Abstract
Gap junctional intercellular communication (GJIC) and connexin (Cx) expression were reported in association with carcinogenesis in various types of tumours. In an earlier histomorphometric study, the protein levels of Cx subtypes 26, 43 and 45 were differentially expressed in oral squamous cell carcinoma (OSCC), corresponding lymph node metastases and dysplasia-free oral mucosa. Moreover, membrane Cx43 acted as an independent prognostic marker in OSCC tissues. This study aimed to confirm the expression of described Cx subtypes at the mRNA level. Hence, a reverse transcription quantitative polymerase chain reaction (RT-qPCR) analysis of Cx26, Cx43 and Cx45 gene expressions was performed in paired carcinoma and mucosa samples of 15 OSCC patients. Additionally, we assessed the interaction between Cx subtype expression and clinicopathological routine parameters. The RT-qPCR analysis revealed that Cx26 was downregulated in OSCC (P=0.01), while Cx43 was marginally upregulated in cancer tissue (P=0.04). Cx45 was significantly overexpressed in OSCC tissue compared with the intraoral mucosa controls (P<0.01), and remained unchanged at different tumour stages. No significant interactions between differential Cx subtype expression and clinicopathological routine parameters were observed. In conclusion, Cx regulation at the transcriptional level appears to be an early event during the initiation and development of OSCC, and is maintained during further progression. However, the mRNA-protein correlation is variable. This may be indicative of post-transcriptional, translational and degradation regulations being associated with the determination of Cx protein concentration during oral carcinogenesis.
Keywords: oral squamous cell carcinoma, gap junctional intercellular communication, connexin 26, connexin 43, connexin 45, connexin regulation
Introduction
Contiguous connexins (Cxs) may form homomeric or heteromeric gap junction hemichannels (connexons) on the cell membrane (1). Two connexons of adjacent cells form a gap junction channel, through which gap junction intercellular communication (GJIC) is possible via the passage of ions and second messengers (1). GJIC plays a crucial role in maintaining cell homeostasis, cell growth control and development (1).
First described in cultured hepatoma cells (2), the association between GJIC and carcinogenesis has since been described in various types of tumours, such as cervical (3,4), mammary (5), bronchial (6) and colorectal carcinoma (7). Depending on tumour progression, GJIC has different functions (8). Cxs affect cell growth by affecting the expression of cell cycle regulatory genes, such as cyclin A, D1 and D2, and cyclin-dependent kinases (8). Cx43 transfection in previously deficient tumour cell lines led to growth inhibition and an accumulation of classical tumour suppressors, such as p27 and Rb protein (8). Lack of GJIC leads to an intracellular accumulation of growth factors (2) and suppressed contact inhibition, causing cell proliferation (9). King et al (4) described a correlation between endogenous Cx43 mRNA and protein expression and increased growth control, with decreased growth capacity in HeLa cervical cancer cells. Cx43 knockout in mice leads to astrocytes exhibiting altered expression of genes associated with apoptosis, cell growth, transcription factors (10,11), and increased susceptibility of mice to pulmonary neoplasia (6). Cx26 and Cx43 expression in MDA-MB-231 breast cancer cells (5) provided similar results. Cx26 is responsible for contact growth inhibition in HeLa and HepG2 cells (3,12). Cx45 may form heteromeric gap junctions along with Cx43, and may affect intercellular contacts during carcinogenesis (13–15). Cx subtypes increase the attachment of tumour cells to the stroma (8) and the endothelial barrier (16), thereby promoting invasion and metastatic spread. Cx26 was identified in melanoma cells and surrounding small vessel endothelia (17), as well as in squamous cell lung carcinoma and its associated lymph node metastases (LMNs) (18). Cx26- and Cx43-negative primary breast cancers developed Cx26- and Cx43-positive LMNs (19). Glioma cells establish functional gap junctions comprising Cx43 with astrocytes in the adult brain, thus facilitating direct parenchymal invasion (20).
For oral squamous cell carcinoma (OSCC), conflicting Cx expression data were reported by Ozawa et al (21) and Villaret et al (22), who described Cx26 and Cx30 expression in OSCC tissues. In a previous histomorphometric study, we analysed the protein expression of Cx subtypes 26, 43 and 45 in tissue samples of OSSC, corresponding LMNs and dysplasia-free oral mucosa in 35 patients (23). In addition to significantly different expression patterns between the studied tissue types, high membrane Cx43 expression in OSCC tissues was found to be associated with poor prognosis (23). Xia et al (24) previously reported reduced Cx43 protein concentration, despite normal mRNA levels, in an induced rat tongue carcinogenesis model. The present study aimed to confirm the expression of the described Cx subtypes at the mRNA level by conducting a reverse transcription quantitative polymerase chain reaction (RT-qPCR) analysis in 15 tissue sample pairs of OSCC and corresponding oral mucosa.
Materials and methods
Patients
Tissue samples from 15 patients suffering from primary OSCC were analysed. All the patients were diagnosed and treated according to the guidelines of the national German Oral Cancer Association (25). The resection margins and presence of LMNs were histologically investigated in all the patients. Metastases to the lung, liver and bone marrow were evaluated by chest radiography, abdominal ultrasound examination and bone scans in all the patients. The patients' characteristics are summarised in Table I. The patients provided written informed consent prior to participating in the study. This study was conducted in line with the ethical standards of the Declaration of Helsinki and was approved by the local Ethics Committee at the George-August-University of Goettingen (vote number 11/6/05).
Table I.
Descriptive statistics and results from multivariate regression models with expression levels as the dependent variable.
| Cx26 | Cx43 | Cx45 | |||||
|---|---|---|---|---|---|---|---|
| Parameters | Descriptive statistics | β ± SE | P-value | β ± SE | P-value | β ± SE | P-value |
| Tumour tissue | −8.4±2.9 | 0.01 | 0.7±0.3 | 0.04 | 10.5±1.5 | <0.01 | |
| Age (years) | 59.0±12.9 | −0.1±0.2 | 0.45 | −0.02±0.02 | 0.22 | −0.1±0.1 | 0.50 |
| Gender | −3.7±6.3 | 0.58 | −0.6±0.6 | 0.33 | 1.8±7.2 | 0.81 | |
| Female | 5 (33%) | ||||||
| Male | 10 (67%) | ||||||
| T status | −10.8±26.2 | 0.69 | 0.7±2.7 | 0.80 | −4.7±27.6 | 0.87 | |
| Is | 1 (7%) | ||||||
| 2 | 5 (33%) | ||||||
| 4 | 8 (53%) | ||||||
| 4a | 1 (7%) | ||||||
| N status | 6.4±3.5 | 0.12 | 0.5±0.4 | 0.17 | 0.5±3.7 | 0.90 | |
| 0 | 9 (60%) | ||||||
| 1 | 3 (20%) | ||||||
| 2b | 2 (13%) | ||||||
| 2c | 1 (7%) | ||||||
| Nicotine abuse | −0.7±9.1 | 0.94 | 0.01±0.9 | 0.99 | −14.3±8.0 | 0.14 | |
| Yes | 14 (93%) | ||||||
| No | 1 (7%) | ||||||
| Alcohol abuse | 6.9±3.5 | 0.22 | −1.0±0.5 | 0.05 | −4.2±4.3 | 0.37 | |
| Yes | 10 (67%) | ||||||
| No | 5 (33%) | ||||||
| Stage | 8.4±24.4 | 0.74 | −0.6±2.5 | 0.82 | 1.3±25.4 | 0.96 | |
| 0 | 1 (7%) | ||||||
| 2 | 5 (33%) | ||||||
| 4 | 9 (60%) | ||||||
| Grade | 1.0±4.8 | 0.84 | −0.7±0.5 | 0.18 | 4.4±4.2 | 0.34 | |
| 1 | 2 (13%) | ||||||
| 2 | 11 (74%) | ||||||
| 3 | 2 (13%) | ||||||
Descriptive statistics are presented as either mean ± standard error (SE) or absolute and relative frequencies. The regression coefficients β may be interpreted as log2-fold changes where appropriate.
Biopsies
One biopsy from OSCC tissue and one from tumour-free oral mucosa were obtained from each patient during tumour ablation. All the biopsies were 2–3 mm in diameter. Sampling was performed according to a predefined, standardized working instructions, based on size and location of sampling, by the same two experienced examiners (F.F. and R.M.G.). The biopsies were incubated in RNAlater® (Ambion/Applied Biosystems, Darmstadt, Germany) overnight and shock-frozen in liquid nitrogen for storage at −80°C until RNA isolation.
RNA isolation
RNA was isolated using the RNeasy Mini kit (Qiagen, Hilden, Germany) according to the manufacturers' recommendations and stored at −80°C. Subsequently, the samples were treated with DNase I to remove genomic DNA contaminations. The RNA quality was determined using the Agilent 2100 Bioanalyzer (Agilent Technologies, Boeblingen, Germany) microfluidic electrophoresis. The analysed tissue sample pairs (OSCC and oral mucosa) had comparable RNA integrity numbers.
RT-qPCR
For verification of Cx subtype expression, RNA samples were converted into cDNA using the Bio-Rad iScript cDNA Synthesis kit (Bio-Rad Laboratories, Munich, Germany) and quantified on a Bio-Rad MyiQ Real-Time PCR Detection system with the Bio-Rad iQ SYBR Green Supermix. The primers are listed below:
GAPDH: 5′-GAGTCAACGGATTTGGTCGT-3′, 5′-GACAAGCTTCCCGTTCTCAG-3′; Cx26: 5′-ACTCCACCAGCATTGGAAAG-3′, 5′-TGGGAGATGGGGAAGTAGTG-3′; Cx43: 5′-AGCAGTCTGCCTTTCGTTGT-3′, 5′-TCTGCTTCAAGTGCATGTCC-3′; and Cx45: 5′-GCACTGCCAGTAGCAAATCA-3′, 5′-CCAACAGCATCCCTGAAGAT-3′.
Relative gene expression was quantified using the ∆∆Cq method. Since GAPDH is not differentially expressed in OSCC tissue (26), it was used as a housekeeping gene, based on which the Cx gene expression was normalized.
Statistical analysis
Relative gene expression normalized to GAPDH and log2-transformed was calculated from Cq values and PCR efficiency (27). The expression change between OSCC and oral mucosa and the effect of clinicopathological routine parameters was separately assessed for Cx26, Cx43 and Cx45 by a multivariate linear regression model. To account for the matched-pair situation (i.e., OSCC and normal mucosa from the same patient), patients were included as random-effects term to each model. For tissue and other dichotomous model parameters, the regression coefficients may be considered as log2 fold changes and are reported with their related standard errors. The significance level was set to α=5% for each test. For a stronger statistical perspective, the P-values for the tissue effects were adjusted by the method of Bonferroni and Holm (28). All the analyses were performed using R software, version 3.1 (www.r-project.org). The multivariate regression models were fitted with the lmer function of the lme4 package for R.
Results
Differential Cx expression in OSCC and normal oral mucosa
The multivariate model analysis yielded a significant differential expression between the two types of tissues (OSCC and oral mucosa) for each of the three Cx subtypes. In detail, Cx45 exhibited a strong overexpression of 10.5-log-fold (95% CI: 7.6–13.4) in OSCC samples and was differentially expressed compared with tumour-free oral mucosa controls (P<0.01). Cx26 exhibited a downregulation of −8.4-log-fold (95% CI: −14.1 to −2.7) in cancer tissues (P=0.01). Compared with oral mucosa controls, the gene expression for Cx43 was marginally upregulated (P=0.04) in OSCC tissues (0.7-log-fold; 95% CI: 0.1–1.3; Fig. 1). Following a Bonferroni/Holm-adjustment, the P-values for the tissue effects remained statistically significant. The gene expression of the described Cx subtypes did not differ between the early and late stages of malignancy, and there were no significant effects by the clinicopathological routine parameters T status, N status and American Joint Committee on Cancer stage. Moreover, Cx subtype expression was not found to be correlated with alcohol and tobacco abuse, gender, or histopathological grade. Only for Cx43, the P-value for the effect of alcohol abuse (P=0.0518) exhibited a trend for a possible downregulation of this Cx in patients abusing alcohol (Table I).
Figure 1.
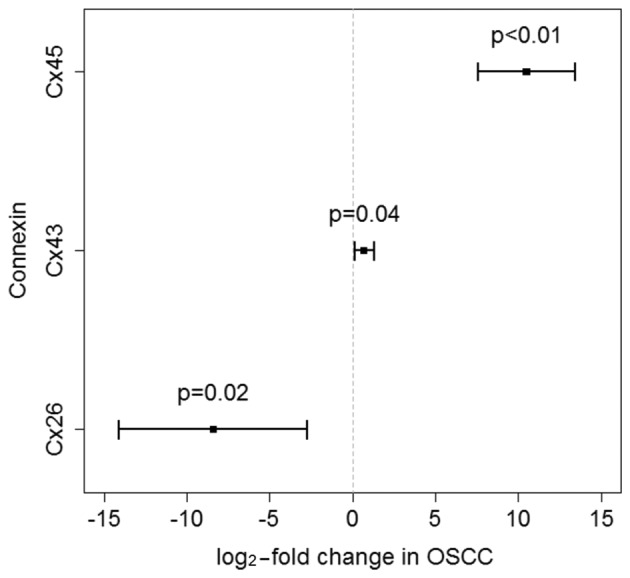
log2-fold changes in OSCC and 95% confidence intervals for the three connexins (Cxs). The displayed P-values are Bonferroni/Holm-adjusted. While Cx43 and Cx45 exhibited an upregulation in OSCC, Cx26 was downregulated.
Discussion
In a previous histomorphometrical analysis (23), we investigated the protein expression patterns of the Cx subtypes 26, 43 and 45 in tissue samples of OSCC, dysplasia-free oral mucosa and LNM in 35 primary OSCC patients and observed differential expression profiles between the tissue types. Moreover, high membrane Cx43 expression in OSSC tissues was associated with poor prognosis, and exhibited a similar prognostic tendency in microscopically unchanged oral mucosa of same patients (23). The present study was performed on tissue samples of tumours at different stages. However, the primary goal was not to investigate the effect of tumour stage, but rather to determine whether the Cx subtype expression differed at the mRNA level between OSSC and oral mucosa. Since differences between the transcriptional and protein levels of Cx43 have been described in an experimental rat tongue carcinogenesis model (24), we further aimed to confirm the expression of Cx subtypes at the mRNA level in OSCC tissues.
Cx45 mRNA has been shown to be more strongly overexpressed in OSCC tissues compared with intraindividual mucosa controls (P<0.01). In contrast to LNM, which exhibited a significant increase in the Cx45 protein level (23), Cx45 protein expression was not found to be significantly different between OSCC tissues and dysplasia-free oral mucosa in our previous investigation (23). There is only limited evidence of the relevance of Cx45 for carcinogenesis in recent publications. Cx45 is variably expressed in human lung fibroblasts and lung carcinoma cells (29), and was detected in normal lung tissue and advanced-stage mouse lung carcinomas (30). Cx45 has been extensively investigated regarding its co-expression with Cx43 resulting in altered gap junctions. Cx45 was found to be upregulated in heart failure, compared with Cx43 (31). The diffusion capacity of cationic fluorescent dyes over heteromeric gap junctions comprising Cx45 and Cx43 was found to be reduced when Cx45 is overexpressed (15), leading to altered intercellular voltage gating mechanisms (14). The relative upregulation of Cx45 in comparison with Cx43 was shown to cause an increased susceptibility to cardiac arrhythmias in vivo (13).
The Cx26 mRNA level was downregulated in OSCC tissues, as opposed to oral mucosa controls (P=0.01). In our histomorphometrical analysis, no Cx26 protein expression was detected in oral mucosa (23); however, it was increased in primary OSCC and exhibited the highest levels in local LNMs. Different studies indicated that Cx26 may be involved in tumour cell invasion and metastasis. Kanczuga-Koda et al (19) described Cx26 overexpression in corresponding LNMs compared with primary mammary carcinoma. Saito-Katsuragi et al (17) demonstrated a significant Cx26 expression in tumour cells and tumour-related microvessel endothelia during metastasis of human malignant melanoma, whereas no Cx26 expression was found in control tissues from either healthy dermis or nevus cell nevi. In this context, it appears likely that neoplastic cells use Cx26 to form homomeric gap junctions with tumour-associated microvessel endothelia, thus improving perivascular accumulation and preparing extravasation.
In the present investigation, Cx43 gene expression was marginally upregulated in OSCC tissues, unlike that in oral mucosa controls (P=0.04). In our previous analysis (23), the cytoplasmic Cx43 protein level was found to be increased in primary OSCC compared with matching oral mucosa. Moreover, membrane Cx43 expression was reduced in OSCC tissues compared with oral mucosa controls, suggesting a loss of gap junctions comprising Cx43, leading to a loss of GJIC. A reduction of Cx43 during carcinogenesis was previously demonstrated (32). However, it has not been fully elucidated whether this loss is due to increased degradation of gap junction channels, or faulty transcription and post-transcriptional modifications within the Cxs. It was recently suggested that post-transcriptional, translational and degradation regulations play a similarly important gene transcription role in the determination of protein concentrations (33). Despite reduced Cx43 protein levels, normal high mRNA levels were detected (24). Budunova et al (34) investigated the expression of Cxs 26, 43 and 31.1 in mouse hyperplastic skin, papilloma and SCC. In addition to the high levels of Cx26 and Cx43 mRNA in most of the SCC, the authors observed decreased protein levels of both Cx subtypes in tumour plasma membranes, and concluded that the expression of these two Cxs in SCC was impaired at the post-translational level (34).
The exact functions of connexins and GJIC during oral carcinogenesis remain unclear. Connexin regulation at the transcriptional level appears to be an early event during the initiation and development of OSCC, and is maintained during tumour progression. However, the mRNA-protein correlation is variable. This may be indicative of post-transcriptional, translational and degradation regulations being relevant for the determination of Cx protein concentration during oral carcinogenesis.
References
- 1.Willecke K, Eiberger J, Degen J, Eckardt D, Romualdi A, Güldenagel M, Deutsch U, Söhl G. Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem. 2002;383:725–737. doi: 10.1515/BC.2002.076. [DOI] [PubMed] [Google Scholar]
- 2.Loewenstein WR, Penn RD. Intercellular communication and tissue growth. II. Tissue regeneration. J Cell Biol. 1967;33:235–242. doi: 10.1083/jcb.33.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mesnil M, Krutovskikh V, Piccoli C, Elfgang C, Traub O, Willecke K, Yamasaki H. Negative growth control of HeLa cells by connexin genes: Connexin species specificity. Cancer Res. 1995;55:629–639. [PubMed] [Google Scholar]
- 4.King TJ, Fukushima LH, Donlon TA, Hieber AD, Shimabukuro KA, Bertram JS. Correlation between growth control, neoplastic potential and endogenous connexin 43 expression in HeLa cell lines: Implications for tumor progression. Carcinogenesis. 2000;21:311–315. doi: 10.1093/carcin/21.2.311. [DOI] [PubMed] [Google Scholar]
- 5.McLachlan E, Shao Q, Wang HL, Langlois S, Laird DW. Connexins act as tumor suppressors in three-dimensional mammary cell organoids by regulating differentiation and angiogenesis. Cancer Res. 2006;66:9886–9894. doi: 10.1158/0008-5472.CAN-05-4302. [DOI] [PubMed] [Google Scholar]
- 6.Avanzo JL, Mesnil M, Hernandez-Blazquez FJ, Mackowiak II, Mori CM, da Silva TC, Oloris SC, Gárate AP, Massironi SM, Yamasaki H, Dagli ML. Increased susceptibility to urethane-induced lung tumors in mice with decreased expression of connexin 43. Carcinogenesis. 2004;25:1973–1982. doi: 10.1093/carcin/bgh193. [DOI] [PubMed] [Google Scholar]
- 7.Dubina MV, Iatckii NA, Popov DE, Vasil'ev SV, Krutovskikh VA. Connexin 43, but not connexin 32, is mutated at advanced stages of human sporadic colon cancer. Oncogene. 2002;21:4992–4996. doi: 10.1038/sj.onc.1205630. [DOI] [PubMed] [Google Scholar]
- 8.Cronier L, Crespin S, Strale PO, Defamie N, Mesnil M. Gap junctions and cancer: New functions for an old story. Antioxid Redox Signal. 2009;11:323–338. doi: 10.1089/ars.2008.2153. [DOI] [PubMed] [Google Scholar]
- 9.Trosko JE, Chang CC, Upham BL, Tai MH. Ignored hallmarks of carcinogenesis: Stem cells and cell-cell communication. Ann N Y Acad Sci. 2004;1028:192–201. doi: 10.1196/annals.1322.023. [DOI] [PubMed] [Google Scholar]
- 10.Iacobas DA, Urban-Maldonado M, Iacobas S, Scemes E, Spray DC. Array analysis of gene expression in connexin-43 null astrocytes. Physiol Genomics. 2003;15:177–190. doi: 10.1152/physiolgenomics.00062.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iacobas DA, Scemes E, Spray DC. Gene expression alterations in connexin null mice extend beyond the gap junction. Neurochem Int. 2004;45:243–250. doi: 10.1016/j.neuint.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Yano T, Hernandez-Blazquez FJ, Omori Y, Yamasaki H. Reduction of malignant phenotype of HEPG2 cell is associated with the expression of connexin 26 but not connexin 32. Carcinogenesis. 2001;22:1593–1600. doi: 10.1093/carcin/22.10.1593. [DOI] [PubMed] [Google Scholar]
- 13.Betsuyaku T, Nnebe NS, Sundset R, Patibandla S, Krueger CM, Yamada KA. Overexpression of cardiac connexin 45 increases susceptibility to ventricular tachyarrhythmias in vivo. Am J Physiol Heart Circ Physiol. 2006;290:H163–H171. doi: 10.1152/ajpheart.01308.2004. [DOI] [PubMed] [Google Scholar]
- 14.Bukauskas FF, Angele AB, Verselis VK, Bennett MV. Coupling asymmetry of heterotypic connexin 45/connexin 43-EGFP gap junctions: Properties of fast and slow gating mechanisms. Proc Natl Acad Sci USA. 2002;99:7113–7118. doi: 10.1073/pnas.032062099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koval M, Geist ST, Westphale EM, Kemendy AE, Civitelli R, Beyer EC, Steinberg TH. Transfected connexin 45 alters gap junction permeability in cells expressing endogenous connexin 43. J Cell Biol. 1995;130:987–995. doi: 10.1083/jcb.130.4.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogawa K, Pitchakarn P, Suzuki S, Chewonarin T, Tang M, Takahashi S, Naiki-Ito A, Sato S, Takahashi S, Asamoto M, Shirai T. Silencing of connexin 43 suppresses invasion, migration and lung metastasis of rat hepatocellular carcinoma cells. Cancer Sci. 2012;103:860–867. doi: 10.1111/j.1349-7006.2012.02228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saito-Katsuragi M, Asada H, Niizeki H, Katoh F, Masuzawa M, Tsutsumi M, Kuniyasu H, Ito A, Nojima H, Miyagawa S. Role for connexin 26 in metastasis of human malignant melanoma: Communication between melanoma and endothelial cells via connexin 26. Cancer. 2007;110:1162–1172. doi: 10.1002/cncr.22894. [DOI] [PubMed] [Google Scholar]
- 18.Ito A, Koma Y, Uchino K, Okada T, Ohbayashi C, Tsubota N, Okada M. Increased expression of connexin 26 in the invasive component of lung squamous cell carcinoma: Significant correlation with poor prognosis. Cancer Lett. 2006;234:239–248. doi: 10.1016/j.canlet.2005.03.049. [DOI] [PubMed] [Google Scholar]
- 19.Kanczuga-Koda L, Sulkowski S, Lenczewski A, Koda M, Wincewicz A, Baltaziak M, Sulkowska M. Increased expression of connexins 26 and 43 in lymph node metastases of breast cancer. J Clin Pathol. 2006;59:429–433. doi: 10.1136/jcp.2005.029272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin JH, Takano T, Cotrina ML, Arcuino G, Kang J, Liu S, Gao Q, Jiang L, Li F, Lichtenberg-Frate H, et al. Connexin 43 enhances the adhesivity and mediates the invasion of malignant glioma cells. J Neurosci. 2002;22:4302–4311. doi: 10.1523/JNEUROSCI.22-11-04302.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozawa H, Matsunaga T, Kamiya K, Tokumaru Y, Fujii M, Tomita T, Ogawa K. Decreased expression of connexin-30 and aberrant expression of connexin-26 in human head and neck cancer. Anticancer Res. 2007;27:2189–2195. [PubMed] [Google Scholar]
- 22.Villaret DB, Wang T, Dillon D, Xu J, Sivam D, Cheever MA, Reed SG. Identification of genes overexpressed in head and neck squamous cell carcinoma using a combination of complementary DNA subtraction and microarray analysis. Laryngoscope. 2000;110:374–381. doi: 10.1097/00005537-200003000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Brockmeyer P, Jung K, Perske C, Schliephake H, Hemmerlein B. Membrane connexin 43 acts as an independent prognostic marker in oral squamous cell carcinoma. Int J Oncol. 2014;45:273–281. doi: 10.3892/ijo.2014.2394. [DOI] [PubMed] [Google Scholar]
- 24.Xia J, Liu X, Tao X, Hong Y, Chen X, Dai Y, Huang Y, Cheng B. Expression of gap junctional protein connexin 43 during 4-nitroquinoline-1-oxide-induced rat tongue carcinogenesis. J Mol Histol. 2009;40:183–188. doi: 10.1007/s10735-009-9229-y. [DOI] [PubMed] [Google Scholar]
- 25.Wolff KD, Follmann M, Nast A. The diagnosis and treatment of oral cavity cancer. Dtsch Arztebl Int. 2012;109:829–835. doi: 10.3238/arztebl.2012.0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fialka F, Gruber RM, Hitt R, Opitz L, Brunner E, Schliephake H, Kramer FJ. CPA6, FMO2, LGI1, SIAT1 and TNC are differentially expressed in early- and late-stage oral squamous cell carcinoma - a pilot study. Oral Oncol. 2008;44:941–948. doi: 10.1016/j.oraloncology.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 27.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holm S. A simple sequentially rejective multiple test procedure. Scandinavian J Stat. 1979;6:65–70. [Google Scholar]
- 29.Zhang ZQ, Hu Y, Wang BJ, Lin ZX, Naus CC, Nicholson BJ. Effective asymmetry in gap junctional intercellular communication between populations of human normal lung fibroblasts and lung carcinoma cells. Carcinogenesis. 2004;25:473–482. doi: 10.1093/carcin/bgh036. [DOI] [PubMed] [Google Scholar]
- 30.Udaka N, Miyagi Y, Ito T. Connexin expression in mouse lung tumor. Cancer Lett. 2007;246:224–229. doi: 10.1016/j.canlet.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 31.Yamada KA, Rogers JG, Sundset R, Steinberg TH, Saffitz J. Up-regulation of connexin 45 in heart failure. J Cardiovasc Electrophysiol. 2003;14:1205–1212. doi: 10.1046/j.1540-8167.2003.03276.x. [DOI] [PubMed] [Google Scholar]
- 32.Xing Y, Xiao Y, Zeng F, Zhao J, Xiao C, Xiong P, Feng W. Altered expression of connexin-43 and impaired capacity of gap junctional intercellular communication in prostate cancer cells. J Huazhong Univ Sci Technolog Med Sci. 2007;27:291–294. doi: 10.1007/s11596-007-0319-3. [DOI] [PubMed] [Google Scholar]
- 33.Vogel C, Marcotte EM. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet. 2012;13:227–232. doi: 10.1038/nrg3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Budunova IV, Carbajal S, Slaga TJ. The expression of gap junctional proteins during different stages of mouse skin carcinogenesis. Carcinogenesis. 1995;16:2717–2724. doi: 10.1093/carcin/16.11.2717. [DOI] [PubMed] [Google Scholar]


