Abstract
Gallic acid (GA) is a natural phenolic compound that possesses various biological effects, including antioxidant, anti-inflammatory, antibiotic, anticancer, antiviral and cardiovascular protection activities. In addition, numerous studies have reported that antioxidants possess antiviral activities. Hepatitis C virus (HCV) is one of the most important causes of chronic liver diseases worldwide, but until recently, only a small number of antiviral agents had been developed against HCV. Therefore, the present study investigated whether GA exhibits an anti-HCV activity. The effects of GA on HCV expression were examined using a subgenomic HCV replicon cell culture system that expressed HCV nonstructural proteins (NSs). In addition, GA cytotoxicity was evaluated at concentrations between 100–600 mg/ml using an MTT assay. Huh-7 replicon cells were incubated with 300 mg/ml GA for different times, and the HCV-RNA and protein levels were measured by reverse transcription-quantitative polymerase chain reaction and western blot analysis, respectively. Pyrrolidine dithiocarbamate (PDTC) was used as an antioxidant control and reactive oxygen species (ROS) production was measured during the exposure. The results indicated that GA did not produce a statistically significant cytotoxicity in parental and HCV replicon cells. Furthermore, GA downregulated the expression levels of NS5A-HCV protein (~55%) and HCV-RNA (~50%) in a time-dependent manner compared with the levels in untreated cells. Notably, GA treatment decreased ROS production at the early time points of exposure in cells expressing HCV proteins. Similar results were obtained upon PDTC exposure. These findings suggest that the antioxidant capacity of GA may be involved in the downregulation of HCV replication in hepatoma cells.
Keywords: gallic acid, antioxidants, hepatitis C virus, oxidative stress
Introduction
Hepatitis C virus (HCV) is a major cause of chronic liver disease, and ~170 million people are infected with this virus worldwide. Patients with persistent HCV infection are at high risk of developing hepatocellular carcinoma, chronic liver diseases and cirrhosis (1). HCV is an enveloped flavivirus, which contains a positive single-stranded RNA of ~9,600 nucleotides (2,3). These nucleotides encode a single polypeptide of ~3,000 amino acids, which is divided into structural (core, E1 and E2) and nonstructural [p7, nonstructural protein (NS)2, NS3, NS4A, NS5A and NS5B] proteins through proteolysis (2,3).
HCV induces cell damage via different mechanisms that remain poorly understood. The generation of reactive oxygen species (ROS) and oxidative stress have been suggested to play major roles in the pathogenesis of chronic HCV infection (4). HCV infection is associated with increasing levels of various oxidative stress markers, including mitochondrial injury, iron overload and chronic inflammation, which are thought to be induced by HCV proteins (1–5). Until recently, the standard of care (SOC) for patients with chronic HCV infection consisted of a combination of pegylated interferon-α and ribavirin (6). At present, a new SOC is used for genotype 1 patients, which includes one protease inhibitor, such as telaprevir, boceprevir or simeprevir, in combination with pegylated interferon-α and ribavirin treatment (7).
A number of studies have reported the beneficial effects of antioxidants, such as glycyrrhizin, catechin, silymarin, phytosterols, N-acetylcysteine and phytochemicals, which are able to decrease HCV replication and liver damage (8). In our previous study, we reported that acetylsalicylic acid reduces the levels of HCV-RNA and viral protein by decreasing cellular oxidative stress and modifying the Cu/Zn superoxide dismutase expression (9). Furthermore, another study reported that carotene, vitamin D2 and linoleic acid inhibited HCV-RNA expression (10).
Gallic acid (GA), also known as 3,4,5-trihydroxybenzoic acid, is a phenolic compound obtained from plants, fruits and vegetables (11). Currently, GA is used in various sectors, for instance as a pharmaceutical, an industrial compound (12,13) and a food additive (14). Previous studies have reported that GA has certain biological effects, such as anti-inflammatory, antibiotic, antiviral, anticancer and cardiovascular protection effects (15,16). These effects result from the fact that GA is a potent antioxidant that is involved in absorbing and neutralizing free radicals produced by cells (17).
GA has also been found to significantly decrease the viability, proliferation and invasion of cancer cells (18–20). In addition, GA isolated from Woodfordia fruticosa flowers exhibited a higher anti-enterovirus 71 activity (21). Another study has demonstrated that GA possess anti-herpes simplex virus type 1 (HSV-1) and anti-human immunodeficiency virus activities (22).
Based on the aforementioned observations, the present study aimed to explore the effect of GA on HCV-RNA expression and further investigate the underlying mechanisms by measuring oxidative stress markers using an HCV subgenomic replicon cell culture system. Furthermore, a potent antioxidant, pyrrolidine dithiocarbamate (PDTC), was used as a control since its effect as an antioxidant has already been reported (23).
Materials and methods
Chemicals
GA, PDTC and H2O2 were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Cell culture
Huh7 hepatocarcinoma cells (donated by Dr. Koromilas; McGill University, Montreal, Canada) were cultured in advanced Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher Scientific, Inc., Grand Island, NY, USA) supplemented with 1% nonessential amino acids, 2% heat-inactivated fetal bovine serum (Hyclone; GE Healthcare Life Sciences, Logan, UT, USA), 1% antibiotics (100 U penicillin G and 100 µg/ml streptomycin; Thermo Fisher Scientific, Inc.) and 1% glutamine, in a humidified atmosphere with 5% CO2 at 37°C. In addition, a genotype 1b HCV subgenomic replicon cell culture system was established (24), which was maintained at the same conditions, but in the presence of 500 µg/ml G418 (Geneticin; Thermo Fisher Scientific, Inc.). Cells grown to 80–85% confluence were trypsinized with 2.5 ml trypsin diluted with fresh medium and counted using a hematocytometer (Marienfield-Superior, Lauda-Königshofen, Germany) with trypan blue (Gibco).
GA treatment and cytotoxic assay
Huh7 parental and HCV replicon cells were seeded onto 96-well plates (2×104 cells/well) and cultured for 24 h. Next, the medium was changed and the cells were treated with different concentrations of GA (100, 300 and 600 µM) dissolved in sterile phosphate buffered saline (PBS) and incubated for 0, 24, 48 and 72 h (25). Following incubation, cell viability was evaluated using an MTT [also known as 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay, according to the standard experimental protocol (26,27).
Total protein extraction
Huh7 HCV replicon cells (~6×105 cells/well) were seeded onto 6-well plates, cultured for 24 h and then treated with 300 µM GA for a duration between 0 and 72 h. After each time point, the cells were harvested and total protein extraction was performed. Briefly, the cells were washed twice with ice-cold 1X PBS/0.5 M EDTA, and proteins were extracted with 1X lysis buffer containing 10 mM Tris-HCl (pH 7.5), 50 mM KCl, 2 mM MgCl2, 1% Triton X-100, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 3 µg/ml aprotinin, 1 µg/ml leupeptin and 1 µg/ml pepstatin. Upon incubation, the cell lysates were centrifuged at 16,438 × g for 5 min at 4°C (28). The supernatant was recovered, the protein concentration was measured using the Bradford method with a Bio-Rad Protein Assay kit (500-0006; Bio-Rad Laboratories, Inc., Hercules, CA, USA), and a standard curve was obtained using bovine serum albumin (Amresco LLC, Solon, OH, USA).
Western blot analysis
Total cellular protein extracts were resolved by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Subsequently, the samples were transferred to Hybond-P polyvinylidene difluoride membranes (GE Healthcare Life Sciences, Little Chalfont, UK), activated with methanol, washed with pure water and equilibrated with 1X transfer buffer. The electrotransfer was performed at 100 V for 1 h at 4°C, and then the membrane was blocked with mouse anti-HCV NS5A monoclonal antibody (MAb; dilution, 1:1,000; AB20342; Abcam, Cambridge, UK) and anti-actin MAb (β-actin; dilution, 1:1,000; MAB1501; EMD Millipore, Billerica, MA, USA). Immunocomplexes on the membranes were detected using an enhanced chemiluminescence assay (Luminol, ImmunoCruz; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) (10). The expression of NS5A relative to β-actin protein was quantified using ImageJ software, version 1.46r (http://imagej.nih.gov/ij/docs/guide/146.html).
RNA extraction
Huh7 HCV replicon cells (~2×105 cells/well) were seeded onto 24-well plates, cultured for 24 h and then treated with 300 µM GA for a duration between 0 and 72 h. Cells were harvested after each time point and total RNA was extracted using TRIzol reagent (Ambion; Thermo Fisher Scientific, Inc.) according to the manufacturer's specifications. RNA precipitates were washed with 75% alcohol and resuspended in 12 µl RNase-free water, and then the samples were stored at −80°C (29).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) for HCV-RNA quantification
The total RNA extracted was subjected to RT to obtain complementary DNA (cDNA) using a SuperScript III RT kit according to the manufacturer's specifications (Applied Biosystems; Thermo Fisher Scientific, Inc., Foster City, CA, USA). Subsequently, 200 ng cDNA was amplified by qPCR to quantify the levels of HCV and GAPDH mRNA using an ABI-7500 Fast Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.). The primers used were as follows: HCV forward (75–93 nt), 5′-GCGTCTAGCCATGGCGTTA-3′, and reverse (138–157 nt), 5′-GGTTCCGCAGACCACTATGG-3′; GAPDH forward, 5′-GTGTTCCTACCCCCAATGTGT-3′, and reverse, 5′-ATTGTCATACCAGGAAATGAGCTT-3′; and the TaqMan probe (94–110 nt), 5′-FAM-CTGCACGACACTCATAC-NFQ-3′. For each PCR reaction, the following were used: 1 µl assay mix, 9 µl cDNA diluted in RNase-free water and 10 µl TaqMan PCR Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.). The thermal cycling conditions were as follows: Initial setup at 50°C for 2 min, then 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 60 sec. GAPDH-RNA expression was used to normalize the cDNA concentration and the amplification plots were analyzed using the ABI-7500 Real-Time PCR System software, version 2.0.1, according to the manufacturer's specifications (Applied Biosystems; Thermo Fisher Scientific, Inc.) (30). The 2−ΔΔCq method was used to calculate relative changes in gene expression determined from RT-qPCR experiments.
ROS measurement
To evaluate the effect of GA on the oxidative stress level, ROS production was measured. Briefly, Huh7 HCV replicon cells (~2×104 cells/well) were seeded onto 96-well plates, cultured for 24 h and then treated with 300 µM GA, 2 µM H2O2 as a damage control or 5 µM PDTC as an antioxidant control, for 0.5, 1, 3, 6, 12 and 24 h at 37°C. Next, 2 µl of 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA; Invitrogen Molecular Probes, Eugene, OR, USA) were added 30 min before the end of the treatment. The fluorescence of cells was measured at room temperature, using 485 nm and 528 nm as the excitation and emission wavelengths, respectively (BioTek Synergy H5; BioTek Instruments, Inc., Winooski, VT, USA) (31,32).
Statistical analysis
All variables were tested in triplicate, and experiments were repeated at least three times. Values were presented as the mean ± standard deviation. Statistically significant differences between control and treated groups were determined by Student's t-test. Differences were considered to be statistically significant for values of P<0.05.
Results
Viability of hepatoma Huh7 cells treated with GA
Initially, the study investigated whether GA induces a cytotoxic effect on treated cells. Huh7 parental and Huh7 HCV replicon cells were exposed to three different concentrations of GA (100, 300 and 600 µM) and incubated for a time between 24 and 72 h. Next, total cell count and viability determinations were performed using an MTT assay. Fig. 1 shows that after 72 h of treatment no statistically significant differences in cell number and viability were detectable between the untreated (cell viability, 100%) and treated cell lines (Fig. 1A, parental cells; Fig. 1B, HCV replicon cells) when using the 100 and 300 µM GA concentrations (cell viability, ~98 and 95%, respectively). By contrast, cells treated with 600 µM GA showed a lower cell survival rate in the two cell lines at all times of exposure. Based on this finding, we selected the concentration of 300 µM GA in all subsequent experiments.
Figure 1.
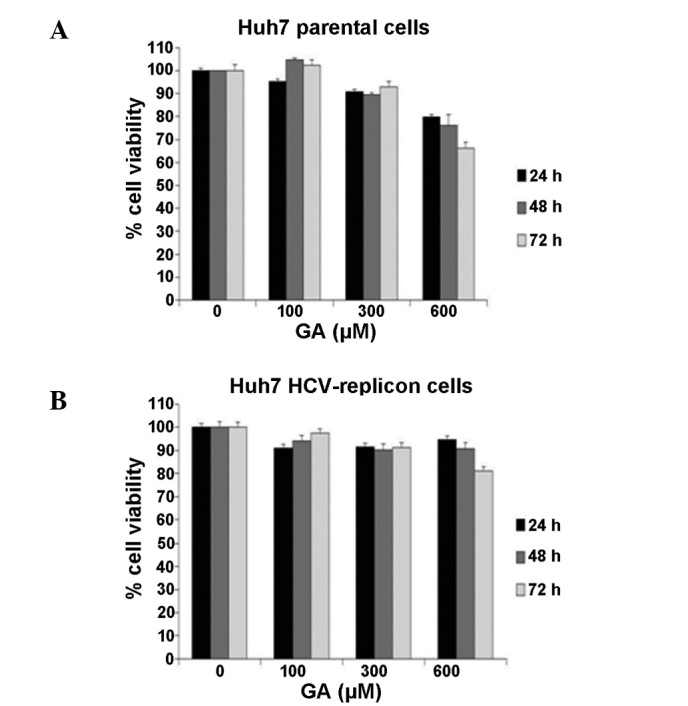
Viability of cells following GA treatment. Effects of GA on the growth of (A) Huh7 parental and (B) Huh7 HCV replicon cells. The two cell lines were treated with different concentrations of GA (100, 300 or 600 µM; ~2×104 cells/well) and were incubated for 0, 24, 48 or 72 h. Cells were assessed using an MTT assay. GA, gallic acid.
GA decreases HCV-RNA replication
To evaluate the effect of GA on HCV-RNA expression in HCV replicon-containing cells, these cells were incubated with 300 µM GA for three different durations (24, 48 and 72 h). Subsequently, the total cellular RNA was extracted and subjected to RT-qPCR for HCV-RNA quantification, as described in the Materials and methods. GA was found to inhibit HCV-RNA expression in a time-dependent manner compared with the untreated cells, showing a higher effect at 72 h post-treatment, at which the lowest HCV-RNA expression was observed (22% inhibition at 48 h and 44% inhibition at 72 h, P<0.01; Fig. 2). Collectively, these results reveal that 300 µM GA is able to decrease HCV expression at the transcriptional level in the HCV replicon cell culture system.
Figure 2.
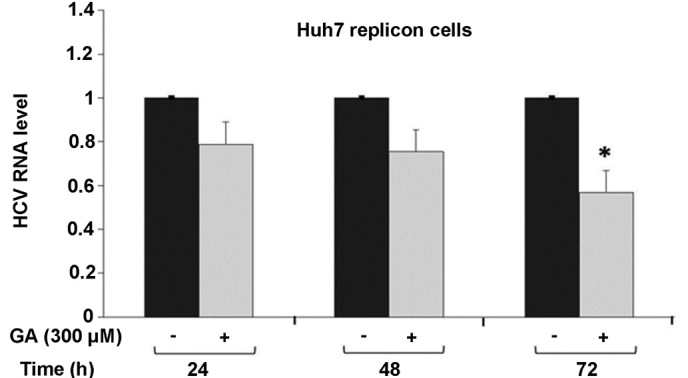
Expression levels of HCV-RNA measured by RT-qPCR (ΔΔCt). Huh7 HCV replicon cells (2×105 cells) were incubated with 300 µM GA or without GA for 0, 24, 48 and 72 h. Next, total cell RNA was extracted at each time point and HCV-RNA levels were quantified by RT-qPCR. RNA viral levels were normalized based on the ratio of HCV/GAPDH-RNA that was amplified in the same plate. HCV-RNA levels are shown relative to the nontreated control, which is defined as 1.0. Data are represented are the mean ± standard deviation of triplicate cultures, and the experiment was repeated three times (*P<0.05 vs. untreated cells). HCV, hepatitis C virus; RT-qPCR, reverse transcription quantitative polymerase chain reaction; GA, gallic acid.
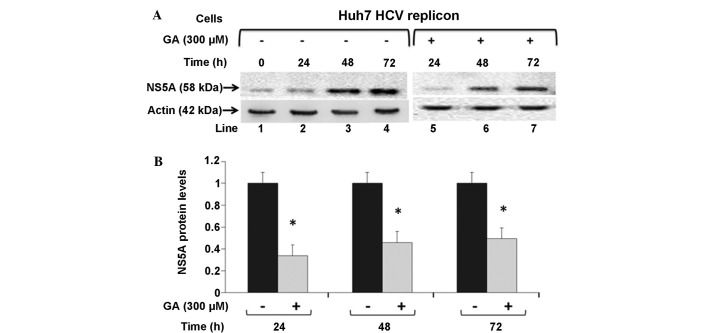
NS5A protein levels are downregulated by GA in Huh7 HCV replicon cells
To investigate whether GA can influence the synthesis of HCV viral proteins, NS5A and actin protein levels were examined by western blot analysis in untreated HCV replicon cells or cells treated with 300 µM GA, and incubated for 24, 48 and 72 h (Fig. 3A). Cells treated with 300 µM GA expressed lower levels of NS5A-HCV proteins, as shown by the lower ratio of NS5A-HCV protein at the three time points after exposure compared with the untreated cells (0.33, 0.45 and 0.49 for the time points 24, 48 and 72 h, respectively, compared with the control value; P<0.05; Fig. 3B). These data suggest that GA treatment may diminish the translational rate of viral proteins or decrease viral protein stability, in addition to the negative effect on HCV-RNA levels.
Figure 3.
HCV NS5A protein levels in Huh7 HCV replicon cells treated with GA. Huh7 HCV replicon cells (6×105 cells) were incubated in the presence (lanes 5–7) or absence (lanes 1–4) of 300 µM GA for different times (0, 24, 48, and 72 h). (A) Cell lysates were prepared, and equal amounts of protein extracts (50 µg) were subjected to immunoblot analysis to detect NS5A (top panel) and actin levels (bottom panel) by western blot analysis. (B) Quantified ratios of NS5A to actin proteins from the immunoblot detection. *P<0.05 vs. control. HCV, hepatitis C virus; NS5A, nonstructural protein 5A; GA, gallic acid.
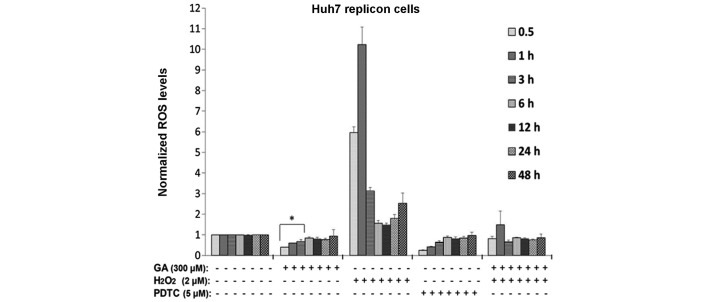
GA decreases ROS production in HCV replicon cells
It has been reported that HCV promotes ROS production in infected hepatocytes, which further promote lipid and protein oxidation, leading to cell death (33). To determine whether GA treatment has an antioxidant activity in HCV replicon cells, ROS levels were evaluated using the H2DCF-DA assay. HCV replicon cells were incubated in the presence or absence of 300 µM GA and incubated for 24, 48 and 72 h, following which the ROS levels were quantified. GA was found to reduce ROS levels in a time-dependent manner (Fig. 4), with a greater effect at earlier time points, including 0.5, 1 and 3 h after treatment (~50, 30 and 20% reduction, respectively; P<0.05). In addition, at 6, 12 and 24 h post-treatment, ROS production was maintained <20% compared with the control cells. As a positive control of antioxidant activity, HCV replicon cells were treated with 5 µM PDTC. Fig. 4 shows that PDTC decreased ROS levels starting at 0.5 h and showing a similar effect as that observed at 3 h after GA treatment (P<0.05). In addition, the present study investigated whether GA is able to decrease the levels of ROS generated by a strong oxidizing agent, such as H2O2. Huh7 replicon cells were treated simultaneously with 2 µM H2O2 and GA, and incubated between 0.5 and 48 h. Subsequently, ROS levels were measured at the end of each time point. It was observed that GA was able to abate the increased ROS levels induced by H2O2, reaching levels similar to those of cells without the oxidizing agent (Fig. 4). This suggested that GA acid was able to mitigate the cellular oxidative stress induced by an oxidizing agent. Therefore, these results confirm that GA was able to decrease oxidative stress markers in the same way the antioxidant agent, PDTC (5 µM).
Figure 4.
Effect of GA on intracellular ROS levels in Huh7 HCV replicon cells. HCV replicon-containing cells (2×104 cells) were incubated with or without 300 µM GA for different times. Next, ROS levels were assessed in total cellular extracts using a 2′,7′-dichlorofluorescein diacetate fluorescence assay and the fluorescence was measured at 485 nm (excitation) and 528 nm (emission). PDTC (5 µM) was used as a control due to its antioxidant effect and H2O2 (2 µM) was used as a positive control of ROS production, and were treated similar to the GA treatment. Data are expressed as the mean ± standard deviation of triplicate cultures, and the experiment was repeated 3 times (*P<0.05). GA, gallic acid; ROS, reactive oxygen species; HCV, hepatitis C virus; PDTC, pyrrolidine dithiocarbamate.
Discussion
Chronic HCV infection is a cause of liver disease worldwide that leads to progressive fibrosis, and may result in cirrhosis, hepatocellular carcinoma, liver failure and mortality (34). The current treatment for HCV is not effective for all patients and causes severe side effects. Therefore, investigations continue to identify alternative therapies for hepatitis C (35). Oxidative stress plays an important role in various diseases, including viral infection and chronic inflammation. HCV gene expression can increase the levels of ROS. Therefore, antioxidants have been found to exert antiviral activities against a variety of viruses by decreasing the oxidative stress generated by the viruses (36,37). In addition, previous studies have demonstrated that GA has an antiviral activity due to its strong radical scavenging activity (38). Numerous studies have shown that interference with the generation of ROS through the use of antioxidants can drastically reduce replication of various viruses (35).
In the present study, we evaluated the GA effect on HCV-RNA and protein expression in a Huh7 replicon cell system. GA was not found to induce cytotoxicity at the concentration used in the present study. These results are in agreement with those of other reports using natural compounds such as silymarin, in which cell viability was not affected upon treatment (35,39).
The results revealed the downregulation of HCV-RNA and viral protein levels that was induced by GA, demonstrating that GA may diminish the translational rate of viral proteins or decrease the stability of viral protein or RNA (Fig. 3). The current results are in agreement with those of other reports showing the antiviral activity of natural compounds with an antioxidant activity (40–42). In addition, a previous study demonstrated that GA has an effect on HSV-1 gD, gC and VP5 viral proteins of HSV-1 in Vero cells, showing that GA suppressed the expression of these proteins (22). Another natural compound, silymarin, has been reported to have an anti-HCV activity by inducing downregulation (80%) of HCV-RNA, core and NS5A viral proteins in the CON1 subgenomic genotype 1b HCV cell line (35).
Recently, an antiviral assay demonstrated that GA possessed good antiviral spectrum against other viruses, including human rhinoviruses in HeLa cells, without inducing cytotoxicity at the concentration used (21). The present study is in agreement with previously published results mentioning that GA is a strong antiviral antioxidant (43–46). The antiviral effect of GA and its derivatives has also been demonstrated in certain RNA viruses, including the vesicular stomatitis virus (Rhabdoviridae family), influenza virus (Orthomyxoviridae family) and poliovirus (Picornaviridae family). Thus, GA inhibits the multiplication of these RNA viruses, which have a different structure, such as enveloped or nonenveloped, and positive- or negative-stranded genome RNA (47).
According to the GA chemical structure, the observed virucidal activity of GA may be due to the hydrophobic interaction between the functional group (hydroxyl) and virion components, providing GA with the capacity to bind free radicals and exert an antioxidative effect. This antioxidant property of GA may explain the antiviral effect observed against HCV in replicon cells, and may be proposed as an alternative therapy for antiviral treatment.
In conclusion, GA treatment was found to diminish the cellular oxidative stress by decreasing ROS production, which in turn was unfavorable for HCV. Thus, GA is suggested to be a promising adjuvant in HCV therapy. Further research is required to elucidate the underlying mechanism(s) of the GA effect on HCV replication.
Acknowledgements
This study was supported in part by grants from CONACYT (no. CB2010-01155082 and SALUD-2008-C01-86996; awarded to Ana M. Rivas-Estilla) and FONCYT-COECYT (no. COAH-2002-C08-C37; awarded to Jesus A. Morlett-Chávez).
Glossary
Abbreviations
- GA
gallic acid
- HCV
hepatitis C virus
- RT-PCR
reverse transcription polymerase chain reaction
- cDNA
complementary DNA
- ROS
reactive oxygen species
- H2DCFDA
2′,7′-dichlorodihydrofluorescein diacetate
- NS5A
nonstructural protein 5A
- MTT
[3-(4,5-dimethlthiazol-2-yl)-2, 5-diphenyl-tetrazolium bromide]
References
- 1.Pahl HL. Signal transduction from the endoplasmic reticulum to the cell nucleus. Physiol Rev. 1999;79:683–701. doi: 10.1152/physrev.1999.79.3.683. [DOI] [PubMed] [Google Scholar]
- 2.Warris G, Siddiqui A. Hepatitis C virus stimulates the expression of cyclooxygenase-2 via oxidative stress: Role of prostaglandin E2 in RNA replication. J Virol. 2005;79:9725–9734. doi: 10.1128/JVI.79.15.9725-9734.2005. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Huang H, Chen Y, Ye J. Inhibition of hepatitis C virus replication by peroxidation of arachidonate and restoration by vitamin E. Proc Natl Acad Sci USA. 2007;104:18666–18670. doi: 10.1073/pnas.0708423104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gong G, Waris G, Tanveer R, Siddiqui A. Human hepatitis C virus NS5A protein alters intracellular calcium levels, induces oxidative stress, and activates STAT-3 and NF-kappa B. Proc Natl Acad Sci USA. 2001;98:9599–9604. doi: 10.1073/pnas.171311298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burra P, Hepatitis C. Semin Liver Dis. 2009;29:53–65. doi: 10.1055/s-0029-1192055. [DOI] [PubMed] [Google Scholar]
- 6.do Kim Y, Ahn SH, Han KH. Emerging therapies for hepatitis C. Gut Liver. 2014;8:471–479. doi: 10.5009/gnl14083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poordad F, McCone J, Jr, Bacon BR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, Reddy KR, Goodman ZD, Boparai N, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195–1206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patrick L, Hepatitis C. epidemiology and review of complementary/alternative medicine treatments. Altern Med Rev. 1999;4:220–238. [PubMed] [Google Scholar]
- 9.Trujillo-Murillo K, Rincón-Sánchez AR, Martinez-Rodriguez H, Bosques-Padilla F, Ramos-Jiménez J, Barrera-Saldaña HA, Rojkind M, Rivas-Estilla AM. Acetylsalicylic acid inhibits hepatitis C virus RNA and protein expression through cyclooxygenase 2 signaling pathways. Hepatology. 2008;47:1462–1472. doi: 10.1002/hep.22215. [DOI] [PubMed] [Google Scholar]
- 10.Yano M, Ikeda M, Abe K, Dansako H, Ohkoshi S, Aoyagi Y, Kato N. Comprehensive analysis of the effects of ordinary nutrients on hepatitis C virus RNA replication in cell culture. Antimicrob Agents Chemother. 2007;51:2016–2027. doi: 10.1128/AAC.01426-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taitzoglou IA, Tsantarliotou M, Zervos I, Kouretas D, Kokolis NA. Inhibition of human and ovine acrosomal enzymes by tannic acid in vitro. Reproduction. 2001;121:131–137. doi: 10.1530/rep.0.1210131. [DOI] [PubMed] [Google Scholar]
- 12.Haslam E, editor. Practical polyphenolics, From structure to molecular recognition and physiological action. Cambridge University Press Cambridge; 1998. pp. 84–177. [Google Scholar]
- 13.Lekha PK, Lonsane BK. Production and application of tannin acyl hydrolase: State of the art. Adv Appl Microbiol. 1997;44:215–260. doi: 10.1016/S0065-2164(08)70463-5. [DOI] [PubMed] [Google Scholar]
- 14.Hocman G. Chemoprevention of cancer: P henolic antioxidants (BHT, BHA) Int J Biochem. 1988;20:639–651. doi: 10.1016/0020-711X(88)90158-9. [DOI] [PubMed] [Google Scholar]
- 15.Sharma S, Wyatt GP, Steele VE. A carcinogen-DNA binding assay as a biomarker screen for identifying potential chemopreventive agents. Methods Cell Sci. 1997;19:4548. doi: 10.1023/A:1009798621899. [DOI] [Google Scholar]
- 16.Kim YJ. Antimelanogenic and antioxidant properties of gallic acid. Biol Pharm Bull. 2007;30:1052–1055. doi: 10.1248/bpb.30.1052. [DOI] [PubMed] [Google Scholar]
- 17.Ji BC, Hsu WH, Yang JS, Hsia TC, Lu CC, Chiang JH, Yang JL, Lin CH, Lin JJ, Suen LJ, et al. Gallic acid induces apoptosis via caspase-3 and mitochondrion-dependent pathways in vitro and suppresses lung xenograft tumor growth in vivo. J Agric Food Chem. 2009;57:7596–7604. doi: 10.1021/jf901308p. [DOI] [PubMed] [Google Scholar]
- 18.Inoue M, Suzuki R, Sakaguchi N, Li Z, Takeda T, Ogihara Y, Jiang BY, Chen Y. Selective induction of cell death in cancer cells by gallic acid. Biol Pharm Bull. 1995;18:1526–1530. doi: 10.1248/bpb.18.1526. [DOI] [PubMed] [Google Scholar]
- 19.Chen HM, Wu YC, Chia YC, Chang FR, Hsu HK, Hsieh YC, Chen CC, Yuan SS. Gallic acid, a major component of Toona sinensis leaf extracts, contains a ROS-mediated anti-cancer activity in human prostate cancer cells. Cancer Lett. 2009;286:161–171. doi: 10.1016/j.canlet.2009.05.040. [DOI] [PubMed] [Google Scholar]
- 20.You BR, Park WH. The effects of mitogen-activated protein kinase inhibitors or small interfering RNAs on gallic acid induced HeLa cell death in relation to reactive oxygen species and glutathione. J Agric Food Chem. 2001;59:763–771. doi: 10.1021/jf103379d. [DOI] [PubMed] [Google Scholar]
- 21.Choi HJ, Song JH, Bhatt LR, Baek SH. Anti-Human Rhinovirus activity of gallic acid possessing antioxidant capacity. Phytother Res. 2010;24:1292–1296. doi: 10.1002/ptr.3101. [DOI] [PubMed] [Google Scholar]
- 22.Kratz JM, Andrighetti-Frohner CR, Kolling DJ, Leal PC, Cirne-Santos CC, Yunes RA, Nunes RJ, Trybala E, Bergström T, Frugulhetti IC, et al. Anti-HSV-1 and anti-HIV-1 activity of gallic acid and pentyl gallate. Mem Inst Oswaldo Cruz. 2008;103:437–442. doi: 10.1590/S0074-02762008000500005. [DOI] [PubMed] [Google Scholar]
- 23.Cuzzocrea S, Chatterjee PK, Mazzon E, Serraino I, Britti D, Dugo L, Mazzullo G, Caputi AP, Thiemermann C. Pyrrolidine dithiocarbamate attenuates the development of acute and chronic inflammation. Br J Pharmacol. 2002;135:496–510. doi: 10.1038/sj.bjp.0704463. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Lohmann V, Körner F, Koch J, Herian U, Theilmann L, Bartenschlager R. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science. 1999;285:110–113. doi: 10.1126/science.285.5424.110. [DOI] [PubMed] [Google Scholar]
- 25.Devasagayam TP, Maurya DK, Nandakumar N. Anticancer property of gallic acid in A549, a human lung adenocarcinoma cell line and possible mechanisms. J Clin Biochem Nutr. 2011;48:85–90. doi: 10.3164/jcbn.11-004FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mosmann T. Rapid colorimetric assay for cellular growth and survival: A pplications to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 27.Yedjou CG, Tchounwou PB. In vitro cytotoxic and genotoxic effects of arsenic trioxide on human leukemia (HL-60) cells using the MTT and alkaline single cell gel electrophoreis (Comet) assays. Mol Cell Biochem. 2007;301:123–130. doi: 10.1007/s11010-006-9403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rivas-Estilla AM, Svitkin Y, Lastra Lopez M, Hatzoglou M, Sherker A, Koromilas AE. PKR-dependent mechanisms of gene expression from a subgenomic hepatitis C virus clone. J Virol. 2002;76:10637–10653. doi: 10.1128/JVI.76.21.10637-10653.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rivas-Estilla AM, Bryan-Marrugo OL, Trujillo-Murillo K, Pérez-Ibave D, Charles-Niño C, Pedroza-Roldan C, RíosIbarra C, Ramírez-Valles E, Ortíz-López R, Islas-Carbajal MC, et al. Cu/Zn superoxide dismutase (SOD1) induction is implicated in the antioxidative and antiviral activity of acetylsalicylic acid in HCV-expressing cells. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1264–G1273. doi: 10.1152/ajpgi.00237.2011. [DOI] [PubMed] [Google Scholar]
- 30.Kopff M, Kopff A, Kowalczyk E. The effect of nonsteroidal anti-inflammatory drugs on oxidative/antioxidative balance. Pol Merkur Lekarski. 2007;23:184–187. (In Polish) [PubMed] [Google Scholar]
- 31.Polat A, Emre MH. Effects of melatonin or acetylsalicylic acid on gastric oxidative stress after bile duct ligation in rats. J Gastroenterol. 2006;41:433–439. doi: 10.1007/s00535-006-1783-4. [DOI] [PubMed] [Google Scholar]
- 32.Chatel-Chaix L, Baril M, Lamarre D. Hepatitis C Virus NS3/4A Protease inhibitors, A light at the end of the Tunnel. Viruses. 2010;2:1752–1765. doi: 10.3390/v2081752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ivanov AV, Bartosh B, Smirnova OA, Isaguliants MG, Kochetkov SN. HCV and oxidative stress in the liver. Viruses. 2013;5:439–469. doi: 10.3390/v5020439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehrab-Mohseni M, Sendi H, Steuerwald N, Ghosh S, Schrum LW, Bonkovsky HL. Legalon-SIL down regulates HCV core and NS5A in human hepatocytes expressing full-length HCV. World J Gastroenterol. 2011;17:1694–1700. doi: 10.3748/wjg.v17.i13.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi HJ, Song JH, Park KS, Baek SH. In vitro anti-enterovirus 71 activity of gallic acid from Woodfordia fruticosa flowers. Lett Appl Microbiol. 2010;50:438–440. doi: 10.1111/j.1472-765X.2010.02805.x. [DOI] [PubMed] [Google Scholar]
- 36.Sriwilaijaroen N, Fukumoto S, Kumagai K, Hiramatsu H, Odagiri T, Tashiro M, Suzuki Y. Antiviral effect of Psidium guajava Linn (guava) tea on the growth of clinical isolated H1N1 viruses: Its role in viral hemagglutination and neuraminidase inhibition. Antiviral Res. 2012;94:139–146. doi: 10.1016/j.antiviral.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 37.de Oliveira A, Adams SD, Lee LH, Murray SR, Hsu SD, Hammond JR, Dickinson D, Chen P, Chu TC. Inhibition of herpes simplex virus type 1 with the modified green tea polyphenol palmitoyl-epigallocatechin gallate. Food Chem Toxicol. 2013;52:207–215. doi: 10.1016/j.fct.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.You BR, Moon HJ, Han YH, Park WH. Gallic acid inhibits the growth of HeLa cervical cancer cells via apoptosis and/or necrosis. Food Chem Toxicol. 2010;48:1334–1340. doi: 10.1016/j.fct.2010.02.034. [DOI] [PubMed] [Google Scholar]
- 39.Polyak SJ, Morishima C, Lohmann V, Pal S, Lee DYW, Liu Y, Graf TN, Oberlies NH. Identification of hepatoprotective flavonolignans from silymarin. Proc Natl Acad Sci USA. 2010;107:5595–5599. doi: 10.1073/pnas.0914009107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee JC, Chen WC, Wu SF, Tseng CK, Chiou CY, Chang FR, Hsu SH, Wu YC. Anti-hepatitis C virus of Acacia confusa extract via suppressing cyclooxygenase-2. Antiviral Res. 2011;89:35–42. doi: 10.1016/j.antiviral.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 41.Ravikumar YS, Ray U, Nandhitha M, Perween A, Naika Raja HR, Khanna N, Das S. Inhibition of hepatitis C virus replication by herbal extract. Phyllanthus amarus as potent natural source. Virus Res. 2011;158:89–97. doi: 10.1016/j.virusres.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 42.Manvar D, Mishra M, Kumar S, Pandey VN. Identification and evaluation of anti Hepatitis C virus phytochemicals from Eclipta alba. J Ethnopharmacology. 2012;144:545–554. doi: 10.1016/j.jep.2012.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahn MJ, Kim CY, Lee JS, Kim TG, Kim SH, Lee CK, Lee BB, Shin CG, Huh H, Kim J. Inhibition of HIV-1 integrase by galloyl glucoses from Terminalia chebula and flavonol glycoside gallates from Euphorbia pekinensis. Planta Med. 2002;68:457–459. doi: 10.1055/s-2002-32070. [DOI] [PubMed] [Google Scholar]
- 44.Duan D, Li Z, Luo H, Zhang W, Chen L, Xu X. Antiviral compounds from traditional Chinese medicines Galla Chinese as inhibitors of HCV NS3 protease. Bioorg Med Chem Lett. 2004;14:6041–6044. doi: 10.1016/j.bmcl.2004.09.067. [DOI] [PubMed] [Google Scholar]
- 45.Uosaki M, Yamasaki H, Katsuyama Y, Higuchi M, Higuti T, Koyama AH. Antiviral effect of octyl gallate against DNA and RNA viruses. Antiviral Res. 2007;73:85–91. doi: 10.1016/j.antiviral.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 46.Nutan Modi M, Goel T, Das T, Malik S, Suri S, Rawat AK, Srivastava SK, Tuli R, Malhotra S, Gupta SK. Ellagic acid & gallic acid from Lagerstroemia speciosa L. inhibit HIV-1 infection through inhibition of HIV-1 protease & reverse transcriptase activity. Indian J Med Res. 2013;137:540–548. [PMC free article] [PubMed] [Google Scholar]
- 47.Liu G, Xiong S, Xiang YF, Guo CW, Ge F, Yang CR, Zhang YJ, Wang YF, Kitazato K. Antiviral activity and possible mechanisms of action of pentagalloylglucose (PGG) against influenza A virus. Arch Virol. 2011;156:1359–1369. doi: 10.1007/s00705-011-0989-9. [DOI] [PubMed] [Google Scholar]