Abstract
Medications to treat cognitive disorders are increasingly needed, yet researchers have had few successes in this challenging arena. Cognitive abilities in primates arise from highly evolved N-methyl-d-aspartate (NMDA) receptor circuits in layer III of the dorsolateral prefrontal cortex. These circuits have unique modulatory needs that can differ from the layer V neurons that predominate in rodents, but they offer multiple therapeutic targets. Cognitive improvement often requires low doses that enhance the pattern of information held in working memory, whereas higher doses can produce nonspecific changes that obscure information. Identifying appropriate doses for clinical trials may be helped by assessments in monkeys and by flexible, individualized dose designs. The use of guanfacine (Intuniv) for prefrontal cortical disorders was based on research in monkeys, supporting this approach. Coupling our knowledge of higher primate circuits with the powerful methods now available in drug design will help create effective treatments for cognitive disorders.
Keywords: schizophrenia, Alzheimer’s disease, acetylcholine, dopamine, norepinephrine
Cognitive disorders are a particular liability in the information age, as effective executive functioning, synthetic capacities, and insightful reasoning are needed to steer through complicated and constant stimulation. Thus, whereas inherent traits of distractibility may have been an advantage in earlier epochs, they are often diagnosed as an attention disorder [attention deficit hyperactivity disorder (ADHD)] in our modern culture. More pointedly, diseases that erode higher reasoning and insight, such as schizophrenia and Alzheimer’s disease (AD), are a profound societal burden, as patients are often unable to care for themselves but may not have the cognitive capacity to realize that they have medical needs. These diseases are particularly tragic because they destroy the person themselves and wreak emotional havoc on the families trying to care for them. Although cognitive disorders are an increasing burden, no truly effective treatments exist. Worse still, many pharmaceutical companies are giving up on the neuroscience arena, given its complexity, expense, and the many failures in translation from preclinical models to clinical success. Some of these failures may arise from a limited understanding of the unique molecular needs of the primate association cortex.
Higher cognitive disorders in humans afflict the association cortices in particular, specifically targeting the most highly evolved pyramidal cell circuits with the most extensive network connections (1–4). As described below, these higher cortical circuits are regulated in a fundamentally different manner from older, sensory-motor cortical and subcortical circuits (5) and thus are difficult to study in standard rodent models, whose brains have very little association cortex (6). This is a particular challenge for pharmaceutical development, in which drug screening is performed traditionally in rodent models. However, nonhuman primates have highly developed association cortices that share many similarities to humans. Thus, guiding drug development with knowledge gained from nonhuman primate research may provide a key bridge in identifying appropriate mechanisms, molecular candidates, and dose ranges for cognitive disorders in humans. This review highlights some of the lessons learned from primate research in creating cognitive enhancers that have translated to human use, as well as some of the many remaining challenges for the field.
COGNITIVE DISORDERS IN HUMANS TARGET THE DORSOLATERAL PREFRONTAL CORTEX
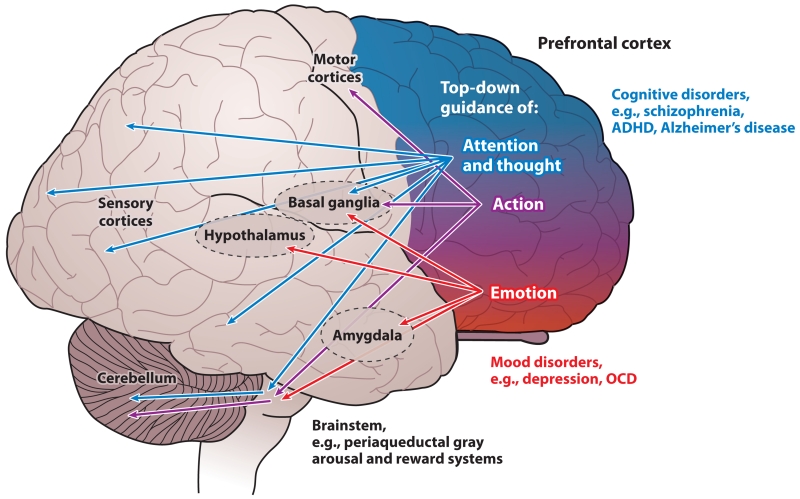
The prefrontal cortex (PFC) composes a third of the human cerebral cortex and is central to conscious cognitive experience and most cognitive disorders in humans (7). The PFC generates mental representations in the absence of sensory stimulation, the foundation of abstract thought (8). This fundamental property underlies the PFC’s involvement in working memory, higher reasoning, decision making, insight, and a variety of so-called executive functions, including regulation of attention, planning, and organizing for the future (7, 9–11). The PFC provides top-down guidance of thought, action, and emotion and does so in a topographically organized manner whereby (in very simple terms) the lateral surface represents the external world, whereas the medial or ventral areas represent our internal, visceral world and emotion (12, 13) (Figure 1). For example, neurons in the dorsolateral PFC (dlPFC) generate persistent representations of visual space (14), and neurons in dorsomedial PFC generate persistent representations of punishment (15). The topographic organization of the PFC is reflected in its connections: The lateral areas have reciprocal projections with visual, auditory, and somatosensory association cortices, and the ventral and medial PFC regions interconnect with olfactory-taste circuitry, insular cortex, and limbic brain areas (12, 13). This topography extends to basal ganglia circuits, whereby the dlPFC projects to the dorsal striatum (caudate), and the ventromedial PFC projects to the ventral striatum. Through all these connections, the PFC is positioned to provide top-down regulation, promoting or arresting inappropriate thoughts, actions, and emotions. The PFC may also be organized in a caudal to rostral manner, such that more rostral areas of the PFC process increasingly more abstract information (16); the most rostral areas are involved in metacognition or “thinking about thinking” (17, 18). The rostral and dorsomedial aspects of the PFC also play important roles in social cognition, including so-called theory of mind, the ability to think about what another person must be thinking (19, 20). Finally, the PFC in humans also appears to be lateralized; the left hemisphere specializes in generative processes (21), whereas the right hemisphere is specialized for inhibiting inappropriate actions, thoughts and emotions (22).
Figure 1.
Topographic organization of PFC functions in primates. The primate PFC provides top-down guidance of attention and thought (blue), action (purple), and emotion (red) through its extensive projections. In general, the PFC is organized topographically, with dorsal and lateral regions regulating thought and attention and ventral and medial regions regulating emotion. This organization is reflected in the PFC projections through the basal ganglia via the dorsal and ventral striatum. There are also parallel projections to the cerebellum via the pontine nuclei. The human brain is lateralized, with the left hemisphere specialized for language and the right hemisphere specialized for inhibition of inappropriate thoughts, actions, and emotions. Dysfunction of the dorsal and lateral PFC is associated with cognitive disorders, whereas more ventral and medial areas are altered in affective disorders. Abbreviations: ADHD, attention deficit hyperactivity disorder; OCD, obsessive-compulsive disorder; PFC, prefrontal cortex.
The topography of the PFC is consistent with the pattern of its dysfunction in clinical disorders (Figure 1). Thus, dysfunction of the lateral PFC results in cognitive disorders such as schizophrenia (4) and AD (2), whereas dysfunction of the ventral and/or medial PFC is associated with mood disorders such as depression (23, 24) or obsessive-compulsive disorder (OCD) (25). Disorders of impulse control have altered function of the right PFC; the right, inferior lateral PFC develops abnormally in ADHD (26), and this same area is hypoactive during the manic phases of bipolar disorder (27). Researchers have also related dysfunction of the PFC to the deficits in social cognition that typify autism spectrum disorders, including theory of mind (28). Although a large body of work links PFC insults to these prevalent mental disorders, the neurobiology of the PFC is rarely taught in medical school curricula, and thus physicians often do not have a sufficient scientific background to appreciate the underlying neuropathology of cognitive disorders and the rationale for treatments.
A closer examination of the neural circuitry within the dlPFC has found that deep layer III pyramidal cells are key for the mental representations that subserve higher cognitive processing (29) (Figure 2). Interestingly, this is the layer and the neurons that expand most in brain evolution (30–33). Rodents not only have a small PFC, but a very sparse layer III (13, 33). There is a very large increase in the number of pyramidal cells in deep layer III in primates, needed to represent a vast mental repertoire. There is also a great expansion in the number of basal dendrites and spines on these layer III pyramidal cells, allowing an immense increase in the number of network connections (30–32). For example, layer III pyramidal cells in monkey dlPFC have about twice as many spines as layer III pyramidal cells in primary visual cortex (V1) (34, 35). Pyramidal cell connections in layer III of dlPFC are made on long, thin spines and have a stable morphology [e.g., a well-developed synapse and spine apparatus (5, 36), which contrasts with thin, so-called learning spines in hippocampus that do not have these features until they enlarge into mushroom spines (37)]. The long, thin geometry of many dlPFC layer III spines may optimize the ability to gate connections, as described below, a key aspect of cognitive strength (5).
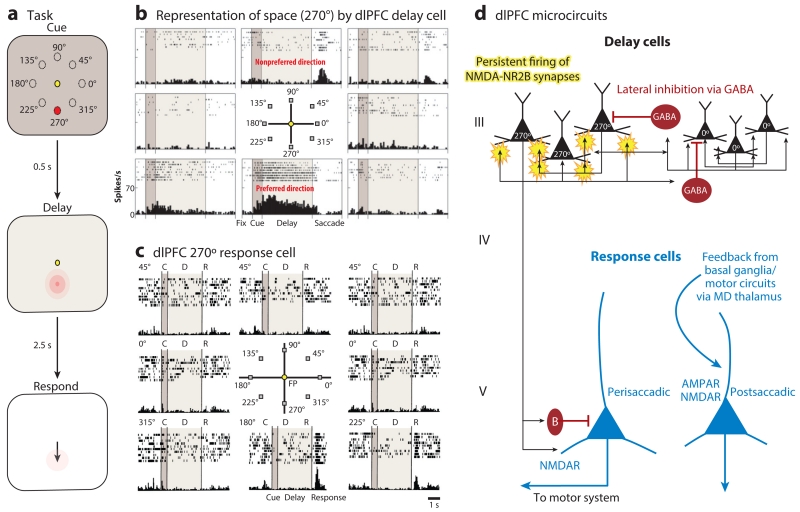
Figure 2.
The delay cells in layer III microcircuits in the dlPFC underlying spatial working memory. (a) Schematic illustration of the ODR task, in which a cue appears briefly (0.5 s) at one of eight locations (e.g., 270°) while the monkey fixates on a central spot. The location must be remembered over a delay period of several seconds until the fixation spot disappears and the monkey can move its eyes to the remembered location for a juice reward. The cued location constantly changes over hundreds of trials, requiring the constant updating of working memory. (b) An example of the firing patterns of a dlPFC delay cell representing the 270° location, the neuron’s preferred direction. This delay cell maintains firing across the delay epoch if the cue had appeared at 270° but not other locations. (c) An example of the firing patterns of a dlPFC response cell that is providing feedback during the eye movement to 270°. Response cells are often inhibited during the delay epoch. (d) The microcircuitry in the primate dlPFC thought to underlie working memory. Microcircuits underlying delay cell firing are thought to reside in deep layer III, the layer that expanded most in primate evolution. Clusters of pyramidal cells with similar preferred directions excite each other to maintain persistent firing across the delay period in the absence of sensory stimulation. This requires glutamate stimulation of NMDAR-NR2B. In contrast, the spatial specificity is refined by lateral inhibition from parvalbumin-containing GABAergic interneurons. In contrast, response cells are thought to reside in layer V. Perisaccadic response cells fire leading up to the motor response, and postsaccadic response cells are thought to carry the corollary discharge feedback that a response has occurred. The postsaccadic response cells are influenced by both NMDAR and AMPAR stimulation. Delay cells likely inhibit response cells during the delay epoch via an inhibitory interneuron. Abbreviations: AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; dlPFC, dorsolateral prefrontal cortex; MD, mediodorsal; NMDA, N-methyl-d-aspartate; NMDAR, NMDA receptor; ODR, oculomotor delayed response.
The pioneering research of Goldman-Rakic (38) revealed the physiology and microcircuitry in the primate dlPFC that underlie spatial working memory and likely apply to other processing domains. Goldman-Rakic and colleagues (14) discovered neurons in the dlPFC that can generate neural representations of visual space in the absence of sensory stimulation, e.g., maintaining firing to the memory of a flash of light at 90° (the preferred direction of the neuron) but not to other spatial locations (nonpreferred directions) (Figure 2b). These neurons are termed delay cells, as they are able to maintain persistent, spatially tuned firing across the delay epoch in a spatial working memory task. Delay cell persistent firing is generated by the recurrent excitation of pyramidal cells with similar spatial tuning (38). These microcircuits are localized in deep layer III, and possibly superficial layer V, of the primate dlPFC (Figure 2d). Delay cells excite each other through N-methyl-d-aspartate (NMDA) receptor (NMDAR) glutamatergic synapses on dendritic spines (39). Both NMDAR with NR2A or NR2B subunits are needed for persistent firing, with only subtle reliance on AMPA receptor (AMPAR) stimulation (39). These findings contrast with classic synapses, e.g., in hippocampus or V1, where NMDAR-NR2Bs are extrasynaptic and AMPAR stimulation is essential for permitting NMDAR actions (40). The spatial tuning of dlPFC delay cells is refined through lateral inhibition from parvalbumin-containing GABA interneurons (38). This also differs from more classic circuits, e.g., in V1, where there is feedforward rather than lateral inhibition (41).
The dlPFC also contains neurons that fire in relationship to the eye movement response, i.e., so-called response cells (Figure 2c). These include neurons that fire immediately prior to the movement and are likely conveying commands to the motor system, as well as neurons that fire during or after the movement and are likely conveying feedback that the response has occurred (corollary discharge) (42). These latter cells may be especially relevant to the symptoms of hallucinations and delusions in schizophrenia, which have been associated with impaired corollary discharge (43). Response cells are likely concentrated in layer V of the dlPFC (39) and appear to be the type of neuron most common in rodent PFC (44) (Figure 2d). Both types of response cells depend on NMDAR stimulation in primate dlPFC, but feedback response cells are also sensitive to AMPAR blockade, perhaps because they are part of a proprioceptive-like sensory circuit (39).
The pyramidal cell networks of the dlPFC are the focus of pathology in both schizophrenia and AD. Postmortem studies of patients with schizophrenia have identified reduced neuropil (45, 46) and loss of dendrites and spines from deep layer III pyramidal cells (47); layer V pyramidal cells appear to be impacted as well (48). GABAergic parvalbumin interneurons also show signs of compensatory weakening (49). Although previous findings from NMDAR antagonist studies in rodents have suggested that pyramidal cells might be disinhibited in schizophrenia and produce a hyperglutamatergic state, recent transcriptome analyses from actual layer III and V pyramidal cells in the dlPFC of patients with schizophrenia show the opposite: Layer III and V dlPFC pyramidal cells in schizophrenia are profoundly underactive, as indicated by mitochondrial markers (4). These new data from patients should have a major impact on drug discovery, emphasizing that the goal of treatment is not to reduce PFC glutamate release but rather to boost the information-processing abilities of NMDAR circuits.
In contrast to schizophrenia, in which dendrites are lost but cell bodies remain, layer III and V dlPFC pyramidal cells degenerate in AD (2). These cells, but not their nearby neighbors, fill with neurofibrillary tangles and die. This degenerative process is also observed in other association cortices that are tightly interconnected with the dlPFC and the entorhinal cortex, which dies early in the disease. AD attacks pyramidal cells with the richest connections but leaves the primary sensory cortices relatively spared, an important clue regarding its etiology (1, 2, 50–52).
COGNITIVE ENHANCERS MUST ENHANCE THE PATTERN OF INFORMATION GENERATED BY HIGHER CORTICAL CIRCUITS
Higher cognitive processes arise from an ever-changing pattern of excitation in complex cortical circuits, creating our mental sketchpad (5). Thus, the challenge for developing effective cognitive enhancers for human use is to learn how to boost the pattern of information represented in these higher cortical circuits by understanding and optimizing their modulatory needs. Enhancement is usually most successful with low-dose drug treatment, in which signals are boosted without generalized effects on neuronal firing. This differs substantially from classic medications, in which higher doses are more effectual as long as there is an acceptable side-effect profile.
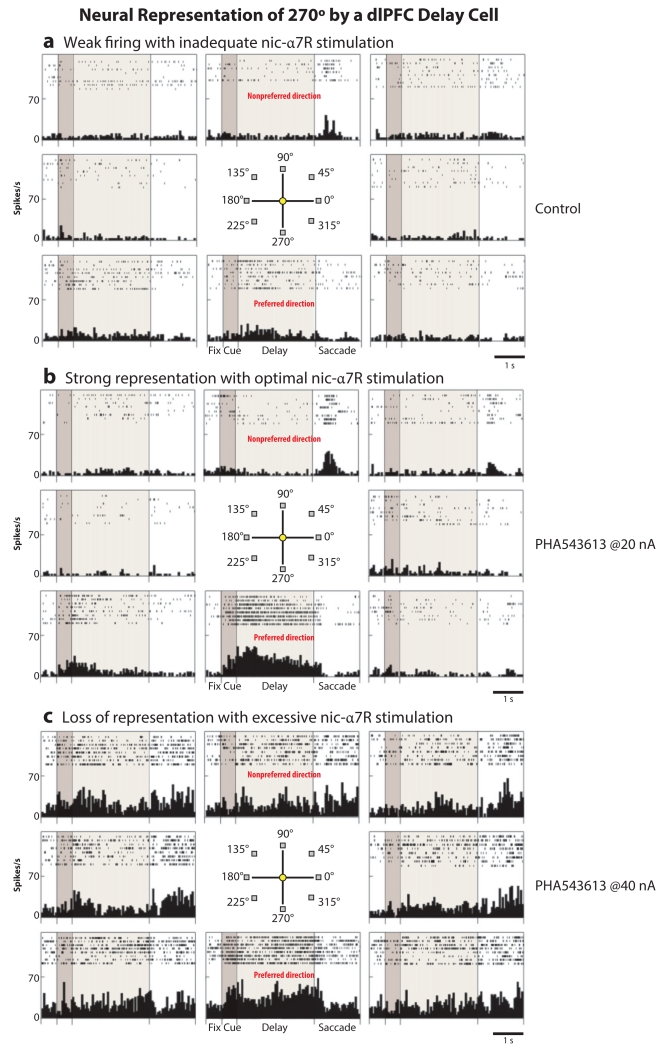
The effects of nicotinic α7 receptor (nic-α7R) stimulation on delay cell firing provide a clear illustration of the importance of correct dosage for enhancing the neural pattern of information in dlPFC circuits. As described above, AMPAR blockade has very subtle effects on delay cell firing, suggesting that additional mechanisms provide the permissive membrane depolarization needed for NMDAR actions. Immuno-electron microscopy data have shown that nic-α7Rs are localized within the postsynaptic density (PSD) of glutamate synapses in layer III of the dlPFC, and physiological data indicate that nic-α7Rs are necessary for NMDAR actions and delay cell firing (53). Thus, blockade of nic-α7Rs markedly reduces delay cell firing, and NMDA is unable to excite delay cells when nic-α7Rs are blocked (53). Iontophoresis of a nic-α7R agonist onto delay cells shows dose-related actions, as shown in Figure 3. Low doses of a nic-α7R agonist enhance the spatially tuned, persistent firing of delay cells, increasing firing for the neuron’s preferred direction but not altering firing for its nonpreferred directions (Figure 3b). However, higher doses of a nic-α7R agonist produce nonspecific increases in neuronal firing, with the neuron increasing its firing to both preferred and nonpreferred directions, thus obscuring the pattern of information (Figure 3c).
Figure 3.
Neural representation of 270° by a dlPFC delay cell. Low, but not high, doses of a nic-α7R agonist enhance the neural representation of visual space by dlPFC delay cells. (a) A dlPFC delay cell has weak, delay-related firing under control conditions, with subtle representation of 270°. (b) Iontophoresis of a low dose of the nic-α7R agonist PHA543613 (at 20 nA) enhances the representation of 270° by increasing persistent firing across the delay period only in trials in which the monkey is remembering 270°. (c) A higher dose of PHA543613 (at 40 nA) increases persistent firing for all directions, thus eroding the information held in working memory stores. Thus, low doses are often essential to be effective cognitive enhancers. Abbreviations: dlPFC, dorsolateral prefrontal cortex; nic-α7R, nicotinic α7 receptor.
This loss of enhancement at higher doses provides several lessons for the successful development of cognitive enhancers. First, low-affinity agonists are needed that better mimic the gentle actions of the native transmitter. This will also minimize receptor desensitization, a problem with several receptor systems [e.g., dopamine (DA) D1 receptors (D1Rs)]. Second, the physiological data shown in Figure 3 caution that finding an effective dose range for cognitive enhancement may require a different approach. If no effect is seen with a drug, it is important to test whether lowering the dose will reveal an enhancing dose range, as excessive doses can have no beneficial effects on cognition even when there are no obvious side effects. This is counterintuitive to typical dosing strategies, in which doses are increased until an effect is seen. Identifying an appropriate dose range is often the greatest challenge in developing compounds that strengthen cognition, a topic discussed more below.
NEWLY EVOLVED PREFRONTAL CIRCUITS ARE MODULATED DIFFERENTLY FROM OLDER CIRCUITS: DYNAMIC NETWORK CONNECTIVITY
The pyramidal cell circuits in deep layer III of the primate dlPFC are modulated differently from classic circuits in the hippocampus and V1 (5) and even differently from response cells within the dlPFC (39). Research is uncovering a variety of molecular mechanisms in layer III pyramidal cells that can rapidly and reversibly alter the strength of synaptic connections, a process termed dynamic network connectivity (DNC) (5, 54). DNC mechanisms promote mental flexibility and coordinate arousal state with cognitive state. For example, the important permissive role of nic-α7Rs for NMDAR actions described above allows dlPFC neurons to connect when we are awake and acetylcholine is released but not when we are in deep sleep and there is no acetylcholine release (55). Thus, conscious cognitive experience may be partly a function of cholinergic stimulation of nic-α7Rs in the dlPFC and interconnected association cortices (53).
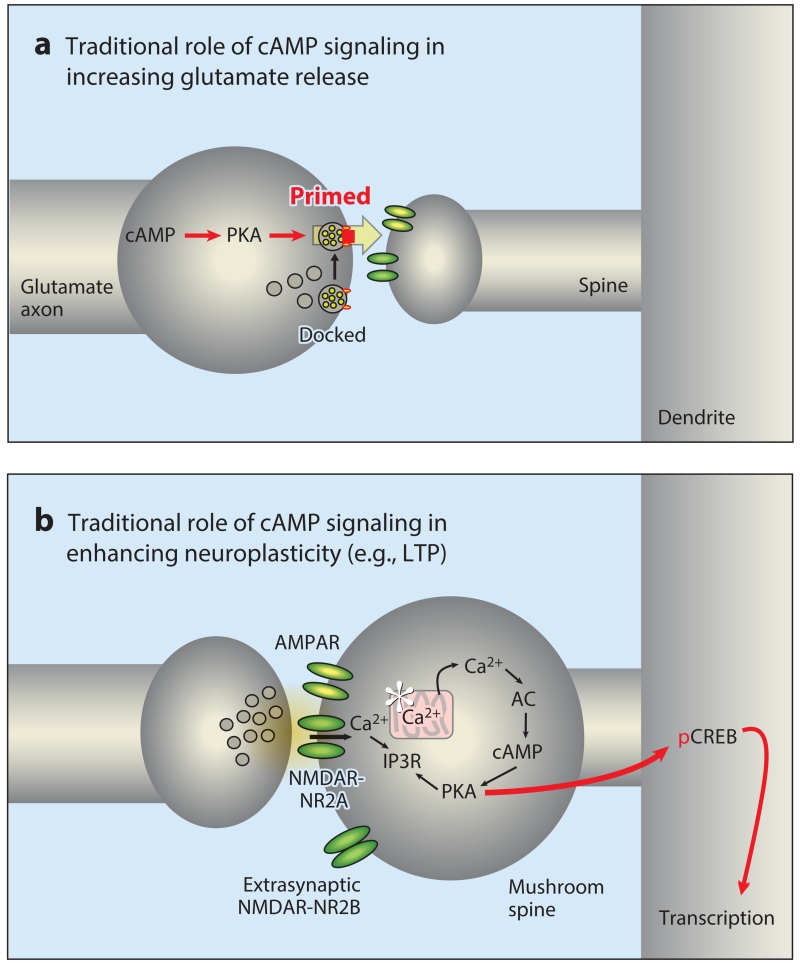
DNC mechanisms also include powerful, feedforward cyclic adenosine monophosphate (cAMP)-calcium signaling actions that rapidly weaken connectivity, opposite to the traditional effects of cAMP signaling in classic circuits (5). Decades of research on hippocampal and sensory circuits have found that increased cAMP–protein kinase A (PKA) and calcium-PKC signaling enhances long-term plasticity, e.g., increasing long-term potentiation or enlarging a mushroom-shaped spine (56, 57) (Figure 4b). These signaling pathways also increase glutamate release from presynaptic terminals, e.g., by priming vesicles for release (58) (Figure 4a). Thus, traditional research on phylogenetically older neural circuits generally finds enhancing effects of calcium-cAMP signaling. In contrast, feedforward calcium-cAMP signaling in newly evolved layer III dlPFC circuits rapidly weakens connections by opening potassium (K+) channels in spines (summarized in Figure 5). For example, the open state of HCN and KCNQ channels is increased by cAMP (59) and PKA (60), respectively, and both channels are concentrated in layer III spines near the synapse and in the spine neck (5, 61). Many thin spines in layer III of the dlPFC contain a spine apparatus, the extension of the smooth endoplasmic reticulum into the spine, that stores and releases calcium (5). cAMP-PKA signaling proteins are anchored near the spine apparatus, where they are positioned to enhance IP3 receptor calcium release (36); increased calcium release can further increase the production of cAMP and thus promote feedforward signaling and the rapid opening of nearby K+ channels. Conversely, the phosphodiesterase PDE4A is anchored near the spine apparatus by Disrupted In SChizophrenia 1 (DISC1) (5, 36, 61), positioned to catabolize cAMP and hold feedforward cAMP-calcium signaling in check. Calcium-cAMP-PKA opening of nearby K+ channels near the synapse contributes to numerous important cellular functions. These actions provide negative feedback to prevent seizures within recurrent excitatory networks, e.g., by opening KCNQ channels. For example, metabotropic glutamate receptor 1a (mGluR1a) and mGluR5 are localized near the synapse (62), where they are positioned to activate feedforward calcium-cAMP-K+ channel signaling when glutamate overflow spills beyond the synapse. Calcium-cAMP opening of K+ channels on a discrete set of spines can also serve to sculpt the contents of working memory, gating out nonpreferred inputs. However, with very high levels of cAMP-calcium signaling, such as what occurs during uncontrollable stress, these actions can take the PFC offline rapidly, a mechanism that may have survival value during danger to switch control of behavior to more primitive, reflexive circuitry. For example, D1Rs are localized in layer III spines, both in the synapse itself (where they may enhance NMDAR insertion into the PSD) and next to the synapse (where they are colocalized with HCN channels) (reviewed in Reference 63). D1R generation of cAMP increases the open state of HCN channels, weakening a synaptic connection (64, 65). During optimal levels of DA release, D1R-HCN channel actions sculpt away noise, preferentially weakening the effects of nonpreferred inputs. However, at higher levels of D1R stimulation during uncontrollable stress, all inputs are weakened and the delay cells stop firing (63). High levels of noradrenergic (NE) α1-adrenoceptor (AR) stimulation also contribute to loss of delay cell firing, via increased calcium-PKC mechanisms (66). These built-in mechanisms to purposefully impair dlPFC function likely help to explain why this cortex dysfunctions so readily in so many different disorders, including those that are aggravated by stress exposure.
Figure 4.
The traditional roles of cAMP-PKA signaling in modulating neurotransmission and neuroplasticity. (a) cAMP-PKA signaling can enhance transmitter release (e.g., glutamate release) from the presynaptic terminal by priming vesicles for release. (b) cAMP-PKA signaling is needed for late-stage LTP in hippocampal neurons. These synapses generally contain AMPAR and NMDAR-NR2A subunits, whereas NMDAR-NR2Bs are found extrasynaptically. This process can involve internal calcium release from a spine apparatus in some synapses. Sufficient activation of PKA can lead to phosphorylation of CREB and transcriptional changes that can lead to spine enlargement (an immature, thin learning spine becomes a mushroom spine) and/or enlargement and stabilization of the PSD. Abbreviations: AC, adenylyl cyclase; AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; cAMP, cyclic adenosine monophosphate; CREB, cAMP response element–binding protein; IP3R, inositol 1,4,5-trisphosphate receptor; LTP, long-term potentiation; NMDA, N-methyl-d-aspartate; NMDAR, NMDA receptor; pCREB, phosphorylated CREB; PKA, protein kinase A; PSD, postsynaptic density.
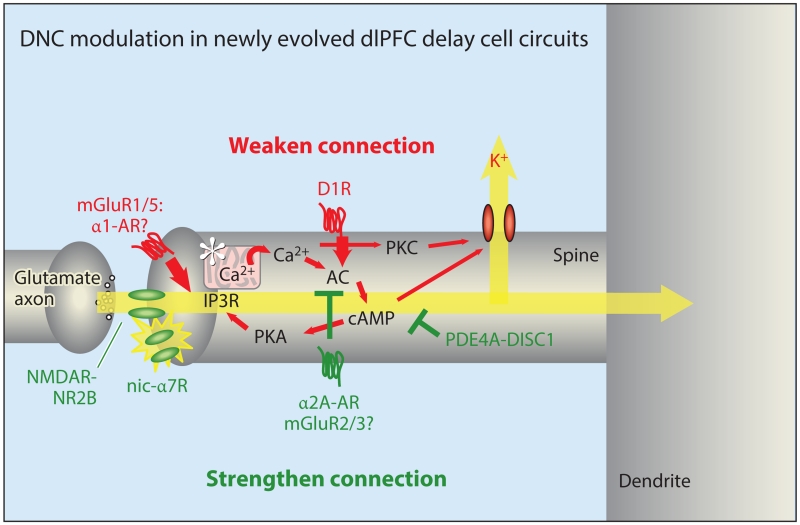
Figure 5.
DNC modulation in newly evolved dlPFC delay cell circuits. DNC in a mature, long, thin spine in layer III of the primate dlPFC alters synapse strength rapidly and reversibly to coordinate cognitive and arousal states. In contrast to traditional synapses, NMDAR-NR2Bs are localized exclusively in the PSD and are not extrasynaptic. These synapses have only a subtle AMPAR component, and the permissive excitation needed for NMDAR opening is instead mediated by cholinergic stimulation of nic-α7R (and possibly muscarinic M1R). Mechanisms that increase feedforward calcium-cAMP-PKA signaling (red, many engaged by stress) weaken synaptic connections by opening nearby K+ channels (HCN and KCNQ) on the spine head, neck, or both. In contrast, inhibiting feedforward calcium-cAMP-PKA signaling (green) strengthens connectivity and enhances delay cell firing. Loss of inhibition, e.g., through genetic insults to DISC1, may contribute to spine loss and impaired dlPFC function. The unique modulation of layer III dlPFC pyramidal cell synapses provides strategies for therapeutic targets for cognitive disorders. Abbreviations: AC, adenylyl cyclase; AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; AR, adrenergic receptor; cAMP, cyclic adenosine monophosphate; D1R, dopamine D1 receptor; DISC1, Disrupted In SChizophrenia 1; dlPFC, dorsolateral prefrontal cortex; DNC, dynamic network connectivity; HCN, hyperpolarization-activated cyclic nucleotide gated channels; IP3R, inositol 1,4,5-trisphosphate receptor; KCNQ, potassium channel, voltage-gated, KQT-like subfamily; M1R, muscarinic M1 receptor; mGluR, metabotropic glutamate receptor; nic-α7R, nicotinic α7 receptor; NMDA, N-methyl-d-aspartate; NMDAR, NMDA receptor; PDE4A, phosphodiesterase 4A; PKA/PKC, protein kinase A/C.
Response cells in the dlPFC are regulated differently from delay cells. For example, response cells are not altered by D1R stimulation but are altered by D2R actions, the exact opposite profile from delay cells (67). Postsaccadic response cells are very sensitive to AMPAR blockade, whereas delay cells show only subtle changes when AMPARs are blocked (39). Studies in progress indicate that delay cells and response cells respond very differently to mGluR2/3 stimulation, with delay cells showing enhanced firing consistent with postsynaptic actions and response cells showing reduced firing consistent with inhibition of presynaptic glutamate release (L.E. Jin, M. Wang & A.F.T. Arnsten, unpublished data). The findings in response cells are similar to what researchers have seen in rodent PFC, where mGluR2 stimulation decreases glutamate excitatory postsynaptic currents on layer V pyramidal cells (68) and cAMP-PKA signaling increases the release of glutamate and enhances the excitation of layer V cells (69). Studies of rodent PFC often focus on layer V pyramidal cells, as they are the numerous [layer V is more than twice the size of layer III in rodents, whereas layer III is more than two times larger than layer V in primates (70)], and the large size of layer V pyramidal cells enables recordings. However, hypotheses based solely on data from layer V in rodent PFC may be misleading, as these neurons are often modulated very differently from the delay cells that generate mental representations in primates.
THE α2A-ADRENOCEPTOR AGONIST GUANFACINE IMPROVES PREFRONTAL CORTICAL FUNCTION IN MONKEYS AND HUMANS: PROOF OF CONCEPT
The α2A-AR agonist guanfacine serves as an example of successful translation from research in animals to cognitive disorders in humans. Although the presynaptic roles of α2A-AR have been the focus of traditional research, the majority of α2A-ARs are actually postsynaptic (71). Norepinephrine has very a high affinity for α2A-AR compared to other adrenergic receptors, and data from monkeys suggest that these are the predominate adrenergic receptors engaged under optimal arousal conditions (72). In the primate dlPFC, α2A-ARs are colocalized with HCN channels in layer III spines near the synapse and in the spine neck (Figure 6a; 73). Stimulation of α2A-ARs, e.g., with the α2A-AR agonist guanfacine, specifically increases firing for the neuron’s preferred direction, thus enhancing mental representations (73) (Figure 6b). In contrast, blockade of α2A-ARs with the antagonist yohimbine causes a complete collapse of dlPFC network firing (74) that can be restored by blocking HCN channels (73). Similar effects are seen on cognitive behavior in monkeys, in which intra-dlPFC infusion of guanfacine improves working memory (75) and systemic administration of guanfacine improves a variety of PFC cognitive functions, including spatial working memory, reversal learning, behavioral inhibition, top-down regulation of attention, and rapid associative learning (reviewed in Reference 76). Importantly, guanfacine improves impulse control, allowing monkeys to inhibit responses to immediate, small rewards and instead be able to wait for a larger reward, a cognitive ability that is especially important for success in life (77). Conversely, infusion of yohimbine into the dlPFC impairs working memory (78) and impulse control (79) and induces locomotor hyperactivity (80). Thus, α2A-AR stimulation strengthens the efficacy of dlPFC connections, enhances delay cell representations, and allows top-down regulation of behavior.
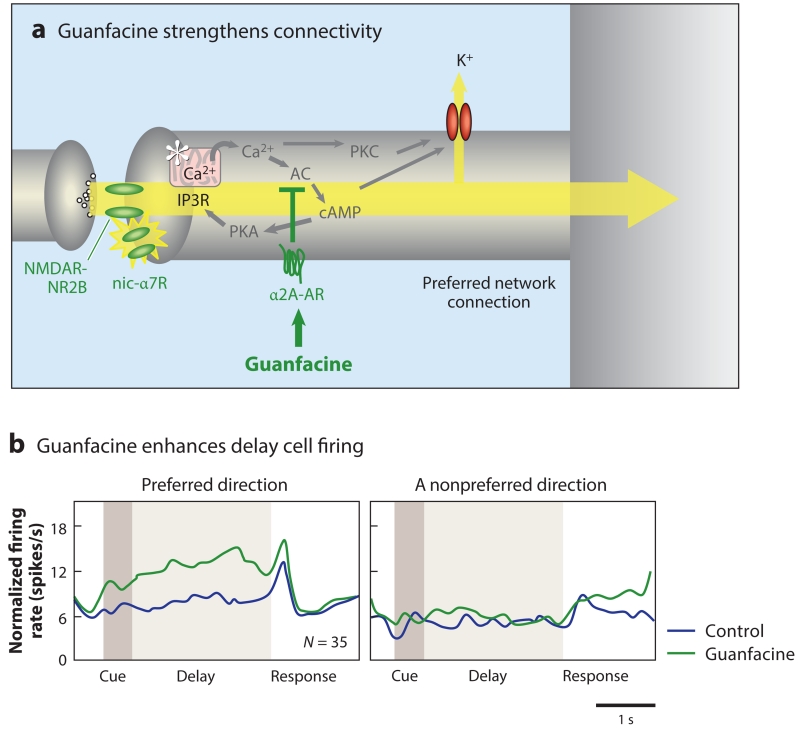
Figure 6.
The α2A-AR agonist guanfacine strengthens dlPFC network connections and enhances dlPFC network firing. (a) A schematic illustration of guanfacine’s actions in the primate dlPFC, engaging postsynaptic α2A-ARs to inhibit feedforward cAMP-calcium-K+ channel signaling and strengthen NMDAR connections. (b) Guanfacine increases the firing of delay cells for their preferred direction. The normalized mean firing rate of 35 delay cells under control conditions (blue) and following iontophoresis of guanfacine (green) are shown, as are the neurons’ preferred direction and their nonpreferred direction opposite to the preferred direction. Guanfacine improves a variety of PFC cognitive functions and is now in widespread clinical use. Abbreviations: AC, adenylyl cyclase; AR, adrenergic receptor; cAMP, cyclic adenosine monophosphate; dlPFC, dorsolateral PFC; IP3R, inositol 1,4,5-trisphosphate receptor; nic-α7R, nicotinic α7 receptor; NMDA, N-methyl-d-aspartate; NMDAR, NMDA receptor; PFC, prefrontal cortex; PKA/PKC, protein kinase A/C.
Studies in rodents have also shown beneficial actions of guanfacine. In vitro recordings from layer II/III pyramidal cells in PFC slices found results similar to monkeys, in which α2A-AR stimulation enhances persistent firing via HCN channel closure (81); however, recordings of layer V neurons show reduced presynaptic glutamate release (82, 83), again emphasizing the differences between PFC lamina. Studies in genetically altered mice found that a functional α2A-AR was needed for guanfacine to produce cognitive improvement (84). However, the enhancing effects on working memory in mice were much less robust than those in monkeys, consistent with the very small number of layer II/III PFC neurons in mice. Recent studies in rats indicate that systemic administration of guanfacine can protect PFC layer II/III dendritic spine loss and cognitive abilities from the detrimental effects of oxygen deprivation (85) or chronic restraint stress (86). Repeated guanfacine administration may actually increase the number of spines on layer II/III neurons under basal conditions (86) and enhance PFC spine maturation in vitro (87). Guanfacine’s protection of spines from chronic stressors may involve several mechanisms, including reducing stress-induced catecholamine release, strengthening PFC connections (as described above), and reducing inflammation by deactivating activated microglia (88).
Based on this research in animals, guanfacine is now being used to treat a variety of PFC disorders in humans. An extended release formulation was approved for the treatment of pediatric ADHD (Intuniv) (89), and it is also commonly used for other pediatric disorders that benefit from stronger PFC function, including the treatment of Tourette’s syndrome (90); autism spectrum illness (91); and emotional trauma, including physical abuse and neglect (92, 93). In adults, guanfacine is being tested or used to treat traumatic brain injury that involves the frontal lobe (94) and to aid top-down control in the treatment of substance abuse (95, 96). Researchers are beginning test it in prodromal schizophrenia, with the hope that it may protect the PFC from the wave of gray matter loss and inflammation that heralds the descent into illness (97).
Several factors facilitated guanfacine’s translation from animals to humans. Extensive guanfacine dose/response curves were available from both young and aged monkeys that allowed identification of a potential dose range (98–100), and indeed, the doses that improved the young monkeys were similar to those used to treat children and young adults with ADHD (100). Guanfacine had also been approved for use in humans as an antihypertensive, thus allowing open-label explorations of effective doses in patients (101, 102). Although this type of exploration is not possible with new compounds, more extensive Phase II testing might achieve similar goals. The research in monkeys and open-label trials also helped to identify the most appropriate methods for easing side effects, by slowly ramping up the dose and allowing subjects to develop tolerance to guanfacine’s sedative actions. This knowledge facilitated the success of the more expensive Phase III trials.
POTENTIAL THERAPEUTIC TARGETS BASED ON DYNAMIC NETWORK CONNECTIVITY
Research on the DNC mechanisms modulating dlPFC delay cells encourages the exploration of several potential therapeutic targets for cognitive disorders in humans; some such studies are already in progress. There is existing interest in nic-α7R agonists for the treatment of schizophrenia (103) and ADHD (104, 105), and the data from monkeys encourage this approach. Schizophrenia has been linked to nic-α7R deficits for many years (103), and the more recent appreciation of NMDAR deficits (106–108) in this illness as well emphasizes the insults to fundamental communication within dlPFC delay cell networks. Most patients with schizophrenia smoke and describe the restorative effects of smoking (109, 110). Thus, nic-α7Rs appear to be an especially important target for this illness (111). The development of nic-α7R agonists has been hampered by the rapid desensitization of these receptors following stimulation and by untoward drug actions in peripheral tissues (112). Very low doses may help to solve all these problems by minimizing desensitization and preferentially altering very sensitive mechanisms in the PFC, where low doses can boost information representation in neural circuits. However, it is important to note that nic-α7R agonists may not be appropriate for cognitive disorders in the aged, as evidence from mouse models of AD suggests that nic-α7R stimulation can cause NMDAR internalization in the presence of beta amyloid (Aβ) oligomers (113).
Additional, cholinergic targets may exist as well, as muscarinic M1Rs are also localized in the PSD of glutamate-like synapses on layer III dlPFC spines (114). Research is needed to learn about their contribution to PFC function, but preliminary data are encouraging. However, selectivity for M1R would be essential, as stimulation of other muscarinic receptors can produce prominent side effects; for example, M2R stimulation slows the heart (115). As described above, a low-affinity but highly selective D1R agonist is needed, as DA’s beneficial effects at this receptor disappear with high levels of stimulation (63). Preliminary data also suggest that low doses of highly selective mGluR2/3 agonists may be useful, as these receptors are prominently localized in spines in layer III of dlPFC, and low doses enhance delay cell firing and improve working memory (L.E. Jin, M. Wang & A.F.T. Arnsten, unpublished data; Figure 5). In contrast, postsynaptic mGluR1/5 stimulation appears to reduce dlPFC delay cell firing, perhaps by increasing feedforward calcium-cAMP signaling. Thus, an agonist must be highly selective for mGluR2/3 over mGluR1/5. This research is still in progress but suggests that the roles and prevalence of mGluR2/3 may have expanded along with the evolution of layer III PFC circuitry. Finally, research could build on the success of guanfacine, designing compounds that preferentially target the configuration of the α2A-AR in its postsynaptic site in the PFC, compared to presynaptic sites. The differences in potency between clonidine and guanfacine at pre- versus postsynaptic sites suggest that this may be possible (116). Although intracellular sites appear attractive, these may likely be both too powerful and too universal, interfering with cellular functions in other circuits. The exception may be disorders in which a pathway is known to be overactive (e.g., PKC signaling in bipolar disorder) and the intracellular action would normalize signaling (e.g., lithium, tamoxifen) (117, 118).
Subtle but important differences between agonists and positive allosteric modulators (PAMs) may also be relevant to drug development for cognitive disorders. PAMS may be able to exaggerate a natural signal and thus facilitate the pattern of information processing, whereas agonists may stimulate receptors more broadly and thus obscure information more readily. However, there are likely important exceptions to this idea, e.g., conditions when the endogenous transmitter is depleted or underactive and unable to provide an endogenous signal, or receptors that are out of reach of a transmitter under normal conditions but have a therapeutic action when engaged. In these cases, an agonist may be more effective than a PAM. Finally, researchers have already shown blockade of detrimental receptor actions in the PFC, such as during stress, to be an effective strategy. For example, the α1-AR antagonist prazosin is in widespread use for the treatment of post-traumatic stress disorder (PTSD) based in part on work in animals (66, 93, 119–121).
CHALLENGES FOR DRUG DEVELOPMENT
Many hurdles must be overcome in the successful development of cognitive enhancers. As the dlPFC is often modulated in a manner opposite to sensory cortex and subcortical structures, it is important to find molecular strategies that can enhance PFC function without interfering with these other circuits. This can sometimes be accomplished by using very low doses, as the PFC appears to be more sensitive than other structures. An exception may be in a disorder such as PTSD, in which global actions are beneficial to simultaneously strengthen dlPFC and weaken amygdala, and thus global actions are useful.
As described above, a major challenge in translating cognition-enhancing drug actions from animals to humans is identifying the appropriate dose range. This is complicated both by narrow, inverted, U-shaped dose response curves, in which there is loss of efficacy when the dose is a little too high, and by the typical variability in optimal dosages between individuals, such as because of variations in levels of the endogenous transmitter. The use of monkeys to identify an approximate dose range prior to Phase II testing in humans may be helpful in facilitating this process. Although monkey research is expensive, human research is more so, so it may actually be considered a cost-effective approach. Similarly, expanding dose-finding in Phase II (especially for low doses) and creating experimental designs that allow optimal individual dosing may facilitate success in this complex space.
IMPORTANT ARENAS FOR FUTURE RESEARCH
Many key areas for future research on the primate cortex would facilitate the development of informed treatments for mental disorders. For example, although we have learned a lot about the molecular needs of the dlPFC, investigators have conducted no or very little research on the needs of other cortical areas that may be modulated in unique ways. Indeed, the gap between molecular biology and studies of the primate cortex is growing, as studies of the primate cortex are generally performed by researchers who have little background or interest in pharmacology or molecular biology, whereas molecular biology is focused on mouse models and cell cultures for which genetic tools can be used. Thus, scientists know almost nothing about the modulation of most of the primate cortex. Research on other cortical areas that have immediate clinical relevance will be especially important, e.g., the subgenual cortex (Brodmann area 25) in regard to depression, the orbital PFC in regard to disorders such as OCD, and the entorhinal cortex and parietal cortices that are afflicted in AD. Some of this work has begun in the marmoset orbital PFC (122, 123), and there have been a few studies in the ventrolateral PFC (124, 125) and the frontal eye fields (126), which are adjacent to the dlPFC and may share mechanistic similarities. However, most of the cortex remains unexplored. It would be particularly interesting to examine the molecular regulation of the primate subgenual cortex (Brodmann area 25), given its enriched serotonin innervation, role as a visceromotor center, and immediate relevance to depression (13, 23, 24). Although mood and cognitive disorders are generally discussed as separate entities, the representation of emotion by medial PFC circuits shares similarities with the representation of visual information by the lateral PFC (127).
A major need for future research is to learn what causes the neurodegeneration of higher cortical circuits in schizophrenia [in which there is loss of dendrites and spines (47)] and in AD [in which pyramidal cells fill with neurofibrillary tangles and die (2)]. In schizophrenia, waves of gray matter loss in the PFC herald the descent into illness (97, 128). What causes this loss of dendritic spines and how does it relate to the normal pruning process and to stress-induced loss of PFC spines? How do increases in feedforward calcium-cAMP signaling during stress lead to increased inflammation and spine loss? Clues from rodent studies suggest that chronic stress may both initiate mechanisms that actively destabilize spines and inhibit new spine formation; for example, calcium-PKC phosphorylation of myristoylated, alanine-rich C-kinase substrate can destabilize the actin cytoskeleton (129, 130), and cAMP-PKA signaling can activate REDD1 and inhibit spinogenesis via mTor signaling (86, 131, 132) or induce constitutive increases in Rac1 signaling (133) that are associated with spine loss (134). But this information is sketchy and hard to study, as it cannot really be replicated in neuronal cultures, which do not mimic the mature PFC. Furthermore, researchers do not know how stress signaling events might interact with the molecular mechanisms involved in healthy spine pruning that begins in teen years, nor how all these events interact with the genetics of schizophrenia. For example, evidence suggests that loss of DISC1 leads to spine loss in rodent PFC when coupled with NMDAR blockade (135). These data suggest that agents that strengthen NMDAR network connections in dlPFC might prevent spine loss, e.g., stimulating nic-α7R or α2A-AR might be protective. The evidence that α2A-AR stimulation protects spines from chronic stress in rat PFC supports this possibility (86).
Our growing understanding of the unique regulation of higher cortical pyramidal cells may also help us understand the origins of degeneration in AD. Although much of the focus on drug development has been aimed at Aβ, and most recently at toxic Aβ oligomers, the work of Braak and colleagues (52) shows the accumulation of phosphorylated tau (pTau) in vulnerable cells quite early in life, and an interaction of Aβ with pTau in the aging association cortices likely contributes to AD pathology in the majority of patients who evidence the disease in advanced age. Recent data indicate that the rhesus monkey shows predegenerative patterns similar to the human association cortex, with pTau building in the dlPFC pyramidal cells with the most cortical-cortical connections (36). Specifically, PDE4A is lost and pTau increases correspondingly with advancing age in the spines of layer III dlPFC pyramidal cells but not in V1. Researchers hypothesize that the greater number of spines and connections in the human association cortex drives this process over the threshold into tangle formation and cellular degeneration (36). The accumulation of pTau on microtubules in distal dendrites suggests there may be a vicious cycle in which pTau traps amyloid precursor protein in beta secretase–containing endosomes and increases the production of Aβ, while Aβ stimulates mGluR5 to increase the production of pTau (36), including generating an inflammatory response that may activate MAPK-activated protein kinase 2 signaling to unanchor PDE4A from DISC1 (136). Interrupting this cycle in middle age may provide opportunities for AD prevention.
It is increasingly evident that treatment must begin early in both AD and schizophrenia to protect the association cortices from degeneration and that treatments initiated later in illness may be too late to be effective. Thus, we must understand what is causing these degenerative processes. Research in monkeys may be helpful in viewing the molecular processes that weaken association cortical circuits. As the dlPFC has built-in mechanisms to take its circuits offline, dysregulation of these events may contribute to their susceptibility to degeneration (5). Recognizing the unique regulation and vulnerabilities of the primate association cortices may help guide future research in protecting higher cognitive functions.
Acknowledgments
A.F.T.A. and M.W. are funded by PHS Pioneer Award DP1AG047744-01, R01AG043430-01A1, R01MH100064-01A1, and R01MH09335401A1 from the US National Institutes of Health.
Footnotes
DISCLOSURE STATEMENT
Yale University and A.F.T.A. receive royalties from the sales of extended-release guanfacine (Intuniv) in the United States from Shire Pharmaceuticals but not from sales of generic or immediate-release guanfacine.
LITERATURE CITED
- 1.Lewis DA, Campbell MJ, Terry RD, Morrison JH. Laminar and regional distributions of neurofibrillary tangles and neuritic plaques in Alzheimer’s disease: a quantitative study of visual and auditory cortices. J. Neurosci. 1987;7:1799–808. doi: 10.1523/JNEUROSCI.07-06-01799.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bussière T, Giannakopoulos P, Bouras C, Perl DP, Morrison JH, Hof PR. Progressive degeneration of nonphosphorylated neurofilament protein-enriched pyramidal neurons predicts cognitive impairment in Alzheimer’s disease: stereologic analysis of prefrontal cortex area 9. J. Comp. Neurol. 2003;463:281–302. doi: 10.1002/cne.10760. [DOI] [PubMed] [Google Scholar]
- 3.Lewis DA, Gonzalez-Burgos GR. Pathophysiologically based treatment interventions in schizophrenia. Nat. Med. 2006;12:1016–22. doi: 10.1038/nm1478. [DOI] [PubMed] [Google Scholar]
- 4.Arion D, Corradi JP, Tang S, Datta D, Boothe F, et al. Distinctive transcriptome alterations of prefrontal pyramidal neurons in schizophrenia and schizoaffective disorder. Mol. Psychiatry. 2015;20:1397–405. doi: 10.1038/mp.2014.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnsten AFT, Wang M, Paspalas CD. Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron. 2012;76:223–39. doi: 10.1016/j.neuron.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Preuss T. Do rats have prefrontal cortex? The Rose-Woolsey-Akert program reconsidered. J. Cogn. Neurosci. 1995;7:1–24. doi: 10.1162/jocn.1995.7.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Fuster JM. The Prefrontal Cortex. Academic; San Diego, CA: 2008. [Google Scholar]
- 8.Arnsten AFT. The neurobiology of thought: the groundbreaking discoveries of Patricia Goldman-Rakic 1937–2003. Cereb. Cortex. 2013;23:2269–81. doi: 10.1093/cercor/bht195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robbins TW. Dissociating executive functions of the prefrontal cortex. Phil. Trans. R. Soc. Lond. 1996;351:1463–71. doi: 10.1098/rstb.1996.0131. [DOI] [PubMed] [Google Scholar]
- 10.Stuss DT, Knight RT, editors. Principles of Frontal Lobe Function. Oxford Univ. Press; New York: 2002. [Google Scholar]
- 11.Thompson-Schill SL, Jonides J, Marshuetz C, Smith EE, D’Esposito M, et al. Effects of frontal lobe damage on interference effects in working memory. Cogn. Affect. Behav. Neurosci. 2002;2:109–20. doi: 10.3758/cabn.2.2.109. [DOI] [PubMed] [Google Scholar]
- 12.Goldman-Rakic PS. Circuitry of the primate prefrontal cortex and the regulation of behavior by representational memory. In: Plum F, editor. Handbook of Physiology, The Nervous System, Higher Functions of the Brain. Am. Physiol. Soc.; Bethesda, MD: 1987. pp. 373–417. [Google Scholar]
- 13.Ongür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb. Cortex. 2000;10:206–19. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 14.Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey’s dorsolateral prefrontal cortex. J. Neurophysiol. 1989;61:331–49. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- 15.Seo H, Lee D. Behavioral and neural changes after gains and losses of conditioned reinforcers. J. Neurosci. 2009;29:3627–41. doi: 10.1523/JNEUROSCI.4726-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Badre D, D’Esposito M. Functional magnetic resonance imaging evidence for a hierarchical organization of the prefrontal cortex. J. Cogn. Neurosci. 2007;19:2082–99. doi: 10.1162/jocn.2007.19.12.2082. [DOI] [PubMed] [Google Scholar]
- 17.Hilgenstock R, Weiss T, Witte OW. You’d better think twice: post-decision perceptual confidence. NeuroImage. 2014;99:323–31. doi: 10.1016/j.neuroimage.2014.05.049. [DOI] [PubMed] [Google Scholar]
- 18.Fleming SM, Huijgen J, Dolan RJ. Prefrontal contributions to metacognition in perceptual decision making. J. Neurosci. 2012;32:6117–25. doi: 10.1523/JNEUROSCI.6489-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat. Rev. Neurosci. 2006;7:268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 20.Seo H, Cai X, Donahue CH, Lee D. Neural correlates of strategic reasoning during competitive games. Science. 2014;346:340–43. doi: 10.1126/science.1256254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson RG, Lipsey JR. Cerebral localization of emotion based on clinical-neuropathological correlations: methodological issues. Psychiatr. Dev. 1985;3:335–47. [PubMed] [Google Scholar]
- 22.Aron AR. From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biol. Psychiatry. 2011;69:e55–68. doi: 10.1016/j.biopsych.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drevets WC, Price JL, Simpson JRJ, Todd RD, Reich T, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–27. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 24.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–60. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 25.Insel TR, Winslow JT. Neurobiology of obsessive compulsive disorder. Psychiatr. Clin. N. Am. 1992;15:813–24. [PubMed] [Google Scholar]
- 26.Shaw P, Lalonde FM, Lepage C, Rabin C, Eckstrand K, et al. Development of cortical asymmetry in typically developing children and its disruption in attention-deficit/hyperactivity disorder. Arch. Gen. Psychiatry. 2009;66:888–96. doi: 10.1001/archgenpsychiatry.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blumberg HP, Stern E, Ricketts S, Martinez D, de Asis J, et al. Rostral and orbital prefrontal cortex dysfunction in the manic state of bipolar disorder. Am. J. Psychiatry. 1999;156:1986–88. doi: 10.1176/ajp.156.12.1986. [DOI] [PubMed] [Google Scholar]
- 28.Sugranyes G, Kyriakopoulos M, Corrigall R, Taylor E, Frangou S. Autism spectrum disorders and schizophrenia: meta-analysis of the neural correlates of social cognition. PLOS ONE. 2011;6:e25322. doi: 10.1371/journal.pone.0025322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kritzer MF, Goldman-Rakic PS. Intrinsic circuit organization of the major layers and sublayers of the dorsolateral prefrontal cortex in the rhesus monkey. J. Comp. Neurol. 1995;359:131–43. doi: 10.1002/cne.903590109. [DOI] [PubMed] [Google Scholar]
- 30.Elston GN. Pyramidal cells of the frontal lobe: all the more spinous to think with. J. Neurosci. 2000;20:RC95. doi: 10.1523/JNEUROSCI.20-18-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elston GN. Cortex, cognition and the cell: new insights into the pyramidal neuron and prefrontal function. Cereb. Cortex. 2003;13:1124–38. doi: 10.1093/cercor/bhg093. [DOI] [PubMed] [Google Scholar]
- 32.Elston GN, Benavides-Piccione R, Elston A, Zietsch B, Defelipe J, et al. Specializations of the granular prefrontal cortex of primates: implications for cognitive processing. Anat. Rec. Part A Discov. Mol. Cell Evol. Biol. 2006;288:26–35. doi: 10.1002/ar.a.20278. [DOI] [PubMed] [Google Scholar]
- 33.DeFelipe J. The evolution of the brain, the human nature of cortical circuits, and intellectual creativity. Front. Neuroanat. 2011;5:29. doi: 10.3389/fnana.2011.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amatrudo JM, Weaver CM, Crimins JL, Hof PR, Rosene DL, Luebke JI. Influence of highly distinctive structural properties on the excitability of pyramidal neurons in monkey visual and prefrontal cortices. J. Neurosci. 2012;32:13644–60. doi: 10.1523/JNEUROSCI.2581-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Young ME, Ohm DT, Dumitriu D, Rapp PR, Morrison JH. Differential effects of aging on dendritic spines in visual cortex and prefrontal cortex of the rhesus monkey. Neuroscience. 2014;274:33–43. doi: 10.1016/j.neuroscience.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carlyle BC, Nairn AC, Wang M, Yang Y, Jin LE, et al. cAMP-PKA phosphorylation of tau confers risk for degeneration in aging association cortex. PNAS. 2014;111:5036–41. doi: 10.1073/pnas.1322360111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bourne J, Harris KM. Do thin spines learn to be mushroom spines that remember? Curr. Opin. Neurobiol. 2007;17:381–86. doi: 10.1016/j.conb.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 38.Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–85. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 39.Wang M, Yang Y, Wang CJ, Gamo NJ, Jin LE, et al. NMDA receptors subserve working memory persistent neuronal firing in dorsolateral prefrontal cortex. Neuron. 2013;77:736–49. doi: 10.1016/j.neuron.2012.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu XB, Murray KD, Jones EG. Switching of NMDA receptor 2A and 2B subunits at thalamic and cortical synapses during early postnatal development. J. Neurosci. 2004;24:8885–95. doi: 10.1523/JNEUROSCI.2476-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gabernet L, Jadhav SP, Feldman DE, Carandini M, Scanziani M. Somatosensory integration controlled by dynamic thalamocortical feed-forward inhibition. Neuron. 2005;48:315–27. doi: 10.1016/j.neuron.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 42.Funahashi S, Bruce CJ, Goldman-Rakic PS. Neuronal activity related to saccadic eye movements in the monkey’s dorsolateral prefrontal cortex. J. Neurophysiol. 1991;65:1464–83. doi: 10.1152/jn.1991.65.6.1464. [DOI] [PubMed] [Google Scholar]
- 43.Ford JM, Mathalon DH, Whitfield S, Faustman WO, Roth WT. Reduced communication between frontal and temporal lobes during talking in schizophrenia. Biol. Psychiatry. 2002;51:485–92. doi: 10.1016/s0006-3223(01)01335-x. [DOI] [PubMed] [Google Scholar]
- 44.Caetano MS, Horst NK, Harenberg L, Liu B, Arnsten AFT, Laubach L. Lost in transition: aging-related changes in executive control by the medial prefrontal cortex. J. Neurosci. 2012;32:3765–77. doi: 10.1523/JNEUROSCI.6011-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Selemon LD, Rajkowska G, Goldman-Rakic PS. Abnormally high neuronal density in the schizophrenic cortex: a morphometric analysis of prefrontal area 9 and occipital area 17. Arch. Gen. Psychiatry. 1995;52:805–18. doi: 10.1001/archpsyc.1995.03950220015005. [DOI] [PubMed] [Google Scholar]
- 46.Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol. Psychiatry. 1999;45:17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- 47.Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch. Gen. Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- 48.Black JE, Kodish IM, Grossman AW, Klintsova AY, Orlovskaya D, et al. Pathology of layer V pyramidal neurons in the prefrontal cortex of patients with schizophrenia. Am. J. Psychiatry. 2004;161:742–44. doi: 10.1176/appi.ajp.161.4.742. [DOI] [PubMed] [Google Scholar]
- 49.Curley AA, Eggan SM, Lazarus MS, Huang ZJ, Volk DW, Lewis DA. Role of glutamic acid decarboxylase 67 in regulating cortical parvalbumin and GABA membrane transporter 1 expression: implications for schizophrenia. Neurobiol. Dis. 2013;50:179–86. doi: 10.1016/j.nbd.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pearson RCA, Esiri MM, Hiorns RW, Wilcock GK, Powell TPS. Anatomical correlates of the distribution of the pathological changes in the neocortex in Alzheimer disease. PNAS. 1985;82:4531–34. doi: 10.1073/pnas.82.13.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Braak H, Braak E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol. Aging. 1995;16:271–78. doi: 10.1016/0197-4580(95)00021-6. [DOI] [PubMed] [Google Scholar]
- 52.Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J. Neuropathol. Exp. Neurol. 2011;70:960–69. doi: 10.1097/NEN.0b013e318232a379. [DOI] [PubMed] [Google Scholar]
- 53.Yang Y, Paspalas CD, Jin LE, Picciotto MR, Arnsten AFT, Wang M. Nicotinic α7 receptors enhance NMDA cognitive circuits in dorsolateral prefrontal cortex. PNAS. 2013;110:12078–83. doi: 10.1073/pnas.1307849110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arnsten AFT, Paspalas CD, Gamo NJ, Yang Y, Wang M. Dynamic Network Connectivity: a new form of neuroplasticity. Trends Cog. Sci. 2010;14:365–75. doi: 10.1016/j.tics.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hobson JA. Sleep and dreaming: induction and mediation of REM sleep by cholinergic mechanisms. Curr. Opin. Neurobiol. 1992;2:759–63. doi: 10.1016/0959-4388(92)90130-d. [DOI] [PubMed] [Google Scholar]
- 56.Abel T, Nguyen PV, Barad M, Deuel TA, Kandel ER, Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:615–26. doi: 10.1016/s0092-8674(00)81904-2. [DOI] [PubMed] [Google Scholar]
- 57.Hongpaisan J, Alkon DL. A structural basis for enhancement of long-term associative memory in single dendritic spines regulated by PKC. PNAS. 2007;104:19571–76. doi: 10.1073/pnas.0709311104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nagy G, Reim K, Matti U, Brose N, Binz T, et al. Regulation of releasable vesicle pool sizes by protein kinase A-dependent phosphorylation of SNAP-25. Neuron. 2004;41:417–29. doi: 10.1016/s0896-6273(04)00038-8. [DOI] [PubMed] [Google Scholar]
- 59.Chen S, Wang J, Siegelbaum SA. Properties of hyperpolarization-activated pacemaker current defined by coassembly of HCN1 and HCN2 subunits and basal modulation by cyclic nucleotide. J. Gen. Physiol. 2001;117:491–504. doi: 10.1085/jgp.117.5.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jentsch TJ. Neuronal KCNQ potassium channels: physiology and role in disease. Nat. Rev. Neurosci. 2000;1:21–30. doi: 10.1038/35036198. [DOI] [PubMed] [Google Scholar]
- 61.Paspalas CD, Wang M, Arnsten AFT. Constellation of HCN channels and cAMP regulating proteins in dendritic spines of the primate prefrontal cortex: potential substrate for working memory deficits in schizophrenia. Cereb. Cortex. 2013;23:1643–54. doi: 10.1093/cercor/bhs152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muly EC, Maddox M, Smith Y. Distribution of mGluR1 α and mGluR5 immunolabeling in primate prefrontal cortex. J. Comp. Neurol. 2003;467:521–35. doi: 10.1002/cne.10937. [DOI] [PubMed] [Google Scholar]
- 63.Arnsten AFT, Wang M, Paspalas CD. Dopamine’s actions in primate prefrontal cortex: challenges for treating cognitive disorders. Pharmacol. Rev. 2015;67:681–96. doi: 10.1124/pr.115.010512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vijayraghavan S, Wang M, Birnbaum SG, Bruce CJ, Williams GV, Arnsten AFT. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat. Neurosci. 2007;10:376–84. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- 65.Gamo NJ, Lur G, Higley MJ, Wang M, Paspalas CD, et al. Stress impairs prefrontal cortical function via D1 dopamine receptor interactions with hyperpolarization-activated cyclic nucleotide-gated channels. Biol. Psychiatry. 2015;78:860–70. doi: 10.1016/j.biopsych.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Birnbaum SB, Yuan P, Wang M, Vijayraghavan S, Bloom A, et al. Protein kinase C overactivity impairs prefrontal cortical regulation of working memory. Science. 2004;306:882–84. doi: 10.1126/science.1100021. [DOI] [PubMed] [Google Scholar]
- 67.Wang M, Vijayraghavan S, Goldman-Rakic PS. Selective D2 receptor actions on the functional circuitry of working memory. Science. 2004;303:853–56. doi: 10.1126/science.1091162. [DOI] [PubMed] [Google Scholar]
- 68.Benneyworth MA, Xiang Z, Smith RL, Garcia EE, Conn PJ, Sanders-Bush E. A selective positive allosteric modulator of metabotropic glutamate receptor subtype 2 blocks a hallucinogenic drug model of psychosis. Mol. Pharmacol. 2007;72:477–84. doi: 10.1124/mol.107.035170. [DOI] [PubMed] [Google Scholar]
- 69.Huang CC, Hsu KS. Presynaptic mechanism underlying cAMP-induced synaptic potentiation in medial prefrontal cortex pyramidal neurons. Mol. Pharmacol. 2006;69:846–56. doi: 10.1124/mol.105.018093. [DOI] [PubMed] [Google Scholar]
- 70.Defelipe J. The evolution of the brain, the human nature of cortical circuits, and intellectual creativity. Front. Neuroanat. 2011;5:29. doi: 10.3389/fnana.2011.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.U’Prichard DC, Bechtel WD, Rouot BM, Snyder SH. Multiple apparent alpha-noradrenergic receptor binding sites in rat brain: effect of 6-hydroxydopamine. Mol. Pharmacol. 1979;16:47–60. [PubMed] [Google Scholar]
- 72.Arnsten AFT, Li B-M. Neurobiology of executive functions: catecholamine influences on prefrontal cortical function. Biol. Psychiatry. 2005;57:1377–84. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 73.Wang M, Ramos BP, Paspalas CD, Shu Y, Simen A, et al. α2A-adrenoceptor stimulation strengthens working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 74.Li B-M, Mao Z-M, Wang M, Mei Z-T. Alpha-2 adrenergic modulation of prefrontal cortical neuronal activity related to spatial working memory in monkeys. Neuropsychopharmacology. 1999;21:601–10. doi: 10.1016/S0893-133X(99)00070-6. [DOI] [PubMed] [Google Scholar]
- 75.Mao Z-M, Arnsten AFT, Li B-M. Local infusion of α-1 adrenergic agonist into the prefrontal cortex impairs spatial working memory performance in monkeys. Biol. Psychiatry. 1999;46:1259–65. doi: 10.1016/s0006-3223(99)00139-0. [DOI] [PubMed] [Google Scholar]
- 76.Arnsten AFT. The use of α-2A adrenergic agonists for the treatment of attention-deficit/hyperactivity disorder. Expert Rev. Neurother. 2010;10:1595–605. doi: 10.1586/ern.10.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim S, Bobeica I, Gamo NJ, Arnsten AFT, Lee D. Effects of α-2A adrenergic receptor agonist on time and risk preference in primates. Psychopharmacology. 2012;219:363–75. doi: 10.1007/s00213-011-2520-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li B-M, Mei Z-T. Delayed response deficit induced by local injection of the α2-adrenergic antagonist yohimbine into the dorsolateral prefrontal cortex in young adult monkeys. Behav. Neural. Biol. 1994;62:134–39. doi: 10.1016/s0163-1047(05)80034-2. [DOI] [PubMed] [Google Scholar]
- 79.Ma C-L, Qi X-L, Peng J-Y, Li B-M. Selective deficit in no-go performance induced by blockade of prefrontal cortical α2-adrenoceptors in monkeys. NeuroReport. 2003;14:1013–16. doi: 10.1097/01.wnr.0000070831.57864.7b. [DOI] [PubMed] [Google Scholar]
- 80.Ma C-L, Arnsten AFT, Li B-M. Locomotor hyperactivity induced by blockade of prefrontal cortical α2-adrenoceptors in monkeys. Biol. Psychiatry. 2005;57:192–95. doi: 10.1016/j.biopsych.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 81.Zhang Z, Cordeiro Matos S, Jego S, Adamantidis A, Séguéla P. Norepinephrine drives persistent activity in prefrontal cortex via synergistic α1 and α2 adrenoceptors. PLOS ONE. 2013;8:e66122. doi: 10.1371/journal.pone.0066122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kamisaki Y, Hamahashi T, Hamada T, Maeda K, Itoh T. Presynaptic inhibition by clonidine of neurotransmitter amino acid release in various brain regions. Eur. J. Pharmacol. 1992;217:57–63. doi: 10.1016/0014-2999(92)90511-2. [DOI] [PubMed] [Google Scholar]
- 83.Yi F, Liu S-S, Luo F, Zhang X-H, Li B-M. Signaling mechanism underlying α2A-adrenergic suppression of excitatory synaptic transmission in the medial prefrontal cortex of rats. Eur. J. Neurosci. 2013;38:2364–73. doi: 10.1111/ejn.12257. [DOI] [PubMed] [Google Scholar]
- 84.Franowicz JS, Kessler L, Dailey Borja CM, Kobilka BK, Limbird LE, Arnsten AFT. Mutation of the α2A-adrenoceptor impairs working memory performance and annuls cognitive enhancement by guanfacine. J. Neurosci. 2002;22:8771–77. doi: 10.1523/JNEUROSCI.22-19-08771.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kauser H, Sahu S, Kumar S, Panjwani U. Guanfacine is an effective countermeasure for hypobaric hypoxia-induced cognitive decline. Neuroscience. 2013;254:110–19. doi: 10.1016/j.neuroscience.2013.09.023. [DOI] [PubMed] [Google Scholar]
- 86.Hains AB, Yabe Y, Arnsten AFT. Chronic stimulation of alpha-2A-adrenoceptors with guanfacine protects rodent prefrontal cortex dendritic spines and cognition from the effects of chronic stress. Neurobiol. Stress. 2015;2:1–9. doi: 10.1016/j.ynstr.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ren W-W, Liu Y, Li B-M. Stimulation of α2A-adrenoceptors promotes the maturation of dendritic spines in cultured neurons of the medial prefrontal cortex. Mol. Cell Neurosci. 2011;49:205–16. doi: 10.1016/j.mcn.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 88.Gyoneva S, Traynelis SF. Norepinephrine modulates the motility of resting and activated microglia via different adrenergic receptors. J. Biol. Chem. 2013;288:15291–302. doi: 10.1074/jbc.M113.458901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Biederman J, Melmed RD, Patel A, McBurnett K, Konow J, et al. A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics. 2008;121:e73–84. doi: 10.1542/peds.2006-3695. [DOI] [PubMed] [Google Scholar]
- 90.Scahill L, Chappell PB, Kim YS, Schultz RT, Katsovich L, et al. A placebo-controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. Am. J. Psychiatry. 2001;158:1067–74. doi: 10.1176/appi.ajp.158.7.1067. [DOI] [PubMed] [Google Scholar]
- 91.McCracken JT, Aman MG, McDougle CJ, Tierney E, Shiraga S, et al. Possible influence of variant of the P-glycoprotein gene (MDR1/ABCB1) on clinical response to guanfacine in children with pervasive developmental disorders and hyperactivity. J. Child Adolesc. Psychopharmacol. 2010;20:1–5. doi: 10.1089/cap.2009.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Connor DF, Grasso DJ, Slivinsky MD, Pearson GS, Banga A. An open-label study of guanfacine extended release for traumatic stress related symptoms in children and adolescents. J. Child Adolesc. Psychopharmacol. 2013;23:244–51. doi: 10.1089/cap.2012.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Arnsten AFT, Raskind M, Taylor FB, Connor DF. The effects of stress exposure on prefrontal cortex: translating basic research into successful treatments for post-traumatic stress disorder. Neurobiol. Stress. 2015;1:89–99. doi: 10.1016/j.ynstr.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McAllister TW, McDonald BC, Flashman LA, Ferrell RB, Tosteson TD, et al. Alpha-2 adrenergic challenge with guanfacine one month after mild traumatic brain injury: altered working memory and BOLD response. Int. J. Psychophysiol. 2011;82:107–14. doi: 10.1016/j.ijpsycho.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fox HC, Seo D, Tuit K, Hansen J, Kimmerling A, et al. Guanfacine effects on stress, drug craving and prefrontal activation in cocaine dependent individuals: preliminary findings. J. Psychopharmacol. 2012;26:958–72. doi: 10.1177/0269881111430746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McKee SA, Potenza MN, Kober H, Sofouglu M, Arnsten AFT, et al. A translational investigation targeting stress-reactivity and prefrontal cognitive control with guanfacine for smoking cessation. J. Psychopharmacol. 2015;29:300–11. doi: 10.1177/0269881114562091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cannon TD, Chung Y, He G, Sun D, Jacobson A, et al. Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol. Psychiatry. 2014;77:147–57. doi: 10.1016/j.biopsych.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Arnsten AFT, Cai JX, Goldman-Rakic PS. The alpha-2 adrenergic agonist guanfacine improves memory in aged monkeys without sedative or hypotensive side effects: evidence for alpha-2 receptor subtypes. J. Neurosci. 1988;8:4287–98. doi: 10.1523/JNEUROSCI.08-11-04287.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Franowicz JCS, Arnsten AFT. The α-2A noradrenergic agonist, guanfacine, improves delayed response performance in young adult rhesus monkeys. Psychopharmacology. 1998;136:8–14. doi: 10.1007/s002130050533. [DOI] [PubMed] [Google Scholar]
- 100.Arnsten AFT, Steere JC, Hunt RD. The contribution of α2-noradrenergic mechanisms to prefrontal cortical cognitive function: potential significance to attention deficit hyperactivity disorder. Arch. Gen. Psychiatry. 1996;53:448–55. doi: 10.1001/archpsyc.1996.01830050084013. [DOI] [PubMed] [Google Scholar]
- 101.Chappell PB, Riddle MA, Scahill L, Lynch KA, Schultz R, et al. Guanfacine treatment of comorbid attention deficit hyperactivity disorder and Tourette’s syndrome: preliminary clinical experience. J. Am. Acad. Child. Adolesc. Psychiatry. 1995;34:1140–46. doi: 10.1097/00004583-199509000-00010. [DOI] [PubMed] [Google Scholar]
- 102.Hunt RD, Arnsten AFT, Asbell MD. An open trial of guanfacine in the treatment of attention deficit hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry. 1995;34:50–54. doi: 10.1097/00004583-199501000-00013. [DOI] [PubMed] [Google Scholar]
- 103.Martin LF, Freedman R. Schizophrenia and the α7 nicotinic acetylcholine receptor. Int. Rev. Neurobiol. 2007;78:225–46. doi: 10.1016/S0074-7742(06)78008-4. [DOI] [PubMed] [Google Scholar]
- 104.Wilens TE, Decker MW. Neuronal nicotinic receptor agonists for the treatment of attention-deficit/hyperactivity disorder: focus on cognition. Biochem. Pharmacol. 2007;74:1212–23. doi: 10.1016/j.bcp.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Levin ED. α7-Nicotinic receptors and cognition. Curr. Drug Targets. 2012;13:602–6. doi: 10.2174/138945012800398937. [DOI] [PubMed] [Google Scholar]
- 106.Kristiansen LV, Bakir B, Haroutunian V, Meador-Woodruff JH. Expression of the NR2B-NMDA receptor trafficking complex in prefrontal cortex from a group of elderly patients with schizophrenia. Schizophr. Res. 2010;119:198–209. doi: 10.1016/j.schres.2010.02.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kristiansen LV, Patel SA, Haroutunian VH, Meador-Woodruff JH. Expression of the NR2B-NMDA receptor subunit and its Tbr-1/CINAP regulatory proteins in postmortem brain suggest altered receptor processing in schizophrenia. Synapse. 2010;64:495–502. doi: 10.1002/syn.20754. [DOI] [PubMed] [Google Scholar]
- 108.Weickert CS, Fung SJ, Catts VS, Schofield PR, Allen KM, et al. Molecular evidence of N-methyl-d-aspartate receptor hypofunction in schizophrenia. Mol. Psychiatry. 2012;18:1185–92. doi: 10.1038/mp.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mexal S, Berger R, Logel J, Ross RG, Freedman R, Leonard S. Differential regulation of α7 nicotinic receptor gene (CHRNA7) expression in schizophrenic smokers. J. Mol. Neurosci. 2010;40:185–95. doi: 10.1007/s12031-009-9233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ahlers E, Hahn E, Ta TM, Goudarzi E, Dettling M, Neuhaus AH. Smoking improves divided attention in schizophrenia. Psychopharmacology. 2014;231:3871–77. doi: 10.1007/s00213-014-3525-2. [DOI] [PubMed] [Google Scholar]
- 111.Hajós M, Rogers BN. Targeting α7 nicotinic acetylcholine receptors in the treatment of schizophrenia. Curr. Pharm. Des. 2010;16:538–54. doi: 10.2174/138161210790361434. [DOI] [PubMed] [Google Scholar]
- 112.Williams DK, Wang J, Papke RL. Positive allosteric modulators as an approach to nicotinic acetylcholine receptor-targeted therapeutics: advantages and limitations. Biochem. Pharmacol. 2011;82:915–30. doi: 10.1016/j.bcp.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Snyder EM, Nong Y, Almeida CG, Paul S, Moran TH, et al. Regulation of NMDA receptor trafficking by amyloid-β. Nat. Neurosci. 2005;8:1051–58. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 114.Mrzljak L, Levey AI, Goldman-Rakic PS. Association of m1 and m2 muscarinic receptor proteins with asymmetric synapses in the primate cerebral cortex: morphological evidence for cholinergic modulation of excitatory neurotransmission. PNAS. 1993;90:5194–98. doi: 10.1073/pnas.90.11.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Davie BJ, Christopoulos A, Scammells PJ. Development of M1 mAChR allosteric and bitopic ligands: prospective therapeutics for the treatment of cognitive deficits. ACS Chem. Neurosci. 2013;4:1026–48. doi: 10.1021/cn400086m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Engberg G, Eriksson E. Effects of α2-adrenoceptor agonists on locus coeruleus firing rate and brain noradrenaline turnover in N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline (EEDQ)-treated rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1991;343:472–77. doi: 10.1007/BF00169548. [DOI] [PubMed] [Google Scholar]
- 117.Manji HK, Lenox RH. Protein kinase C signaling in the brain: molecular transduction of mood stabilization in the treatment of manic-depressive illness. Biol. Psychiatry. 1999;46:1328–51. doi: 10.1016/s0006-3223(99)00235-8. [DOI] [PubMed] [Google Scholar]
- 118.Zarate CAJ, Singh JB, Carlson PJ, Quiroz JA, Jolkovsky L, et al. Efficacy of a protein kinase C inhibitor (tamoxifen) in the treatment of acute mania: a pilot study. Bipolar. Disorders. 2007;9:561–70. doi: 10.1111/j.1399-5618.2007.00530.x. [DOI] [PubMed] [Google Scholar]
- 119.Birnbaum SG, Gobeske KT, Auerbach J, Taylor JR, Arnsten AFT. A role for norepinephrine in stress-induced cognitive deficits: α-1-adrenoceptor mediation in prefrontal cortex. Biol. Psychiatry. 1999;46:1266–74. doi: 10.1016/s0006-3223(99)00138-9. [DOI] [PubMed] [Google Scholar]
- 120.Raskind MA, Peskind ER, Kanter ED, Petrie EC, Radant A, et al. Reduction in nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am. J. Psychiatry. 2003;160:371–73. doi: 10.1176/appi.ajp.160.2.371. [DOI] [PubMed] [Google Scholar]
- 121.Taylor FB, Lowe K, Thompson C, McFall MM, Peskind ER, et al. Daytime prazosin reduces psychological distress to trauma specific cues in civilian trauma posttraumatic stress disorder. Biol. Psychiatry. 2006;59:577–81. doi: 10.1016/j.biopsych.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 122.Clarke HF, Walker SC, Dalley JW, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion is behaviorally and neurochemically specific. Cereb. Cortex. 2007;17:18–27. doi: 10.1093/cercor/bhj120. [DOI] [PubMed] [Google Scholar]
- 123.Walker SC, Robbins TW, Roberts AC. Differential contributions of dopamine and serotonin to orbitofrontal cortex function in the marmoset. Cereb. Cortex. 2009;19:889–98. doi: 10.1093/cercor/bhn136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Puig MV, Miller EK. The role of prefrontal dopamine D1 receptors in the neural mechanisms of associative learning. Neuron. 2012;74:874–86. doi: 10.1016/j.neuron.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Puig MV, Miller EK. Neural substrates of dopamine D2 receptor modulated executive functions in the monkey prefrontal cortex. Cereb. Cortex. 2015;25:2980–87. doi: 10.1093/cercor/bhu096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Noudoost B, Moore T. Control of visual cortical signals by prefrontal dopamine. Nature. 2011;474:372–75. doi: 10.1038/nature09995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Opler LA, Opler MGA, Arnsten AFT. Ameliorating treatment-refractory depression with intranasal ketamine: potential NMDA receptor actions in the pain circuitry representing mental anguish. CNS Spectr. 2015 doi: 10.1017/S1092852914000686. In press. doi: 10.1017/S1092852914000686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cannon TD, Thompson PM, van Erp TG, Toga AW, Poutanen VP, et al. Cortex mapping reveals regionally specific patterns of genetic and disease-specific gray-matter deficits in twins discordant for schizophrenia. PNAS. 2002;99:3228–33. doi: 10.1073/pnas.052023499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Calabrese B, Halpain S. Essential role for the PKC target MARCKS in maintaining dendritic spine morphology. Neuron. 2005;48:77–90. doi: 10.1016/j.neuron.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 130.Hains AB, Vu MAT, Maciejewski PK, van Dyck CH, Gottron M, Arnsten AFT. Inhibition of protein kinase C signaling protects prefrontal cortex dendritic spines and cognition from the effects of chronic stress. PNAS. 2009;106:17957–62. doi: 10.1073/pnas.0908563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ota KT, Liu RJ, Voleti B, Maldonado-Aviles JG, Duric V, et al. REDD1 is essential for stress-induced synaptic loss and depressive behavior. Nat. Med. 2014;20:531–35. doi: 10.1038/nm.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yanagawa Y, Hiraide S, Matsumoto M, Togashi H. Rapid induction of REDD1 gene expression in macrophages in response to stress-related catecholamines. Immunol. Lett. 2014;158:109–15. doi: 10.1016/j.imlet.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 133.Bachmann VA, Riml A, Huber RG, Baillie GS, Liedl KR, et al. Reciprocal regulation of PKA and Rac signaling. PNAS. 2013;110:8531–36. doi: 10.1073/pnas.1215902110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hayashi-Takagi A, Araki Y, Nakamura M, Vollrath B, Duron SG, et al. PAKs inhibitors ameliorate schizophrenia-associated dendritic spine deterioration in vitro and in vivo during late adolescence. PNAS. 2014;111:6461–66. doi: 10.1073/pnas.1321109111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hayashi-Takagi A, Takaki M, Graziane N, Seshadri S, Murdoch H, et al. Disrupted-in-Schizophrenia 1 (DISC1) regulates spines of the glutamate synapse via Rac1. Nat. Neurosci. 2010;13:327–32. doi: 10.1038/nn.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.MacKenzie KF, Wallace DA, Hill EV, Anthony DF, Henderson DJP, et al. Phosphorylation of cAMP-specific PDE4A5 (phosphodiesterase-4A5) by MK2 (MAPKAPK2) attenuates its activation through protein kinase A phosphorylation. Biochem. J. 2011;435:755–69. doi: 10.1042/BJ20101184. [DOI] [PubMed] [Google Scholar]