Abstract
Objective
To assess the prognostic impact of weight loss on clinical outcomes in patients with coronary artery disease (CAD). The effect of such weight loss on prognosis is unclear and controversial.
Methods
We performed a systematic review and meta-analysis of the prognostic effects of weight loss in patients with CAD on a composite outcome of all-cause mortality, cardiovascular mortality, and major adverse cardiac events considering studies published from January 1, 1946 through August 8, 2013.We considered weight loss “intentional” when it occurred in the presence of programmed therapeutic lifestyle changes (TLC), and “observational” when no such intervention was specified.
Results
We searched 1,218 abstracts of which 12 studies with 14 cohorts met inclusion criteria. A total of 35,335 patients (mean age 64 years, 72% male, BMI 30, 3.2 years of follow-up) were included. Overall, weight loss was associated with a greater risk of the composite outcome, RR (95% CI), 1.30 (1.00, 1.69, p = 0.05). However, heterogeneity was high (I2 = 90%) and was substantially explained by weight loss intentionality. Presumed intentional weight loss (4 cohorts) was associated with improved outcomes (RR of 0.67 [0.56, 0.80], p < 0.001), whereas observational weight loss (10 cohorts) was associated with worsened outcomes (RR 1.62 [1.26, 2.08], p <0.001; interaction p < 0.001.)
Conclusions
While observational weight loss is associated with increased adverse cardiovascular events, intentional weight loss is associated with lower clinical events. These results suggest that the underlying mechanism of weight loss (i.e., intentional or unintentional) affects its impact on subsequent risk in persons with known CAD.
Keywords: Obesity, Weight loss, Coronary Artery Disease, Mortality, Outcomes
Introduction
Obesity is an independent risk factor for coronary artery disease (CAD).1,2 Consequently, an initial 10% body weight loss is recommended in American Heart Association and American College of Cardiology practice guidelines for patients with CAD who are overweight or obese, with the goal of achieving a body mass index (BMI) <25 kg/m2.3 These recommendations are primarily based upon the consistent beneficial effects of weight loss on intermediate risk markers such as hypertension,4 diabetes control,5 metabolic syndrome,6 and blood lipid levels.7 It is generally thought that such improvements will lead to improved long-term outcomes.8,9
However, weight loss is not uniformly associated with improved long-term outcomes. Specifically, it is well-established that among general adult patients, weight loss can be an important risk marker for the subsequent development of cancer, diabetes, or other life-threatening systemic illness,10–12 particularly when the weight loss is unintentional. Even patients with intentional weight loss do not always have improved long-term cardiovascular outcomes.13,14 Additionally, the recent Look AHEAD study found that patients with diabetes randomized to a lifestyle intervention designed for purposeful weight loss did not have improved long-term outcomes.15
To further complicate the issue, several studies have suggested that among patients with CAD, weight loss appears to be associated with worse long term survival.16,17 Reasons for this association are unclear, but may be rooted in the obesity paradox, a finding where obese patients with CAD have better long-term survival compared to their normal weight counterparts.18 Consequently, this set of controversial findings casts doubt on current clinical practice guidelines and leaves clinicians with substantial uncertainty regarding the value of weight loss in patients with CAD.
Consequently, we undertook a systematic review and meta-analysis to summarize the literature, explore possible reasons for these conflicting results, and guide future research on the long term effects of weight loss on prognosis in patients with CAD. We specifically hypothesized that weight loss intentionality might be an important discriminator between studies that show harmful vs. beneficial effects of weight loss in patients with CAD.
Methods
Data Sources and Searches
We performed a literature search for all articles that included 1) patients with clinical CAD, 2) measures of achieved weight loss/change, 3) a comparison to a non-weight loss group, and 4) long term clinical outcomes. We identified potentially relevant articles through a search of PubMed and EMBASE from January 1st, 1946 through August 8th, 2013 using a search strategy developed with the assistance of a medical librarian (AMF, see eAppendix 1.) Web of Science was searched from March 1st 2008 to March 1st 2013 for meeting abstracts from cardiology, endocrinology, and obesity society meetings. Bibliographies of selected articles were reviewed for additional potentially relevant articles. As no individual patient data were analyzed, ethical approval was not required.
Study Selection
In mixed populations, we required that >50% of the cohort have documented CAD and the remainder be at high risk with another form of vascular disease or diabetes. If the population was <50% CAD, we included studies only if the CAD sub-group outcomes were reported and analyzed separately. We required that each analysis directly assess the impact of achieved weight loss on outcomes as well. We also required that the study account for weight change due to medications (such as a sibutramine) present in the original randomized trial. We included studies regardless of the study sample’s baseline BMI or proportion classified as overweight or obese.
We excluded studies evaluating children, cardiac cachexia, heart failure not directly preceded by a coronary artery disease diagnosis/event, bariatric surgery, and isolated diabetes, isolated peripheral vascular disease, or isolated cerebrovascular disease where CAD was not a co-morbidity. We excluded all reviews, commentaries, letters to the editor, or non-English abstracts.
Data Extraction and Quality Assessment
Two authors (QP, JPRE) independently reviewed all titles, abstracts, and selected full-text articles. Data abstraction was done by QP and verified by JPRE. All disagreements were resolved by FLJ. When not reported directly, data for meta-analysis were estimated from reported outcomes. Missing data were obtained from study authors as needed.
Quality assessment was done in duplicate (QP and JPRE) and utilized the Newcastle-Ottawa quality assessment scale for cohort studies.19 Although some studies were originally randomized controlled trials testing pharmacologic interventions, the weight loss studies were uniformly secondary or ad-hoc analyses, and as such were treated as cohorts for the purpose of quality assessment. We noted which studies reported evaluating, controlling, or adjusting for the effects of age, smoking status, sex, and pre-existing cancer diagnosis or cancer development on their outcomes. We considered secondary analyses of randomized controlled trials and cardiac rehabilitation studies to be at risk for selection bias.
Data Synthesis and Analysis
We pre-defined a 5% body weight loss as the primary predictor. As not all studies utilized this definition, we further classified studies into low, medium, and high weight loss with weight loss definitions of <2.5%, 2.5% to 4.9%, and ≥5% body weight loss, respectively. We considered a 5 kg threshold to be approximately equivalent to a 5% body weight change. When articles reported dividing patients by median weight change, we utilized the difference in means as the body weight change in kg. When studies reported a per-unit hazard ratio, we scaled this to 5% (or 5 kg) body weight change to increase comparability between studies.
The primary endpoint was a combination of all-cause mortality, cardiovascular mortality, or a composite outcome called “major adverse cardiac events” (MACE). Per the original articles, MACE usually included measures of mortality plus one or more of the following additional outcomes: non-fatal myocardial infarction, coronary revascularization (either percutaneous coronary intervention or coronary artery bypass grafting surgery), non-fatal stroke, sudden cardiac death, survival after cardiopulmonary resuscitation, unstable angina, cardiac hospitalization, and hospitalization for heart failure. When more than one outcome was reported, we included the results from the highest order, most adjusted outcome for the meta-analysis primary outcome (i.e., adjusted all-cause mortality > raw all-cause mortality > cardiovascular mortality > MACE.) When necessary, we treated a hazard ratio (HR) as a relative risk (RR) for the purpose of the primary outcome.
The main secondary outcome was adjusted all-cause mortality measured utilizing HRs, which excluded studies reporting only raw event rates or RR. We also explored a dose-response for the effects of differing amounts of weight loss on outcomes. Additionally, we evaluated the effect of weight gain compared with weight stability.
As part of our a priori hypothesis, studies were divided according to weight loss intention. We considered weight loss “presumably intentional” when it occurred in the presence of programmed therapeutic lifestyle changes (TLC), and “observational” when no such intervention was specified. Specifically, we defined programmed TLC as interventions in which components of exercise, healthy diet, or both were specified and monitored. For studies in which the intervention (for example, simvastatin or losartan) would not ordinarily be expected to change a patient’s weight or lifestyle habits, weight loss was considered “observational.”
We utilized a random-effects model in all analyses. Heterogeneity was assessed by I2 using values of 25%, 50%, and 75% to indicate low, moderate and high heterogeneity, respectively. Publication bias was assessed using a funnel plot. All analyses were performed on RevMan 5.2 (Cochrane IMS, Oxford, UK). Statistical significance was set at α = 0.05, and all tests were two-tailed.
Results
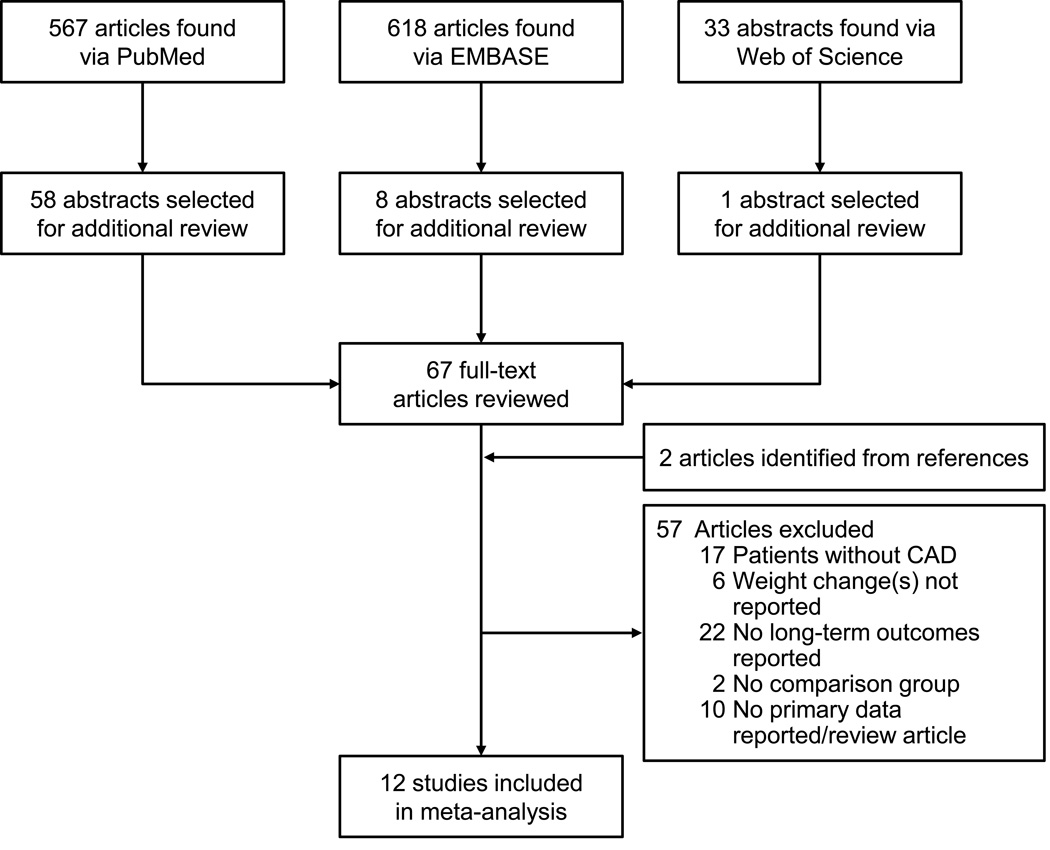
We identified 1,218 potentially relevant articles of which 67 full text articles were reviewed and 12 articles were selected for meta-analysis (Figure 1). One article17 reported on 3 independent cohorts, for a total of 14 cohorts. The selected studies and their characteristics are shown in Table 1.8,9,16,17,20–27
Figure 1.
Study Selection Flow Diagram
Table 1.
Included Studies and Characteristics
| Author, year, studya | Site | Patients | Sample Size |
F/u (yrs.) |
Wt. Δ Def. |
Wt. Δ time (mo.) |
Mean BMI (±SD) |
% with BMI>25 |
TLC | Original Intervention |
|---|---|---|---|---|---|---|---|---|---|---|
| Caterson, 2012, SCOUT21 | Multi-national | CAD ± DM | 9802 | 3.4 | 5 kg | 1.5 | 35±5 | 100% | + | Sibutramine (RCT) |
| Doehner, 2011, PROactive22 | Europe | CAD +DM | 5202 | 2.9 | 5% | 12 | 31±5 | 90% | - | Pioglitazone (RCT) |
| Kang, 201223 | Korea | AMI +PCI | 545 | 1.0 | 5%b | 10b | 24±3 | 42% | - | AMI with PCI |
| Kennedy, 2006, OPTIMAAL17 | Europe | CAD +HF | 4360 | 2.7 | 3% | 3 | 27±4 | 66% | - | Losartan vs. captopril (RCT) |
| Kennedy, 2006, CONSENSUS17 | Scandinavia | AMI | 4012 | 0.22 | 3% | 3 | 27±4 | 66% | - | Enalapril (RCT) |
| Kennedy, 2006, 4S17 | Scandinavia | CAD | 4178 | 4.4 | 3% | 12 | 26±3 | 62% | - | Simvastatin (RCT) |
| Kocz, 201224 | USA | CABG | 899 | 4.7b | 5% | 12 | 29±5 | 80% | - | CABG |
| Lavie, 20099 | USA | CAD | 393c | 3.5 | 7% | 3 | 30±4 | 100% | + | Cardiac rehabilitation |
| Lopez-Jimenez, 200816 | USA | AMI | 1676 | 2.4 | 5% | 6 | 29±6 | 75% | - | CBT for depression in AMI |
| Myers, 201127 | USA | CAD | 976c | 6.8 | 0.5 kg | 3 | 29±5 | 79% | - | Exercise test |
| Robins, 2008, VA-HIT25 | USA | CAD | 1966 | 5.1 | 0.5 kg | 12 | 29±5 | 80% | - | Gemfibrozil (RCT) |
| Sierra-Johnson, 20088 | USA | CAD | 377 | 6.4 | 5 kg | 3 | 28±5 | 76% | + | Cardiac rehabilitation |
| Singh, 199626 | India | CAD | 294c | 3 | 6kg | 36 | 24±2 | 37% | + | Diet and exercise |
| Walker, 199520 | England | CAD | 655c | 6.5 | 4% | 60 | 26±3 | 50% | - | Epidemiologic cohort |
Abbreviations: AMI = acute myocardial infarction; BMI = body mass index; CABG = coronary artery bypass graft; CAD = coronary artery disease; CBT = cognitive behavioral therapy; DM = diabetes mellitus; F/u = follow-up; HF = heart failure; NR = not recorded; PCI = percutaneous coronary intervention; RCT = randomized controlled trial; SD = Standard deviation; TLC = Therapeutic Lifestyle Change; USA = United States of America; Wt. Δ Def. = weight change definition; Wt. Δ Interval = weight change interval time.
Data obtained from study authors.
These numbers are the eligible subgroup of patients with CAD within the study.
A total of 35,335 patients were included, with a mean follow-up of 3.2 years. The average population age was 64 years, 72% male, BMI 30 ± 4 kg/m2, and studies were primarily based in the United States or Europe. There were 7 different definitions of weight change and 9 weight change time intervals. There were 6 different definitions among the 8 articles reporting MACE. Follow-up times ranged from 0.2 years to 6.4 years. Of note, the two studies23,26 with the lowest percentage of patients with BMI >25 were located in Korea and India and utilized lower BMI cut-points and alternate definitions of central obesity according to their population-specific definitions of obesity and overweight.
Study quality, adjustments, and reported outcomes of the selected studies are shown in Table 2. With the exception of Lopez et al.,16 no articles described the methods of weight measurements, such as the presence of clothing or shoes, or the use of, accuracy, or reproducibility of the scales. Few articles adjusted for our pre-specified confounders of age, gender, smoking cessation, and baseline cancer or subsequent cancer development. Eight studies reported adjusted HRs (aHR) for all-cause mortality. Only 2 studies were considered population-based.23,24
Table 2.
Study Quality, Adjustments, and Reported Outcomes
| Quality by Newcastle-Ottawa Scaleb | Specified Adjustments | Reported Outcomes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author, year, study, reference | Selection | Comparability | Outcome | Total Score |
Age | Sex | Cancer | Tob. | All-Cause Mortality |
CV Mortality |
MACE | Cause of Non-CV Mortality |
| Caterson, 2012, SCOUT21 | *** | * | ** | 6 | - | - | - | - | - | X | X | - |
| Doehner, 2011, PROactive22 | *** | ** | *** | 8 | + | + | + | + | X | - | - | X |
| Kang, 201223 | *** | * | * | 5 | + | - | - | - | X | X | X | - |
| Kennedy, 2006, OPTIMAAL17 | *** | ** | *** | 8 | + | + | + | + | X | X | - | - |
| Kennedy, 2006, CONSENSUS21 | *** | * | ** | 6 | + | - | - | - | X | - | - | - |
| Kennedy, 2006, 4S21 | *** | ** | *** | 8 | + | + | - | + | X | X | - | - |
| Kocz, 201224 | **** | * | 5 | - | - | - | - | X | - | X | - | |
| Lavie, 20099 | *** | *** | 6 | - | - | - | - | X | - | - | - | |
| Lopez-Jimenez, 200816 | *** | ** | *** | 8 | + | + | + | + | X | X | X | - |
| Myers, 201127 | *** | * | ** | 6 | + | - | + | - | X | X | - | X |
| Robins, 2008, VA-HIT25 | *** | *** | 6 | - | - | - | - | - | - | X | - | |
| Sierra-Johnson, 20088 | *** | ** | *** | 8 | + | + | - | + | X | - | X | - |
| Singh, 199626 | *** | ** | *** | 8 | - | - | - | - | X | - | X | - |
| Walker, 199520 | *** | ** | 5 | - | - | - | - | - | - | X | - | |
Abbreviations: CV = Cardiovascular; MACE = Major adverse cardiac events; Tob. = Tobacco
The Newcastle Ottawa Scale allows 4 stars for selection, 2 for comparability, and 3 for outcomes, for a maximum of 9 total.
Four studies reported weight loss associated with therapeutic lifestyle changes. Sierra-Johnson et al.8 and Lavie et al.9 utilized comprehensive outpatient cardiac rehabilitation based in the United States. This generally included observed exercise of 1–3×/week for 8–12 weeks, additional home exercise, individualized and group dietary counseling, stress management and risk factor education. Singh et al. provided patient counseling designed to achieve >400 g/day of low energy high nutrient fruits and vegetable intake, coupled with >300kcal/day of moderate intensity exercise over 12 week period followed by a 3 year follow-up.26 Caterson et al.21 as part of a randomized controlled trial of sibutramine, provided a 6-week run-in weight management program to all patients designed to ensure >150 minutes of exercise (walking or cycling) per week and a diet designed to create a 600 kcal deficit per day with a mean 3.4 year follow-up.28
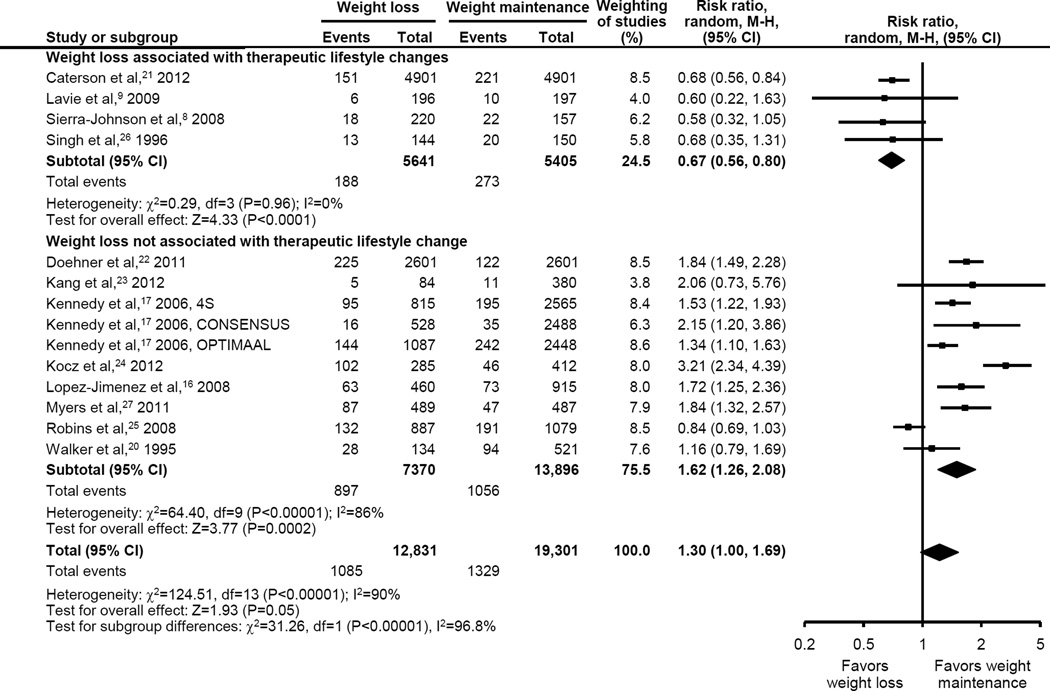
The primary composite outcome demonstrated that an approximate 5% weight loss (range 0.5%–7%), when compared with weight stability, was marginally associated with worse long-term outcomes, (RR [95% CI] 1.30 [1.00 to 1.69], p = 0.05, Figure 2). However, heterogeneity was very high (I2 = 90%) and substantially explained by weight loss intention. While observational weight loss (10 studies, n = 21,266) was associated with worsened outcomes (RR 1.62 [1.26 to 2.08], p <0.001, I2 = 86%), presumed intentional weight loss (4 studies, n = 10,866) was associated with uniformly improved outcomes and low heterogeneity (RR of 0.67 [0.56 to 0.80], p < 0.001, I2 = 0%; interaction p < 0.001). There was no evidence for publication bias by funnel plot analysis (eFigure 1.)
Figure 2.
Effect of Weight Loss on Clinical Outcomes in Patients with Coronary Artery Disease According to Association with Therapeutic Lifestyle Changes
Among the observational weight loss studies, outcomes varied somewhat according to observed weight loss in a dose-response fashion. For patients losing <2.5%, 2.5% to 4.9%, ≥5% body weight, the primary composite outcome showed HRs of 1.23 (0.57 to 2.66), 1.42 (1.21 to 1.67), and 2.14 (1.55 to 2.95), respectively (p = 0.07 for subgroup differences).
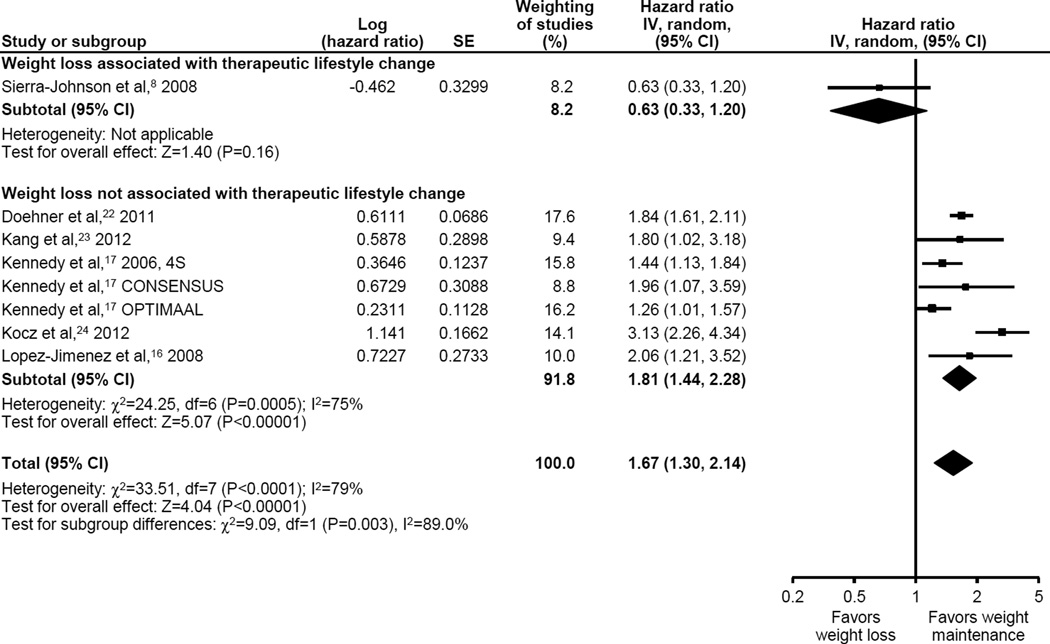
Weight loss was associated with a 67% increased adjusted all-cause mortality (8 studies, n=21,249, aHR [95% CI] 1.67 [1.30 to 2.14], p < 0.0001, Figure 3), but with high heterogeneity (I2 = 79%.) Observational weight loss demonstrated an aHR of 1.81 (1.44 to 2.28, p <0.001) contrasting with an aHR 0.63 (0.33 to 1.20, p = 0.16) in the single study with presumed intentional weight loss (interaction p = 0.003).
Figure 3.
Adjusted All-Cause Mortality Associated with Weight Loss By Association with Therapeutic Lifestyle Change
Six studies (all observational) reported adjusted all-cause mortality associated with weight gain compared to weight stability. In each article, identical definitions of percent body weight change (range +3–5%) were utilized for both weight loss and weight gain (see Table 1). A weight gain of approximately 3–5% of body weight was associated with a non-significant decrease in adjusted all-cause mortality (aHR 0.94 [0.81 to 1.10], p = 0.44, I2 = 24%, eFigure 2.)
Discussion
In this study, we found that weight loss intentionality and its association with TLC are major factors that determine the prognosis of weight loss among patients with CAD. When exercise and dietary improvements are programmed and purposeful, achieved weight loss appears to be associated with improved long-term prognosis. However, when weight loss is observational or “unintentional”, it should serve as a sign of increased risk to clinicians because it portends a substantially worse long-term prognosis.
Although our study is the first to demonstrate the importance of weight loss intentionality in patients with CAD, the importance of weight loss intentionality in separating beneficial vs. harmful weight loss has been well documented in the general medical literature. When weight loss is unintentional, it is a strong marker of increased risk12 usually because of underlying occult disease.27 On the other hand, when weight loss occurs intentionally, the effects are usually neutral to beneficial.10,13,14,29–32
The exact mechanism by which TLC-associated weight loss improves outcomes in patients with CAD is unclear, but it is likely related to exercise training and healthy dietary changes as each of the TLC-related studies contained both of these interventions. In addition, however, these programs may also favorably impact unmeasured factors such as medication adherence,33 mental stress34 or other factors which influence long term outcomes. Similarly, the mechanism behind harm in observational weight loss is unclear. This harm is likely related to patients having more severe disease at baseline (CAD with heart failure) or an occult systemic illness (malignancy) which manifested itself later in the disease course and resulted in harmful weight loss.27 Unfortunately, causes of non-cardiovascular death were uncommonly described in the studies contributing to this systematic review.
Our findings shed important light on the obesity paradox, a finding that patients with an elevated BMI and incident CAD have an improved long-term prognosis when compared to patients with a normal BMI.35 Although this paradox is clearly modulated by factors such as central adiposity,36,37 amount of lean mass,38 physical fitness,39 and the presence of heart failure,40 our results suggest that purposeful weight loss should be beneficial among obese patients.
There were several important studies that did not meet our inclusion criteria.13,14,41,42 The primary reason for exclusion was that each cohort had fewer than 50% of their patients with established CAD or did not specifically report on CAD subgroups. Kalantar-Zadeh et al.42 and Barba et al.41 found that observational weight loss was associated with worse outcomes in populations at high vascular risk (end-stage renal disease and established mixed vascular disease.) In addition, Williams et al., in two separate articles, found that prognosis associated with weight loss in a mixed risk population was dependent upon weight loss intentionality,13,14 consistent with the findings in this meta-analysis.
Our results differ from the subgroup analysis in the Look-AHEAD trial which assessed the effect of weight loss in patients with type II diabetes and CAD, which found no effect on the primary outcome in the weight loss arm.15 Our meta-analysis included articles describing groups according to achieved weight loss versus no weight loss, while the Look-AHEAD trial assessed outcomes on an intention to treat basis. Future analysis by weight loss intensity in the Look-AHEAD subgroup of CAD patients will help to clarify this issue. Additionally, the Look-AHEAD trial included only patients with type II diabetes. Whether having diabetes or not modulates the potential effect of purposeful weight loss in patients with CAD is yet to be determined.
Furthermore, it is important to note that TLC is the intervention and weight loss is an outcome. As a result, a patient cannot be randomized to weight loss per se. Rather, a patient can be randomized to TLC with the expectation of weight loss. However, even with TLCs, some patients will have greater success in achieving weight loss than others. Consequently, there is likely some personal behavior, underlying physiology, or genetic predisposition that allows certain patients to succeed in achieving weight loss with TLC while others experience minimal weight loss. Such an unmeasured confounder may actually be responsible for the mortality benefits seen in this meta-analysis, rather than the weight loss per se. As a result, it appears possible that in a patient undergoing TLC, weight loss may act more like a prognostic marker rather than a mediator of the long term benefits from TLC.
A well designed prospective study appears necessary to conclusively test the importance of weight loss in patients with established CAD. Such a study would be adequately powered for long-term outcomes, carefully assess body composition changes,43 employ behavioral weight loss strategies,44 encourage high-caloric (walk often, walk far) expenditure exercise,45 and carefully control for cancer development and smoking cessation. Such a study would provide more conclusive evidence on the effect of intentional weight loss on the prognosis of patients with CAD and would answer multiple questions, increase confidence in weight management recommendations for patients with CAD, and further clarify the obesity paradox. In addition, given the known benefits of bariatric surgery in the general population, a randomized controlled trial of bariatric surgery in patients with CAD might also be appropriate.
An important limitation of this meta-analysis was the heterogeneity across study results and that only 4 studies assessed intentional weight loss. Although weight loss intentionality accounted for some heterogeneity, substantial residual heterogeneity remained. In particular, there were important variations in weight loss definition, weight loss interval time period definitions, population percentage that was obese/overweight, and reported outcomes and their definitions. Data on important subgroups such as minorities and women were reported infrequently enough to preclude analysis. In addition, adjustments for important co-variates such as age, gender, smoking cessation, and cancer development were infrequently performed. Future research on weight loss outcomes in patients with CAD should carefully control for these factors including the well-known weight gain associated with successful tobacco cessation.
Conclusions
We found that observational weight loss in patients with CAD is associated with worse long-term outcomes, but that when weight loss occurs intentionally in the setting of lifestyle changes it is protective. A randomized controlled trial appears necessary to test the importance of weight loss in patients with established CAD. In the meantime, our findings support national guidelines that TLC for weight loss can be confidently recommended to overweight and obese patients with CAD.
Supplementary Material
Acknowledgments
We would like to thank our librarian Ann M. Farrell, MS for help with our search strategy and article selection.
Funding
This meta-analysis was supported in part by CTSA Grant Number TL1 TR000137 from the National Center for Advancing Translational Science (NCATS).
Abbreviations
- aHR
Adjusted Hazard Ratio
- BMI
Body Mass Index
- CAD
Coronary Artery Disease
- HR
Hazard Ratio
- MACE
Major Adverse Cardiac Events
- RR
Relative Risk
- TLC
Therapeutic Lifestyle Changes
Footnotes
Disclosures and Conflicts of Interest: None for all authors
References
- 1.Whitlock G, Lewington S, Sherliker P, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009 Mar 28;373(9669):1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berrington de Gonzalez A, Hartge P, Cerhan JR, et al. Body-mass index and mortality among 1.46 million white adults. New Engl J Med. 2010 Dec 2;363(23):2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith SC, Jr, Benjamin EJ, Bonow RO, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association. J Am Coll Cardiol. 2011 Nov 29;58(23):2432–2446. doi: 10.1016/j.jacc.2011.10.824. [DOI] [PubMed] [Google Scholar]
- 4.Horvath K, Jeitler K, Siering U, et al. Long-term effects of weight-reducing interventions in hypertensive patients: systematic review and meta-analysis. Arch Intern Med. 2008 Mar 24;168(6):571–580. doi: 10.1001/archinte.168.6.571. [DOI] [PubMed] [Google Scholar]
- 5.Anderson JW, Kendall CWC, Jenkins DJA. Importance of weight management in type 2 diabetes: review with meta-analysis of clinical studies. J Am Coll Nutr. 2003 Oct;22(5):331–339. doi: 10.1080/07315724.2003.10719316. [DOI] [PubMed] [Google Scholar]
- 6.Malin SK, Niemi N, Solomon TP, et al. Exercise training with weight loss and either a high- or low-glycemic index diet reduces metabolic syndrome severity in older adults. Ann Nutr Metab. 2012;61(2):135–141. doi: 10.1159/000342084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelley GA, Kelley KS, Roberts S, Haskell W. Comparison of aerobic exercise, diet or both on lipids and lipoproteins in adults: a meta-analysis of randomized controlled trials. Clin Nutr. 2012 Apr;31(2):156–167. doi: 10.1016/j.clnu.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sierra-Johnson J, Romero-Corral A, Somers VK, et al. Prognostic importance of weight loss in patients with coronary heart disease regardless of initial body mass index. Eur J Cardiovasc Prev Rehabil. 2008 Jun;15(3):336–340. doi: 10.1097/HJR.0b013e3282f48348. [DOI] [PubMed] [Google Scholar]
- 9.Lavie CJ, Milani RV, Artham SM, Patel DA, Ventura HO. The obesity paradox, weight loss, and coronary disease. Am J Med. 2009 Dec;122(12):1106–1114. doi: 10.1016/j.amjmed.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Harrington M, Gibson S, Cottrell RC. A review and meta-analysis of the effect of weight loss on all-cause mortality risk. Nutr Res Rev. 2009 Jun;22(1):93–108. doi: 10.1017/S0954422409990035. [DOI] [PubMed] [Google Scholar]
- 11.Ostergaard JN, Gronbaek M, Schnohr P, Sorensen TIA, Heitmann BL. Combined effects of weight loss and physical activity on all-cause mortality of overweight men and women. Int J Obes (Lond) 2010 Apr;34(4):760–769. doi: 10.1038/ijo.2009.274. [DOI] [PubMed] [Google Scholar]
- 12.Breeze E, Clarke R, Shipley MJ, Marmot MG, Fletcher AE. Cause-specific mortality in old age in relation to body mass index in middle age and in old age: follow-up of the Whitehall cohort of male civil servants. Int J Epidemiol. 2006 Feb;35(1):169–178. doi: 10.1093/ije/dyi212. [DOI] [PubMed] [Google Scholar]
- 13.Williamson DF, Pamuk E, Thun M, Flanders D, Byers T, Heath C. Prospective study of intentional weight loss and mortality in overweight white men aged 40–64 years. Am J Epidemiol. 1999 Mar 15;149(6):491–503. doi: 10.1093/oxfordjournals.aje.a009843. [DOI] [PubMed] [Google Scholar]
- 14.Williamson DF, Pamuk E, Thun M, Flanders D, Byers T, Heath C. Prospective study of intentional weight loss and mortality in never-smoking overweight US white women aged 40–64 years.[Erratum appears in Am J Epidemiol 1995 Aug 1;142(3):369] Am J Epidemiol. 1995 Jun 15;141(12):1128–1141. doi: 10.1093/oxfordjournals.aje.a117386. [DOI] [PubMed] [Google Scholar]
- 15.Wing RR, Bolin P, Brancati FL, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013 Jul 11;369(2):145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopez-Jimenez F, Wu CO, Tian X, et al. Weight change after myocardial infarction--the Enhancing Recovery in Coronary Heart Disease patients (ENRICHD) experience. Am Heart J. 2008 Mar;155(3):478–484. doi: 10.1016/j.ahj.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kennedy LMA, Dickstein K, Anker SD, et al. Weight-change as a prognostic marker in 12 550 patients following acute myocardial infarction or with stable coronary artery disease. Eur Heart J. 2006 Dec;27(23):2755–2762. doi: 10.1093/eurheartj/ehl182. [DOI] [PubMed] [Google Scholar]
- 18.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009 May 26;53(21):1925–1932. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- 19.Wells G, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. Ottawa Hospital Research Institute. [Accessed March 28, 2013]; Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 20.Walker M, Wannamethee G, Whincup PH, Shaper AG. Weight change and risk of heart attack in middle-aged British men. Int J Epidemiol. 1995 Aug;24(4):694–703. doi: 10.1093/ije/24.4.694. [DOI] [PubMed] [Google Scholar]
- 21.Caterson ID, Finer N, Coutinho W, et al. Maintained intentional weight loss reduces cardiovascular outcomes: results from the Sibutramine Cardiovascular OUTcomes (SCOUT) trial. Diabetes Obes Metab. 2012 Jun;14(6):523–530. doi: 10.1111/j.1463-1326.2011.01554.x. [DOI] [PubMed] [Google Scholar]
- 22.Doehner W, Erdmann E, Cairns R, et al. Inverse relation of body weight and weight change with mortality and morbidity in patients with type 2 diabetes and cardiovascular co-morbidity: an analysis of the PROactive study population. Int J Cardiol. 2012 Dec 15;162(1):20–26. doi: 10.1016/j.ijcard.2011.09.039. [DOI] [PubMed] [Google Scholar]
- 23.Kang WY, Hwang SH, Hwang SH, et al. Effects of weight change on clinical outcomes in overweight and obese patients with acute myocardial infarction who underwent successful percutaneous coronary intervention. Chonnam Med J. 2012 Apr;48(1):32–38. doi: 10.4068/cmj.2012.48.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kocz R, Hassan MA, Perala PR, Negargar S, Javadzadegan H, Nader ND. The effect of weight loss on the outcome after coronary artery bypass grafting in obese patients. Ann Card Anaesth. 2012 Jul-Sep;15(3):190–198. doi: 10.4103/0971-9784.97975. [DOI] [PubMed] [Google Scholar]
- 25.Robins SJ, Collins D, McNamara JR, Bloomfield HE. Body weight, plasma insulin, and coronary events with gemfibrozil in the Veterans Affairs High-Density Lipoprotein Intervention Trial (VA-HIT) Atherosclerosis. 2008 Feb;196(2):849–855. doi: 10.1016/j.atherosclerosis.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 26.Singh RB, Rastogi V, Rastogi SS, Niaz MA, Beegom R. Effect of diet and moderate exercise on central obesity and associated disturbances, myocardial infarction and mortality in patients with and without coronary artery disease. J Am Coll Nutr. 1996 Dec;15(6):592–601. doi: 10.1080/07315724.1996.10718635. [DOI] [PubMed] [Google Scholar]
- 27.Myers J, Lata K, Chowdhury S, McAuley P, Jain N, Froelicher V. The obesity paradox and weight loss. Am J Med. 2011 Oct;124(10):924–930. doi: 10.1016/j.amjmed.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 28.James WP, Caterson ID, Coutinho W, et al. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. New Engl J Med. 2010 Sep 2;363(10):905–917. doi: 10.1056/NEJMoa1003114. [DOI] [PubMed] [Google Scholar]
- 29.Wannamethee SG, Shaper AG, Lennon L. Reasons for intentional weight loss, unintentional weight loss, and mortality in older men. Arch Intern Med. 2005 May 9;165(9):1035–1040. doi: 10.1001/archinte.165.9.1035. [DOI] [PubMed] [Google Scholar]
- 30.Eilat-Adar S, Eldar M, Goldbourt U. Association of intentional changes in body weight with coronary heart disease event rates in overweight subjects who have an additional coronary risk factor. Am J Epidemiol. 2005 Feb 15;161(4):352–358. doi: 10.1093/aje/kwi045. [DOI] [PubMed] [Google Scholar]
- 31.French SA, Folsom AR, Jeffery RW, Williamson DF. Prospective study of intentionality of weight loss and mortality in older women: the Iowa Women's Health Study. Am J Epidemiol. 1999 Mar 15;149(6):504–514. doi: 10.1093/oxfordjournals.aje.a009844. [DOI] [PubMed] [Google Scholar]
- 32.Williamson DF, Thompson TJ, Thun M, Flanders D, Pamuk E, Byers T. Intentional weight loss and mortality among overweight individuals with diabetes. Diabetes Care. 2000 Oct;23(10):1499–1504. doi: 10.2337/diacare.23.10.1499. [DOI] [PubMed] [Google Scholar]
- 33.Shah ND, Dunlay SM, Ting HH, et al. Long-term medication adherence after myocardial infarction: experience of a community. Am J Med. 2009 Oct;122(10):961, e967-913. doi: 10.1016/j.amjmed.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allison DB, Zannolli R, Faith MS, et al. Weight loss increases and fat loss decreases all-cause mortality rate: results from two independent cohort studies. Int J Obes Relat Metab Disord. 1999 Jun;23(6):603–611. doi: 10.1038/sj.ijo.0800875. [DOI] [PubMed] [Google Scholar]
- 35.Romero-Corral A, Montori VM, Somers VK, et al. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet. 2006 Aug 19;368(9536):666–678. doi: 10.1016/S0140-6736(06)69251-9. [DOI] [PubMed] [Google Scholar]
- 36.Coutinho T, Goel K, Correa de Sa D, et al. Central obesity and survival in subjects with coronary artery disease: a systematic review of the literature and collaborative analysis with individual subject data. J Am Coll Cardiol. 2011 May 10;57(19):1877–1886. doi: 10.1016/j.jacc.2010.11.058. [DOI] [PubMed] [Google Scholar]
- 37.Coutinho T, Goel K, Correa de Sa D, et al. Combining body mass index with measures of central obesity in the assessment of mortality in subjects with coronary disease: role of "normal weight central obesity". J Am Coll Cardiol. 2013 Feb 5;61(5):553–560. doi: 10.1016/j.jacc.2012.10.035. [DOI] [PubMed] [Google Scholar]
- 38.Lavie CJ, De Schutter A, Patel DA, Romero-Corral A, Artham SM, Milani RV. Body composition and survival in stable coronary heart disease: impact of lean mass index and body fat in the "obesity paradox". J Am Coll Cardiol. 2012 Oct 9;60(15):1374–1380. doi: 10.1016/j.jacc.2012.05.037. [DOI] [PubMed] [Google Scholar]
- 39.McAuley PA, Artero EG, Sui X, et al. The obesity paradox, cardiorespiratory fitness, and coronary heart disease. Mayo Clin Proc. 2012 May;87(5):443–451. doi: 10.1016/j.mayocp.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oreopoulos A, Padwal R, Kalantar-Zadeh K, Fonarow GC, Norris CM, McAlister FA. Body mass index and mortality in heart failure: a meta-analysis. Am Heart J. 2008 Jul;156(1):13–22. doi: 10.1016/j.ahj.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 41.Barba R, Bisbe J, Pedrajas JNA, et al. Body mass index and outcome in patients with coronary, cerebrovascular, or peripheral artery disease: findings from the FRENA registry. Eur J Cardiovasc Prev Rehabil. 2009 Aug;16(4):457–463. doi: 10.1097/HJR.0b013e32832b1818. [DOI] [PubMed] [Google Scholar]
- 42.Kalantar-Zadeh K, Kopple JD, Kilpatrick RD, et al. Association of morbid obesity and weight change over time with cardiovascular survival in hemodialysis population. Am J Kidney Dis. 2005 Sep;46(3):489–500. doi: 10.1053/j.ajkd.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 43.Pack QR, Rodriguez-Escudero JP, Thomas RJ, et al. Diagnostic performance of weight loss to predict body fatness improvement in cardiac rehabilitation patients. J Cardiopulm Rehabil Prev. 2013 Mar;33(2):68–76. doi: 10.1097/HCR.0b013e31827fe7e3. [DOI] [PubMed] [Google Scholar]
- 44.Aggarwal S, Arena R, Cuda L, et al. The independent effect of traditional cardiac rehabilitation and the LEARN program on weight loss: a comparative analysis. J Cardiopulm Rehabil Prev. 2012 Jan-Feb;32(1):48–52. doi: 10.1097/HCR.0b013e31823f2da1. [DOI] [PubMed] [Google Scholar]
- 45.Ades PA, Savage PD, Toth MJ, et al. High-calorie-expenditure exercise: a new approach to cardiac rehabilitation for overweight coronary patients. Circulation. 2009 May 26;119(20):2671–2678. doi: 10.1161/CIRCULATIONAHA.108.834184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.