Abstract
Solitary fibrous tumors (SFTs) are uncommon, with the pleura as a site of predilection. Central nervous system SFTs, particularly intracranial SFTs, are extremely rare. The lesions are generally benign and localized, and surgery is the main therapeutic solution. The current study reports the cases of a patient who presented with right haunch pain, right leg weakness and paresthesias for several months, and a patient with a history of unexpected loss of consciousness. Magnetic resonance imaging revealed the presence of lesions, with a spindle cell morphology evident on pathological examination. The immunohistochemical examination demonstrated a strong immunoreaction for cluster of differentiation 34, which supported the diagnosis of an SFT. Following a near-total resection, the patients had a good neural prognosis. The present study also provides a literature review, discussing the imageological and pathological characteristics of SFT, and the diagnostic methods that aid in distinguishing the entity from other spindle-cell central nervous system tumors.
Keywords: solitary fibrous tumor, extrapleural, central nervous system
Introduction
Solitary fibrous tumors (SFTs) are rare spindle-cell mesenchymal neoplasms that are predominantly found in the pleura (1). Extrapleural SFTs are uncommon and occur in various locations that are associated with the lungs, pericardium, mediastinum, upper respiratory tract, bones and soft tissue of any site. Central nervous system SFTs, first described by Carneiro et al (2), are extremely rare. Over the past 10 years, a few studies on central nervous system SFTs have been reported, with the majority of cases located in the spinal cord. Intracranial lesions, however, are not always observed. It is reported that central nervous system SFTs account for ~0.09% of meningeal tumors (3). Forming a pre-operative diagnosis for intracranial SFTs is difficult due to the atypical symptoms and imaging manifestations, and therefore, it mainly depends on the post-operative pathological examination. To date, the clinical course, histogenesis, cytogenetics and prognosis for intracranial SFTs remain largely unknown (4). The treatment for the tumors mainly depends upon a surgical resection and the prognosis for patients with SFTs is good when the tumors are completely resected. The current study reports two cases of primary SFTs of the brain and reviews the previously reported cases, with a discussion of the possible differential diagnosis. The patients provided written informed consent.
Case report
Case one
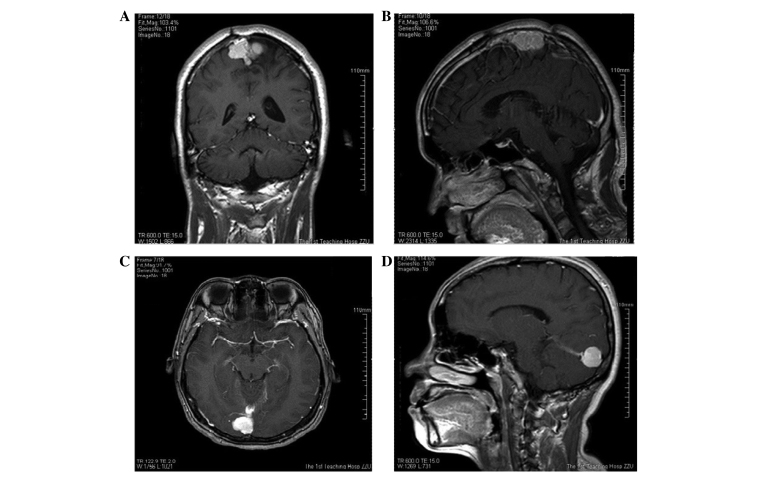
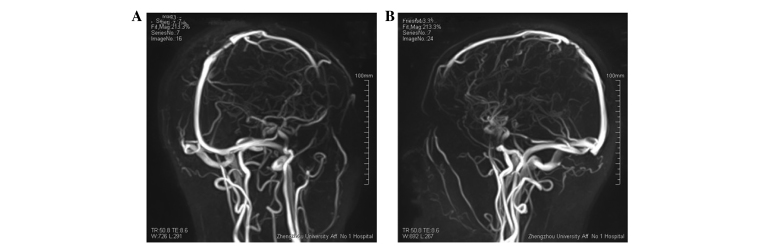
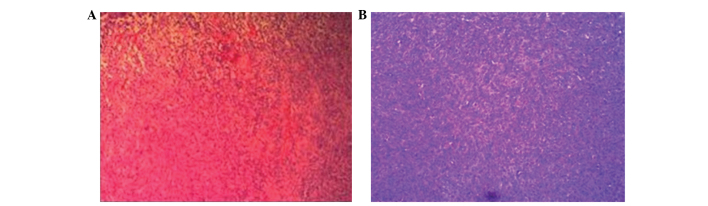
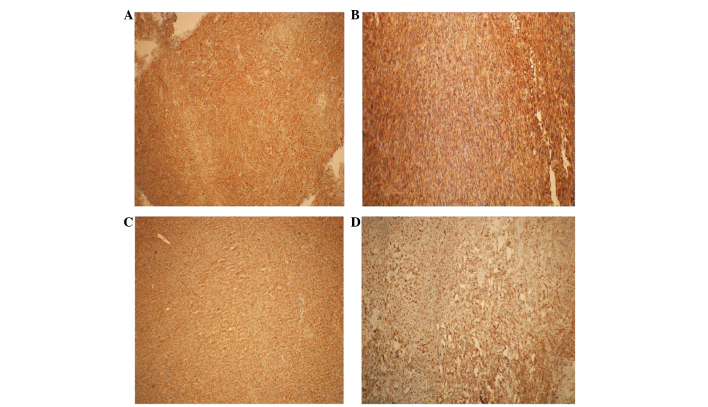
A 39-year-old male presented to the Department of Neurosurgery (The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China) on March 20, 2014, with right haunch pain, right leg weakness and paresthesia that had been apparent for 3 months. The patient had been diagnosed with a meningioma on the left frontal lobe and was treated successfully by tumor resection 13 years earlier. Magnetic resonance imaging (MRI) showed a relapsing, dumbbell-like, relatively well-defined lesion, 34×27 mm in size, located on the frontal and temporal lobes. The mass crossed the superior sagittal sinus and the cerebral falx (Fig. 1). The lesion exhibited a moderate signal intensity on T1-weighted imaging, a relatively high signal intensity on T2-weighted imaging and homogeneous enhancement on T1-weighted imaging with gadolinium. MR venography disclosed external compression and infiltration into the superior sagittal sinus, with moderate surrounding brain tissue edema (Fig. 2). The tumor was visualized via a cross-midline craniectomy, where it was observed that the tumor had invaded the cerebral falx and the wall of the superior sagittal sinus. The tumor was almost totally resected away from the surrounding brain tissue and sinus, with a little tumor tissue remaining in the sinus. A lesion with a spindle cell morphology was determined on pathological examination (Fig. 3). Microscopic immunohistochemical examination demonstrated a strong immunoreaction for cluster of differentiation (CD)34, vimentin and B-cell lymphoma 2 (Bcl-2), but a negative reaction for epithelial membrane antigen (EMA) and S-100 (Fig. 4). The frozen section analysis confirmed the presence of spindle cells and confirmed a diagnosis of an SFT. The post-operative neurological status was substantially improved and regular follow-up examinations for 6 months post-surgery have shown that the patient is currently disease-free.
Figure 1.
Magnetic resonance imaging showing (A and B) the dumbbell-like tumor across the falx in case one, and (C and D) the tumor located at the torcular herophili and tentorium cerebelli in case two.
Figure 2.
(A and B) Magnetic resonance venography showing the clear oppression of the superior sagittal sinus in case one.
Figure 3.
Pathological examination results showing the spindle cell morphology in cases (A) one and (B) two. (hematoxylin & eosin; magnification, ×20).
Figure 4.
Tumor sections showing (A and C) strong immunoreactivity for cluster of differentiation 34 in cases one and two, and positive staining for (B) B-cell lymphoma 2 and (D) vimentin in case one. Magnification, ×400.
Case two
A 42-year-old female presented with a history of unexpected loss of consciousness with no limb twitches and high fever for 20 days, was admitted to hospital on February 25, 2014. MRI showed a quasi-circular lesion in the occipital lobe, which was 13×18 mm in size; the lesion exhibited a clear boundary with the brain and it was observed to stretch across the torcular herophili and the tentorium cerebelli. The lesion exhibited a moderate signal intensity on T1-weighted imaging, a high signal intensity on T2-weighted imaging and homogeneous enhancement on T1-weighted imaging with gadolinium (Fig. 1). During surgery, it was disclosed that the tumor was compressing the torcular herophili and had an abundant blood supply. A near-total resection was performed, with only a little of the tissue that had invaded into the sinus remaining. The pathological examination revealed an SFT with a spindle cell morphology, high density and obvious nuclear fission (Fig. 3). The tumor was distinctively positive for CD34, but not for S-100, cytokeratin (CK) and EMA (Fig. 4). The patient achieved a good outcome, with no epilepsy or other neurological symptoms experienced on a regular 6-month follow-up. The patient is currently disease free.
Discussion
An SFT is a form of benign and circumscribed lesion that was first defined by Klemperer and Rabin in 1931 (5). The tumor originates from the mesenchymal cells rather than the mesothelial cells and mainly affects the pleura and mediastinum. The microscopic histology of SFTs reveals that the tissue exhibits a proliferation of spindle cells with a variety of growth patterns (3). Central nervous system SFTs account for only 0.09% of all meningeal tumors, while intracranial SFTs are extremely rare (6). The majority of intracranial SFTs are found in females. The tumors grow slowly and certain patients may develop the symptoms of episodic headaches, gait imbalance, dizziness, sensory disturbance, hemiplegic paralysis or epileptic seizure, while other patients may be asymptomatic, with no distinctive local symptoms (7). Only when the lesions become large enough or infringe into the important functional areas, will clear clinical symptoms arise.
Currently, the diagnosis of intracranial SFTs depends on immunohistochemical staining. Staining for CD34, CD99, Bcl-2 and vimentin is almost always strongly positive. CD34 is expressed in hematopoietic stem cells, the endothelium, hematopoietic stem cells, endothelial progenitor cells, myeloid progenitor cells and certain mesenchymal cells in the dermis, and can be found in nearly all cases. CD34 is therefore the most significant and sensitive index property (8). However, the deficiency of CD34 has also been observed in certain cases (9). SFTs are immunoreactive for Bcl-2, with a positive expression rate of 80–100% (10,11). CD99 is also strongly exhibited in certain cases of SFT, with a positive expression rate of 75–100% (12). At the same time, other referential elements exhibit no expression, including EMA, CK, S-100 and α-smooth muscle actin, as reported by previous studies (2,13–16). SFT is difficult to distinguish from meningeal hemangiopericytoma (HPC), which exhibits a high rate of recurrence and late extracranial metastases (17). A recent study has revealed the difference between HPC and SFT in terms of behavioral biology (18). Unlike SFTs, which originate from mesenchymal cells, HPC is the most common dural-based mesenchymal tumor. Identical to SFT, the immunohistochemistry of HPC also reveals a high percentage of cases with vimentin (85%) expression, but few cases with CD34 (25%) expression (19). In addition, HPC is markedly different compared with SFT with regard to dense laminae, which are highly cellular vascularized monotonous spindle cell proliferations (20).
SFTs are frequently misdiagnosed as other tumors, including neurofibroma, fibrous meningioma, hemangiopericytoma, malignant fibrous histiocytoma and fibrosarcoma, on MRI (21,22). In one study, more than half (66.7%) of SFTs exhibited an isointense signal on T1-weighted imaging, while heterogeneous and hypointense cases accounted for nearly one-third of cases (20.8 and 11.1%, respectively) (23). However, hyperintensity on T1-weighted imaging is not frequently reported (3). The most common status on T2-weighted imaging is a hypointense signal (62.2%) with the remaining signals attributed as hyperintense or heterogeneous. In one study, the majority of cases exhibited homogenous enhancement with gadolinium administration (78%) (24). MR venography and angiography are of great significance in order to reveal associations between the blood supply and the intracranial artery prior to surgery (25).
SFTs are generally localized lesions. Extremely few cases may be malignant or undergo malignant transformation (26). The tumors are frequently confused with other meningeal tumors on imageological diagnosis. The use of chemotherapy or radiotherapy for the treatment of SFTs does not show much promise (17). The main principle of treating the disease is early surgical excision, which greatly alleviates the neurological symptoms. Due to the limitations of the lesions, the majority can be completely removed. The current study presents two cases of SFTs in which the patients accepted surgical therapy and achieved a good prognosis on the 6-month follow-up.
In conclusion, an SFT is a type of benign tumor that originates from the mesenchymal cells. The diagnosis of an SFT relies mainly on immunohistochemical staining, which is characterized by the positive expression of CD34 and Bcl-2. Radiotherapy and chemotherapy to the tumor is dissatisfactory. As a benign and localized disease, SFT has a good neurological outcome following early surgical treatment, and recurrences are rarely recorded.
References
- 1.Suzuki SO, Fukui M, Nishio S, Iwaki T. Clinicopathological features of solitary fibrous tumor of the meninges, An immunohistochemical reappraisal of cases previously diagnosed to be fibrous meningioma or hemangiopericytoma. Pathol Int. 2000;50:808–817. doi: 10.1046/j.1440-1827.2000.01120.x. [DOI] [PubMed] [Google Scholar]
- 2.Carneiro SS, Scheithauer BW, Nascimento AG, Hirose T, Davis DH. Solitary fibrous tumor of the meninges, A lesion distinct from fibrous meningioma. Histopathology. Am J Clin Pathol. 1996;106:217–224. doi: 10.1093/ajcp/106.2.217. [DOI] [PubMed] [Google Scholar]
- 3.Suster S, Nascimento AG, Miettinen M, Sickel JZ, Moran CA. Solitary fibrous tumors of soft tissue. Histopathology. Am J Surg Pathol. 1995;19:1257–1266. doi: 10.1097/00000478-199511000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Gold JS, Antonescu CR, Hajdu C, Ferrone CR, Hussain M, Lewis JJ, Brennan MF, Coit DG. Clinicopathologic correlates of solitary fibrous tumors. Cancer. 2002;94:1057–1068. doi: 10.1002/cncr.10328. [DOI] [PubMed] [Google Scholar]
- 5.Klemperer P, Rabin CB. Primary neoplasia of the pleura: A report of five cases. Arch Pathol. 1931;1:385–412. [Google Scholar]
- 6.Brunori A, Cerasoli S, Donati R, Giangaspero F, Chiappetta F. Solitary fibrous tumor of the meninges, Two new cases and review of the literature. Surg Neurol. 1999;51:636–640. doi: 10.1016/S0090-3019(98)00115-3. [DOI] [PubMed] [Google Scholar]
- 7.Fargen KM, Opalach KJ, Wakefield D, Jacob RP, Yachnis AT, Lister JR. The central nervous system solitary fibrous tumor, A review of clinical, imaging and pathologic findings among all reported cases from 1996 to 2010. Clin Neurol Neurosurg. 2011;113:703–710. doi: 10.1016/j.clineuro.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 8.Fina L, Molgaard HV, Robertson D, Bradley NJ, Monaghan P, Delia D, Sutherland DR, Baker MA, Greaves MF. Expression of the CD34 gene in vascular endothelial cells. Blood. 1990;75:2417–2426. [PubMed] [Google Scholar]
- 9.Ng HK, Choi PC, Wong CW, To KF, Poon WS. Metastatic solitary fibrous tumor of the meninges. Histopathology. J Neurosurg. 2000;93:490–493. doi: 10.3171/jns.2000.93.3.0490. [DOI] [PubMed] [Google Scholar]
- 10.Alawi F, Stratton D, Freedman PD. Solitary fibrous tumor of the oral soft tissues, A clinicopathologic and immunohistochemical study of 16 cases. Am J Surg Pathol. 2001;25:900–910. doi: 10.1097/00000478-200107000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Chen HJ, Zhang HY, Li X, Guo LX, Wei B, Guo H, Bu H, Yang K, Liu BL. Solitary fibrous tumor, The clinicopathologic and immunohistochemical characteristics of 26 cases. Sichuan Da Xue Xue Bao Yi Xue Ban. 2004;35:675–679. (In Chinese) [PubMed] [Google Scholar]
- 12.Zhang H, Lucas DR, Pass HI, Che M. Disseminated malignant solitary fibrous tumor of the pleura. Pathol Int. 2004;54:111–115. doi: 10.1111/j.1440-1827.2004.01591.x. [DOI] [PubMed] [Google Scholar]
- 13.Artlich A, Schmidt D. Immunohistochemical profile of meningiomas and their histological subtypes. Hum Pathol. 1990;21:843–849. doi: 10.1016/0046-8177(90)90054-9. [DOI] [PubMed] [Google Scholar]
- 14.Das A, Tan WL, Smith DR. Expression of extracellular matrix markers in benign meningiomas. Neuropathology. 2003;23:275–281. doi: 10.1046/j.1440-1789.2003.00512.x. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi K, Imagama S, Ito Z, Ando K, Ukai J, Muramoto A, Shinjo R, Matsumoto T, Nakashima H, Matsuyama Y, Ishiguro N. Recurrence of solitary fibrous tumor of the cervical spinal cord. Nagoya J Med Sci. 2014;76:217–223. [PMC free article] [PubMed] [Google Scholar]
- 16.Ishii K, Nakamura M, Matsumoto M, Mukai M, Toyama Y, Chiba K. Intramedullary solitary fibrous tumor of the spinal cord. J Orthop Sci. 2009;14:450–454. doi: 10.1007/s00776-009-1339-6. [DOI] [PubMed] [Google Scholar]
- 17.Munks S. Solitary fibrous tumor (SFT) of the nasal mucosa. Laryngorhinootologie. 2003;82:655–658. doi: 10.1055/s-2003-42683. (In German) [DOI] [PubMed] [Google Scholar]
- 18.Ambrosini-Spaltro A, Eusebi V. Meningeal hemangiopericytomas and hemangiopericytoma/solitary fibrous tumors of extracranial soft tissues: A comparison. Virchows Arch. 2010;456:343–354. doi: 10.1007/s00428-010-0888-6. [DOI] [PubMed] [Google Scholar]
- 19.Perry A, Scheithauer BW, Nascimento AG. The immunophenotypic spectrum of meningeal hemangiopericytoma, A comparison with fibrous meningioma and solitary fibrous tumor of meninges. Am J Surg Pathol. 1997;21:1354–1360. doi: 10.1097/00000478-199711000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen GP, O'Connell JX, Dickersin GR, Rosenberg AE. Solitary fibrous tumor of soft tissue, A report of 15 cases, including 5 malignant examples with light microscopic, immunohistochemical and ultrastructural data. Mod Pathol. 1997;10:1028–1037. [PubMed] [Google Scholar]
- 21.Suzuki SO, Fukui M, Nishio S, Iwaki T. Clinicopathological features of solitary fibrous tumor of the meninges, An immunohistochemical reappraisal of cases previously diagnosed to be fibrous meningioma or hemangiopericytoma. Pathol Int. 2000;50:808–817. doi: 10.1046/j.1440-1827.2000.01120.x. [DOI] [PubMed] [Google Scholar]
- 22.Prayson RA, McMahon JT, Barnett GH. Solitary fibrous tumor of the meninges. Histopathology. J Neurosurg. 1997;86:1049–1052. doi: 10.3171/jns.1997.86.6.1049. [DOI] [PubMed] [Google Scholar]
- 23.Koçak A, Cayli SR, Saraç K, Aydin NE. Intraventricular solitary fibrous tumor: An unusual tumor with radiological, ultrastructural and immunohistochemical evaluation, Case report. Neurosurgery. 2004;54:213–216. doi: 10.1227/01.NEU.0000097555.75899.C3. discussion 216–217. [DOI] [PubMed] [Google Scholar]
- 24.Caroli E, Salvati M, Orlando ER, Lenzi J, Santoro A, Giangaspero F. Solitary fibrous tumors of the meninges, Report of four cases and literature review. Neurosurg Rev. 2004;27:246–251. doi: 10.1007/s10143-004-0331-z. [DOI] [PubMed] [Google Scholar]
- 25.Ahn JY, Shim JY, Yang WI, Kim TS. Meningeal solitary fibrous tumor as an unusual cause of exophthalmos, Case report and review of the literature. Neurosurgery. 2001;48:1362–1366. doi: 10.1097/00006123-200106000-00039. [DOI] [PubMed] [Google Scholar]
- 26.Gidwani AL, Mullan FJ, Kenny B. Solitary fibrous tumour of the falciform ligament containing multiple foci of malignant transformation. J Clin Pathol. 2004;57:546–547. doi: 10.1136/jcp.2003.008821. [DOI] [PMC free article] [PubMed] [Google Scholar]