Abstract
Many people with major depressive disorder (MDD) show evidence of systemic inflammation, including elevations in inflammatory factors, but the cause is unclear. The purpose of this analysis was to determine if obesity might contribute to the pro-inflammatory state in MDD patients. Blood was obtained from 135 MDD patients and 50 controls. Serum was extracted and assayed for interleukin (IL) −1β, IL-2, IL-5, IL-6, IL-8, IL-10, IL-12p70, IL-17, interferon-γ (IFNγ), tumor necrosis factor α (TNFα), C-reactive protein (CRP), leptin, and adiponectin using single- or multi-plex human immunoassay kits. The primary analysis contrasted IL-6, TNFα, and CRP between MDD and control groups with body mass index (BMI) as a covariate. The other analytes were compared in an exploratory fashion. IL-6 (but not TNFα or CRP) showed significant differences between MDD and controls even after covarying for BMI. Obese controls and obese MDD groups were significantly higher in IL-6 than both lean groups, but the two obese groups did not differ from each other. In the exploratory analyses, the IL-2 level showed robust and significant differences between MDD and controls even after covarying for BMI. Both lean and obese MDD were higher than lean and obese controls. Adiponectin levels were also lower in the MDD sample than controls. Prior findings of higher IL-6, and CRP in MDD patients may be explained, at least in part, based on obesity. High IL-2, however, was associated with depression and not obesity. The results have significant implications for the understanding of pathophysiology and, potentially treatment of MDD.
Keywords: Depression, inflammation, cytokine, C-reactive protein, leptin, adiponectin
Introduction
Evidence for systemic inflammation in a subset of depressed patients has been long recognized (Maes et al., 1993, Maes et al., 1995, Maes et al., 1994). Many people with depression are found to have elevations of inflammatory cytokines including interleukin (IL) 6 and tumor necrosis factor α (TNFα) (Dowlati et al., 2010, Raison et al., 2006), acute phase reactants such as C-reactive protein (CRP), chemokines, and cell adhesion molecules (Raison et al., 2006). In spite of decades of research, however, the specific etiologies of systemic inflammation in depressed patients remain elusive. While the mechanistic paths from inflammatory cytokines to depression, such as depressive states induced by interferon (IFN) α treatment, are reasonably well understood (Raison et al., 2006), the reverse is not true. There are many depressed patients who show evidence of a sustained pro-inflammatory state in the absence of known systemic disease.
A number of hypotheses have ventured to explain this phenomenon. For example, psychological stress stimulates the release of cytokines and activates cellular immune mechanisms in both animal and human studies (Bierhaus et al., 2003, Garate et al., 2013, Pace et al., 2006). As well, early life adversity, which is known to increase the risk for depression, is also associated with systemic inflammation (Danese et al., 2009, Pace et al., 2006). In one prospective study, people who experienced early life abuse were more likely to have depression and show increased CRP in adult life (Danese et al., 2009, Danese et al., 2008). However, that study also showed that early life adversity was associated with cardiometabolic risk factors such as hypertension, elevated total cholesterol and reduced HDL cholesterol, and higher glycated hemoglobin, phenomena that are associated with obesity, especially increased intra-abdominal adipose tissue (IAAT) (Wajchenberg, 2000). This is consistent with findings that early trauma is also associated with increased risk of general obesity and elevated IAAT in adult life (Thomas et al., 2008).
Both cross-sectional (Papakostas et al., 2005, Simon et al., 2001) and longitudinal (Rotella and Mannucci, 2013) studies have shown increased risk for obesity in depressed patients. What has received considerably less attention, however, is the association between obesity and systemic inflammation in depressed patients. Miller et al. (Miller et al., 2002) assessed the association of depression and cardiometabolic risk factors including systemic inflammation. Depressed participants exhibited higher CRP and IL-6 and meditational analyses indicated that adiposity accounted for a portion of the relationship between depression and increased inflammatory markers. Another recent study assessed IL-2, IL-4, IL-5, IL-10, IL-12, IL-13, granulocyte macrophage colony-stimulating factor (GM-CSF), interferon γ (IFNγ), and TNFα and found higher IL-5, IL-12, IL-13, GM-CSF, IFNγ and TNFα levels in obese depressed participants compared to non-obese depressed and control subjects. (Schmidt et al., 2014)
The primary objective of the current study was to compare the systemic inflammation, particularly IL-6, TNFα, and CRP in a set of obese (body mass index [BMI] ≥ 30) versus non-obese depressed patients and both obese and non-obese controls. Secondary exploratory analyses sought to assess a range of inflammatory cytokines, leptin, and adiponectin across obese and non-obese depressed and controls. The primary hypothesis was that obese depressed and controls would show higher inflammatory factors than non-obese groups, but that the obese depressed participants would show the highest levels of systemic inflammation.
Material and Methods
Samples for this analysis were obtained from two sources. The first set of serum samples were obtained from participants with major depressive disorder (MDD) obtained at baseline in multi-site study of the augmentation of SSRI antidepressants with l-methylfolate previously reported (n=75) (Papakostas et al., 2012). The second was a set of patients with MDD (n=53) and normal volunteer controls (n=50) without history of mental or substance abuse disorder obtained at the University of Alabama Medical Center in Birmingham, AL (UAB). Controls were selected to approximate the proportions of MDD participants with BMI≥30. The research was conducted in accordance with the Declaration of Helsinki (6th Revision). The studies were reviewed and approved by local institutional review boards and written informed consent was obtained from all subjects. Participants were males and females ages 19–66 and were physically healthy or had stable medical conditions. None had evidence of systemic inflammatory diseases such as rheumatoid arthritis, lupus, or similar conditions. Diagnosis was confirmed using the Structured Clinical Interview for DSM-IV (First et al., 2002) or the Mini International Neuropsychiatric Interview (Sheehan et al., 1998). The 17-item Hamilton Rating Scale for Depression (HRSD) (Hamilton, 1960) was obtained on all participants collected in the l-methylfolate study, and the Montgomery-Åsberg Depression Rating Scale (Montgomery and Asberg, 1979) was obtained on the participants collected at UAB.
Blood samples were obtained from all participants and serum extracted using standard methods and stored at −80°C until assayed (Tuck et al., 2009). The timing of the blood sample was not controlled in the study and the participants were not fasting. Anthropometrics were collected using National Health and Nutrition Examination Survey (NHANES) methods (NCHS, 2007). BMI was calculated in kg/m2 from height and weight measures. Obesity was defined as a BMI≥30.
Immunoassays
Plasma samples were analyzed for IL-1β, IL-2, IL-5, IL-6, IL-8, IL-10, IL-12p70, IL-17, IFNγ, and TNFα, using single- or multi-plex human immunoassay kits (Meso Scale Discovery, Gaithersburg, MD). CRP (minimum sensitivity=0.5 mg/l) was analyzed using immunoassay on a Stanbio Sirrus Analyzer (Stanbio Laboratory, Boeme, TX) using a Pointe Scientific (Canton, MI) turbidimetric CRP reagent. Minimum sensitivity, intra-assay, and inter-assay coefficient of variation (%) were as follows: IL-1β: 0.03 pg/ml, 17.11, 6.36; IL-2: 0.16 pg/ml, 10.62, 10.86; IL-5: 0.11 pg/ml, 13.23, 4.04; IL-6: 0.25 pg/ml, 7.12, 5.77; IL-8: 0.09 pg/ml, 1.21, 1.37; IL-10: 0.07 pg/ml, 6.61, 2.06; IL-12p70: 0.09 pg/ml, 1.96, 12.32; IL-17: 0.91 pg/ml, 3.66, 11.40; IFNγ: 0.56 ng/ml, 4.75, 2.83, TNFα: 0.08 pg/ml, 5.72, 5.61; CRP: 0.5 mg/L, 7.49, 2.13. Leptin (minimum sensitivity=1.04 ng/ml, intra-assay CV=1.128%) and adiponectin (minimum sensitivity=1.24 µg/ml, intra-assay CV=3.98%) were analyzed using single human radioimmunoassay kits (Millipore, Billerica, MA). All samples were run in duplicate and the mean of the duplicate samples were reported. If the duplicate value was >5% higher or lower than the other value the assay was repeated in duplicate; if the duplicate varied on a second analysis by more than 5%, the sample was excluded.
Study data were collected and managed using REDCap (Research Electronic Data Capture) electronic data capture tools hosted at the University of Alabama at Birmingham (Harris et al., 2009). REDCap is a secure, web-based application designed to support data capture for research studies.
Statistical analysis
Demographic data including age, sex distribution, BMI, weight, and waist and hip circumference, and waist-to-hip ratio were compared between MDD and controls using t-tests for continuous and Chi2 for categorical data. The primary analysis tested the differences in levels of IL-6, TNFα, and CRP between the MDD and control groups with univariate analysis of variance (ANOVA) with BMI as a covariate and Bonferroni correction. Secondary exploratory analyses also tested the other cytokines, leptin, and adiponectin via ANOVA with BMI as a covariate. A final analysis step assessed a four-group comparison via ANOVA followed by Tamhane’s T2 test (Tamhane, 1977) for unequal variances for all analytes: 1. Non-obese controls; 2. Obese controls; 3. Non-obese MDD, and 4. Obese MDD groups. The overall primary hypothesis was that differences between MDD and control groups on the three primary analytes would be accounted for by obesity status.
The associations between BMI, waist and hip circumference (cm), and waist to hip ratio with cytokines, CRP, leptin, and adiponectin were assessed using the Pearson product-moment correlation coefficient. Analyses were conducted using SPSS version 22.
Results
A total of 135 patients with MDD and 50 controls were included. Demographic data are included in Table 1. Only age was significantly different between groups (t=−2.454, df=163, p=0.015). There were no significant differences between controls and MDD patients in anthropometrics, including weight, waist circumference, hip circumference, or waist-to-hip ratio for the total sample, males, or females. For the MDD participants collected in the l-methylfolate trial (Papakostas et al., 2012) the mean (SD) HRSD score was 21.3 (3.9) and the mean (SD) MADRS scores for the UAB MDD sample was 28.2 (13.4).
Table 1.
Demographics by group
| Controls |
Depressed |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Sample (n=50) |
Non-obese (n=28) |
Obese (n=21) |
Total Sample (N=135) |
Non-obese (n=72) |
Obese (n=64) |
|||||||
| - | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Age | 38.4 | 10.2 | 38.8 | 10.4 | 39.1 | 11.0 | 45.0* | 12.5 | 39.2 | 13.1 | 47.9** | 11.6 |
| BMI | 30.4 | 7.8 | 24.4 | 3.2 | 36.4 | 3.7 | 30.9 | 8.3 | 24.9 | 3.3 | 38.1 | 7.3 |
| Weight (kg) | 85.0 | 21.3 | 69.7 | 13.4 | 97.5 | 9.6 | 84.3 | 19.6 | 72.1 | 14.0 | 100.0 | 17.8 |
| −Males | 84.3 | 14.4 | 80.7 | 14.1 | 94.2 | 18.7 | 85.9 | 16.8 | 79.0 | 12.9 | 109.7 | 11.9 |
| −Females | 85.2 | 22.9 | 64.6 | 9.9 | 98.1 | 8.5 | 83.5 | 21.1 | 65.8 | 9.4 | 98.6 | 18.3 |
| Waist (cm) | 92.5 | 16.4 | 82.3 | 10.1 | 100.8 | 10.5 | 94.3 | 17.7 | 84.7 | 12.2 | 102.6 | 18.8 |
| −Males | 95.2 | 10.8 | 92.9 | 10.5 | 103.5 | 9.9 | 93.2 | 19.6 | 89.2 | 13.1 | 95.0 | 42.4 |
| −Females | 91.4 | 17.4 | 77.4 | 4.9 | 100.3 | 11.0 | 94.9 | 16.7 | 79.8 | 9.3 | 103.9 | 13.4 |
| Sex | N | % | N | % | N | % | N | % | N | % | N | % |
| Males | 10 | 20.0 | 8 | 28.6 | 2 | 9.5 | 46 | 34.1 | 31 | 43.1 | 15 | 23.4 |
| Females | 40 | 80.0 | 20 | 71.4 | 19 | 90.5 | 89 | 65.9 | 41 | 56.9 | 49 | 76.6 |
| Race | ||||||||||||
| −White | 24 | 48.0 | 13 | 46.6 | 10 | 47.6 | 81 | 56.8 | 46 | 56.3 | 36 | 56.3 |
| −African-American | 24 | 48.0 | 13 | 46.6 | 11 | 52.4 | 52 | 38.5 | 26 | 36.1 | 26 | 40.6 |
| −Hispanic-Latino | 1 | 2.0 | 1 | 4.5 | 0 | 0 | 1 | 0.7 | 0 | 0 | 1 | 1.6 |
| −Asian | 1 | 2.0 | 1 | 4.5 | 0 | 0 | 1 | 0.7 | 0 | 0 | 1 | 1.6 |
| Obese (BMI≥30) | 22 | 44.0 | 63 | 46.7 | ||||||||
| Severely obese (BMI≥35) | 14 | 28.0 | 35 | 25.9 | ||||||||
p=0.001, MDD vs. controls.
Temhane T2 p=0.01 versus non-obese and obese controls; p=0.07 versus non-obese depressed. All other comparisons NS.
Between-groups analysis
The assay data for all groups and analytes is summarized in Table 2. The between groups contrast comparing MDD and control groups for CRP showed significant main effects for BMI (F=40.81, df=1,181, p<0.0001) but not group (F=0.82, df=1,181, p=NS). IL-6 showed significant differences for both group (F=5.46, df=1,179, p=0.021) and BMI (F=36.70, df=1,179, p<0.0001). TNFα showed significant effects for BMI (F=8.35, df=1,182, p=0.004) but not group (F=1.78, df=1,182, p=NS). The group effect for IL-6 survived correction for multiple comparisons only at a trend level (p=0.063). The BMI effect survived correction for all three analytes.
Table 2.
Cytokines, CRP, Leptin, and Adiponectin by study group.
| Controls |
Depressed |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Sample (n=50) |
Non-obese (n=28) |
Obese (n=22) |
Total Sample (N=135) |
Non-obese (n=72) |
Obese (n=64) |
|||||||
| - | Mean |
SD |
Mean |
Mean |
SD |
Mean |
SD |
Mean |
SD |
Mean |
SD |
|
| CRPa | 4.554 | 4.566 | 2.838 | 2.849 | 6.528e | 5.453 | 5.464 | 5.989 | 3.176 | 3.045 | 8.092f | 7.282 |
| IL-6b | 1.027 | 0.693 | 0.744 | 0.562 | 1.406g | 0.700 | 1.455h | 1.240 | 1.069 | 0.801 | 1.902i | 1.485 |
| TNFα | 2.667 | 1.329 | 2.334 | 0.921 | 3.137 | 1.668 | 3.026 | 1.655 | 2.787 | 1.288 | 3.279j | 1.960 |
| IL-1β | 0.151 | 0.092 | 0.141 | 0.098 | 0.169 | 0.082 | 0.136 | 0.073 | 0.135 | 0.068 | 0.135 | 0.079 |
| IL-2 | 0.180 | 0.067 | 0.192 | 0.083 | 0.163 | 0.031 | 0.322k | 0.210 | 0.297l | 0.192 | 0.349m | 0.227 |
| IL-5 | 0.695 | 1.542 | 0.716 | 1.788 | 0.686 | 1.205 | 0.416 | 0.310 | 0.421 | 0.332 | 0.407 | 0.282 |
| IL-8 | 6.911 | 3.818 | 7.345 | 3.613 | 6.454 | 4.158 | 7.948 | 4.082 | 7.722 | 4.197 | 8.140 | 3.966 |
| IL-10 | 1.270 | 1.758 | 0.952 | 0.990 | 1.737 | 2.416 | 1.026 | 1.022 | 0.904 | 0.637 | 1.156 | 1.323 |
| IL-12p70 | 1.933 | 3.193 | 2.210 | 3.406 | 1.630 | 2.985 | 1.493 | 2.638 | 1.644 | 2.831 | 1.300 | 2.389 |
| IL-17 | 1.470 | 1.489 | 1.466 | 1.555 | 1.511 | 1.465 | 1.129 | 1.897 | 1.139 | 2.224 | 1.114 | 1.458 |
| IFNγ | 4.330 | 3.055 | 4.940 | 3.525 | 3.410 | 1.973 | 4.560 | 2.835 | 4.890 | 3.083 | 4.160 | 2.466 |
| Leptinc | 21.580 | 19.087 | 15.470 | 11.823 | 28.450 | 22.114 | 25.941 | 20.097 | 22.546 | 17.759 | 30.506n | 22.550 |
| Adiponectind | 23.452o | 20.258 | 19.113 | 15.451 | 31.406p | 25.287 | 11.760 | 10.215 | 11.185 | 6.185 | 12.387 | 13.397 |
mg/L
AII cytokines pg/ml.
ng/ml.
µg/ml.
p=0.05 versus non-obese controls.
p<0.001 versus non-obese controls and non-obese depressed.
p=0.008 versus non-obese controls.
p=0.021 versus controls.
p<0.002 versus non-obese controls and non-obese depressed.
p=0.013 versus non-obese controls.
p<0.0001 versus controls.
p<0.003 versus non-obese and obese controls.
p<0.003 versus non-obese and obese controls.
p<0.001 versus non-obese controls.
p<0.0001 versus depressed.
p=0.05 versus non-obese and non-obese depressed.
The exploratory analyses for the other analytes were as follows. Significant effects were found for group and/or BMI for IL-2 (group: F=17.03, df=1,166, p<0.0001; BMI: F=5.62, df=1,164, p=0.019), IL-10 (BMI: F=7.80, df=1,181, p=0.006), leptin (BMI: F=15.16, df=1,175, p<0.0002), and adiponectin (group: F=26.38, df=1,175, p<0.0001), and at a trend level for group for IL-5 (F=3.85, df=1,177, p=0.051).
The between groups contrasts for the total MDD and control groups not stratified by BMI are shown in Supplemental Table 1. Significant group differences were shown for IL-2 (t=3.49, df=145, p=0.001), IL-6 (t=2.06, df=159, p<0.05), and adiponectin (t=−4.48, df=154, p<0.001). Of the three, only IL-2 and adiponectin survived correction for BMI.
Four-group comparisons
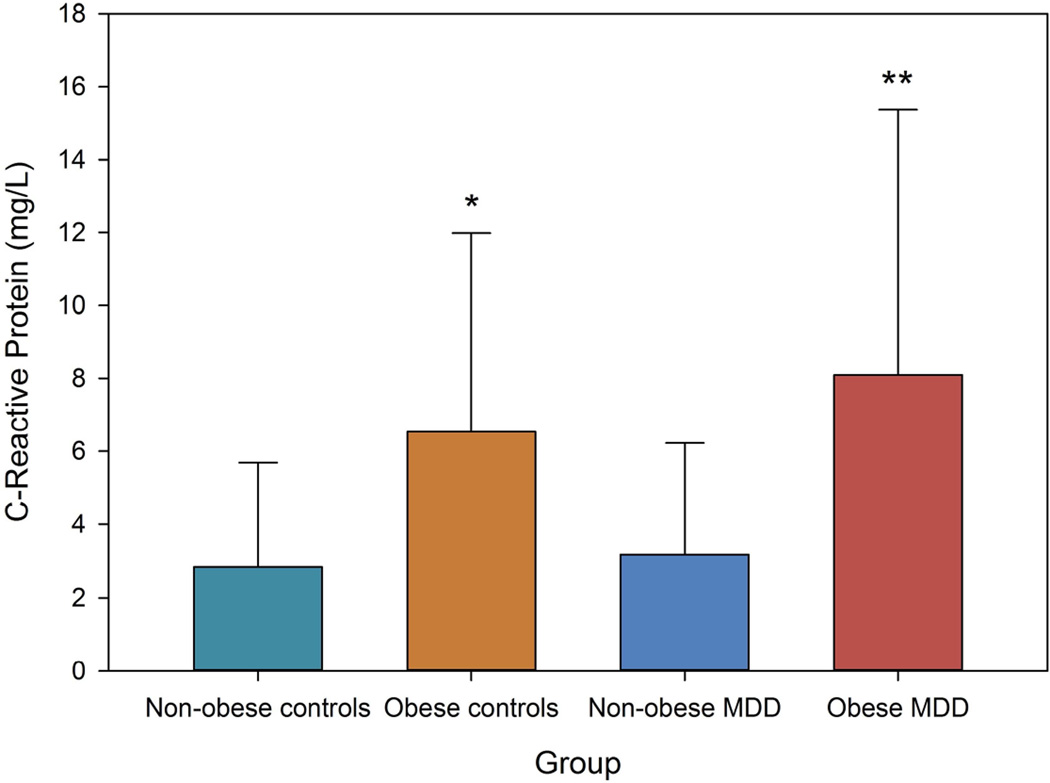
The four-group comparison (1. Non-obese controls; 2. Obese controls; 3. Non-obese MDD, and 4. Obese MDD) ANOVA was significant for IL-6 (F[3,156]=9.780, p<0.001) and CRP (F[3,156]=11.319, p<0.001), and marginally significant for IL-1β (F[3,156]=2.385, p=0.071), and TNFα (F[3,156]=2.191, p=0.091). Post-hoc Tamhane’s T2 test showed a mixture of effects. For IL-6, the obese MDD group was significantly higher than non-obese MDD and non-obese controls (both p<0.002), but not obese controls. Non-obese MDD were not significantly higher than non-obese MDD or controls. Similarly, for CRP, obese MDD was significantly higher than both non-obese MDD and controls (p<0.001) but not obese controls. Again, obese controls were not significantly higher than non-obese groups. These data support the idea that high IL-6 and CRP in MDD patients may be a product of obesity. TNFα did not show any differences between groups. Adding age as a covariate in the analyses did not change the results except that the multivariate test for TNFα became non-significant (p=0.148).
The four-groups comparison for the remaining analytes indicates that the most robust effect for group was for IL-2, for which both the non-obese and obese MDD patients were significantly higher than both groups of controls (all Tamhane’s T2 p<0.03). Other significant effects include the observations that the leptin levels in obese MDD sample were higher than non-obese controls (p<0.001), but not obese controls or non-obese MDD. Adiponectin levels were higher in obese controls than both non-obese and obese MDD patients (Tamhane’s p<0.05) but not non-obese controls.
Although most of the analyses described above were considered exploratory, two analyses, overall group differences for IL-2 and adiponectin survived correction for multiple comparisons.
Correlations
Correlational analyses are summarized in Supplemental Table 2. The controls showed significant correlations between CRP and BMI, waist, and hip circumference; IL-6 and BMI, waist, and hip circumference; IFNγ and BMI; leptin and BMI; and adiponectin and BMI, waist, and hip circumference (all p<0.05). MDD participants showed significant correlations between CRP and BMI; IL-2 and BMI and waist circumference; IL-6 and BMI; TNFα and BMI, waist circumference, and waist-to-hip ratio; and Leptin and BMI (all p<0.05).
Discussion
Increased IL-6, TNFα, and CRP have been consistently demonstrated in MDD patients compared to normal controls (Dowlati et al., 2010, Howren et al., 2009). This study suggests that the association of these three indicators of systemic inflammation with depression is associated in large part with obesity, not just depression per se. Key findings are that both CRP and TNFα levels did not differ between MDD and βcontrol groups when controlling for BMI. BMI, on the other hand is strongly correlated with both (CRP p<0.00001, TNFα p<0.01). IL-6 showed significant main effects for both group and BMI, but the effects for BMI were much stronger. The four-group (non-obese control, obese control, non-obese MDD and obese MDD) contrast showed that with all three analytes, non-obese MDD patients were numerically slightly higher than non-obese controls and obese MDD patients were slightly higher than obese controls, but in no instance were there significant differences. Therefore, for this set of biomarkers, the dominant driving factor for group differences was BMI.
While meta-analyses have suggested that CRP, IL-6, and TNFα are elevated in MDD patients (Dowlati et al., 2010, Howren et al., 2009), there was heterogeneity in the earlier studies, with some studies showing significant differences and many others not (Dowlati et al., 2010). Our data suggest that some of this variability may be accounted for by obesity. Our samples were well matched on BMI, waist circumference, hip circumference, and waist-to hip ratio. We hypothesize that the outcome variations in the prior studies could be related to different proportions of obese participants in the MDD and control samples.
The Howren et al. (2009) meta-analysis reported effect sizes for several of the analytes measured in this study and found significant differences between MDD and control groups for CRP (d=0.15), IL-6 (d=0.25), and IL-1 (d=0.35), which are close to the effect sizes in the current study in the overall MDD versus control comparison (CRP, d=0.22; IL-6, d=0.39; IL-1β, d=0.21) (Supplemental Table 1). While IL-6 showed significant differences between groups not corrected for BMI, this study was underpowered to show significant differences for CRP and IL-β. For example, CRP would require a sample size of about 280 participants to achieve significant results. Similarly to the present study, Howren et al. found that the effect sizes diminished after entering BMI as a covariate for CRP (d=0.11) and IL-6 (d=0.08), although not IL-1 (d=0.39).
There is a well-established link between obesity and elevations in IL-6, TNFα and CRP (Sutherland et al., 2004). As fat mass accumulates, adipocytes release IL-6 and TNFα, which stimulate production of CRP in the liver, as well as factors such as chemokine (C-C motif) ligand 2 (CCL2), also referred to as monocyte chemotactic protein 1 (MCP1). The release of CCL2 attracts macrophages, T lymphocytes, and dendritic cells to adipose tissue that also release cytokines into the systemic circulation (Shelton and Miller, 2010). This process of expansion of adipose tissue leading to a systemic inflammatory state is a known mechanism for metabolic diseases such as cardiovascular disease, hypertension, and type 2 diabetes (Sutherland et al., 2004).
Previous studies have implicated obesity as a possible causal process for elevated IL-6, CRP, and other inflammatory factors in MDD patients (Benson et al., 2008, Capuron et al., 2010). For example, Capuron et al. found that IL-6 and CRP correlated with baseline BMI and with both the depression and anxiety facets of neuroticism on the Revised NEO Personality Inventory in a group of women waiting for gastric bypass surgery. Weight loss was associated with reduced BMI, depression, IL-6, and CRP, and the change in depression over time was mediated by change in the inflammatory factors. As noted previously by Miller and colleagues, “the association between increased BMI and inflammation represents a complicating factor in the relationship among inflammation, depression, and antidepressant treatment” (Miller et al., 2009).
The association between BMI and systemic inflammation in MDD does not mean that the inflammation is merely artifactual and not related to the pathophysiology of depression. A large number of prior studies have shown that induction of inflammation with, for example, interferon-α (IFNα) therapy, will produce depression in 30–50% of patients (Raison et al., 2006). However, the fact that not all people treated with IFNα become depressed indicates that interferon-induced depression is likely to occur in people who are otherwise predisposed to depression. In the current context, obesity-induced elevations in inflammatory factors such as IL-6 in people vulnerable to depression for other reasons may result in depression, even when there are no absolute differences in cytokine levels from obese people without depression.
BMI is only moderately correlated with IAAT, which is more directly associated with altered levels of systemic inflammatory factors, even within obese populations. Prior data indicate that MDD patients have higher IAAT levels than matched controls (Everson-Rose et al., 2009) and that baseline depression leads to increased accumulation of IAAT in contrast to non-depressed populations (Vogelzangs et al., 2008). Therefore, visceral fat mass may be even more important than BMI per se in explaining the increased systemic inflammation in MDD. Future studies should include direct measures of IAAT and other fat compartments to assess these relationships.
For the remaining analytes, the results with IL-2 are the most significant. IL-2 was significantly elevated in the overall ANOVA after controlling for BMI, and in the four-groups comparison in which both non-obese and obese MDD groups were higher than both control groups. Further, the associations with depression were much more robust (p<0.0001) than obesity (p<0.02). The overall group differences survived correction for multiple comparisons. While the correlation between IL-2 and BMI were significant, the association was weak (r=.193). These results suggest that in contrast to most other inflammatory and metabolic factors measured in this study, high IL-2 was more strongly associated with MDD rather than obesity. This is surprising given the relative absence of significant differences in prior studies in at least one meta-analysis (Dowlati et al., 2010). However, both the methods and results were highly heterogeneous in prior studies and one study showed significant differences between MDD and controls (Jozuka et al., 2003).
These findings have significant implications with regard to the treatment of depressed patients. Both obesity (Kloiber et al., 2007, Oskooilar et al., 2009) and systemic inflammation, including elevated IL-6 and CRP (Eller et al., 2008, Lanquillon et al., 2000, Maes et al., 1997, Vogelzangs et al., 2014), are associated with poorer response to antidepressant treatment. Therefore, targeting obesity as a component of the treatment of overweight depressed patients may result in greater improvement than the use of antidepressants or psychotherapy alone. Consistent with this, exercise interventions have demonstrated beneficial effects on depressive symptoms (Fabricatore et al., 2011), although few studies have specifically targeted clinically depressed patients. One such study by Trivedi et al. (Trivedi et al., 2011) indicated that either 16 or 4 kcal per kg per week (KKW) of exercise expenditure for 12 weeks reduced depressive symptoms, and there was a trend for higher remission rates in the 16 KKW group (28.3% versus 15.5%). Moreover, many of the treatments used for depression, including some antidepressants and the atypical antipsychotics cause weight gain, which may contribute to the development of treatment resistance. These findings argue for expanding the repertoire of depression treatment to include methods to reduce overweight and obesity.
The results of this study should be viewed with caution. Most significantly, the majority of the analyses were conducted in an exploratory manner. While the results suggest important associations between obesity and systemic inflammation in depressed patients, the results must be replicated before they can be accepted. Future studies should not only assess obesity with BMI, waist, and hip circumferences, but also conduct direct measures of total fat mass and fat compartments including IAAT, subcutaneous abdominal, and other fat compartments. Further, although all participants were healthy and without unstable medical conditions, information on medical co-morbidity was not systematically collected. The relationship between inflammation and co-morbid medical conditions would have been a valuable addition. Other limitations include the fact that all participants were recruited in tertiary medical centers and the findings may not correspond well with other settings. In addition, depressed participants were recruited from a variety of centers across the U.S. while all the controls were recruited in Birmingham, AL. It should also be noted that while there were no statistically significant differences between obese MDD and obese controls in IL-6 and CRP, mean levels were numerically higher in obese MDD participants (IL-6=2.172 pg/ml, CRP=8.241 mg/L) than controls (IL-6=1.332 pg/ml, CRP=6.770). It is therefore possible that a larger sample size could have detected significant differences between these groups, although it is likely that they both would have remained significantly higher than the non-obese groups. Even if this were true it would indicate that both IL-6 and CRP would be significantly higher in obese MDD than other groups including non-obese MDD. Finally, almost all of the MDD participants were on either an SSRI or SNRI antidepressant; SSRIs appear to have anti-inflammatory effects in depressed patients with significant effects previously demonstrated for IL-6 (Basterzi et al., 2005), which could have affected the results of this study.
Summary
The main findings from this study were that most indicators of systemic inflammation such as IL-6, TNFα, and CRP were associated with obesity in both MDD and control samples. Of the three, only IL-6 showed significant main effects that were independent of BMI. This indicates that the consistently replicated findings of high IL-6, TNFα, and CRP in MDD patients, at least in part, may be related to obesity, not depression per se. These findings have serious implications for both treatment and prevention of depression given the ample evidence that systemic inflammation may be both a causal and perpetuating factor in depression (Miller et al., 2009). Clearly more research is needed to determine if obesity, particularly high visceral fat, and the systemic inflammation associated with visceral obesity might be appropriate targets for treatment. Although prior studies have assessed change in depression associated with exercise and weight loss, few have targeted bona fide clinically depressed populations. There are even more serious implications for prevention. There is now strong evidence that baseline obesity and metabolic disease increase risk for subsequent depression in both adults (Akbaraly et al., 2009, Hryhorczuk et al., 2013, Milaneschi et al., 2012, Molyneaux et al., 2014, Vogelzangs et al., 2010) (including older adults (Almeida et al., 2009, Herbst et al., 2007, Milaneschi et al., 2012, Roberts et al., 2003, Vogelzangs et al., 2010)) and children (Erermis et al., 2004, Goodman and Whitaker, 2002). This creates a strategic opportunity for secondary prevention strategies to reduce risk for depression across the lifespan.
Supplementary Material
Figure.
* p=0.05 versus non-obese controls
** fp<0.001 versus non-obese controls and non-obese depressed
Highlights.
There is strong evidence of systemic inflammation in some people with major depression, but without known cause.
We hypothesized that obesity may account for this inflammation, at least in part.
The cytokine IL-6 was elevated in depressed patients relative to controls; there were significant effects for both group (MDD vs. controls) and BMI.
Obese MDD patients had higher IL-6 than non-obese MDD and controls, but not obese controls
TNFα and CRP show significant effects for BMI but not group, indicating that group differences in prior research might be accounted for by obesity.
Acknowledgments
Support for the sample collection and analysis was provided by the Brain & Behavior Research Foundation and Pamlab, Inc. Funding for the REDCAP database was provided by the NIH National Center for Research Resources as part of its Clinical and Translational Science Award Program (5UL1RR025777-03, 5KL2RR025776-03, 5TL1RR025775-03). None of the sponsors had any role in the analysis, interpretation, or other aspects of the manuscript.
Role of Funding Source
Support for the sample collection and analysis was provided by the Brain & Behavior Research Foundation and Pamlab, Inc. The sponsors had no role in the design, execution, analysis, interpretation of the data, or decisions about where to submit the article. Funding for the REDCAP database was provided by the NIH National Center for Research Resources as part of its Clinical and Translational Science Award Program (5UL1RR025777-03, 5KL2RR025776-03, 5TL1RR025775-03), which similarly provided no input
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
All authors have contributed materially to the design, planning, execution, analysis, and interpretation of the data. All have reviewed and approved the final draft of the manuscript. Richard C. Shelton, MD
| Design | Planning | Data Collection |
Analysis | Interpretation | Writing | Final Approval |
|
|---|---|---|---|---|---|---|---|
| Richard C. Shelton, MD | X | X | X | X | X | X | X |
| Michael Falola, M.D., MPH | X | X | X | X | |||
| Li Li, M.D., Ph.D. | X | X | X | ||||
| John Zajecka, MD | X | X | |||||
| Maurizio Fava, MD | X | X | X | ||||
| George I. Papakostas, MD | X | X | X |
Literature cited
- Akbaraly TN, Kivimaki M, Brunner EJ, Chandola T, Marmot MG, Singh-Manoux A, et al. Association between metabolic syndrome and depressive symptoms in middle-aged adults: results from the Whitehall II study. Diabetes Care. 2009;32:499–504. doi: 10.2337/dc08-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida OP, Calver J, Jamrozik K, Hankey GJ, Flicker L. Obesity and metabolic syndrome increase the risk of incident depression in older men: the health in men study. Am J GeriatrPsychiatry. 2009;17:889–898. doi: 10.1097/JGP.0b013e3181b047e3. [DOI] [PubMed] [Google Scholar]
- Basterzi AD, Aydemir C, Kisa C, Aksaray S, Tuzer V, Yazici K, et al. IL-6 levels decrease with SSRI treatment in patients with major depression. HumPsychopharmacol. 2005;20:473–476. doi: 10.1002/hup.717. [DOI] [PubMed] [Google Scholar]
- Benson S, Janssen OE, Hahn S, Tan S, Dietz T, Mann K, et al. Obesity, depression, and chronic low-grade inflammation in women with polycystic ovary syndrome. Brain BehavImmun. 2008;22:177–184. doi: 10.1016/j.bbi.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Poitou C, Machaux-Tholliez D, Frochot V, Bouillot JL, Basdevant A, et al. Relationship between adiposity, emotional status and eating behaviour in obese women: role of inflammation. PsycholMed. 2010;20:1–12. doi: 10.1017/S0033291710001984. [DOI] [PubMed] [Google Scholar]
- Danese A, Moffitt TE, Harrington H, Milne BJ, Polanczyk G, Pariante CM, et al. Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Arch PediatrAdolescMed. 2009;163:1135–1143. doi: 10.1001/archpediatrics.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Moffitt TE, Pariante CM, Ambler A, Poulton R, Caspi A. Elevated Inflammation Levels in Depressed Adults With a History of Childhood Maltreatment. Archives of General Psychiatry. 2008;65:409–415. doi: 10.1001/archpsyc.65.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biological Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Eller T, Vasar V, Shlik J, Maron E. Pro-inflammatory cytokines and treatment response to escitalopram in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:445–450. doi: 10.1016/j.pnpbp.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Erermis S, Cetin N, Tamar M, Bukusoglu N, Akdeniz F, Goksen D. Is obesity a risk factor for psychopathology among adolescents? PediatrInt. 2004;46:296–301. doi: 10.1111/j.1442-200x.2004.01882.x. [DOI] [PubMed] [Google Scholar]
- Everson-Rose SA, Lewis TT, Karavolos K, Dugan SA, Wesley D, Powell LH. Depressive symptoms and increased visceral fat in middle-aged women. Psychosom Med. 2009;71:410–416. doi: 10.1097/PSY.0b013e3181a20c9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabricatore AN, Wadden TA, Higginbotham AJ, Faulconbridge LF, Nguyen AM, Heymsfield SB, et al. Intentional weight loss and changes in symptoms of depression: a systematic review and meta-analysis. Int J Obes (Lond) 2011 doi: 10.1038/ijo.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) New York, NY Biometrics Research: New York State Psychiatric Institute; 2002. [Google Scholar]
- Garate I, Garcia-Bueno B, Madrigal JL, Caso JR, Alou L, Gomez-Lus ML, et al. Stress-induced neuroinflammation: role of the Toll-like receptor-4 pathway. Biol Psychiatry. 2013;73:32–43. doi: 10.1016/j.biopsych.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Goodman E, Whitaker RC. A Prospective Study of the Role of Depression in the Development and Persistence of Adolescent Obesity. Pediatrics. 2002;110:497–504. doi: 10.1542/peds.110.3.497. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. JNeurolNeurosurgPsychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. JBiomedInform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst S, Pietrzak RH, Wagner J, White WB, Petry NM. Lifetime major depression is associated with coronary heart disease in older adults: results from the National Epidemiologic Survey on Alcohol and Related Conditions. PsychosomMed. 2007;69:729–734. doi: 10.1097/PSY.0b013e3181574977. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-Reactive Protein, IL-1, and IL-6: A meta-analysis. Psychosomatic Medicine. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Hryhorczuk C, Sharma S, Fulton SE. Metabolic disturbances connecting obesity and depression. Frontiers in neuroscience. 2013;7:177. doi: 10.3389/fnins.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jozuka H, Jozuka E, Takeuchi S, Nishikaze O. Comparison of immunological and endocrinological markers associated with major depression. J Int Med Res. 2003;31:36–41. doi: 10.1177/147323000303100106. [DOI] [PubMed] [Google Scholar]
- Kloiber S, Ising M, Reppermund S, Horstmann S, Dose T, Majer M, et al. Overweight and obesity affect treatment response in major depression. Biol Psychiatry. 2007;62:321–326. doi: 10.1016/j.biopsych.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Lanquillon S, Krieg JC, Bening-Abu-Shach U, Vedder H. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacol. 2000;22:370–379. doi: 10.1016/S0893-133X(99)00134-7. [DOI] [PubMed] [Google Scholar]
- Maes M, Bosmans E, De Jongh R, Kenis G, Vandoolaeghe E, Neels H. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine. 1997;9:853–858. doi: 10.1006/cyto.1997.0238. [DOI] [PubMed] [Google Scholar]
- Maes M, Bosmans E, Meltzer HY, Scharpe S, Suy E. Interleukin-1 beta: a putative mediator of HPA axis hyperactivity in major depression? AmJPsychiatry. 1993;150:1189–1193. doi: 10.1176/ajp.150.8.1189. [DOI] [PubMed] [Google Scholar]
- Maes M, Meltzer HY, Bosmans E, Bergmans R, Vandoolaeghe E, Ranjan R, et al. Increased plasma concentrations of interleukin-6, soluble interleukin-6, soluble interleukin-2 and transferrin receptor in major depression. J AffectDisord. 1995;34:301–309. doi: 10.1016/0165-0327(95)00028-l. [DOI] [PubMed] [Google Scholar]
- Maes M, Scharpe S, Meltzer HY, Okayli G, Bosmans E, D’Hondt P, et al. Increased neopterin and interferon-gamma secretion and lower availability of L-tryptophan in major depression: further evidence for an immune response. Psychiatry Res. 1994;54:143–160. doi: 10.1016/0165-1781(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Milaneschi Y, Simonsick EM, Vogelzangs N, Strotmeyer ES, Yaffe K, Harris TB, et al. Leptin, abdominal obesity, and onset of depression in older men and women. J Clin Psychiatry. 2012 doi: 10.4088/JCP.11m07552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and Its Discontents: The Role of Cytokines in the Pathophysiology of Major Depression. Biological Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Stetler CA, Carney RM, Freedland KE, Banks WA. Clinical depression and inflammatory risk markers for coronary heart disease. Am J Cardiol. 2002;90:1279–1283. doi: 10.1016/s0002-9149(02)02863-1. [DOI] [PubMed] [Google Scholar]
- Molyneaux E, Poston L, Ashurst-Williams S, Howard LM. Obesity and Mental Disorders During Pregnancy and Postpartum: A Systematic Review and Meta-analysis. Obstetrics and gynecology. 2014;123:857–867. doi: 10.1097/AOG.0000000000000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- NCHS. National Health and Nutrition Examination Protocol. Hyattsville, MD: Centers for Disease Control and Prevention (CDC), U.S. Department of Health and Human Services; 2007. National Center for Health Statistics. [Google Scholar]
- Oskooilar N, Wilcox CS, Tong ML, Grosz DE. Body mass index and response to antidepressants in depressed research subjects. J ClinPsychiatry. 2009;70:1609–1610. doi: 10.4088/JCP.09l05226blu. [DOI] [PubMed] [Google Scholar]
- Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. AmJPsychiatry. 2006;163:1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Petersen T, Iosifescu DV, Burns AM, Nierenberg AA, Alpert JE, et al. Obesity among outpatients with major depressive disorder. Int J Neuropsychopharmacol. 2005;8:59–63. doi: 10.1017/S1461145704004602. [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Shelton RC, Zajecka JM, Etemad B, Rickels K, Clain A, et al. l-Methylfolate as Adjunctive Therapy for SSRI-Resistant Major Depression: Results of Two Randomized, Double-Blind, Parallel-Sequential Trials. Am J Psychiatry. 2012;169:1267–1274. doi: 10.1176/appi.ajp.2012.11071114. [DOI] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RE, Deleger S, Strawbridge WJ, Kaplan GA. Prospective association between obesity and depression: evidence from the Alameda County Study. IntJObesRelat Metab Disord. 2003;27:514–521. doi: 10.1038/sj.ijo.0802204. [DOI] [PubMed] [Google Scholar]
- Rotella F, Mannucci E. Depression as a risk factor for diabetes: a meta-analysis of longitudinal studies. J Clin Psychiatry. 2013;74:31–37. doi: 10.4088/JCP.12r07922. [DOI] [PubMed] [Google Scholar]
- Schmidt FM, Lichtblau N, Minkwitz J, Chittka T, Thormann J, Kirkby KC, et al. Cytokine levels in depressed and non-depressed subjects, and masking effects of obesity. J Psychiatr Res. 2014;55:29–34. doi: 10.1016/j.jpsychires.2014.04.021. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 4–57. [PubMed] [Google Scholar]
- Shelton RC, Miller AH. Eating ourselves to death (and despair): The contribution of adiposity and inflammation to depression. Progress in Neurobiology. 2010;91:275–299. doi: 10.1016/j.pneurobio.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon GE, Ludman EJ, Linde JA, Operskalski BH, Ichikawa L, Rohde P, et al. Association between obesity and depression in middle-aged women. General Hospital Psychiatry. 2001;30:32–39. doi: 10.1016/j.genhosppsych.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland JP, McKinley B, Eckel RH. The metabolic syndrome and inflammation. Metab SyndrRelat Disord. 2004;2:82–104. doi: 10.1089/met.2004.2.82. [DOI] [PubMed] [Google Scholar]
- Tamhane AC. Multiple comparisons in model i one-way ANOVA with unequal variances. Communications in Statistics-Theory and Methods. 1977;A6:15–32. [Google Scholar]
- Thomas C, Hypponen E, Power C. Obesity and type 2 diabetes risk in midadult life: the role of childhood adversity. Pediatrics. 2008;121:e1240–e1249. doi: 10.1542/peds.2007-2403. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Greer TL, Church TS, Carmody TJ, Grannemann BD, Galper DI, et al. Exercise as an augmentation treatment for nonremitted major depressive disorder: a randomized, parallel dose comparison. J Clin Psychiatry. 2011;72:677–684. doi: 10.4088/JCP.10m06743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuck MK, Chan DW, Chia D, Godwin AK, Grizzle WE, Krueger KE, et al. Standard operating procedures for serum and plasma collection: early detection research network consensus statement standard operating procedure integration working group. Journal of proteome research. 2009;8:113–117. doi: 10.1021/pr800545q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelzangs N, Beekman AT, van Reedt Dortland AK, Schoevers RA, Giltay EJ, de Jonge P, et al. Inflammatory and metabolic dysregulation and the 2-year course of depressive disorders in antidepressant users. Neuropsychopharmacology. 2014;39:1624–1634. doi: 10.1038/npp.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelzangs N, Kritchevsky SB, Beekman AT, Brenes GA, Newman AB, Satterfield S, et al. Obesity and onset of significant depressive symptoms: results from a prospective community-based cohort study of older men and women. J Clin Psychiatry. 2010;71:391–399. doi: 10.4088/JCP.08m04743blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelzangs N, Kritchevsky SB, Beekman AT, Newman AB, Satterfield S, Simonsick EM, et al. Depressive symptoms and change in abdominal obesity in older persons. Arch Gen Psychiatry. 2008;65:1386–1393. doi: 10.1001/archpsyc.65.12.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocrine reviews. 2000;21:697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.