Abstract
Glioma is a type of tumor derived from glial cells, which is associated with a high level of incidence and mortality. At present, the generation of a fast and efficient method to evaluate the malignancy grade of glioma is required. Cancer stem cells (CSCs) are currently attracting attention in oncological studies; therefore, the present study aimed to investigate novel biomarkers of glioma CSCs, in order to provide new criteria for the grading of glioma. The mRNA expression levels of CD133, (sex determining region Y)-box 2, nestin, vascular endothelial growth factor (VEGF) and phosphoinositide-3-kinase (PI3K) were detected in 15 human samples of high-malignancy glioma and 12 human samples of low-malignancy glioma in vitro. The mRNA expression levels of VEGF and PI3K were higher in the high-malignancy group, as compared with in the low-malignancy group. In conclusion, the mRNA expression levels of VEGF and PI3K in glioma CSCs may be considered a novel criteria for the grading of glioma.
Keywords: glioma, cancer stem cells, disease grading
Introduction
Gliomas, which are tumors that originate from neuroglial cells, commonly occur in the brain and occasionally in the spinal cord (1). Gliomas account for 80% of primary tumors in the brain and central nervous system, and for 30% of malignant tumors in the brain (2). Patients with low-malignancy gliomas [World Health Organization (WHO) grade II] have good prognoses, with a 10-year survival rate of up to 47% (3), and reportedly as long as 17 years (4). By contrast, high-malignancy gliomas (WHO grades III–IV) are rich in vessels and their size increases rapidly. Hypoxic and necrotic tissues in the tumor center can damage the surrounding tissues and destroy the blood-brain barrier; thus, these tumors may relapse even when completely resected. The 1- and 2-year survival rates of high-malignancy gliomas are only 50 and 25%, respectively (5).
Cancer stem cells (CSCs) have the same characteristics as other types of stem cells, but can also differentiate into various types of cells in the tumor tissue (6,7). Thus, CSCs closely affect tumor recurrence and metastasis. Glioma CSCs are important in the development of invasive tumor growth, insensitivity to chemotherapy and radiotherapy, and poor prognosis (8,9). Since glioma CSCs were successfully isolated and cultured in vitro in 2004 (10), their characteristics have been studied extensively. Currently known glioma CSC markers include CD133, nestin, (sex determining region Y)-box 2 (SOX2), ATP-binding cassette sub-family G member 2 (ABCG2) and musashi-1 (11–15), with CD133 considered to be the most important of these markers. CD133+ glioma CSCs form cell spheres in culture medium that contains growth factors (16), and differentiate into neurons and glial cells following the removal of the growth factors (17–19). However, certain CD133− glioma cells also exhibit similar characteristics to CSCs (20). In addition, certain types of cells, such as endothelial cells, are also CD133+ (21). Therefore, CD133, even alongside other biomarkers, may not be a specific biomarker for glioma CSCs.
Vascular endothelial growth factor (VEGF) is highly expressed in glioma cells (22–24), and its expression is directly associated with the malignancy and prognosis of gliomas (25). Phosphoinositide-3-kinase (PI3K) is a lipid second messenger associated with intracellular signal transduction that can catalyze the formation of phosphoinositide-3 phosphate, which is the phosphorylated product of the third hydroxyl of inositol phosphate (26). PI-3,4,5-P3 is the phosphorylated product of PI3K, which is gathered in the inner surface of the cell membrane. Protein kinase B (Akt) combines with PI-3,4,5-P3 and is subsequently activated. The activated Akt then enters the cell membrane, where it is phosphorylated by PDK1 and PDK2, and regulates a series of functions, including the cell cycle, growth and survival (27). PI3K comprises of five subtypes, including p55α, p55γ, p85α, p85β and p50α, all of which are expressed in neuronal cells in the rat brain, indicating that PI3K is important in signal transduction in the brain (28). However, the association between PI3K/Akt gene expression and glioma remains unclear.
In the present study, specimens from glioma patients were divided into the following two groups according to clinical grading: Low-malignancy (WHO grade II) and high-malignancy (WHO grades III–IV) groups (29). Stem cells were extracted from fresh tumor tissues, and the expression levels of CD133, nestin, SOX2, VEGF and PI3K were detected by reverse transcription-quantitative polymerase chair reaction (RT-qPCR), in order to identify the association of glioma CSCs with the VEGF and PI3K signal transduction systems. To the best of our knowledge, this is the first study investigating the expression levels of VEGF and PI3K in glioma CSCs obtained from glioma patients. The results will provide first-hand information for further study of drugs that target glioma.
Materials and methods
Sample collection
Samples were collected in strict accordance with the scientific research sample collection guidelines of the Department of Neurosurgery the Affiliated Hospital of Beihua University (Jilin, Jilin). Glioma samples were successfully collected from 27 patients with glioma who were undergoing resection surgery at the Department of Neurosurgery, between 2010 and 2013. Tissue samples were diagnosed by pathological section and classified into 12 low- and 15 high-malignancy gliomas, according to the WHO guidelines (29–33). The tissue samples immediately underwent tissue digestion and cell isolation. This study was conducted in accordance with the declaration of Helsinki and was approved by the Ethics Committee of Jilin University (Changchun, China). Written informed consent was obtained from all the participants.
Isolation and purification of glioma CSCs
Several 6-well plates were coated with 20 µg/ml poly-ornithine (Sigma-Aldrich, Carlsbad, CA, USA) and incubated in a cell incubator at 37°C for at least 2 h. Subsequently, the poly-ornithine was removed by washing once with deionized water, the plates were rinsed once with phosphate-buffered saline (PBS), and were then incubated with a final concentration of 5 µg/ml laminine (Sigma-Aldrich) for 1 h.
Fresh glioma tumor tissues were cut into small sections (7 µm) and rinsed twice with Hank's balanced salt solution (HBSS; Gibco Life Technologies, Grand Island, NY, USA) containing 20% fetal bovine serum (FBS; Thermo Fisher Scientific, Inc., Pittsburgh, PA, USA). The specimens were then rinsed three times with HBSS without FBS in order to remove blood cells and then digested with 0.25% trypsin (Gibco Life Technologies) at 37°C for 10 min. Subsequently, the digestion was terminated using 3 ml HBSS containing 20% FBS, and the samples were centrifuged at 400 × g at 4°C for 3 min. The supernatant was then discarded, and the cells were resuspended using the neural stem cell culture medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The cell suspension was filtered through a 70-µm nylon mesh (Mumford Industries, Inc., CA, USA) and the cells were transferred to the 6-well plates that had been prepared previously. To further evaulate biomarker expression, U251 human glioma cells (Sigma-Aldrich) were used as a control. The U251 and primary neural stem cells were cultured in medium containing 10% FBS, 40 ng/ml fibroblast growth factor 2, 20 ng/ml epidermal growth factor (EGF) and 20 ng/ml platelet-derived growth factor (all from Gibco Life Technologies) on the first day. On the second day, the medium was replaced with fresh medium without FBS, and thereafter, half the medium was changed every day, with the addition of fresh growth factors. The cells were observed and images were captured under 200× magnification using a microscope (BX51-32H01; Olympus Corporation, Tokyo, Japan).
Detection of glioma CSCs using RT-qPCR
The total RNA of glioma CSCs and U251 cells was extracted using an RNeasy kit (Qiagen, Hilden, Germany). Next, 1 µg total RNA was reverse transcribed into cDNA using iScript cDNA Synthesis kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA), and PCR was performed using PCR Master Mix (Applied Biosystems, Life Technologies, Rockville, MD, USA). The PCR protocol comprised of initial denaturation at 95°C for 10 min, 35 cycles at 95°C for 30 sec, 54°C for 30 sec and 72°C for 30 sec, and then extension at 72°C for 10 min. The primers used in the present study are listed in Table I. PCR products were seperated by 1% agarose gel electrophoresis, and results were quantified using model-driven agility software (Kepler 4.3; Eclipse Foundation, Inc., Ottawa, ON, Canada).
Table I.
Primers used in the present study.
| Gene | Forward primer (5′→3′) | Reverse primer (5′→3′) |
|---|---|---|
| CD133 | acactgctggtgtgctgac | cccaaggaccacttcacagt |
| SOX2 | cacaactcggagatcagcaa | ctccgggaagcgtgtactta |
| Nestin | aggctgagaactctcgcttg | attaggcaagggggaagaga |
| VEGF | cacgaacgagtccctagagc | atggtgatgcggttttcttc |
| PI3K | attacgctagttacactgca | tggacctggccatcgactga |
| GAPDH | accacagtccatgccatcac | tccaccaccctgttgctgta |
SOX2, (sex determining region Y)-box 2 (SOX2); VEGF, vascular endothelial growth factor; PI3K, phosphoinositide-3-kinase.
Statistical analysis
VEGF and PI3K mRNA expression levels were normalized to GAPDH. Student's t-test was used to analyze the VEGF and PI3K expression levels between the two glioma groups. All analyses were conduced using SPSS 20 software (IBM SPSS, Armonk, NY, USA). P<0.05 was considered to indicate a statistically significance difference.
Results
Comparison of clinical conditions
All the glioma patients included in the present study had not received any treatment prior to surgery. The patients were divided into the low-malignancy (WHO grade II; n=12) and high-malignancy groups (WHO grades III–IV; n=15). No statistically significant differences in the gender, age, tumor site or pathological type were observed between the two groups (P>0.05; Table II).
Table II.
Comparison of clinical characteristics of patients with glioma.
| Characteristic | Low-malignancy group (n=12) | High-malignancy group (n=15) |
|---|---|---|
| Mean age, years | 42 | 45 |
| Age range, years | 33–52 | 28–56 |
| Male: female ratio | 1.8:1 | 1.6:1 |
| Tumor site | ||
| Frontal lobe | 5 | 5 |
| Temporal lobe | 4 | 6 |
| Parietal lobe | 3 | 4 |
| Pathological type | ||
| Glioblastoma | 5 | 4 |
| Oligodendroglioma | 2 | 4 |
| Diffuse astroglioma | 5 | 7 |
Patients were divide into the low-malignancy (WHO grade II; n=12) and high-malignancy groups (WHO grades III–IV; n=15). No statistically significant differences between the two groups were observed in the gender, age, tumor site or pathological type (P>0.05).
Morphology of glioma CSCs
Glioma CSCs were observed in the suspended and adherent state (Fig. 1). These two cell states display different shapes. The suspended cells formed spheres, whereas the adherent cells exhibited a spindle shape. Notably, the amount of sphere-shaped CSCs was increased in the high-malignancy group, as compared with in the low malignancy group. Taken together, various states of CSCs were detected in the cell culture.
Figure 1.
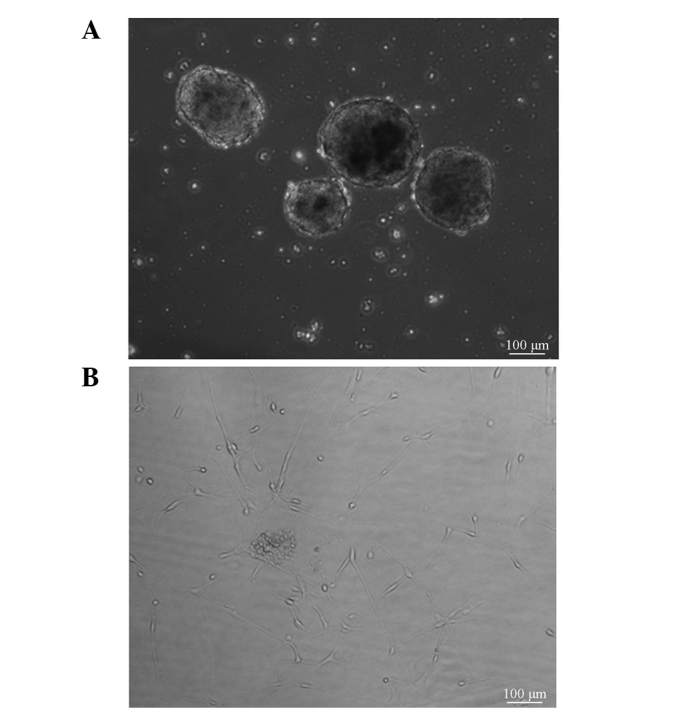
Morphology of glioma stem cells. (A) Glioma CSCs formed spheres in the suspended state, (B) whereas the appearance of cells in the adherent state was similar to that of neural stem cells. Bar, 100 µm. CSCs, cancer stem cells.
Identification of glioma stem cell markers
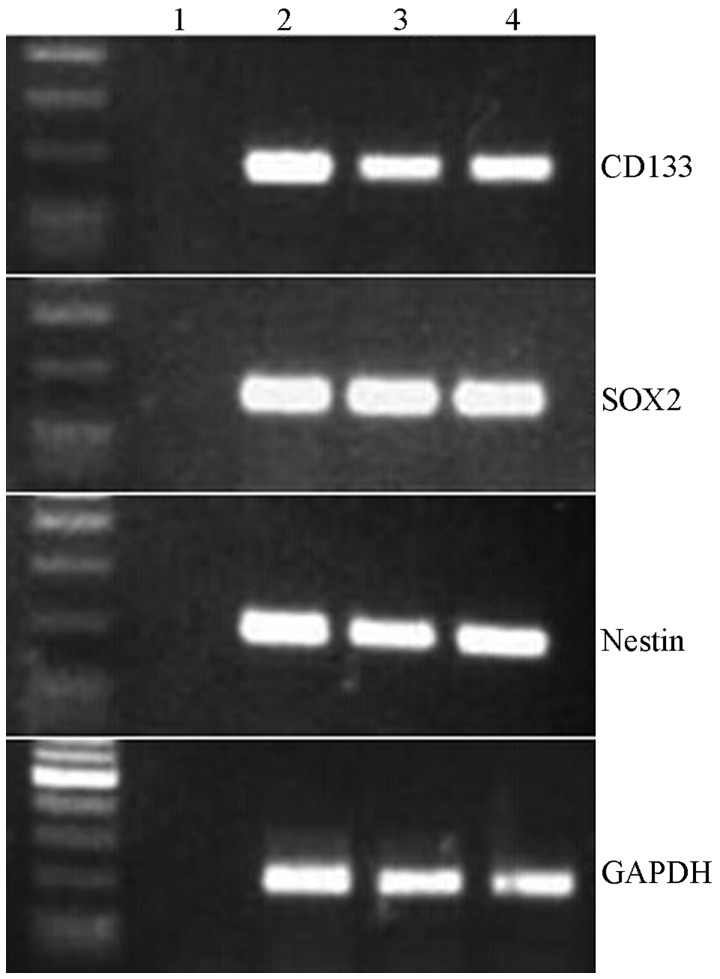
To validate the expression levels of conventional CSC biomarkers, the mRNA expression levels of CD133, SOX2 and nestin were detected in the high- and low-malignancy glioma CSCs. CD133 expression levels were markedly lower in the low-malignancy group, as compared with in the high-malignancy group; however, there was no significant difference with the U251 cells (Fig. 2). Furthermore, neither SOX2 nor nestin expression levels differed between the glioma CSCs and U251 cells. These results suggest that the transcriptional levels of CD133, SOX2 and nestin may not be able to efficiently evaluate the malignant stage of glioma CSCs.
Figure 2.
Gene expression levels for CD133, SOX2 and nestin in glioma cancer stem cells (CSCs). Levels were similar to those in CSCs extracted from U851 glioma cells. Lane 1, negative control; lanes 2 and 3, two glioma CSC samples; lane 4, U251 cell line. SOX2, (sex determining region Y)-box 2.
Expression levels of VEGF and PI3K
The present study further investigated the VEGF and PI3K mRNA expression levels between the two glioma groups. VEGF was significantly upregulated in the high-malignancy group, as compared with the low-malignancy group (T value=12.655, P=0.002). In addition, the mRNA expression levels of PI3K exhibited a similar tendency (T value=15.948, P=0.001) (Table III; Fig. 3A and B). These results suggest that the transcriptional levels of VEGF and PI3K may significantly differ in the various malignant stages of glioma.
Table III.
Differential expression of VEGF and PI3K in the low- and high-malignancy glioma CSCs.
| A, Low-malignancy glioma CSCs (n=12) | ||
|---|---|---|
| Patient | VEGF levels | PI3K levels |
| 1 | 14.5±1.0 | 4.6±0.2 |
| 2 | 10.9±0.6 | 6.4±0.1 |
| 3 | 5.0±0.2 | 2.6±0.6 |
| 4 | 4.7±0.5 | 5.8±0.7 |
| 5 | 13.2±2.0 | 6.8±0.6 |
| 6 | 9.5±1.5 | 1.7±0.1 |
| 7 | 11.7±1.2 | 1.0±0.1 |
| 8 | 7.8±0.4 | 3.6±0.2 |
| 9 | 12.6±1.7 | 2.4±0.1 |
| 10 | 10.3±1.6 | 4.7±0.3 |
| 11 | 6.9±0.3 | 2.4±0.7 |
| 12 | 7.2±0.3 | 4.2±0.8 |
| B, High-malignancy glioma CSCs (n=15) | ||
| Patient | VEGF levels | PI3K levels |
| 1 | 28.4±2.5 | 15.7±0.9 |
| 2 | 18.9±1.7 | 18.5±1.2 |
| 3 | 37.9±2.6 | 25.8±2.7 |
| 4 | 15.4±1.2 | 28.4±3.0 |
| 5 | 19.5±1.3 | 8.5±0.5 |
| 6 | 32.5±2.0 | 26.8±0.4 |
| 7 | 45.2±2.4 | 9.8±0.5 |
| 8 | 26.3±2.9 | 20.0±2.1 |
| 9 | 35.8±2.4 | 15.8±1.9 |
| 10 | 24.7±1.3 | 13.8±1.4 |
| 11 | 38.9±2.1 | 23.3±1.8 |
| 12 | 19.9±1.2 | 24.8±2.1 |
| 13 | 32.4±2.3 | 28.4±1.9 |
| 14 | 15.3±0.5 | 23.0±1.2 |
| 15 | 23.6±1.6 | 17.2±2.1 |
Data are presented as the mean ± standard deviation. VEGF, vascular endothelial growth factor; PI3K, phosphoinositide-3-kinase; CSCs, cancer stem cells.
Figure 3.
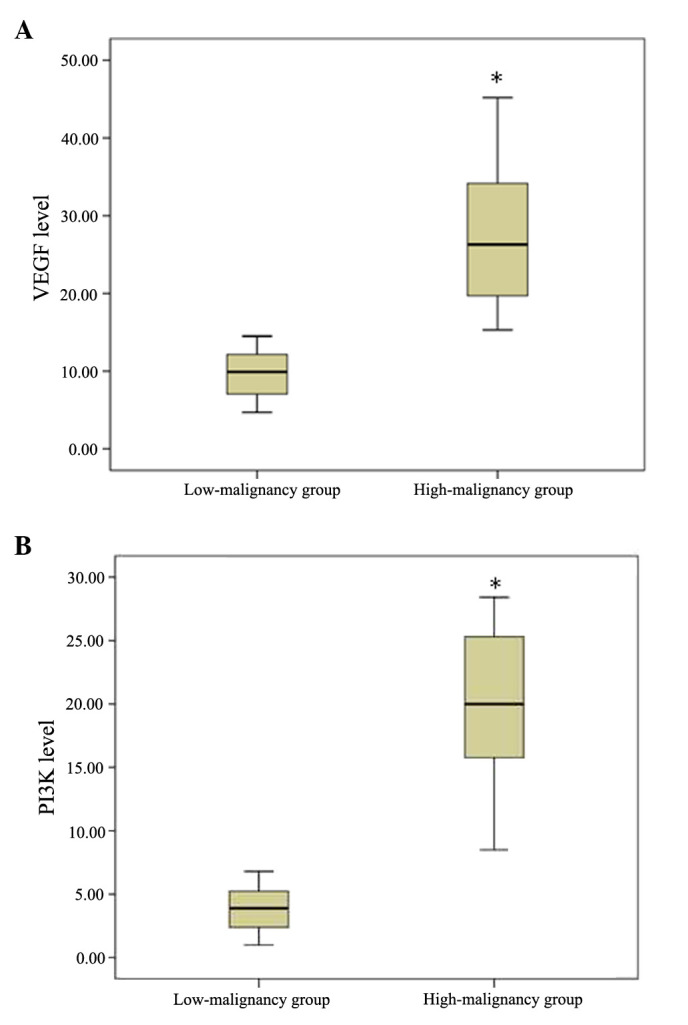
Comparison of expression levels of (A) VEGF and (B) PI3K in the low- and high-malignancy groups. Compared with the low-malignancy group, the expression levels of VEGF and PI3K were significantly higher in the high-malignancy group (*P=0.001). VEGF, vascular endothelial growth factor; PI3K, phosphoinositide-3-kinase.
Discussion
The incidence of glioma is 3.8/100,000 for men and 3.1/100,000 for women in developing countries, and 5.8/100,000 for men and 4.4/100,000 for women in developed countries (34). In the United States, malignant glioma accounts for 70% of newly-diagnosed cases of glioma, of which glioblastoma accounts for 60–70% (35,36). The glioma classification system of the World Health Organization (WHO) includes four grades, based on the pathological atypia, mitotic condition and anaplasia. The present study aimed to detect the differences in the transcriptional expression of CD133, SOX2, nestin, VEGF and PI3K between high-malignancy and low-malignancy glioma CSCs. The results of the present study suggested a robust association among tumor grade, invasion of CSCs and prognosis. In addition, the expression levels of VEGF and PI3K in WHO grade III–IV samples were found to be significantly higher compared with those in grade II samples, which implies that the expression levels of these two biomarkers may be used as criteria for the clinical grading of gliomas. However, a larger-scale study is required to further confirm this finding.
Mutation in the isocitrate dehydrogenase (IDH) gene is widely detected in low-grade gliomas and is generally considered to be an important predictor of prognosis in glioma patients (37,38). The IDH mutation may induce abnormal formation of 2-hydroxyglutarate, which inhibits ketoglutarate-dependent dioxygenase, resulting in abnormal histone and DNA methylation, and thus to poor prognosis for glioma patients (38,39).
Methylation of the O6-alkylguanine DNA alkyltransferase (MGMT) gene promoter is also associated with glioma prognosis (40). Hegi et al confirmed that MGMT gene promoter methylation was associated with long-term survival in glioma patients who received only chemotherapy treatment (41). Furthermore, Brandes et al revealed that MGMT gene demethylation increased the vascular permeability of glioblastomas (42). MGMT promoter demethylation plays different roles in gliomas of different grades and pathological types. For instance, the types of gene mutations in WHO grade IV gliomas differ from those in other grades of glioma. Mutations of the IDH and p53 genes are rare, whereas the mutation of EGF receptor (EGFR) is common (43). Further experiments with larger-scale samples are required to detect the significance of mutations in the IDH, MGMT, EGFR and p53 genes.
CSCs not only have the same characteristics as other types of stem cells, but can also differentiate into various types of cells in tumor tissue, and greatly affect tumor recurrence and metastasis. Since Bonnet and Dick isolated leukemia stem cells in 1997 (44), researchers have identified CSCs in specimens of brain, lung, colon, ovary, pancreas and prostate cancer (45–50). Neurosphere assay is a primary in vitro method for the culture of neural stem cells, which can then differentiate into neurons and glial cells (51–53). Neural stem cells are gathered to form neurospheres subsequent to cell division, and the size of neurospheres increases along with the increase of cell division times, so as to achieve a rise in the number of cells. Since glioma CSCs were first isolated and successfully cultured in vitro in 2004 (10), thorough research has been conducted on these cells. Glioma CSCs have been found to express CD133, SOX2, nestin and other markers. However, the role of glioma CSCs in tumor grading has not received considerable attention.
In conclusion, the results of the present study revealed that the expression levels of VEGF and PI3K mRNA in glioma CSCs from patients with grade III–IV glioma were significantly higher compared with those in glioma CSCs of patients with grade II disease. Thus, the expression of VEGF and PI3K mRNA in glioma stem cells may play a key role in the clinical grading of glioma and the accurate prediction of the disease prognosis.
References
- 1.Mamelak AN, Jacoby DB. Targeted delivery of antitumoral therapy to glioma and other malignancies with synthetic chlorotoxin (TM-601) Expert Opin Drug Deliv. 2007;4:175–186. doi: 10.1517/17425247.4.2.175. [DOI] [PubMed] [Google Scholar]
- 2.Goodenberger ML, Jenkins RB. Genetics of adult glioma. Cancer Genet. 2012;205:613–621. doi: 10.1016/j.cancergen.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Smoll NR, Gautschi OP, Schatlo B, Schaller K, Weber DC. Relative survival of patients with supratentorial low-grade gliomas. Neuro Oncol. 2012;14:1062–1069. doi: 10.1093/neuonc/nos144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olson JD, Riedel E, DeAngelis LM. Long-term outcome of low-grade oligodendroglioma and mixed glioma. Neurology. 2000;54:1442–1448. doi: 10.1212/WNL.54.7.1442. [DOI] [PubMed] [Google Scholar]
- 5.Ohgaki H, Kleihues P. Population-based studies on incidence survival rates and genetic alterations in astrocytic and oligodendroglial glioma. J Neuropathol Exp Neurol. 2005;64:479–489. doi: 10.1093/jnen/64.6.479. [DOI] [PubMed] [Google Scholar]
- 6.Plaks V, Kong N, Werb Z. The cancer stem cells niche, How essential is the niche in regulating stemness of tumor cells? Cell Stem Cell. 2015;16:225–238. doi: 10.1016/j.stem.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang L, Xue Y, Shen Y, Li W, Cheng Y, Yan X, Shi W, Wang J, Gong Z, Yang G, et al. Claudin 6: A novel surface marker for characterizing mouse pluripotent stem cells. Cell Res. 2012;22:1082–1085. doi: 10.1038/cr.2012.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilbertson RJ, Rich JN. Making a tumour's bed: Glioblastoma stem cells and the vascular niche. Nat Rev Cancer. 2007;7:733–736. doi: 10.1038/nrc2246. [DOI] [PubMed] [Google Scholar]
- 9.Chen L, Chen XR, Chen FF, Liu Y, Li P, Zhang R, Yan K, Yi YJ, Xu ZM, Jiang XD. MicroRNA-107 inhibits U87 glioma stem cells growth and invasion. Cell Mol Neurobiol. 2013;33:651–657. doi: 10.1007/s10571-013-9927-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 11.Gaedicke S, Braun F, Prasad S, Machein M, Firat E, Hettich M, Gudihal R, Zhu X, Klingner K, Schüler J, et al. Noninvasive positron emission tomography and fluorescence imaging of CD133+ tumor stem cells. Proc Natl Acad Sci USA. 2014;111:E692–E701. doi: 10.1073/pnas.1314189111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lubanska D, Market-Velker BA, deCarvalho AC, Mikkelsen T. Fidalgo daS ilva E and Porter LA: The cyclin-like protein Spy1 regualtes growth and division characteristics of the CD133+ population in human glioma. Cancer Cell. 2014;25:64–76. doi: 10.1016/j.ccr.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Jin X, Jin X, Jung JE, Beck S, Kim H. Cell surface nestin is a biomarker for glioma stem cells. Biochem Biophys Res Commun. 2013;433:496–501. doi: 10.1016/j.bbrc.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 14.Dell'Albani P. Stem cell markers in gliomas. Neurochem Res. 2008;33:2407–2415. doi: 10.1007/s11064-008-9723-8. [DOI] [PubMed] [Google Scholar]
- 15.Strojnik T, Røsland GV, Sakariassen PO, Kavalar R, Lah T. Neural stem cell markers, nestin and musashi proteins, in the progrssion of human glioma, Correlation of nestin with prognosis of patient survival. Surg Neurol. 2007;68:133–143. doi: 10.1016/j.surneu.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 16.Raso A, Negri F, Gregorio A, Nozza P, Mascelli S, De Marco P, Merello E, Milanaccio C, Ravegnani M, Cama A, et al. Successful isolation and long-term establishment of a cell line with stem cell-like features from an anaplastic medulloblastoma. Neuropathol Appl Neurobiol. 2008;34:306–315. doi: 10.1111/j.1365-2990.2007.00896.x. [DOI] [PubMed] [Google Scholar]
- 17.Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser M, Kornblum HI. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci USA. 2003;100:15178–15183. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ignatova TN, Kukekov VG, Laywell ED, Suslov ON, Vrionis FD, Steindler DA. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia. 2002;39:193–206. doi: 10.1002/glia.10094. [DOI] [PubMed] [Google Scholar]
- 19.Dolgova EV, Alyamkina EA, Efremov YR, Nikolin VP, Popova NA, Tyrinova TV, Kozel AV, Minkevich AM, Andrushkevich OM, Zavyalov EL, et al. Identification of cancer stem cells and a strategy for their elimination. Cancer Biol Ther. 2014;15:1378–1394. doi: 10.4161/cbt.29854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beier D, Hau P, Proescholdt M, Lohmeier A, Wischhusen J, Oefner PJ, Aigner L, Brawanski A, Bogdahn U, Beier CP. CD133(+) and CD133(−) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 2007;67:4010–4015. doi: 10.1158/0008-5472.CAN-06-4180. [DOI] [PubMed] [Google Scholar]
- 21.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 22.Ma C, Li Y, Zhang X, Zhao G, Xu H. Levels of vascular endothelial growth factor and matrix metalloproteinase-9 proteins in patients with glioma. J Int Med Res. 2014;42:198–204. doi: 10.1177/0300060513481924. [DOI] [PubMed] [Google Scholar]
- 23.Li Q, Qiao G, Ma J, Li Y. Downregulation of VEGF expression attenuates maligant biological behavior of C6 glioma stem cells. Int J Oncol. 2014;44:1581–1588. doi: 10.3892/ijo.2014.2331. [DOI] [PubMed] [Google Scholar]
- 24.Plate KH, Breier G, Millauer B, Ullrich A, Risau W. Up-regulation of vascular endothelial growth factor and its cognate receptors in a rat glioma model of tumor angiogenesis. Cancer Res. 1993;53:5822–5827. [PubMed] [Google Scholar]
- 25.Das S, Marsden PA. Angiogenesis in glioblastoma. N Engl J Med. 2013;369:1561–1563. doi: 10.1056/NEJMcibr1309402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whitman M, Downes CP, Keeler M, Keller T, Cantley L. Type I phosphatidylinositol kinase makes a novel inositol phospholipid, phosphatidylinositol-3-phosphate. Nature. 1988;332:644–646. doi: 10.1038/332644a0. [DOI] [PubMed] [Google Scholar]
- 27.Vara Fresno JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, González-Barón M. PI3K/Akt signaling pathway and cancer. Cancer Treat Rev. 2004;30:193–204. doi: 10.1016/j.ctrv.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 28.Shin BC, Suzuki M, Inukai K, Anai M, Asano T, Takata K. Multiple isoforms of the regulatory subunit for phosphatidylinositol 3 kinase (PI3-kinase) are expressed in neurons in the rat brain. Biochem Biophys Res Commun. 1998;246:313–319. doi: 10.1006/bbrc.1998.8606. [DOI] [PubMed] [Google Scholar]
- 29.Weller M, Weber RG, Willscher E. Molecular classification of diffuse cerebral WHO grade II/III gliomas using genome- and transcriptome-wide profiling improves stratification of prognostically distinct patient groups. Acta Neuropathol. 2015;129:679–693. doi: 10.1007/s00401-015-1409-0. [DOI] [PubMed] [Google Scholar]
- 30.Simonetti G, Gaviani P, Botturi A, Innocenti A, Lamperti E, Silvani A. Clinical management of grade III oligodendroglioma. Cancer Manag Res. 2015;7:213–223. doi: 10.2147/CMAR.S56975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang C, Bao Z, Zhang W, Jiang T. Progress on molecular biomarkers and classification of malignant gliomas. Front Med. 2013;7:150–156. doi: 10.1007/s11684-013-0267-1. [DOI] [PubMed] [Google Scholar]
- 32.Kahramancetin N, Tihan T. Aggressive behavior and anaplasia in pleomorphic xanthoastrocytoma a plea for a revision of the current WHO classification. CNS Oncol. 2013;2:523–530. doi: 10.2217/cns.13.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schafer ML, Maurer MH, Synowitz M, Wustefeld J, Marnitz T, Streitparth F, Wiener E. Low- (WHO II) and anaplastic (WHO III) gliomas, Differences in morphology and MRI signal intensities. Eur Radiol. 2013;23:2846–2853. doi: 10.1007/s00330-013-2886-y. [DOI] [PubMed] [Google Scholar]
- 34.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 35.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neurophthol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report Primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro Oncol 15 Suppl. 2013;2:ii1–ii56. doi: 10.1093/neuonc/not151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olar A, Aldape KD. Biomarkers classification and therapeutic decision-making for malignant gliomas. Curr Treat Options Oncol. 2012;13:417–436. doi: 10.1007/s11864-012-0210-8. [DOI] [PubMed] [Google Scholar]
- 38.Ichimura K. Molecular pathogenesis of IDH mutations in gliomas. Brain Tumor Pathol. 2012;29:131–139. doi: 10.1007/s10014-012-0090-4. [DOI] [PubMed] [Google Scholar]
- 39.Birner P, Pusch S, Christov C, Mihaylova S, Toumangelova-Uzeir K, Natchev S, Schoppmann SF, Tchorbanov A, Streubel B, Tuettenberg J, Guentchev M. Mutant IDH1 inhibits PI3K/Akt signaling in human glioma. Cancer. 2014;120:2440–2447. doi: 10.1002/cncr.28732. [DOI] [PubMed] [Google Scholar]
- 40.Olson RA, Brastianos PK, Palma DA. Prognostic and predictive value of epigenetic silencing of MGMT in patients with high grade gliomas, A systematic review and meta-analysis. J Neuroncol. 2011;105:325–335. doi: 10.1007/s11060-011-0594-5. [DOI] [PubMed] [Google Scholar]
- 41.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Eng J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 42.Brandes AA, Franceschi E, Tosoni A, Blatt V, Pession A, Tallini G, Bertorelle R, Bartolini S, Calbucci F, Andreoli A, et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol. 2008;26:2192–2197. doi: 10.1200/JCO.2007.14.8163. [DOI] [PubMed] [Google Scholar]
- 43.Ohgaki H, Kleihues P. Genetic pathways to primary and secondary glioblastoma. Am J Pathol. 2007;170:1445–1453. doi: 10.2353/ajpath.2007.070011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 45.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 46.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumor growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 48.Zhang S, Balch C, Chan MW, Lai HC, Matei D, Schilder JM, Yan PS, Huang TH, Nephew KP. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68:4311–4320. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 50.Maitland NJ, Collins AT. Prostate cancer stem cells: A new target for therapy. J Clin Oncol. 2008;26:2862–2870. doi: 10.1200/JCO.2007.15.1472. [DOI] [PubMed] [Google Scholar]
- 51.Temple S. Division and differentiation of isolated CNS blast cells in microculture. Nature. 1989;340:471–473. doi: 10.1038/340471a0. [DOI] [PubMed] [Google Scholar]
- 52.Reynolds BA, Tetzlass W, Weiss S. A Multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J Neurosci. 1992;12:4564–4574. doi: 10.1523/JNEUROSCI.12-11-04565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reynolds BA, Weiss A. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]