Abstract
Aim
Determine the long-term associations of a sex- and race/ethnicity-specific MetS-severity Z-score from childhood and adulthood with future diagnosis of type 2 diabetes mellitus (T2DM).
Methods
We performed a prospective cohort study with evaluations from the Lipids Research Clinic (LRC) 1973–1976 and Princeton Follow-up Study (PFS) 1998–2003 and further disease status from Princeton Health Update (PHU) 2010–2014. We assessed MetS severity as a predictor of incident T2DM among 629 cohort participants assessed at both LRC and PFS and 354 participants at PHU.
Results
Cohort participants had a mean age of 12.9y at baseline (LRC), 38.4y at PFS and 49.6y at most recent follow-up. Childhood MetS-Z-scores were associated with adult MetS-Z-scores (p<0.01). Compared to individuals disease-free at all time points, those who developed T2DM by 1998–2003 and 2010–2014 had higher MetS severity scores in childhood (p<0.05). For every 1-unit elevation in childhood MetS-Z-score, the OR of future T2DM was 2.7 for incident disease by mean age 38.5y (p<0.01) and 2.8 for incident disease by mean age 49.6y (p<0.05). In assessing associations with the change in Z-score from childhood to adulthood, for every 1-unit increase in MetS-Z-score over time, the OR of incident T2DM by mean age 49.6 was 7.3 (p<0.01).
Conclusions
The severity of MetS in childhood was associated with incidence of adult T2DM and the degree of increase in this severity also predicted future disease. This provides evidence of potential clinical utility in assessing MetS severity to detect risk and follow clinical progress over time.
Keywords: insulin resistance, metabolic syndrome, risk, type 2 diabetes mellitus
Introduction
The high morbidity and mortality from type 2 diabetes mellitus world-wide [1] highlights the need to identify and track risk in children and adults and to motivate patients toward lifestyle interventions [2, 3]. One factor associated with increased future disease risk is the metabolic syndrome (MetS), a cluster of cardiovascular risk factors including central obesity, high blood pressure (BP), elevated triacylglycerol, low HDL-cholesterol and elevated fasting glucose [4]. MetS is linked to insulin resistance [5] and obesity [6] and is associated with underlying abnormalities in cellular function [3, 7, 8]. MetS has traditionally been classified based on an individual exhibiting abnormal values for ≥3 of the 5 individual components [4]. Using such criteria, MetS has utility in predicting future disease among children [9, 10] and adults [11–15] that in some [12, 13] but not all cases [14, 15] equated to more than the sum of the individual CVD risk factors.
Use of a binary score for MetS has made it difficult to monitor changes in MetS over time [16, 17]. In addition, there appear to be differences in how MetS is manifest on the basis of sex and race/ethnicity, with African American males in particular being classified as having a low prevalence of MetS despite having high rates of type 2 diabetes and death from CVD [18–24]. We thus formulated a sex- and race/ethnicity-specific MetS severity score that takes into account differences in how MetS components segregate by sex and race/ethnicity, both for adolescents and adults [25, 26]. While other continuous MetS scores have been formulated [27, 28], none other takes into account that the individual MetS components may require different weights in their contribution to MetS.
Such a metric of MetS severity raises potential benefits both for identifying individuals at particularly high risk based on the severity of their MetS score and for following the score over time to track disease progress and response to treatment. We recently reported the potential utility of this score as a predictor of cardiovascular disease (CVD), revealing linear associations of the MetS severity score during childhood with risk for CVD 36.7 years later [29]. It remains unclear whether an increase in MetS severity over time also confers additional risk for future type 2 diabetes. We hypothesized that as opposed to standard MetS classification criteria, variations in the severity of MetS—both assessed at baseline and as in increase in severity over time—would be important predictors of later type 2 diabetes. We assessed this on long-term data from the Princeton (OH) Lipid Research Study, a group of white and African American individuals evaluated for MetS components in childhood/adolescence, with long-term follow-up 25 and 36 years later as adults.
Methods
Participants were originally recruited as part of the Cincinnati Clinic of the National Heart Lung and Blood Institute Lipid Research Clinic (LRC) Prevalence Program (1972–1978), a multistage survey of lipids and other CVD risk factors [30, 31]. In 1973–1976, the LRC enrolled students in grades 1–12 in the Princeton School District and a random sample of their parents. The Institutional Review Boards of NHLBI, the University of Cincinnati, West Virginia University and the University of Virginia approved the study and/or its analysis. The Princeton Follow-up Study (PFS, 1998–2003) was a 25–30-year follow-up of these student and parent-participants to prospectively assess changes in CVD risk factors from childhood into the 4th–5th decades of life [9]. PFS eligibility required participation in LRC visits where lipoproteins were measured and participation of a first-degree relative at those same visits, with a 70% recruitment of eligible participant families. The Princeton Health Update (PHU, 2010–2014) was performed 8–14 years after the PFS to assess updated disease status of PFS participants. Data were obtained by telephoning or mailing participants and first-degree relatives using a standardized questionnaire and by examining death certificates from the National Death Index for cause of death. Inclusion criteria for the primary analysis of these participants were: LRC age < 20 years old and complete information on metabolic syndrome and its components and both the LRC and PFS visits. Participants with triacylglycerol values > 2.26 mmol/l (200 mg/dl), LDL values > 4.66 mmol/l (180 mg/dl), or glucose values > 7.0 mmol/l (126 mg/dl) at the LRC visit were excluded from the analysis.
Clinical Measures
In both the LRC and PFS studies, data were collected via standard protocols [9, 30, 31], including measures of height and weight in LRC [32] and height, weight, and waist circumference (WC) in PFS [9]. WC was measured in PFS at the level of the umbilicus following normal expiration. In the LRC and PFS, BP was measured on a participant’s right arm with a standard sphygmomanometer after sitting for 5 minutes. In LRC and PFS fasting blood was drawn and tested for lipid profiles in LRC–Centers for Disease Control and Prevention (CDC) standardized laboratories. In the LRC, glucose was measured on the ABA-100 by a hexokinase method.[33] In the PFS, glucose was measured on the Dade Dimension Xpand (Dade Behring, Deerfield, IL), by the hexokinase-glucose-6- phosphate-dehydrogenase method.[34] Diabetes was classified based on self-report or fasting glucose ≥7.0 (126 mg/dl) at all three studies.
Traditional MetS was defined using the ATP-III criteria for adults [4]; participants had to meet ≥3 of the following 5 criteria: concentration of triacylglycerol ≥1.69 mmol/l (150 mg/dl), HDL-C <1.04 mmol/l (40 mg/dL) for men and <1.3 mmol/L (50 mg/dL) for women, WC ≥102 cm for males and 88 cm for females, glucose concentration ≥5.55 mmol/l (100 mg/dl), and systolic BP ≥130 mmHg or diastolic BP ≥85 mmHg. MetS in childhood was defined using a modification of these criteria [9, 35] in which participants had to meet ≥3 of the following: concentration of triacylglycerol ≥1.24 mmol/l (110 mg/dl), HDL-C ≤1.04 mmol/l (40 mg/dl), BMI ≥90th percentile, glucose ≥5.55 mmol/l (100 mg/dl) and systolic or diastolic BP ≥90th percentile (age, height, and sex-specific)[36].
MetS severity Z-score was calculated for adolescents at their LRC visit and then again as adults during their PFS visit using formulas published elsewhere [25, 26]. Briefly, these scores were formed using confirmatory factor analysis of the 5 traditional components of MetS (as above) to determine the weighted contribution of each of these components to a latent MetS “factor” on a sex- and race/ethnicity-specific basis. Confirmatory factor analysis was performed on data from the National Health and Nutrition Examination Survey (NHANES) for adolescents age 12–19 years [25] and adults age 20–64 years [26], with both adolescents and adults divided into six sub-groups based on sex and the following self-identified race/ethnicities: non-Hispanic white, non-Hispanic black and Hispanic. For each of these six population sub-groups, loading coefficients for the 5 MetS components were determined toward a single MetS factor. The loading coefficients were then used to generate equations to calculate a standardized MetS severity score for each sub-group (http://publichealth.hsc.wvu.edu/biostatistics/metabolic-syndrome-severity-calculator/). These MetS severity scores are Z-scores (ranging from negative infinity to positive infinity) of relative MetS severity on a sex- and race/ethnicity-specific basis and are highly correlated to other surrogate markers of MetS risk, including hsCRP, uric acid and the homeostasis model of insulin resistance [25, 26]. In calculating these scores in the present study, individual measures of participants from LRC and PHS were entered into these equations to calculate MetS severity as children and adults, respectively. For the LRC visit, BP was missing for 185 of the 629 participants; for these individuals, systolic BP was estimated to be the 50th percentile of normal based on published equations for sex, age, and height percentile [36]. A sensitivity analysis of the data eliminating these individuals with imputed data did not alter the study’s findings.
Statistical analysis
All statistical analyses were performed using SAS 9.4. Comparisons between groups for continuous and categorical variables were performed using t-tests and chi-square tests, respectively. Pearson’s r correlation was calculated to estimate linear associations between MetS severity Z-scores at LRC and PFS. Mean (SD) severity Z-scores were calculated at both LRC visits and PFS visits, and compared between those participants who did not self-report with diabetes at either visit, and those who self-reported diabetes at each of the two later visits. Given the lack of information on the precise date of the event (i.e., interval-censored data) and the primary interest in a time-varying predictor (MetS score), as well as an interest in clinical interpretation of odds of future disease, logistic regression was chosen over survival methods. Logistic models were fit estimating odds of incident diabetes at PFS and then again at PHU (excluding those individuals who reported disease at PFS). These models included MetS again as traditionally defined as well as the MetS severity Z-score, with comparisons between the two using the Akaike Information Criterion (AIC). Smaller AIC values indicate a better model fit of the outcomes. Since the intent was to determine the predictive value of future disease/outcomes, models that included MetS severity scores included MetS scores at “baseline” (LRC) and the change in score from LRC to PFS. Such models would allow for interpretation of the predictive value of a childhood score measured in a clinical setting and monitoring of the score over time. Likewise, for models of type 2 diabetes by PHU as an outcome, individuals who developed disease by the PFS were excluded. For models of incident disease that included the severity score as a predictor, receiver operating characteristic (ROC) curves were produced to display the ability of this new score to discriminate between those who developed future disease and those who did not, quantified by the area under the curve (AUC). An AUC value of 0.5 indicates no discriminative ability and a value of 1.0 indicates perfect predictive ability.
Results
Participant characteristics
We evaluated data from 629 participants with adequate data from LRC and PFS for analysis of MetS severity, 354 of whom were able to be contacted or had NDI data available for disease status update in PHU (Table 1, with further breakdown by sex and race/ethnicity in Supplementary Table 1). The remainder of participants in the analytic cohort was lost to follow-up. Compared to those not evaluated in PHU, those with updated data were more likely to be female (60% vs. 52% p=0.039) and white (76% vs. 61%, p<0.001) but had similar baseline MetS scores (mean scores −0.47 vs. −0.49, p=0.776). Participants had a mean age of 12.9 years in LRC, 38.4 years in PFS and 49.6 years at PHU. There was a low prevalence of overweight (12.1%) and obesity (9.2%) during LRC, compared to PFS (32.4% and 34.6%, respectively). As expected, each of the components of MetS was more abnormal during PFS than during LRC. During LRC, participants had a childhood/adolescent MetS severity Z-score of −0.5, while during PFS participants had an adult MetS severity Z-score of 0.1. During childhood, participants had a low prevalence of MetS by traditional criteria (2.7%) that increased greatly by PFS (32.0%). At PFS and PHU respectively, 5% and 13.6% of individuals had diabetes and 0.9% and 5.8% had CVD. By PHU, 1.5% of cohort members accounted for had died.
Table 1. Descriptive Statistics.
Participants with valid MetS severity scores at LRC and PFS visits, ability to safely classify MetS status at LRC and PFS visits, and < 20 years old at LRC
| LRC 1973–1976 |
PFS 1998–2003 |
PHU 2010–2014 |
|
|---|---|---|---|
| N | 629 | 629 | 354 |
| Mean (SD): | |||
| Age (years) | 12.9 (3.3) | 38.4 (3.5) | 49.6 (3.5) |
| BMI (kg/m2) | 19.9 (4.3) | 28.7 (6.8) | -- |
| Waist (cm) | -- | 97.2 (16.7) | -- |
| Glucose (mmol/l) (mg/dl) |
4.72 (0.44) 85.1 (8.0) |
5.0 (1.51) 90.1 (27.2) |
-- |
| HDL-C (mmol/l) (mg/dl) |
1.41 (0.30) 54.5 (11.7) |
1.18 (0.37) 45.6 (14.3) |
-- |
| Triacylglycerol (mmol/l) (mg/dl) |
0.81 (0.33) 71.5 (29.0) |
1.49 (1.25) 132.3 (110.3) |
-- |
| Systolic blood pressure (mm Hg) | 102.7 (12.7) | 120.4 (14.9) | -- |
| Diastolic blood pressure (mm Hg) | 63.3 (11.0) | 79.6 (10.9) | -- |
| Metabolic syndrome severity score (z-score) | −0.5 (0.7) | 0.1 (1.1) | -- |
| Frequency (Percent): | |||
| Male | 275 (43.7%) | 275 (44.5%) | 142 (40.1%) |
| White | 437 (69.5%) | 451 (69.5%) | 269 (76.0%) |
| Overweighta | 76 (12.1%) | 204 (32.4%) | -- |
| Obese* | 58 (9.2%) | 217 (34.6%) | -- |
| Metabolic syndrome | 17 (2.7%) | 201 (32.0%) | -- |
| Type 2 diabetesb | -- | 30 (5.0%) | 48 (13.6%) |
| Myocardial Infarctionb | -- | 3 (0.5%) | 9 (2.6%) |
| Stroke** | -- | 1 (0.2%) | 8 (2.4%) |
| Angioplasty, Stent, Bypass, or other Heart Surgeryb | -- | 1 (0.2%) | 10 (2.9%) |
| Cardiovascular Diseaseb | 5 (0.9%) | 20 (5.8%) | |
| Deceased** | -- | 0 | 5 (1.5%) |
Overweight = BMI ≥ 85th percentile for LRC, ≥ 25 for PFS; Obese = BMI ≥ 95th percentile for LRC, ≥ 30 for PFS
Cumulative frequencies by the designated visit
MetS severity predicting type 2 diabetes
There was a high degree of correlation in MetS severity score between childhood at LRC and mid-adulthood at PFS: r=0.41 (p<0.01)(Figure 1). Figure 2 provides MetS severity scores at the LRC and PFS visits by diabetes disease status for three groups of individuals: 1) those who did not have diabetes at any of the three visits; 2) those who developed T2DM between LRC and PFS; and 3) those who developed T2DM between PFS and PHU. In each case, MetS severity scores in childhood and adulthood were lowest among those who never had disease, highest among those who developed diabetes by PFS (mean age 38.4 years), and intermediate among those who developed diabetes later (between PFS and PHU, by mean age 49.6 years)(for p-values, see Figure 2).
Figure 1. Correlation of MetS Severity Scores within Individuals over Time.
MetS severity Z-scores on the x-axis during childhood (Lipids Research Clinic, 1973–1976) and on the y-axis during adulthood (Princeton Follow-up Study, 1998–2003). Pearson’s r=0.41 (p<0.01).
Figure 2. Mean MetS Severity Scores within Individuals by Later Diabetes Status.
MetS severity score (mean, 95-percent confidence interval) by disease status for diabetes. Scores shown are those during childhood (Lipid Research Clinic) and adulthood (Princeton Follow-up Study) among individuals who: 1) remained disease-free at LRC, PFS and Princeton Health Update (light grey circle, n=310), 2) those who were disease-free at PFS but later developed disease by PHU (dark grey square, n=17), and 3) those with incident disease between LRC and PFS (black triangle, n=30). Comparison with disease-free group: * p<0.05, ** p<0.01. Comparison with incident disease between PFS and PHU: †† p<0.01.
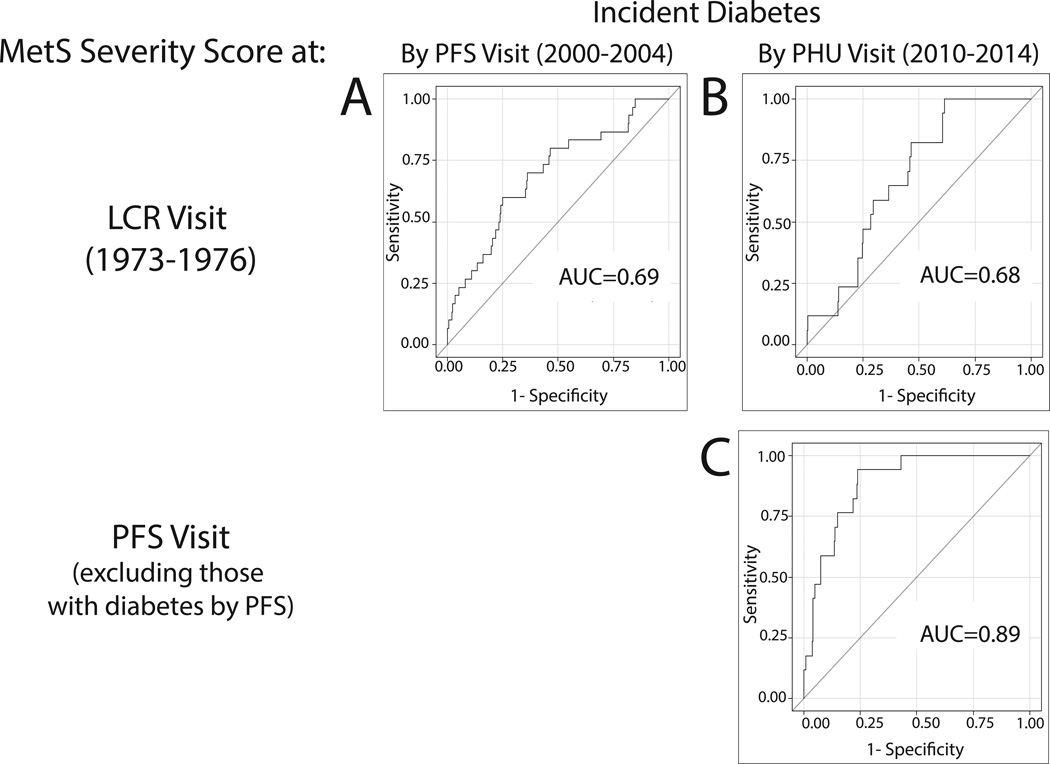
Figure 3 displays ROC curves evaluating risk of incident diabetes, using either MetS severity from LRC or PFS. As a linear measure, childhood MetS severity was significantly associated with risk of diabetes 24–41 years later (AUC=0.69 and 0.68 by PFS and PHU visits, respectively)(Figure 3). MetS severity in adulthood at PFS was also linked to diabetes incidence over the ensuing 6–14 years (AUC=0.89).
Figure 3. ROC Curves Displaying Predictive Value of MetS Severity Scores for Future Diabetes.
a–b.. Childhood MetS severity score (Lipid Research Clinic, 1973–1976) predicting incident diabetes by (a) Princeton Follow-up Study (PFS, 2000–2004)(area-under-the-curve [AUC]=0.69) and (b) Princeton Health Update (2010–2014)(AUC=0.68). c. Adult MetS severity score (PFS) predicting incident diabetes by PHU (AUC=0.89).
Using logistic regression, each 1.0 Z-score increase in childhood MetS severity score carried elevated odds ratios (OR’s) of 2.7 for incident diabetes by PFS (both p<0.001), and 2.8 for incident diabetes between PFS and PHU (both p<0.05)(Table 2). When change in MetS Severity Score from LRC to PFS was added to baseline LRC MetS severity score in the model, this further carried elevated OR’s of 7.3 for incident diabetes by PHU (p<0.01). AIC values indicate that compared to the use of traditional MetS criteria as a predictor of diabetes, utilizing MetS severity scores provide a better fit of outcomes at both time points, particularly when using LRC scores and the change from LRC to PFS.
Table 2. Odds of Future Disease Using Traditional MetS Criteria and MetS Severity Score.
| Incident Disease by PFS Visit: | Incident Disease by PHU Visita: | |||
|---|---|---|---|---|
| Diabetes | Diabetes | |||
| Odds Ratio (95% CI) |
p-value | Odds Ratio (95% CI) |
p-value | |
| MetS at LRC: | 4.4 (1.2, 16.4) | 0.0249 | 7.8 (1.4, 43.8) | 0.0189 |
| -- Model AIC | 238.95 | 132.39 | ||
| MetS at both time points: | ||||
| LRC | 3.5 (0.6, 20.8) | 0.1745 | ||
| PFS | 5.2 (1.8, 15.0) | 0.0023 | ||
| -- Model AIC | 124.87 | |||
| MetS Severity Score at LRC: | 2.7 (1.6, 4.4) | 0.0001 | 2.8 (1.3, 6.0) | 0.0093 |
| -- Model AIC | 227.68 | 129.01 | ||
| MetS Severity Score at: | ||||
| LRC | 7.6 (3.0, 19.4) | < 0.0001 | ||
| Change in Score (PFS – LRC) | 7.3 (3.0, 17.3) | < 0.0001 | ||
| -- Model AIC | 101.98 | |||
Abbreviations: AIC = Akaike Information Criterion
Excluding Prevalent Cases of Diabetes at PFS
Discussion
We found that the degree of severity of MetS as a linear measure in childhood and adulthood served as a predictor of future diabetes over 36-year and 11-year periods, respectively. This association was overall modest during childhood (AUC on the ROC curve of 0.68) and stronger during adulthood (AUC of 0.89). Moreover, the change in this MetS severity score from adolescence to adulthood conveyed further disease risk. In this sense the MetS severity score functioned similarly to that for the prediction of CVD [29]. Given that the current obesity epidemic begins early in life, tools are needed both to identify those at highest risk for future disease and to motivate individuals toward lifestyle changes [3]. These MetS severity scores—possibly calculated automatically using the electronic medical record—hold potential for clinical use as such a screening tool to identify individuals in childhood and mid-adulthood who are at high risk for future diabetes and would particularly benefit for preventative interventions [37]. Moreover, as a linear measure, these scores could be used to follow for within-individual changes in MetS severity, including assessing for the effectiveness of treatments to decrease MetS severity.
The majority of previous work on MetS utilized dichotomous criteria for MetS for which MetS was classified when an individual exceeded population-based cut-off values for ≥3 of the individual MetS components [4, 11, 38]. There has been some controversy regarding which of these criteria to use, both in adults[39] and among children [40]. We elected to use either the commonly employed ATP-III criteria (for adults [4]) or a version of these adapted for use in adolescents [35]—though clearly our results may have differed had we have chosen a different set of criteria. While these traditional MetS criteria were effective in the present cohort in identifying increased risk of future diabetes, with OR of 4.4 for MetS in childhood (based on relatively small numbers) and 9.7 in mid-adulthood compared to individuals without MetS, these sets of criteria are limited by 1) an inability to assess for changes in MetS over time (with the exception of its presence or absence); 2) an inability to assess risk among individuals who have measures of multiple MetS components that lie just shy of the cut-offs; and 3) attribution of equal importance to each individual MetS component, despite evidence that certain components such as elevated WC or high triacylglycerol levels are more highly associated with MetS risk over time [41, 42] and may be more tightly linked to the abnormal cellular processes underlying MetS [7]. This MetS severity score accounts for these limitations of traditional criteria, and produces a score that is linearly associated with risk for diabetes.
An additional problem with current binary criteria for MetS has been racial/ethnic variation in this score, particularly among African American males, who have a high prevalence of diabetes and death from CVD but paradoxically low prevalence of MetS by traditional criteria [18–22, 24]. It is primarily for this reason that we took race/ethnicity into account in formulating these MetS severity scores. Nevertheless, while the current cohort had both white and African American participants, there were too few study participants overall to assess for any added benefit of this score regarding racial/ethnic differences in MetS. Thus, further analyses in larger multi-ethnic cohorts are needed to confirm any potential benefit of this score on a race/ethnicity-specific basis.
While the durability of MetS within an individual has been disputed when using traditional criteria [16, 17], we found a moderate degree of association (R2 =0.18) of MetS severity scores between childhood and 26 years later. It is important to note that the equations to calculate these childhood and adult severity scores were derived separately from NHANES data, producing Z-scores of MetS severity relative to a group of adolescents age 12–19 years and a group of adults age 20–64 years. Using these measures, the present cohort at baseline (mean age 12.9 years) had a below-average MetS severity Z-score of −0.5. This lower score may have been related to the younger age of this cohort at baseline or to the lower degree of obesity seen when the cohort was evaluated in the early 1970’s (9.7%) compared to the derivation population in 1999–2010 (19.2%). As adults with mean age 38.4 years, there remained a relatively low severity Z-score of 0.1 (i.e. near the mean for all adults) that may again reflect that participants had ages in the middle of the adult range, and potentially that they had completed their childhood growth in the mid-1980s prior to the current U.S. obesity epidemic. MetS appears to be produced by genetic factors and multiple pathophysiological processes including cellular dysfunction, oxidative stress, and low-grade inflammation—processes that are also associated with insulin resistance [3, 7, 8]. These MetS severity scores had previously been shown to be associated within individuals with surrogates for these processes, including uric acid, hsCRP and fasting insulin [25, 26]. The high correlation between these scores over a 26-year span suggests a degree of durability of MetS in a given individual over time or a genetic susceptibility that is manifest already in childhood.
As a predictor of future disease, the MetS severity score functioned overall similarly in predicting diabetes as in predicting CVD [29]. Odds ratios were 2.7 and 9.8 for each 1.0 increase in the childhood MetS severity scores in predicting diabetes and CVD, respectively, by mean age 38.4 and 2.8 and 2.4 for predicting T2DM and CVD, respectively, by mean age 49.6. The improved performance in predicting CVD is in some ways surprising given that MetS has been viewed as being more strongly associated with incidence of type 2 diabetes than CVD [11]. Nevertheless, the links between childhood MetS and future disease for both diagnoses underscores the potential utility for this score in assessing future risk and motivating toward early lifestyle change.
While a key benefit of linear MetS severity scores is in following for change over time, the clinical use of cut-off values can provide added utility to determine elevated risk. We were unfortunately unable to determine cut-off values due to limitations in sample size. Further evaluations of MetS severity scores in other longitudinal cohorts are needed to generate cut-off levels corresponding to high risk for future disease. These cut-offs could then be used to guide the initiation of targeted adjunct therapies, while less severe elevations in MetS severity could serve on a larger-scale basis as a trigger for insulin-sensitizing lifestyle therapy for optimal prevention overall..
This study had several additional limitations. We lacked BP data on 185 children in LRC and imputed BP data for these participants; however, eliminating these participants from the analysis did not alter the study’s findings. Our analysis was based on an outcome (incidence of diabetes) that had occurred in only 30 individuals by PFS and 48 individuals by PHU. For the PHU study, we were unable to obtain complete follow-up and relied on self-report of outcomes in PHU, without adjudicated outcomes or in-person assessments of MetS severity status. While the cohort had both white and African American participants, Hispanic participants were lacking, reflecting the population base from which they were drawn in the early 1970’s. And. However, the study also had several strengths, including 37-year follow-up in a biracial cohort originally studied as children in the 1970’s.
In summary, we found that a sex- and race/ethnicity-specific MetS severity Z-score had modest within-person durability over a 26-year period and predicted future diabetes both based on baseline values in childhood and adulthood and based on the change in score between these time points. These data provide evidence for a role for MetS severity as a marker of disease risk and suggest potential clinical utility in following MetS severity over time. Future research in determining risk thresholds in the score is still needed.
Supplementary Material
Acknowledgements
None
Funding: This work was supported by NIH grants K08HD060739 (MDD), U54GM104942 (MJG), 1R21DK085363 (MJG and MDD), 1R01HL120960 (MJG and MDD), and NHLBI N01HV22914; a CCHMC Heart Institute Research Core grant (Cincinnati, OH); a Schmidlapp Women’s Scholar’s Award (Cincinnati, OH)(JGW); and American Heart Association grant 9750129 (Chicago, IL)(JAM). All authors were independent of these funding agencies. The funding agencies had no role in the analysis of the data or writing of the manuscript.
Abbeviations
- BP
blood pressure
- CVD
cardiovascular disease
- LRC
Lipid Research Clinic
- MetS
metabolic syndrome
- NHANES
National Health and Nutrition Examination Survey
- NHLBI
National Heart Lung and Blood Institute Lipid Research Clinic (LRC)
- PFS
Princeton Follow-up Study
- PHU
Princeton Health Update
- T2DM
type 2 diabetes mellitus
- WC
waist circumference
Footnotes
Duality of interest: The authors of this work do not have any conflicts of interest to report.
Author contributions: MDD, MJG, JGW and John A. Morrison designed the study. JGW and JAM oversaw participant recruitment and data collection. MDD and MJG planned the analysis. MJG performed the analysis. MDD, MJG, JGW and JAM wrote the manuscript. All authors have read and given final approval of the manuscript. MDD is responsible for the integrity of the work as a whole.
References
- 1.Popkin BM. Global nutrition transition and the pandemic of obesity in developing countries (vol 70, pg 3, 2012) Nutrition Reviews. 2012;70:256–256. doi: 10.1111/j.1753-4887.2011.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cefalu WT. Steps Toward the Meaningful Translation of Prevention Strategies for Type 2 Diabetes. Diabetes Care. 2012;35:663–665. doi: 10.2337/dc12-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeBoer MD. Obesity, systemic inflammation, and increased risk for cardiovascular disease and diabetes among adolescents: A need for screening tools to target interventions. Nutrition. 2013;29:379–386. doi: 10.1016/j.nut.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome - An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 5.Hunt KJ, Resendez RG, Williams K, Haffner SM, Stern MP. National Cholesterol Education Program versus World Health Organization metabolic syndrome in relation to all-cause and cardiovascular mortality in the San Antonio Heart Study. Circulation. 2004;110:1251–1257. doi: 10.1161/01.CIR.0000140762.04598.F9. [DOI] [PubMed] [Google Scholar]
- 6.Weiss R, Dziura J, Burgert TS, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350:2362–2374. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 7.de Ferranti S, Mozaffarian D. The perfect storm: obesity, adipocyte dysfunction, and metabolic consequences. Clin Chem. 2008;54:945–955. doi: 10.1373/clinchem.2007.100156. [DOI] [PubMed] [Google Scholar]
- 8.Tilg H, Moschen AR. Inflammatory mechanisms in the regulation of insulin resistance. Mol Med. 2008;14:222–231. doi: 10.2119/2007-00119.Tilg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morrison JA, Friedman LA, Wang P, Glueck CJ. Metabolic syndrome in childhood predicts adult metabolic syndrome and type 2 diabetes mellitus 25 to 30 years later. J Pediatr. 2008;152:201–206. doi: 10.1016/j.jpeds.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Morrison JA, Friedman LA, Gray-McGuire C. Metabolic syndrome in childhood predicts adult cardiovascular disease 25 years later: The Princeton Lipid Research Clinics follow-up study. Pediatrics. 2007;120:340–345. doi: 10.1542/peds.2006-1699. [DOI] [PubMed] [Google Scholar]
- 11.Wannamethee SG, Shaper AG, Lennon L, Morris RW. Metabolic syndrome vs Framingham Risk Score for prediction of coronary heart disease, stroke, and type 2 diabetes mellitus. Arch Intern Med. 2005;165:2644–2650. doi: 10.1001/archinte.165.22.2644. [DOI] [PubMed] [Google Scholar]
- 12.Golden SH, Folsom AR, Coresh J, Sharrett AR, Szklo M, Brancati F. Risk factor groupings related to insulin resistance and their synergistic effects on subclinical atherosclerosis - The Atherosclerosis Risk in Communities Study. Diabetes. 2002;51:3069–3076. doi: 10.2337/diabetes.51.10.3069. [DOI] [PubMed] [Google Scholar]
- 13.Simons LA, Simons J, Friedlander Y, McCallum J. Is Prediction of Cardiovascular Disease and All-cause Mortality Genuinely Driven by the Metabolic Syndrome, and Independently from its Component Variables? The Dubbo Study. Heart Lung and Circulation. 2011;20:214–219. doi: 10.1016/j.hlc.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 14.McNeill AM, Schmidt MI, Rosamond WD, et al. The metabolic syndrome and 11-year risk of incident cardiovascular disease in the atherosclerosis risk in communities study. Diabetes Care. 2005;28:385–390. doi: 10.2337/diacare.28.2.385. [DOI] [PubMed] [Google Scholar]
- 15.Malik S, Wong ND, Franklin SS, et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110:1245–1250. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- 16.Gustafson JK, Yanoff LB, Easter BD, et al. The stability of metabolic syndrome in children and adolescents. J Clin Endocrinol Metab. 2009;94:4828–4834. doi: 10.1210/jc.2008-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li C, Ford ES, Huang TTK, Sun SS, Goodman E. Patterns of change in cardiometabolic risk factors associated with the metabolic syndrome among children and adolescents: the Fels Longitudinal Study. The Journal of pediatrics. 2009;155:S5.e9–S16.e9. doi: 10.1016/j.jpeds.2009.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker SE, Gurka MJ, Oliver MN, Johns DW, DeBoer MD. Racial/ethnic discrepancies in the metabolic syndrome begin in childhood and persist after adjustment for environmental factors. Nutrition Metabolism and Cardiovascular Diseases. 2012;22:141–148. doi: 10.1016/j.numecd.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeBoer MD, Dong L, Gurka MJ. Racial/Ethnic and Sex Differences in the Ability of Metabolic Syndrome Criteria to Predict Elevations in Fasting Insulin Levels in Adolescents. Journal of Pediatrics. 2011;159:975-U141. doi: 10.1016/j.jpeds.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeBoer MD, Gurka MJ, Sumner AE. Diagnosis of the Metabolic Syndrome Is Associated With Disproportionately High Levels of High-Sensitivity C-Reactive Protein in Non-Hispanic Black Adolescents: An analysis of NHANES 1999–2008. Diabetes Care. 2011;34:734–740. doi: 10.2337/dc10-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeBoer MD, Gurka MJ. Low sensitivity for the metabolic syndrome to detect uric acid elevations in females and non-Hispanic-black male adolescents: An analysis of NHANES 1999–2006. Atherosclerosis. 2012;220:575–580. doi: 10.1016/j.atherosclerosis.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111:1233–1241. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 23.Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988–2006. Diabetes Care. 2010;33:562–568. doi: 10.2337/dc09-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sumner AE. Ethnic Differences in Triglyceride Levels and High-Density Lipoprotein Lead to Underdiagnosis of the Metabolic Syndrome in Black Children and Adults. Journal of Pediatrics. 2009;155:e7–e11. doi: 10.1016/j.jpeds.2009.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gurka MJ, Ice CL, Sun SS, DeBoer MD. A confirmatory factor analysis of the metabolic syndrome in adolescents: an examination of sex and racial/ethnic differences. Cardiovascular Diabetology. 2012;11 doi: 10.1186/1475-2840-11-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gurka MJ, Lilly CL, Oliver MN, DeBoer MD. An examination of sex and racial/ethnic differences in the metabolic syndrome among adults: A confirmatory factor analysis and a resulting continuous severity score. Metabolism-Clinical and Experimental. 2014;63:218–225. doi: 10.1016/j.metabol.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eisenmann JC. On the use of a continuous metabolic syndrome score in pediatric research. Cardiovascular Diabetology. 2008;7:17. doi: 10.1186/1475-2840-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okosun IS, Lyn R, Davis-Smith M, Eriksen M, Seale P. Validity of a Continuous Metabolic Risk Score as an Index for Modeling Metabolic Syndrome in Adolescents. Annals of Epidemiology. 2010;20:843–851. doi: 10.1016/j.annepidem.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 29.DeBoer MD, Gurka MJ, Woo JG, Morrison JA. Severity of Metabolic Syndrome as a Predictor of Cardiovascular Disease Between Childhood and Adulthood: The Princeton Lipid Research Cohort Study. J Amer Coll Card. 2015 doi: 10.1016/j.jacc.2015.05.061. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morrison J, Degroot I, Kelly K, Mellies M, Glueck CJ. PARENT-CHILD ASSOCIATIONS - CHOLESTEROL AND TRIGLYCERIDE. Circulation. 1977;56:20–20. [Google Scholar]
- 31.Woo JG, Morrison JA, Stroop DM, Aronson Friedman L, Martin LJ. Genetic architecture of lipid traits changes over time and differs by race: Princeton Lipid Follow-up Study. J Lipid Res. 2014;55:1515–1524. doi: 10.1194/jlr.M049932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laskarzewski P, Morrison JA, Mellies MJ, et al. RELATIONSHIPS OF MEASUREMENTS OF BODY-MASS TO PLASMA-LIPOPROTEINS IN SCHOOL-CHILDREN AND ADULTS. American Journal of Epidemiology. 1980;111:395–406. doi: 10.1093/oxfordjournals.aje.a112914. [DOI] [PubMed] [Google Scholar]
- 33.Barthelmai W, Czok R. Enzymatische Bestimmungen der Glucose in Blut, Liquor und Harn. Klin Wochschr. 1965;40:585. [Google Scholar]
- 34.Sacks DB. Carbohydrates. WB Saunders: Philadelphia; 1999. [Google Scholar]
- 35.Ford ES, Li C, Cook S, Choi HK. Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation. 2007;115:2526–2532. doi: 10.1161/CIRCULATIONAHA.106.657627. [DOI] [PubMed] [Google Scholar]
- 36.The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 37.Bassi N, Karaqodin I, Wang S, et al. Lifestyle Modification for Metabolic Syndrome: A Systematic Review. Am J Med. 2014 doi: 10.1016/j.amjmed.2014.06.035. e-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 38.Mottillo S, Filion KB, Genest J, et al. The Metabolic Syndrome and Cardiovascular Risk A Systematic Review and Meta-Analysis. Journal of the American College of Cardiology. 2010;56:1113–1132. doi: 10.1016/j.jacc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 39.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 40.DeBoer MD, Gurka MJ. Ability among adolescents for the metabolic syndrome to predict elevations in factors associated with type 2 diabetes and cardiovascular disease: data from the national health and nutrition examination survey 1999–2006. Metab Syndr Relat Disord. 2010;8:343–353. doi: 10.1089/met.2010.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janiszewski PM, Janssen I, Ross R. Does waist circumference predict diabetes and cardiovascular disease beyond commonly evaluated cardiometabolic risk factors? Diabetes Care. 2007;30:3105–3109. doi: 10.2337/dc07-0945. [DOI] [PubMed] [Google Scholar]
- 42.Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. Jama-Journal of the American Medical Association. 2007;298:299–308. doi: 10.1001/jama.298.3.299. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.