Abstract
Background and Aims
The severity of the metabolic syndrome (MetS) is linked to future cardiovascular disease. However, it is unclear whether MetS severity increases among individuals followed over time.
Methods
We assessed changes in a sex- and race/ethnicity-specific MetS severity Z-score over a 10-year period (visits 1–4) among 9,291 participants of the Atherosclerosis Risk in Communities study cohort. We compared sex- and racial/ethnic subgroups for the rate of change in the MetS severity score and MetS prevalence as assessed using traditional ATP-III MetS criteria. We further examined effects of use of medications for hypertension, diabetes and dyslipidemia.
Results
Over the 10 years of follow-up, MetS severity Z-scores increased in 76% of participants from an overall mean of 0.08 ± 0.77 at baseline to 0.48 ± 0.96 at visit 4 with the greatest progression in scores observed among African-American women. Baseline MetS severity scores predicted the time until ATP-III MetS diagnosis, with a model-predicted 77.5% of individuals with a visit 1 MetS severity score of 0.75 progressing to ATP-III MetS within 10 years. The rate of increase in MetS severity score was higher among those younger at baseline but was independent of baseline MetS status or the use of medications to treat blood pressure, lipids and diabetes.
Conclusion
The severity of metabolic derangements as measured using this MetS severity score increases over time within individuals and predicts diagnosis of ATP-III MetS. These data may have implications for tracking MetS related risk within individuals over time.
Keywords: Metabolic syndrome x, cardiovascular disease, type 2 diabetes mellitus, minority health
Introduction
The metabolic syndrome (MetS) is a cluster of metabolic risk factors that are associated with increased risk for cardiovascular disease (CVD) and type 2 diabetes mellitus (T2DM).1 MetS is typically classified based on a person exhibiting abnormalities beyond specific cut-off levels for the individual MetS components of waist circumference, blood pressure (BP), fasting glucose, fasting triglycerides, and HDL-cholesterol. There is an overall tendency for these component levels to worsen in an individual over time;2 thus, it is not surprising that in longitudinal cohorts there is an increase in the prevalence of MetS over time as more individuals exceed the cut off levels for the individual components.3 Based on current criteria such as that of the Adult Treatment Panel III (ATP-III), an individual must have abnormalities in at least 3 of these 5 components to be classified as having MetS.4 Nevertheless, use of medications to treat specific components such as elevated fasting glucose and dyslipidemia may contribute to overall reductions in these values.5,6 Because of the binary nature of traditional MetS criteria, other longitudinal studies have shown the propensity of some individuals to toggle back and forth between a MetS classification over time7 —which has been seen as a limitation to current criteria.8
An additional limitation to the current MetS criteria is that they appear to exhibit racial/ethnic discrepancies in that African-Americans are less likely to be classified as having MetS,9–13 despite having higher rates of T2DM14 and death from CVD15 —conditions with which MetS is closely associated. Similarly, use of ATP-III MetS definition in other populations has been questioned.16 We have formulated a MetS severity score that is sex- and race/ethnicity-specific and can follow changes in MetS characteristics w ithin a given individual.17,18 We recently reported use of this score in predicting future CVD based on MetS severity in childhood and mid-adulthood.19,20 While there was a strong correlation between MetS severity in childhood and adulthood, it remains unclear how MetS severity tracks within an individual, as related to race/ethnicity and medical treatment.
Our goal in the current study was to evaluate for variation in MetS severity within individuals over time using this sex- and race/ethnicity-specific MetS severity score. We hypothesized that use of this score would reveal a gradual worsening of MetS severity in a population over time—and that this worsening would itself vary by sex and race/ethnicity and by treatment with MetS-related medications. In addition, we wished to determine the ability of this MetS severity score to predict ATP-III defined MetS. We hypothesized that this score would provide a more sensitive early measure of a person’s risk of MetS. We evaluated longitudinal data from the Atherosclerosis Risk in Communities Study (ARIC), with potential implications to tracking MetS clinically over time in an individual.
Methods
Study Population
ARIC study is a large community-based epidemiological cohort study that started in 1987–89 across 4 field centers in the US. A total of 15,792 participants aged 45–64 years were recruited. Of these 15,397 consented to be included in this study – 5,359 white men, 5,943 white women, 1,585 African-American men, and 2,464 African-American women and 46 participants of other races. Details of the study design and objectives are published elsewhere.21 From this sample, we excluded those who reported presence of CVD or DM at the baseline visit, who missed any of the follow- up visits up to visit 4 (1996–98), participants other than African Americans and whites, or those with missing data on any of the components of MetS. Thus, a total of 9,291 participants were included in the current analyses.
Measurement of metabolic syndrome components
Previous reports have published details of procedures for blood collection and analysis for lipids22 and serum glucose.23 Briefly, participants fasted overnight for 12 hours before the examination. Phlebotomy was performed, blood sample was centrifuged and serum was sent to a central laboratory for examination. Triglycerides were measured by enzymatic methods, and HDL cholesterol was measured after dextran-magnesium precipitation. LDL cholesterol was calculated using the Friedewald equation. Serum glucose was measured by the hexokinase -6 phosphate dehydrogenase method.24 Trained clinical staff measured waist circumference at the umbilical level to the nearest cm. BP was examined in sitting position with a random-zero sphygmomanometer – of the three measurements performed, the average of the last two measurements were used for analysis. Similar procedures were followed at all study sites over the 4 visits.
MetS severity was calculated as a Z-score for participants at all four visits using sex and race based formulae. As described elsewhere,17 these scores were derived using a confirmatory factor analysis approach for the 5 traditional components of MetS to determine the weighted contribution of each of these components to a latent MetS “factor” on a sex- and race/ethnicity-specific basis. Confirmatory factor analysis was performed among adults 20–64 years from the National Health and Nutrition Examination Survey (NHANES) with categorization into six sub-groups based on sex and the following self-identified race/ethnicities: non-Hispanic white, non-Hispanic black and Hispanic. For each of these six population sub-groups, loading coefficients for the 5 MetS components were determined toward a single MetS factor. These loading coefficients were used to generate equations to calculate a standardized MetS severity score for each sub-group (http://publichealth.hsc.wvu.edu/biostatistics/metabolic-syndrome-severity-calculator/). The resulting MetS severity scores are Z-scores (ranging from theoretical negative infinity to theoretical positive infinity) of relative MetS severity on a sex- and race/ethnicity-specific basis. These scores are highly correlated to other surrogate markers of MetS risk, including hsCRP, uric acid and the homeostasis model of insulin resistance17 and were recently shown to correlate with long-term CVD and T2DM risk in the Princeton Lipid Research Cohort Study.19,20
MetS was defined using the criteria established by the Adult Treatment Panel III (ATP III), i.e. presence of three or more of the following criteria – elevated waist circumference (≥102 cm for men, ≥88 cm for women), elevated triglycerides (≥150 mg/dl or drug treatment for elevated triglycerides), reduced HDL (<40 mg/dl for men, <50 mg/dl for women or drug treatment for reduced HDL), elevated BP (≥130 mmHg systolic or ≥85 mmHg diastolic or drug treatment for hypertension) and elevated blood glucose (≥100 mg/dl or drug treatment for elevated glucose).4 Leisure time physical activity was self-reported at baseline using questionnaire designed by Baecke.25
Statistical analysis
All analyses were performed using SAS Version 9.4 (Cary, NC) with statistical significance set to α = 0.05. Descriptive statistics on baseline characteristics were calculated for all included participants and compared with those that were excluded from the current study. Prevalence of MetS and mean MetS severity scores we re calculated across the four visits, and by age group (< 50, 50–59, and ≥ 60 years), sex, and race (white vs. African-American). For trends that appeared linear, generalized estimating equations (GEE’s) were fit to model linear trends over time to compare prevalence (MetS) and mean (MetS severity score) as well as the linear increases across the four sex/race groups of interest (white males, white females, African-American males, African-American females) by age category stated above. An unstructured working correlation matrix was used to account for the repeated observations across individuals.
To determine the sensitivity of the MetS severity score in changing prior to actual development of traditionally defined MetS, mean MetS severity scores were modeled using GEE’s, separately by categories defined by the timing of initial diagnosis of MetS (visit 1, 2, 3, or 4), adjusting for baseline age. This analysis only looked at those individuals who were consistently classified as having MetS in subsequent visits, eliminating those individuals whose diagnosis changed repeatedly during the study (n=1,974, 21.2%). Further insight into the discriminative ability of the MetS severity score was determined by using an accelerated failure time model (assuming a Weibull distribution) to model “time to MetS” as a function of baseline MetS severity score (excluding those individuals with MetS at visit 1), again adjusting for baseline age. This model accounts for the interval censoring that occurred in this study (i.e., there is not a specific date of definitive diagnosis of MetS, only that we know it developed between two visits).
Finally, to determine the impact of medication use on MetS severity, mean MetS scores were modeled across visits using GEE’s, as a function of medication status. Separate analyses were performed for blood pressure medications, lipid medications, and diabetes medications, and categories were defined by the start of medication use, again limiting to those individuals who remained on medications for subsequent visits.
Results
Participant baseline characteristics
We assessed data from 9,291 ARIC participants with complete data for visits 1–4. Compared to those included in the analysis, excluded participants were slightly older (54.8 ± 5.9 vs. 53.8 ± 5.7 years, p<0.05), more likely to be African-American (38.8% vs. 18.3%, p<0.05) and male (47.2% vs. 43.9%, p<0.05), and had higher rates of smoking (33.5% vs. 21.5%, p<0.05) and higher baseline MetS severity scores (0.81±1.60 vs. 0.08±0.77, p<0.05). Participant characteristics of those included in the analysis are shown in Table 1.
Table 1.
Metabolic Syndrome and descriptive characteristics of the ARIC study population*
| Whites (n=7593) |
African-Americans (n=1698) |
|||
|---|---|---|---|---|
| Male (n=3,443) |
Female (n=4,150) |
Male (n=639) |
Female (n=1,059) |
|
| MetS Components | ||||
| Systolic BP (mmHg) | 119.3 (15.2) | 115.7 (16.8) | 125.8 (18.5) | 124.4 (18.0) |
| Diastolic BP (mmHg) | 73.6 (9.7) | 69.8 (9.5) | 81.4 (11.7) | 77.9 (10.7) |
| Waist circumference (cm) | 99.0 (10.0) | 92.0 (14.1) | 96.2 (11.6) | 98.4 (15.9) |
| HDL-cholesterol (mg/dl) | 43.7 (12.2) | 58.7 (16.7) | 50.3 (14.8) | 59.8 (17.1) |
| Triglycerides (mg/dl) | 139.2 (83.9) | 119.9 (71.8) | 111.9 (67.2) | 99.2 (46.3) |
| Glucose (mg/dl) | 101.5 (10.1) | 96.9 (9.6) | 100.4 (11.2) | 98.8 (11.4) |
| Descriptive Characteristics | ||||
| Age (years) | 54.4 (5.6) | 53.8 (5.6) | 53.1 (5.8) | 52.7 (5.6) |
| T. Cholesterol (mg/dl) | 210.0 (37.5) | 216.4 (40.5) | 212.4 (44.2) | 214.9 (43.5) |
| LDL-cholesterol (mg/dl) | 139.1 (34.7) | 133.8 (38.0) | 140.4 (42.2) | 135.4 (42.1) |
| BMI, (kg/m2) | 27.3 (3.8) | 26.3 (5.1) | 27.5 (4.4) | 30.2 (6.3) |
| Hypertension (%) | 23.6 | 22.2 | 44.4 | 50.4 |
| Physical activity† | 2.4 (0.5) | 2.5 (0.5) | 2.1 (0.6) | 2.1 (0.6) |
| Smoking status (%) | ||||
| Current | 20.1 | 21.1 | 31.7 | 22.8 |
| Former | 48.0 | 25.2 | 36.1 | 18.1 |
| Never | 31.9 | 53.6 | 32.0 | 59.9 |
| Alcohol drinking (%) | ||||
| Current | 72.7 | 64.0 | 50.6 | 24.2 |
| Former | 16.9 | 11.9 | 23.0 | 18.1 |
| Never | 10.4 | 24.0 | 26.3 | 57.7 |
| Education (%) | ||||
| Basic | 13.6 | 12.1 | 33.2 | 33.6 |
| Intermediate | 39.4 | 51.6 | 28.6 | 30.0 |
| Advanced | 47.0 | 36.2 | 38.3 | 36.4 |
| Medications (%) | ||||
| Cholesterol | ||||
| At baseline | 17.2 | 22.0 | 21.9 | 33.3 |
| After baseline | 26.0 | 21.2 | 23.0 | 27.4 |
| Blood-pressure | ||||
| At baseline | 13.5 | 13.5 | 25.0 | 35.1 |
| After baseline | 16.1 | 15.0 | 19.7 | 21.2 |
| Diabetes | ||||
| At baseline‡ | NA | NA | NA | NA |
| After baseline | 4.2 | 2.7 | 9.3 | 9.6 |
Those with loss to follow-up by visit 4, with baseline diabetes and CHD were excluded from the analysis. Values are mean (SD), unless otherwise mentioned
Leisure-time physical activity was measured on a scale of 1 to 4.5 using Baecke questionnaire.{Baecke, 1982 #1966}
Those taking diabetes medications were excluded from the analysis
At baseline, 33% of the participants met criteria for ATP-III MetS. Baseline MetS severity score (mean ± SD) was higher among those with ATP-III MetS (0.85 ± 0.52) than those without (−0.29 ± 0.59) (p<0.001)(Supplementary Table 1). In addition to having higher levels of the individual MetS components, those with ATP-III MetS had worse profile on multiple other cardiovascular risk factors including age, LDL-c, and socioeconomic status. African-American females had the highest prevalence of ATP-III MetS at baseline (39.7%, p<0.01 as compared to other groups), with white males next at 35.9%, while the prevalence was relatively low among African-American males (28.4%) and white females (29.5%).
MetS severity scores by age, sex, and racial/ethnic sub-group
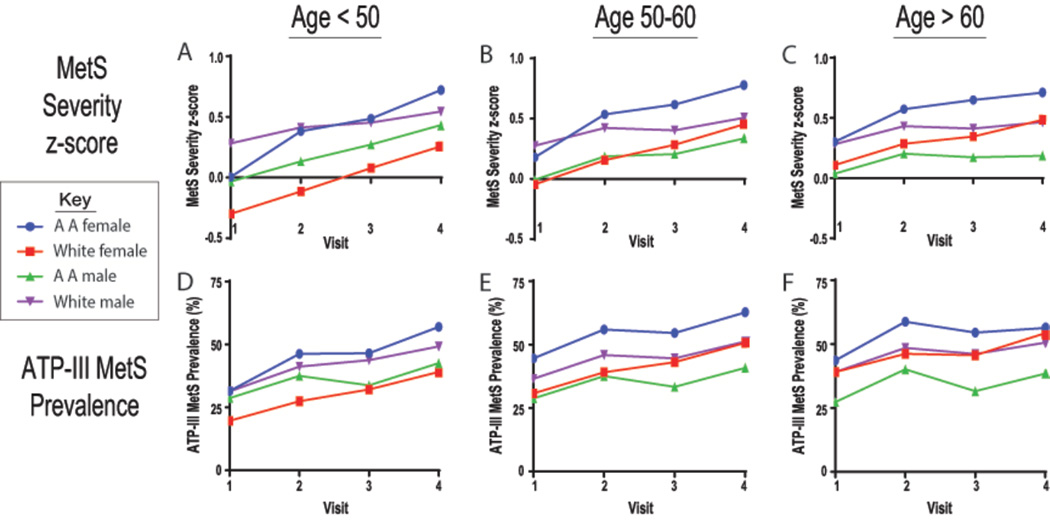
Over the average 10-year follow-up period MetS severity scores increased overall from (0.08 ±0.77) to (0.48 ±0.96), increasing in 76% of the participants. In contrast, the prevalence of ATP-III MetS increased from 32.9% to 49.9%. Similar r ace/sex-specific trends in MetS severity scores were observed after stratification by baseline age (<50 years, 50– <60 years, and ≥60 years), as were seen before stratification (Table 2 and Figure 1). Overall, MetS severity score progression was notably higher among women (0.165 ±0.004 per visit) than among men (0.077 ±0.004) (p<0.05). Among women, the progression was higher among those <50 years at baseline than older age groups. In addition, with successive baseline age-categories, we observed progressively higher baseline MetS severity scores among women as compared to men; while the baseline scores for both African-American and white men remained unchanged across the age-categories, the baseline scores for both African-American and white women were higher for successive age categories. Similar trends were observed with the prevalence of ATP-III MetS over time. Further, when evaluated as age categories, participants who were <50 years were more likely to be current smokers (24.4% vs. 16.9% of those ≥60 years) and more likely to be current drinkers (66.0% vs. 58.4%). The results did not change materially when we further adjusted for baseline level of leisure-time physical activity (supplementary table 2).
Table 2.
Estimates of Linear Increases in MetS Prevalence and MetS Severity over Time: Overall and By Age Group for White and African American (AA) Participants
| Model Estimates (Standard Errors) of MetS Prevalence | ||||||||
| Overall | Age < 50 | Age 50 – < 60 | Age ≥60 | |||||
| Visit 1 Rate | Ratio* | Visit 1 Rate | Ratio* | Visit 1 Rate | Ratio* | Visit 1 Rate | Ratio* | |
| White Males | 0.38 (0.01) | 1.10 (0.01) | 0.33 (0.01) | 1.14 (0.02) | 0.38 (0.01) | 1.10 (0.01) | 0.42 (0.02) | 1.07 (0.01) |
| White Females | 0.30 (0.01)a | 1.17 (0.01)a | 0.21 (0.01)a | 1.23 (0.02)a | 0.32 (0.01)a | 1.17 (0.01)a | 0.40 (0.02) | 1.10 (0.01) |
| AA Males | 0.30 (0.02)a | 1.10 (0.02)b | 0.30 (0.03)b | 1.11 (0.03)b | 0.30 (0.02)a | 1.10 (0.03)b | 0.31 (0.04)ab | 1.07 (0.05) |
| AA Females | 0.43 (0.01)abc | 1.12 (0.01)b | 0.35 (0.02)b | 1.18 (0.02) | 0.47 (0.02)abc | 1.10 (0.02)b | 0.49 (0.03)bc | 1.06 (0.03) |
| Model Estimates (Standard Errors) of MetS Severity | ||||||||
| Overall | Age < 50 | Age 50 – < 60 | Age ≥60 | |||||
| Visit 1 Mean | Difference** | Visit 1 Mean | Difference** | Visit 1 Mean | Difference** | Difference** | Difference** | |
| White Males | 0.30 (0.01) | 0.07 (0.00) | 0.30 (0.03) | 0.09 (0.01) | 0.30 (0.02) | 0.07 (0.01) | 0.33 (0.03) | 0.05 (0.01) |
| White Females | −0.08 (0.01)a | 0.16 (0.00)a | −0.30 (0.02)a | 0.18 (0.01)a | −0.03 (0.02)a | 0.16 (0.01)a | 0.13 (0.03)a | 0.12 (0.01)a |
| AA Males | 0.02 (0.03)ab | 0.11 (0.02)ab | −0.03 (0.06)ab | 0.16 (0.04) | −0.02 (0.04)a | 0.11 (0.02)b | 0.10 (0.07)a | 0.03 (0.02)b |
| AA Females | 0.21 (0.03)abc | 0.18 (0.01)ac | 0.06 (0.05)ab | 0.23 (0.03)a | 0.26 (0.04)bc | 0.17 (0.02)ac | 0.38 (0.06)bc | 0.10 (0.02)ac |
Ratio = Multiplicative increase in MetS rate between any two successive visits (bold indicates different than 1 (p < 0.05))
Difference = Additive increase in mean MetS severity between any two visits (bold indicates different than 0 (p < 0.05))
Statistical comparisons (white male as referent):
Different than white male (p < 0.05)
Different than white female (p < 0.05)
Different than African-American male (p < 0.05)
Figure 1. Metabolic syndrome (MetS) severity and ATP-III MetS prevalence over time.
Mean MetS severity Z-scores (A–C) and prevalence of ATP-III MetS (D–F) are shown by sex/racial sub-groups and visit number for ARIC participants with age at baseline <50, 50 – <60 and ≥60 years. AA = African-American.
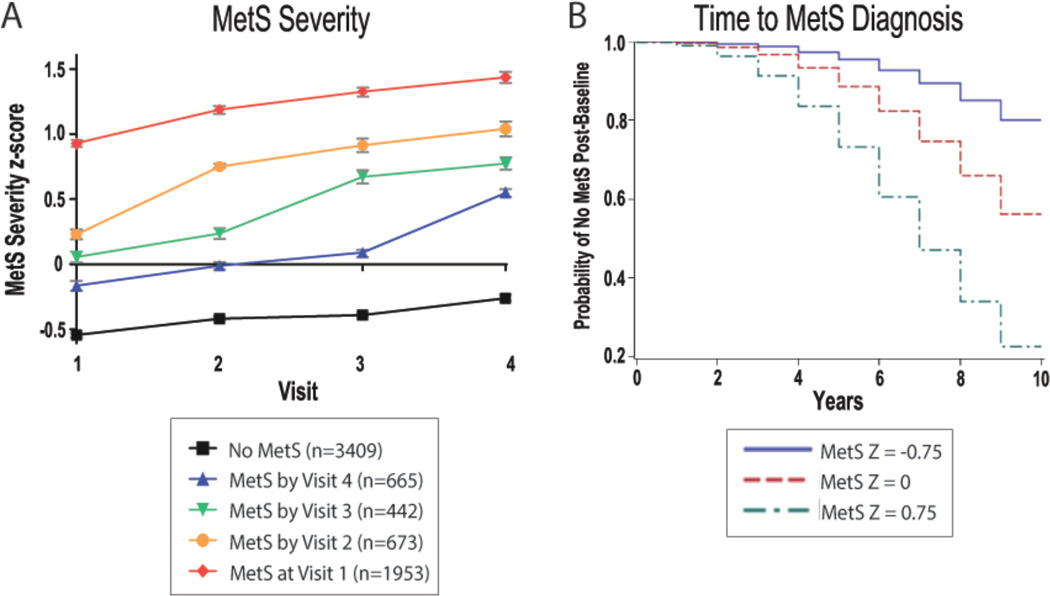
MetS severity score by MetS diagnosis
We next assessed MetS severity scores by the follow-up visit at which ATP-III MetS was first diagnosed (Figure 2). Participants with baseline ATP-III MetS had the highest MetS severity scores across all visits, for those diagnosed with ATP-III MetS in subsequent visits had progressively higher baseline MetS severity scores in relationship to their time until ATP-III MetS diagnosis (all p<0.05; Figure 2a). Using model-based predicted probabilities of MetS-free “survival” among those 50 years old at baseline, we found that the MetS severity score predicted time to development of ATP-III MetS during the follow-up. For example, the predicted rate of individuals without ATP-III MetS at 10 years was 80.0% among those with a baseline MetS severity score of −0.75, 56.2% with a baseline MetS severity score of 0.0, while it was only 22.5% among those with a baseline score of 0.75. (Figure 2b)
Figure 2. Metabolic syndrome (MetS) severity by timing of ATP-III MetS diagnosis.
A. Mean MetS severity Z-scores and 95% confidence intervals among ARIC participants without ATP-III MetS throughout visit 1–4 and those diagnosed with MetS at each of the individual visit. B. Model-based predicted probabilities of time-until-MetS-diagnosis among individuals without ATP-III MetS and 50 years old at baseline with starting MetS Z scores as shown.
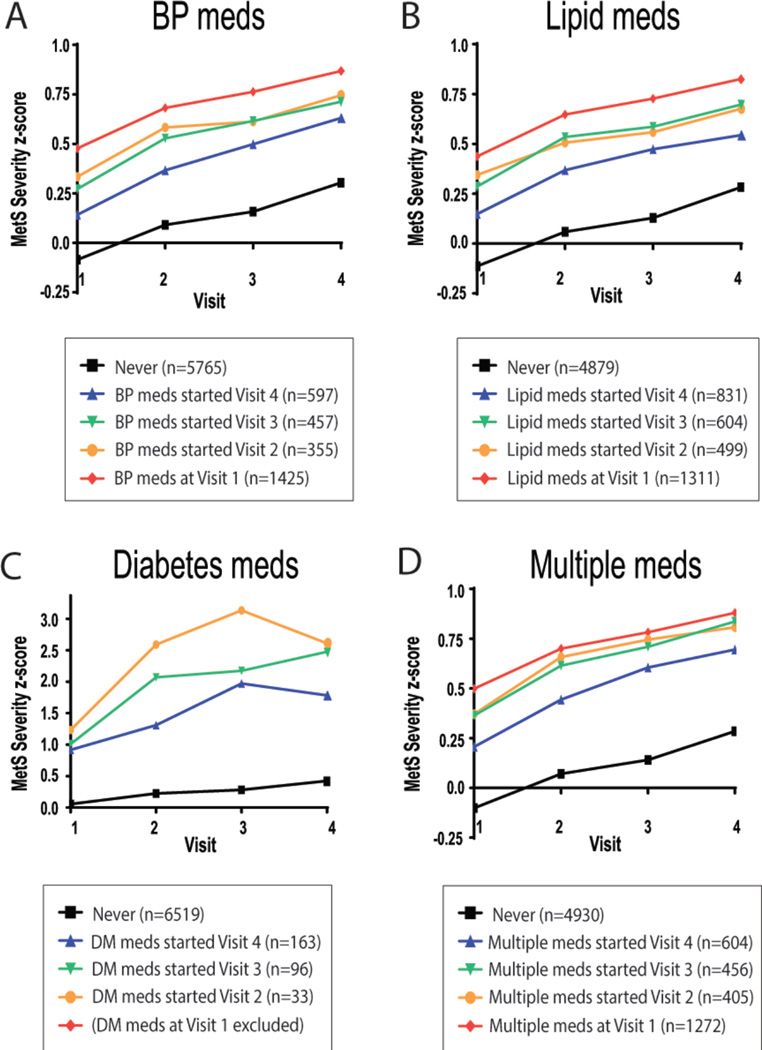
Medication Use and MetS severity
Figure 3 displays MetS severity scores by the timing of start of medications for correcting abnormalities in one or more of the MetS components (hypertension, dyslipidemia and diabetes). Participants taking these medications had higher overall MetS severity scores than those not taking such medications, with a gradual increase observed that was inversely related to the time of starting medication. MetS severity score continued to increase even after starting such medications, with no difference in the rate of change of the MetS severity score between the visit prior to and the visit after starting either one medication or a combination of medications (Figure 3).
Figure 3. Metabolic syndrome (MetS) severity by timing of initiation of medications.
Mean MetS severity Z-score by use of medications to treat blood pressure (A), lipids (B), diabetes (C) or a combination of these treatments (D) among ARIC participants by use of who never took the individual class of medication or who started the medication (or combination) at visit 1–4, as shown. In each case, individuals with baseline diabetes were excluded.
Discussion
We used a sex- and race/ethnicity-specific MetS severity score to assess for changes in MetS over time, finding that the severity of MetS worsened within individuals over a 10 year period, with increased MetS severity scores from baseline to follow-up in 76% of individuals. This increase in MetS severity was similar among participants with and without ATP-III MetS classification, suggesting that the burden of MetS increased in the majority of the cohort. By contrast, use of traditional MetS criteria to assess for changes in the burden of MetS during this time period was only able to document an increase in prevalence by 17%, missing assessment of any ongoing worsening of metabolic derangements within individuals already classified with MetS. Given recent findings relating the change in this score over time as a predictor of future CVD26 these data may have implications for following MetS in clinical settings.
As a demonstration of potential utility of a MetS severity score in clinical settings, we found that individuals without ATP-III MetS at baseline who developed incident MetS at visits 2, 3 and 4 had increasingly higher baseline MetS severity scores that were inversely related to their time to ATP-III progression (Figure 2). This is significant given prior longitudinal studies that demonstrated that over a 10-year period, individuals with ATP-III MetS (compared to those without MetS) had an OR of 1.8 for development of CVD27 and 4.1 for development of T2DM.28 While it was outside the scope of the current study to assess the relationship between this score and future disease diagnosis, our prior report described a significant relationship between baseline score and risk for future diagnosis of CVD, with higher scores being implicated in risk for earlier disease diagnosis.26 These data support potential use of MetS severity as a screening tool and a means of following a particular individual’s trajectory toward worsening MetS severity and eventual diagnosis of traditional MetS, and potential disease risk.
The pace of progression of MetS severity over time differed by sex- and racial/ethnic-subgroup, with more rapid progression among women, and particularly among African-American women. Except at baseline, African-American women had highest MetS severity scores at all visits; they also progressed sharply over the four visits, with a change in severity that outpaced both white and African-American males. This may not be completely surprising given that in a standardized analysis of T2DM prevalence in the US, African-American women had overall the highest prevalence of T2DM,29 and in an analysis of lifetime risk of T2DM, African-American females had the highest likelihood of future T2DM of the four sex- and racial/ethnic subgroups studied here.30 The progression in metabolic severity appeared sharper than trends in the change in prevalence of ATP-III MetS. For example, in the ≥60 age group, African-American women had a decrease in prevalence of ATP-III MetS during visits 3 and 4 at the same time that the overall MetS severity scores co ntinued to increase—again suggesting a worsening in scores within individuals. Overall, these rapid increases in MetS severity may have implications as a trigger for which individuals to follow more closely for MetS-related sequelae.
Baseline age group also had significant effects on MetS severity scores, with more rapid progression among younger age groups and higher baseline severity scores by age group (lower half of Table 2). This could indicate that this type of score could be more useful in determining risk among younger individuals of intermediate CVD risk.31,32 The relationship between baseline age and progression was again stronger among women as compared to men, with gradual increases in MetS scores with increasing age groups in both white and African-American women. This may be related to the worsening of individual components of MetS over time that has been noted previously.2 The reason that the increase in baseline MetS severity with increasing age category was not observed in men is unclear, though this may have been due to a survival bias among those in the higher age categories, given that we required data from all 4 visits for inclusion in the analytic data set. This may have resulted in overall healthier participants among those in the higher age categories, with these participants being more likely to practice healthier lifestyle habits. Indeed, participants in the older categories (≥60 years) had lower rates of current smoking (24.4% vs. 16.9% of those ≥60 years) and current drinking (66.0% vs. 58.4%) than those in the <50 year age category. Despite these differences, baseline MetS severity scores that were unexpectedly similar between age groups, we noted gradual increases in MetS severity in each group over time, consistent with our hypothesis.
Use of medications that improve measures of MetS components would be expected to lower MetS severity scores. However, in looking at MetS severity scores for individuals by the timing of initiation of medications for BP, lipids and diabetes we found an inverse relationship between the timing of initiation of medications and both baseline and later MetS severity scores. This likely suggests a tendency to start medications only after an individual has exhibited extremes in one or more components of MetS. The lack of significant improvement in MetS scores after initiating each of these medications may be due to these medications targeting limited components of the severity score. BP-lowering medications would be expected to decrease systolic BP by approximately 6%,33 though it should be acknowledged that systolic BP levels have a relatively low percent contribution to MetS severity score values,17 and may not have contributed to significant changes in scores. Lipid lowering medications such as HMG-co-A reductase inhibitors have their strongest effects on lowering LDL cholesterol, itself not a component of MetS. However, they also have effects on lowering triglycerides (by 10–14% for early-generation statins)34 and slightly raising HDL cholesterol (by 3–6%),35 contributing to lower MetS severity scores. Other lipid treatment medications (as may have been more commonly used, given the time frame of this cohort, 1986–1999) could have had overall similar effects on triglycerides and/or HDL, including niacin, nicotinic acid and fibrates.36 With respect to diabetes medications, there is a higher likelihood that effective treatment of blood sugar elevations would have contributed better to reductions in MetS Z-scores. Unfortunately, we were limited by a lack of data related to diabetes medications, which were used at very low rates in this cohort (<5%). This low rate of use was in part due to exclusion of individuals with diabetes at baseline but may also reflect fewer diabetes treatment options at the time of the study or a misunderstanding of participants regarding what constituted a diabetes medication. Overall, the rate of increase in MetS severity scores did not differ significantly by the timing of when these medications were started, demonstrating a lack of dramatic effects of individual or combined medications (Figure 3). This may emphasize the need for a more concerted approach toward treatment of these metabolic derangements, with both combinations of medications and change in lifestyle to lower MetS scores over time. While it remains unclear how subsequent changes in the MetS severity score following medication use are related to the individual’s cardiometabolic risk, it remains likely that the true MetS severity score for an individual taking such medications would be higher in the absence of treatment.
Strengths and Limitations
Results from our study should be interpreted keeping in mind certain study limitations. We excluded those individuals from the analysis who were lost to follow-up by visit 4. These participants were more likely to be older, have a lower educated level, be African-American, and have MetS at baseline—introducing the potential for bias. However, inclusion of this healthier population sample from baseline allowed us to examine the score progression in a healthier sample–a population likely to benefit from clinical use of the score as a screening tool. It remains unknown whether MetS severity scores predict CVD or T2DM in this population; examination of MetS severity score as a predictor of CVD is our logical next step.
Conclusion
We found worsening MetS severity over time in both individuals with and without ATP-III MetS that persisted despite medication treatment. Baseline levels of MetS severity score predicted time to ATP III MetS diagnosis. These data suggest the potential for clinical application in following MetS severity scores within individuals as a marker of metabolic risk— and potentially as a way of tracking response to treatment. Future research is needed to link specific thresholds in this score to risk for later disease, as a means of better targeting individuals most likely to benefit from interventions. In addition, documentation of score response to treatment in controlled c onditions could provide guidance in the best approach to lowering overall metabolic risk over time.
Supplementary Material
Manuscript Highlights.
Metabolic syndrome, when examined as a continuous severity Z-score, exhibits gender and race/ethnic differences in progression over time.
Africa-American women have higher rate of progression of metabolic syndrome severity as compared to other groups.
Higher metabolic syndrome severity Z-scores at baseline predict earlier onset of ATP-III metabolic syndrome.
Acknowledgements
Funding: This work was supported by NIH grants 1R01HL120960 (MJG and MDD) and U54GM104942 (MJG). The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). The authors thank the staff and participants of the ARIC study for their important contributions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors of this work do not have any conflicts of interest to report.
Reference
- 1.Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288(21):2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Srinivasan SR, Li S, Xu J, Berenson GS. Clustering of long-term trends in metabolic syndrome variables from childhood to adulthood in Blacks and Whites: the Bogalusa Heart Study. Am J Epidemiol. 2007;166(5):527–533. doi: 10.1093/aje/kwm105. [DOI] [PubMed] [Google Scholar]
- 3.Wilson PW, D'Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112(20):3066–3072. doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- 4.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome - An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 5.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beltran-Sanchez H, Harhay MO, Harhay MM, McElligott S. Prevalence and trends of metabolic syndrome in the adult U.S. population, 1999–2010. J Am Coll Cardiol. 2013;62(8):697–703. doi: 10.1016/j.jacc.2013.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodman E, Daniels SR, Meigs JB, Dolan LM. Instability in the diagnosis of metabolic syndrome in adolescents. Circulation. 2007;115(17):2316–2322. doi: 10.1161/CIRCULATIONAHA.106.669994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kahn R, Buse J, Ferrannini E, Stern M. The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2005;28(9):2289–2304. doi: 10.2337/diacare.28.9.2289. [DOI] [PubMed] [Google Scholar]
- 9.Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2003;163(4):427–436. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker SE, Gurka MJ, Oliver MN, Johns DW, DeBoer MD. Racial/ethnic discrepancies in the metabolic syndrome begin in childhood and persist after adjustment for environmental factors. Nutr Metab Cardiovasc Dis. 2012;22(2):141–148. doi: 10.1016/j.numecd.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anuurad E, Chiem A, Pearson TA, Berglund L. Metabolic syndrome components in african americans and European-american patients and its relation to coronary artery disease. Am J Cardiol. 2007;100(5):830–834. doi: 10.1016/j.amjcard.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 12.DeBoer MD, Gurka MJ. Low sensitivity for the metabolic syndrome to detect uric acid elevations in females and non-Hispanic-black male adolescents: an analysis of NHANES 1999–2006. Atherosclerosis. 2012;220(2):575–580. doi: 10.1016/j.atherosclerosis.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeBoer MD, Dong L, Gurka MJ. Racial/Ethnic and Sex Differences in the Ability of Metabolic Syndrome Criteria to Predict Elevations in Fasting Insulin Levels in Adolescents. Journal of Pediatrics. 2011;159(6) doi: 10.1016/j.jpeds.2011.05.023. 975-U141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988–2006. Diabetes Care. 2010;33(3):562–568. doi: 10.2337/dc09-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111(10):1233–1241. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 16.Patel A, Huang KC, Janus ED, et al. Is a single definition of the metabolic syndrome appropriate?--A comparative study of the USA and Asia. Atherosclerosis. 2006;184(1):225–232. doi: 10.1016/j.atherosclerosis.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Gurka MJ, Lilly CL, Norman OM, DeBoer MD. An Examination of Sex and Racial/Ethnic Differences in the Metabolic Syndrome among Adults: A Confirmatory Factor Analysis and a Resulting Continuous Severity Score. Metabolism. 2014;63(2):218–225. doi: 10.1016/j.metabol.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gurka MJ, Ice CL, Sun SS, DeBoer MD. A confirmatory factor analysis of the metabolic syndrome in adolescents: an examination of sex and racial/ethnic differences. Cardiovascular Diabetology. 2012;11 doi: 10.1186/1475-2840-11-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeBoer MD, Gurka MJ, Woo JG, Morrison JA. Severity of Metabolic Syndrome as a Predictor of Cardiovascular Disease Between Childhood and Adulthood: The Princeton Lipid Research Cohort Study. J Am Coll Cardiol. 2015;66(6):755–757. doi: 10.1016/j.jacc.2015.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeBoer MDG MJ, Woo JG, Morrison JA. Severity of Metabolic Syndrome as a Predictor of Type 2 Diabetes Between Childhood and Adulthood: The Princeton Lipid Research Cohort Study. Diabetologia. 2015 doi: 10.1007/s00125-015-3759-5. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 22.Sharrett AR, Ballantyne CM, Coady SA, et al. Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B. andHDL density subfractions: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2001;104(10):1108–1113. doi: 10.1161/hc3501.095214. [DOI] [PubMed] [Google Scholar]
- 23.Folsom AR, Szklo M, Stevens J, Liao F, Smith R, Eckfeldt JH. A prospective study of coronary heart disease in relation to fasting insulin, glucose, and diabetes. The Atherosclerosis Risk in Communities (ARIC) Study. Diabetes Care. 1997;20(6):935–942. doi: 10.2337/diacare.20.6.935. [DOI] [PubMed] [Google Scholar]
- 24.McNeill AM, Schmidt MI, Rosamond WD, et al. The metabolic syndrome and 11-year risk of incident cardiovascular disease in the atherosclerosis risk in communities study. Diabetes Care. 2005;28(2):385–390. doi: 10.2337/diacare.28.2.385. [DOI] [PubMed] [Google Scholar]
- 25.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36(5):936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 26.DeBoer MD, Gurka MJ, Woo JG, Morrison JA. Severity of Metabolic Syndrome as a Predictor of Cardiovascular Disease Between Childhood and Adulthood: The Princeton Lipid Research Cohort Study. J. Amer. Coll. Card. 2015 doi: 10.1016/j.jacc.2015.05.061. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dekker JM, Girman C, Rhodes T, et al. Metabolic syndrome and 10-year cardiovascular disease risk in the Hoorn Study. Circulation. 2005;112(5):666–673. doi: 10.1161/CIRCULATIONAHA.104.516948. [DOI] [PubMed] [Google Scholar]
- 28.Hanley AJ, Karter AJ, Williams K, et al. Prediction of type 2 diabetes mellitus with alternative definitions of the metabolic syndrome: the Insulin Resistance Atherosclerosis Study. Circulation. 2005;112(24):3713–3721. doi: 10.1161/CIRCULATIONAHA.105.559633. [DOI] [PubMed] [Google Scholar]
- 29.Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health And Nutrition Examination Survey 1999–2002. Diabetes Care. 2006;29(6):1263–1268. doi: 10.2337/dc06-0062. [DOI] [PubMed] [Google Scholar]
- 30.Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. Jama. 2003;290(14):1884–1890. doi: 10.1001/jama.290.14.1884. [DOI] [PubMed] [Google Scholar]
- 31.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics-2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 32.Yeboah J, McClelland RL, Polonsky TS, et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308(8):788–795. doi: 10.1001/jama.2012.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Law MR, Wald NJ, Morris JK, Jordan RE. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003;326(7404):1427. doi: 10.1136/bmj.326.7404.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335(14):1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 35.Jones PH, Davidson MH, Stein EA, et al. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial) Am J Cardiol. 2003;92(2):152–160. doi: 10.1016/s0002-9149(03)00530-7. [DOI] [PubMed] [Google Scholar]
- 36.Knopp RH. Drug treatment of lipid disorders. N Engl J Med. 1999;341(7):498–511. doi: 10.1056/NEJM199908123410707. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.