- 6.Lorent N, De Leyn P, Lievens Y, Verbeken E, Nackaerts K, Dooms C, Van Raemdonck D, Anrys B, Vansteenkiste J. Leuven Lung Cancer Group: Long-term survival of surgically staged IIIA-N2 non-small-cell lung cancer treated with surgical combined modality approach: Analysis of a 7-year prospective experience. Ann Oncol. 2004;15:1645–1653. doi: 10.1093/annonc/mdh435. [DOI] [PubMed] [Google Scholar]
- 7.Rosell R, Gómez-Codina J, Camps C, et al. A randomized trial comparing preoperative chemotherapy plus surgery with surgery alone in patients with non-small-cell lung cancer. N Engl J Med. 1994;330:153–158. doi: 10.1056/NEJM199401203300301. [DOI] [PubMed] [Google Scholar]
- 8.Pataer A, Kalhor N, Correa AM, et al. University of Texas M. Histopathology. J Thorac Oncol. 2012;7:825–832. doi: 10.1097/JTO.0b013e318247504a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Betticher DC, Schmitz Hsu SF, Tötsch M, et al. Mediastinal lymph node clearance after docetaxel-cisplatin neoadjuvant chemotherapy is prognostic of survival in patients with stage IIIA pN2 non-small-cell lung cancer: a multicenter phase II trial. J Clin Oncol. 2003;21:1752–1759. doi: 10.1200/JCO.2003.11.040. [DOI] [PubMed] [Google Scholar]
- 10.Kim YN, Yi CA, Lee KS, et al. A proposal for combined MRI and PET/CT interpretation criteria for preoperative nodal staging in non-small-cell lung cancer. Eur Radiol. 2012;22:1537–1546. doi: 10.1007/s00330-012-2388-3. [DOI] [PubMed] [Google Scholar]
- 11.Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 12.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–214. doi: 10.1002/1097-0142(19810101)47:1<207::AID-CNCR2820470134>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 13.Naruke T, Suemasu K, Ishikawa S. Lymph node mapping and curability at various levels of metastasis in resected lung cancer. J Thorac Cardiovasc Surg. 1978;76:832–839. [PubMed] [Google Scholar]
- 14.Pass HI, Pogrebniak HW, Steinberg SM, Mulshine J, Minna J. Randomized trial of neoadjuvant therapy for lung cancer, Interim analysis. Ann Thorac Surg. 1992;53:992–998. doi: 10.1016/0003-4975(92)90373-C. [DOI] [PubMed] [Google Scholar]
- 15.Roth JA, Fossella F, Komaki R, et al. A randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. J Natl Cancer Inst. 1994;86:673–680. doi: 10.1093/jnci/86.9.673. [DOI] [PubMed] [Google Scholar]
- 16.Depierre A, Milleron B, Moro-Sibilot D, et al. French Thoracic Cooperative Group: Preoperative chemotherapy followed by surgery compared with primary surgery in resectable stage I (except T1N0), II, and IIIa non-small-cell lung cancer. J Clin Oncol. 2002;20:247–253. doi: 10.1200/JCO.20.1.247. [DOI] [PubMed] [Google Scholar]
- 17.Nagai K, Tsuchiya R, Mori T, Tada H, Ichinose Y, Koike T, Kato H. Lung Cancer Surgical Study Group of the Japan Clinical Oncology Group. A randomized trial comparing induction chemotherapy followed by surgery with surgery alone for patients with stage IIIA N2 non-small cell lung cancer (JCOG 9209) J Thorac Cardiovasc Surg. 2003;125:254–260. doi: 10.1067/mtc.2003.15. [DOI] [PubMed] [Google Scholar]
- 18.van Meerbeeck JP, Kramer GW, Van Schil PE, et al. European Organisation for Research and Treatment of Cancer-Lung Cancer Group: Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst. 2007;99:442–450. doi: 10.1093/jnci/djk093. [DOI] [PubMed] [Google Scholar]
- 19.Silvestri GA, Gould MK, Margolis ML, et al. Noninvasive staging of non-small cell lung cancer: ACCP evidenced-based clinical practice guidelines (2nd edition) Chest. 2007;132((Suppl 3)):178S–201S. doi: 10.1378/chest.07-1360. [DOI] [PubMed] [Google Scholar]
- 20.Kim HK, Choi YS, Kim K, Shim YM, Park K, Ahn YC, Lee KS, Choi JY, Kim J. Outcomes of mediastinoscopy and surgery with or without neoadjuvant therapy in patients with non-small cell lung cancer who are N2 negative on positron emission tomography and computed tomography. J Thorac Oncol. 2011;6:336–342. doi: 10.1097/JTO.0b013e318201212e. [DOI] [PubMed] [Google Scholar]
- 21.Al-Sarraf N, Aziz R, Gately K, Lucey J, Wilson L, McGovern E, Young V. Pattern and predictors of occult mediastinal lymph node involvement in non-small cell lung cancer patients with negative mediastinal uptake on positron emission tomography. Eur J Cardiothorac Surg. 2008;33:104–109. doi: 10.1016/j.ejcts.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 22.Eberhardt W, Wilke H, Stamatis G, et al. Preoperative chemotherapy followed by concurrent chemoradiation therapy based on hyperfractionated accelerated radiotherapy and definitive surgery in locally advanced non-small-cell lung cancer: mature results of a phase II trial. J Clin Oncol. 1998;16:622–634. doi: 10.1200/JCO.1998.16.2.622. [DOI] [PubMed] [Google Scholar]
- 23.Port JL, Korst RJ, Lee PC, et al. Surgical resection for residual N2 disease after induction chemotherapy. Ann Thorac Surg. 2005;79:1686–1690. doi: 10.1016/j.athoracsur.2004.10.057. [DOI] [PubMed] [Google Scholar]