Abstract
The aim of the present study was to investigate the inhibitory effects of 90Sr-90Y β-irradiation in a rat model of alkali burn-induced corneal neovascularization (CNV). Alkali burn-induced CNV was induced in the right eyes of 30 female Wistar rats, which were randomly divided into the following three groups (n=10/group): i) The alkali burn control group, which received a balanced salt solution treatment; ii) group 1, which received treatment with angiogenesis inhibitors; and iii) group 2, which received 90Sr-90Y β-irradiation treatment. A further 10 female Wistar rats comprised a blank control group and received only balanced salt solution. Digital photographs of the corneas were acquired and the area of NV was calculated. In addition, the expression levels of matrix metalloproteinase (MMP)-9, vascular endothelial growth factor (VEGF), VEGF receptor (VEGFR)-1 and VEGFR-2 in alkali-burned rat corneas were determined using western blot analysis. The results suggested that the number of new vessels and the area of CNV were significantly decreased in groups 1 and 2, as compared with the alkali burn group at each time point (P<0.05). In addition, the number of inflammatory cells and the degree of edema were decreased in groups 1 and 2, as compared with the alkali burn group, with group 2 exhibiting the most marked reduction. Western blot analysis demonstrated that the expression levels of MMP-9, VEGF, VEGFR-1 and VEGFR-2 were significantly decreased in groups 1 and 2, as compared with the alkali burn control group, with group 2 exhibiting the most significant reduction (P<0.05). The results of the present study suggested that 90Sr-90Y β-irradiation and angiogenesis inhibitor treatments were able to inhibit alkali burn-induced CNV, although 90Sr-90Y β-irradiation may be more effective.
Keywords: corneal, 90Sr-90Y, alkali burn, neovascularization, β-irradiation
Introduction
Corneal neovascularization (CNV) is typically associated with inflammatory, infectious, traumatic, toxic, degenerative or immunological disorders of the ocular surface and cornea (1,2). CNV may result in significant visual impairment and blindness, due to edema, scar formation or lipid deposition (3,4). The regulation of corneal angiogenesis is known to be a complex multistep process controlled by stimulatory and inhibitory factors (5). Among various other proangiogenic factors, the vascular endothelial growth factor (VEGF) family serves a crucial function in stimulating the multiplication of endothelial cells and the formation of new blood vessels (6). Furthermore, VEGF inhibitors have exhibited potential for the treatment of CNV through the direct inhibition of angiogenesis at a molecular level (3). In addition, numerous pharmacological agents, including glucocorticosteroids, interleukin-1 receptor antagonist, cyclosporine A, plasminogen fragments, doxycycline and triamcinolone acetonide, appear to exhibit anti-angiogenic activity (7–12). However, there is currently no clear consensus regarding the most effective treatment option for CNV, which underlines the requirement for novel therapies for the treatment of CNV.
90Sr-90Y β-irradiation has been widely used for the treatment of various diseases, including coronary artery in-stent restenosis, post-operative scar hyperplasia and skin hemangioma (13–15). Notably, a previous study demonstrated that the inhibition of the budding process of CNV pathogenesis via β-irradiation may limit the formation and development of new corneal blood vessels (16); however, the effect of 90Sr-90Y β-irradiation in the cornea, and specifically in animal models of CNV, has not yet been described. The aim of the present study was to evaluate the safety and efficacy of 90Sr-90Y β-irradiation in an experimental rat model of alkali burn-induced CNV.
Materials and methods
Materials
The 90Sr-90Y ophthalmic applicator (SSR9013) used in the present study was provided by the China Institute of Atomic Energy (Beijing, China). The slit-lamp microscope was purchased from Topcon Corporation (Tokyo, Japan) and the BX41-72H02 binocular optical microscope was obtained from Olympus Corporation (Tokyo, Japan).
Animals
A total of 40 female Wistar rats (age, 55–60 weeks; weight, 200–250 g) obtained from the Experimental Animal Center of Changchun Institute for Biological Sciences (Changchun, China) were used in the present study. Approval of the experimental protocol was obtained from the Jilin University Medical School Research Committee (Changchun, China). The rats were treated and maintained in accordance with the guidelines of the Statement for the Use of Animals in Ophthalmic and Visual Research by the Association for Research in Vision and Ophthalmology (17). The present study was approved by the Ethics Committee of Jilin University. The rats were stored in a specific pathogen-free facility, within filter-topped cages, under a 12 h light/dark cycle at room temperature and 50–60% humidity. The rats had ad libitum access to standard rodent chow and water throughout the study.
Alkali-induced corneal injury model and drug treatment protocol
A study population of 30 female Wistar rats were anesthetized with an intraperitoneal injection of ketamine hydrochloride (25 mg/kg) and xylazine hydrochloride (5 mg/kg; both Sigma-Aldrich, St. Louis, MO, USA). All eyes were examined under a binocular microscope to exclude corneal scaring, opacity and NV prior to the study. Corneal injury was induced by placing a monolayer filter saturated with 1 mol/l NaOH onto the right eye of the rat for 2 min, as previously described (18–20). Following the establishment of the alkali burn corneal injury, the 30 alkali-injured rats were allocated at random into three groups: Alkali burn control group, which received, 3 drops of balanced salt solution (Sigma-Aldrich) 3 times a day for 7 days in the alkali-treated eyes; group 1, which received 1% cyclosporine (Sigma-Aldrich) from day 1 following alkali injury, 3 drops 3 times a day for 7 days in the alkali-treated eyes; and group 2, which received 90Sr-90Y β-irradiation from day 1 following alkali injury, 1 Gy once a day for 7 days in the alkali-treated eyes. In addition, 10 Wistar rats which did not receive any treatment were selected as the alkali burn control group, receiving 3 drops of the balanced salt solution, 3 times a day for 7 days).
Evaluation of CNV
The CNV and edema formation in each group under anesthesia was observed using the slit-lamp microscope on days 2, 5 and 7 following the experiment. The average NV length (VL), corneal radius (r) and corneal hours (CH) were calculated. The NV area was measured according to the following formula (21): Area (mm2) = CH/12 × 3.14[r2-(r-VL)2].
Photographic analysis
All rats were sacrificed by exsanguination on day 7 immediately followed by observation using the slit-lamp microscope. Briefly, the eyes were enucleated and the globes were fixed in freshly prepared 4% paraformaldehyde. Following fixation for 24 h, corneal samples were prepared by macroscopic incisions from limbus to limbus passing through the central cornea to include the region with the highest NV intensity. Subsequently, fixed tissues were sectioned serially in the horizontal plane at 4 µm. In the majority of sections, the NV density was obtained from the central region of the cornea. The sections were stained with hematoxylin and eosin (H&E; Sigma-Aldrich). The degree of CNV was evaluated histomorphometrically using the optical microscope, as described in a previous study (22). In addition, the inflammatory index was evaluated using slit-lamp biomicroscopy, and inflammatory cells that had infiltrated into the cornea tissue were detected by histological analysis at days 1, 7 and 14 following the alkali burn, as previously described (23).
Western blot analysis
The rats were sacrificed by exsanguination and the corneas harvested from the treated eyes were dissected and frozen at −70°C, then homogenized in ice-cold RIPA lysis buffer solution (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). Following centrifugation for 5 min at 12,000 × g, the supernatants were collected and the protein concentrations were determined using the Bradford reagent (Sigma-Aldrich Chemie GmbH, Steinheim, Germany). Equal quantities of protein (15 µg/lane) from the cell lysates were separated using 8–15% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose membranes (Santa Cruz Biotechnology, Inc.). Membranes were incubated for 2 h in phosphate-buffered saline plus 0.1% Tween-20 and 5% non-fat skim milk to block non-specific binding. The membranes were then incubated overnight at 4°C with the following antibodies: Goat monoclonal anti-mouse VEGF (1:2,000; sc-7269; Santa Cruz Biotechnology, Inc.), goat monoclonal anti-mouse VEGF receptor (VEGFR)-1 (1:3,000; 4762; Sigma-Aldrich), goat monoclonal anti-mouse VEGFR-2 (1:1,000; 3817; Cell Signaling Technology, Danvers, MA, USA.), goat monoclonal anti-mouse matrix metalloproteinase (MMP)-9 (1:2,000; sc-21733; Santa Cruz Biotechnology, Inc.), and goat monoclonal anti-mouse β-actin (1:10,000; sc-47778; Santa Cruz Biotechnology, Inc.), which was used as a loading control. Subsequently, the membranes were incubated with the anti-mouse horseradish peroxidase-conjugated immunoglobulin G (1:10,000; sc-2005; Santa Cruz Biotechnology, Inc.), and protein bands were visualized using a SuperSignal chemiluminescence detection kit (Thermo Fisher Scientific, Inc., Rockford, Illinois, USA).
Statistical analysis
Data are presented as the mean ± standard deviation. The statistical comparison among >2 groups was performed using one-way analysis of variance followed by the Tukey's post-hoc test. Statistical analysis was performed using SPSS software, version 16.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism, version 5.01 (GraphPad Software Inc., San Diego, CA, USA) for Windows. P<0.05 was considered to indicate a statistically significant difference.
Results
Anti-NV effects of 90Sr-90Y β-irradiation in the cornea
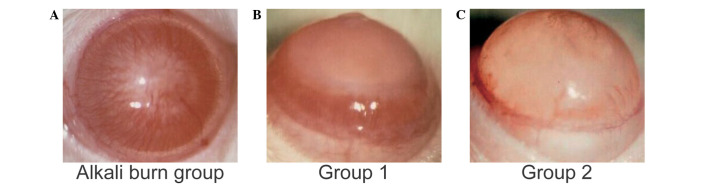
The clinical indication of CNV was examined first. The results showed that numerous new vessels had invaded the suture area from the limbal region in the alkali burn control group on day 7 (Fig. 1A). The rats treated with angiogenesis inhibitors (group 1) and 90Sr-90Y β-irradiation (group 2) exhibited reduced CNV (Fig. 1B and C), as compared with the alkali burn control group (Fig. 1A). The results for the NV length and area revealed that angiogenesis inhibitors (group 1) and 90Sr-90Y β-irradiation (group 2) treatment significantly reduced the CNV at the various time points, compared with the alkali burn control group (Tables I and II); however, the CNV length and area in group 1 were significantly decreased, as compared with group 2 on day 2 (P<0.05; Tables I and II), and increased on days 5 and 7 (P<0.05; Tables I and II).
Figure 1.
Effect of 90Sr-90Y β-irradiation treatment on alkali burn-induced corneal neovascularization (CNV) in rats. Following the induction of alkali burn injury in the corneas of the rats, NV was monitored using a slip-lamp microscope. Representative photographs of alkali burn-induced CNV at 7 days following induction in the (A) alkali burn control group, (B) group 1 and (C) group 2. The Alkali burn control group was treated with balanced salt solution. Group 1 received angiogenesis inhibitors treatment and group 2 received 90Sr-90Y β-irradiation treatment.
Table I.
Average length of CNV among the different groups at each time-point (n=10 per group).
| Average length of CNV (mm) | |||
|---|---|---|---|
| Group | Day 2 | Day 5 | Day 7 |
| Alkali burn group | 0.410±0.024 | 1.980±0.015 | 2.580±0.037 |
| Group 1 | 0.278±0.025a | 1.678±0.017a | 2.178±0.032a |
| Group 2 | 0.352±0.021ab | 1.482±0.030ab | 1.882±0.033ab |
Data are presented as the mean ± standard deviation.
P<0.05 vs. the alkali burn control group;
P<0.05 vs. group 1. CNV, corneal neovascularization.
Table II.
Average area of CNV among the different groups at each time-point (n=10 per group).
| Average area of CNV (mm2) | |||
|---|---|---|---|
| Group | Day 2 | Day 5 | Day 7 |
| Alkali burn group | 4.596±0.184 | 14.516±0.112 | 21.739±0.209 |
| Group 1 | 2.167±0.181a | 12.465±0.132a | 17.851±0.199a |
| Group 2 | 3.471±0.178ab | 10.499±0.202ab | 14.471±0.211ab |
Data are presented as the mean ± standard deviation.
P<0.05 vs. the alkali burn control group;
P<0.05 vs. group 1. CNV, corneal neovascularization.
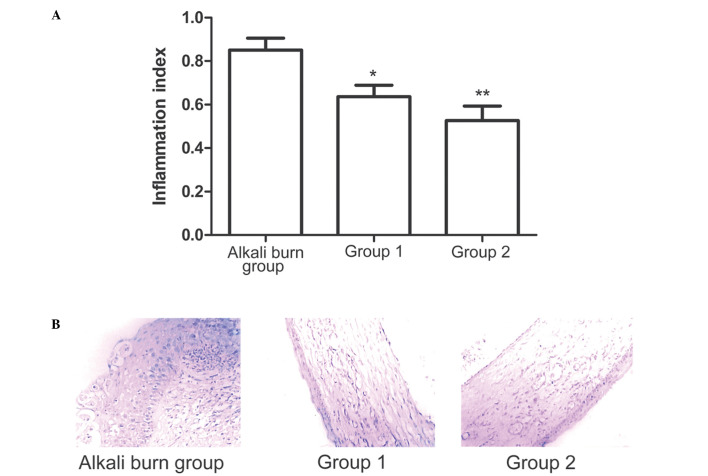
Anti-inflammatory effects of 90Sr-90Y β-irradiation in the cornea
Corneal inflammation was detected and analyzed simultaneously. The inflammatory index results demonstrated that the treatments administered in groups 1 and 2 significantly reduced inflammation on day 7 compared with the alkali burn control group (Fig. 2A). The the most marked reduction in the number of inflammatory cells and the degree of edema was observed in group 2. Histological examination of H&E staining showed that the alkali burn control group exhibited increased inflammatory cell infiltration in the corneal stroma compared with the other groups (Fig. 2B), while the treatments administered in groups 1 and 2 decreased inflammatory cell infiltration (Fig. 2B). In addition, the angiogenesis inhibitors and 90Sr-90Y β-irradiation treatment resulted in reduced corneal edema compared with the alkali burn control group.
Figure 2.
Anti-inflammatory effects of β-irradiation treatment in the cornea. (A) Statistical analysis of clinical inflammatory index data among the three groups on day 7 following treatment. The alkali burn control group was treated with balanced salt solution. Group 1 received angiogenesis inhibitors treatment and group 2 received 90Sr-90Y β-irradiation treatment. (B) Representative images of hematoxylin and eosin staining of the central cornea on day 7. *P<0.05 and **P<0.01 vs. alkali burn control group.
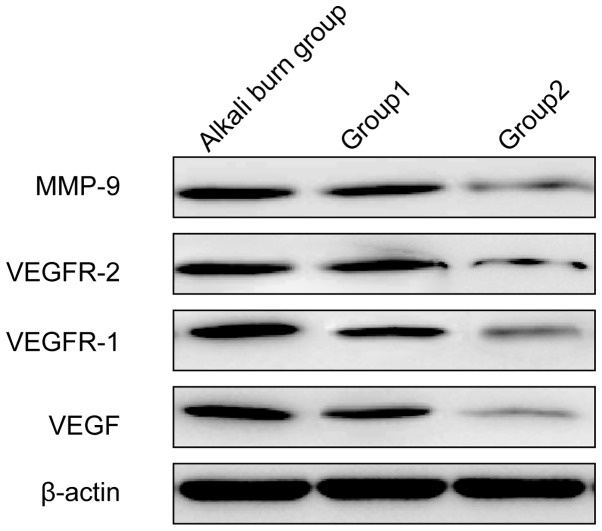
Inhibition of angiogenic factor expression in alkali-injured corneas by 90Sr-90Y β-irradiation
The balance of proangiogenic factors is crucial for angiogenesis. Therefore, the effect of 90Sr-90Y β-irradiation on the expression levels of a number of proangiogenic factors in alkali-burn corneal wounds was examined. The expression of MMP-9, VEGF, VEGFR-1 and VEGFR-2 was measured using western blot analysis. The angiogenesis inhibitors (group 1) and 90Sr-90Y β-irradiation (group 2) appeared to produce marked reductions in the expression levels of MMP-9, VEGF, VEGFR-1 and VEGFR-2 compared with the alkali burn control group (Fig. 3). Compared with the treatment in group 1, the treatment in group 2 appeared to produce reductions in the protein expression levels of MMP-9, VEGF, VEGFR-1 and VEGFR-2.
Figure 3.
Effect of 90Sr-90Y β-irradiation treatment on the protein expression of neovascularization-related signal molecules in the cornea. β-Actin served as an internal standard. The alkali burn control group was treated with balanced salt solution. Group 1 received angiogenesis inhibitors treatment and group 2 90Sr-90Y β-irradiation treatment. MMP-9, matrix metalloproteinase-9; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor.
Discussion
CNV is able to cause edema, scar formation or lipid deposition, resulting in significant visual impairment and blindness (3). Therefore, treating this potentially blinding condition is of clinical significance (24). Previous studies have shown that thalidomide, photodynamic therapy, steroids, cyclosporine, conjunctival limbal allograft and fine needle diathermy may exert inhibitory effects against CNV (25–29); however, these drugs are not always effective and may result in complications (30). Radiation therapy is widely used to treat post-operative scar hyperplasia and skin hemangioma (14,15), which suggests that it may exert an inhibitory effect against CNV. The aim of the present study was to compare the treatment efficacy of 90Sr-90Y β-irradiation with that of angiogenesis inhibitors in a rat model of alkali burn-induced CNV, and to evaluate the inhibitory effects of 90Sr-90Y β-irradiation on CNV. The present results showed that 90Sr-90Y β-irradiation exhibits inhibitory effects on alkali burn-induced CNV, which were found to be superior to those of the angiogenesis inhibitor cyclosporine.
Alkali burn may result in corneal endothelial cell division and angiogenesis (31). The growth trend of NV in the angiogenesis inhibitors and 90Sr-90Y β-irradiation groups were similar to that of the alkali burn control group, but with a limited degree of inhibition at different time points (P<0.05). The curative effect of angiogenesis inhibitors was more marked compared with that of 90Sr-90Y β-irradiation in the early stage of treatment (P<0.05), while the opposite occurred in the later stage (P<0.05). These results suggested that 90Sr-90Y β-irradiation was more effective compared with angiogenesis inhibitors in inhibiting alkali burn-induced CNV. The phenomenon in the early stage of treatment could be explained by the interference of 1% cyclosporine with certain cytokines associated with angiogenesis, thus achieving a rapid biological effect (32).
The corneal of rats in the alkali burn control group exhibited marked NV and numerous inflammatory cells, and presented obvious edema. These pathological manifestations were consistent with the results of a previous study (33). In the 90Sr-90Y β-irradiation group, the degree of NV and edema, in addition to the number of inflammatory cells, were reduced compared with the angiogenesis inhibitors group. These results implied that 90Sr-90Y β-irradiation was more effective in inhibiting CNV compared with angiogenesis inhibitors. This may be explained by the biological effects of ionizing radiation, which is able to damage DNA in endothelial cells of cornea angiogenesis, inhibit cell proliferation and accelerate apoptosis, which caused the vascular permeability to decrease and capillaries to atrophy. Furthermore, the superiority of 90Sr-90Y β-irradiation may be a result of the ability of ionizing radiation to reduce plasma leakage and corneal inflammation, thus reducing cytokine expression and leading to a subsequent reduction in CNV (34).
To investigate the effects of the 90Sr-90Y β-irradiation on mediators responsible for angiogenic activity, the expression levels of VEGF, VEGFR-1, VEGFR-2 and MMP-9 were investigated in the present study. It is generally acknowledged that an upregulation of angiogenic factors occurs during CNV (35). VEGF is a major mediator of the process of angiogenesis and is crucially involved in the development of NV (36). Two high-affinity receptor tyrosine kinases, soluble VEGFR-1 and VEGFR-2 have been shown to regulate the angiogenic activity of VEGF (37). Therapeutic strategies involving the targeting of VEGF, in order to inhibit the cascade of neovascular formation, have recently been investigated (3). Abnormal MMPs are potent proangiogenic factors and are known to be involved in CNV progression (38). The present study demonstrated that 90Sr-90Y β-irradiation suppressed the expression of VEGF, VEGFR-1, VEGFR-2 and MMP-9, suggesting that 90Sr-90Y β-irradiation may inhibit CNV by downregulating the expression of angiogenic factors.
In conclusion, the results of the present study indicate that 90Sr-90Y β-irradiation and angiogenesis inhibitors have inhibitory effects on CNV induced by alkali burn, with the former being more effective, suggesting that 90Sr-90Y β-irradiation possesses therapeutic potential for CNV. Although the short-term inhibiting effect of 90Sr-90Y β-irradiation in Wistar rats was promising, its long-term effect and mechanism remains to be elucidated.
Acknowledgements
The authors thank the National Natural Science Foundation of China projects (grant no. 81271606) and Research Fund of the Science and Technology Department of Jilin Province (grant no. 201015185 and 201201041) for the financial support.
References
- 1.Siemerink MJ, Augustin AJ, Schlingemann RO. Mechanisms of ocular angiogenesis and its molecular mediators. Dev Ophthalmol. 2010;46:4–20. doi: 10.1159/000320006. [DOI] [PubMed] [Google Scholar]
- 2.Maddula S, Davis DK, Maddula S, Burrow MK, Ambati BK. Horizons in therapy for corneal angiogenesis. Ophthalmology. 2011;118:591–599. doi: 10.1016/j.ophtha.2011.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang JH, Garg NK, Lunde E, Han KY, Jain S, Azar DT. Corneal neovascularization, An anti-VEGF therapy review. Surv Ophthalmol. 2012;57:415–429. doi: 10.1016/j.survophthal.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang JH, Gabison EE, Kato T, Azar DT. Corneal neovascularization. Curr Opin Ophthalmol. 2001;12:242–249. doi: 10.1097/00055735-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Hsu CC, Chang HM, Lin TC, Hung KH, Chien KH, Chen SY, Chen SN, Chen YT. Corneal neovascularization and contemporary antiangiogenic therapeutics. J Chin Med Assoc. 2015;78:323–330. doi: 10.1016/j.jcma.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Morabito A, De Maio E, Di Maio M, Normanno N, Perrone F. Tyrosine kinase inhibitors of vascular endothelial growth factor receptors in clinical trials: Current status and future directions. Oncologist. 2006;11:753–764. doi: 10.1634/theoncologist.11-7-753. [DOI] [PubMed] [Google Scholar]
- 7.Phillips K, Arffa R, Cintron C, Rose J, Miller D, Kublin CL, Kenyon KR. Effects of prednisolone and medroxyprogesterone on corneal wound healing, ulceration, and neovascularization. Arch Ophthalmol. 1983;101:640–643. doi: 10.1001/archopht.1983.01040010640024. [DOI] [PubMed] [Google Scholar]
- 8.Dana R. Comparison of topical interleukin-1 vs tumor necrosis factor-alpha blockade with corticosteroid therapy on murine corneal inflammation neovascularization, and transplant survival (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2007;105:330–343. [PMC free article] [PubMed] [Google Scholar]
- 9.Lipman RM, Epstein RJ, Hendricks RL. Suppression of corneal neovascularization with cyclosporine. Arch Ophthalmol. 1992;110:405–407. doi: 10.1001/archopht.1992.01080150103037. [DOI] [PubMed] [Google Scholar]
- 10.Li C, Li L, Cheng R, Dai Z, Li C, Yao Y, Zhou T, Yang Z, Gao G, Yang X. Acidic/neutral amino acid residues substitution in NH2 terminal of plasminogen kringle 5 exerts enhanced effects on corneal neovascularization. Cornea. 2013;32:680–688. doi: 10.1097/ICO.0b013e3182781ec9. [DOI] [PubMed] [Google Scholar]
- 11.Jovanovic V, Nikolic L. The effect of topical doxycycline on corneal neovascularization. Curr Eye Res. 2014;39:142–148. doi: 10.3109/02713683.2013.833246. [DOI] [PubMed] [Google Scholar]
- 12.Mehrjardi HZ, Ghaffari R, Mahbod M, Hashemi H. Triamcinolone acetonide as an adjunct to bevacizumab for prevention of corneal neovascularization in a rat model. J Ophthalmic Vis Res. 2014;9:162–168. [PMC free article] [PubMed] [Google Scholar]
- 13.Schiele TM, Herbst J, Pӧllinger B, Rieber J, Kӧnig A, Sohn HY, Krötz F, Leibig M, Belka C, Klauss V. Late and very late catch-up after 90Sr/90Y beta-irradiation for the treatment of coronary in-stent restenosis. Acute Card Care. 2011;13:9–13. doi: 10.3109/17482941.2010.532221. [DOI] [PubMed] [Google Scholar]
- 14.Shi CB, Yuan B, Lu JR, Xu JL, Yang WD, Deng JL, Wang J. Continuous low-dose-rate radiation of radionuclide phosphorus-32 for hemangiomas. Cancer Biother Radiopharm. 2012;27:198–203. doi: 10.1089/cbr.2011.0995. [DOI] [PubMed] [Google Scholar]
- 15.Huang CM, Lee KW, Huang CJ. Radiation therapy for life-threatening huge laryngeal hemangioma involving pharynx and parapharyngeal space. Head Neck. 2013;35:E98–E101. doi: 10.1002/hed.21919. [DOI] [PubMed] [Google Scholar]
- 16.Feng K, Yang J, Li B. An investigation on morphology of experimental corneal neovascularization. Zhonghua Yan Ke Za Zhi. 2001;37:384–386. (In Chinese) [PubMed] [Google Scholar]
- 17.British Photobiology Society; Association for Research in Vision and Ophthalmology. Proceedings of a meeting on visual sensitivity and adaptation. 1979;19:351–440. Sponsored by the British Photobiology Society and the Association for Research in Vision and Ophthalmology Inc. held at the University of Surrey Guildford, 19-23 September, 1978. Vision research. [PubMed] [Google Scholar]
- 18.Lu P, Li L, Mukaida N, Zhang X. Alkali-induced corneal neovascularization is independent of CXCR2-mediated neutrophil infiltration. Cornea. 2007;26:199–206. doi: 10.1097/01.ico.0000248385.16896.34. [DOI] [PubMed] [Google Scholar]
- 19.Zhou WJ, Liu GQ, Li LB, Zhang XG, Lu PR. Inhibitory effect of CCR3 signal on alkali-induced corneal neovascularization. Int J Ophthalmol. 2012;5:251–257. doi: 10.3980/j.issn.2222-3959.2012.03.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Q, Yang L, Qu M, Wang Y, Chen P, Wang Y, Shi W. Role of senescent fibroblasts on alkali-induced corneal neovascularization. J Cell Physiol. 2012;227:1148–1156. doi: 10.1002/jcp.22835. [DOI] [PubMed] [Google Scholar]
- 21.Needleman P, Turk J, Jakschik BA, Morrison AR, Lefkowith JB. Arachidonic acid metabolism. Annu Rev Biochem. 1986;55:69–102. doi: 10.1146/annurev.bi.55.070186.000441. [DOI] [PubMed] [Google Scholar]
- 22.Nakayama M, Iejima D, Akahori M, Kamei J, Goto A, Iwata T. Overexpression of HtrA1 and exposure to mainstream cigarette smoke leads to choroidal neovascularization and subretinal deposits in aged mice. Invest Ophthalmol Vis Sci. 2014;55:6514–6523. doi: 10.1167/iovs.14-14453. [DOI] [PubMed] [Google Scholar]
- 23.Peng LH, Shen W, Yong W, Lu L, Liu L. Effects of AMD3100 subconjunctival injection on alkali burn induced corneal neovascularization in mice. Int J Ophthalmol. 2011;4:44–48. doi: 10.3980/j.issn.2222-3959.2011.01.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bachmann B, Taylor RS, Cursiefen C. The association between corneal neovascularization and visual acuity, A systematic review. Acta Ophthalmol. 2013;91:12–19. doi: 10.1111/j.1755-3768.2011.02312.x. [DOI] [PubMed] [Google Scholar]
- 25.Kruse FE, Joussen AM, Rohrschneider K, Becker MD, Vӧlcker HE. Thalidomide inhibits corneal angiogenesis induced by vascular endothelial growth factor. Graefes Arch Clin Exp Ophthalmol. 1998;236:461–466. doi: 10.1007/s004170050106. [DOI] [PubMed] [Google Scholar]
- 26.Gohto Y, Obana A, Kanai M, Nagata S, Nakajima S, Miki T. Treatment parameters for selective occlusion of experimental corneal neovascularization by photodynamic therapy using a water soluble photosensitizer, ATX-S10(Na) Exp Eye Res. 2001;72:13–22. doi: 10.1006/exer.2000.0931. [DOI] [PubMed] [Google Scholar]
- 27.Haynes WL, Proia AD, Klintworth GK. Effect of inhibitors of arachidonic acid metabolism on corneal neovascularization in the rat. Invest Ophthalmol Vis Sci. 1989;30:1588–1593. [PubMed] [Google Scholar]
- 28.Daya SM, Ilari FA. Living related conjunctival limbal allograft for the treatment of stem cell deficiency. Ophthalmology. 2001;108:126–134. doi: 10.1016/S0161-6420(00)00475-9. [DOI] [PubMed] [Google Scholar]
- 29.Faraj LA, Elalfy MS, Said DG, Dua HS. Fine needle diathermy occlusion of corneal vessels. Br J Ophthalmol. 2014;98:1287–1290. doi: 10.1136/bjophthalmol-2014-304891. [DOI] [PubMed] [Google Scholar]
- 30.Williams KA, Irani YD, Klebe S. Novel therapeutic approaches for corneal disease. Discov Med. 2013;15:291–299. [PubMed] [Google Scholar]
- 31.Giacomini C, Ferrari G, Bignami F, Rama P. Alkali burn versus suture-induced corneal neovascularization in C57BL/6 mice, An overview of two common animal models of corneal neovascularization. Exp Eye Res. 2014;121:1–4. doi: 10.1016/j.exer.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 32.Navarro-Antolín J, Redondo-Horcajo M, Zaragoza C, Alvarez-Barrientos A, Fernández AP, León-Gómez E, Rodrigo J, Lamas S. Role of peroxynitrite in endothelial damage mediated by Cyclosporine A. Free Radic Biol Med. 2007;42:394–403. doi: 10.1016/j.freeradbiomed.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 33.Li X, Li NC, Wang G, Na YQ. Effect and safety of transrectal 137 CS gamma-rays in the treatment of benign prostatic hyperplasia. Zhonghua Nan Ke Xue. 2006;12:525–527. (In Chinese) [PubMed] [Google Scholar]
- 34.Onoda JM, Kantak SS, Diglio CA. Radiation induced endothelial cell retraction in vitro, Correlation with acute pulmonary edema. Pathol Oncol Res. 1999;5:49–55. doi: 10.1053/paor.1999.0049. [DOI] [PubMed] [Google Scholar]
- 35.Martin G, Schlunck G, Hansen LL, Agostini HT. Differential expression of angioregulatory factors in normal and CNV-derived human retinal pigment epithelium. Graefes Arch Clin Exp Ophthalmol. 2004;242:321–326. doi: 10.1007/s00417-003-0838-y. [DOI] [PubMed] [Google Scholar]
- 36.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 37.Philipp W, Speicher L, Humpel C. Expression of vascular endothelial growth factor and its receptors in inflamed and vascularized human corneas. Invest Ophthalmol Vis Sci. 2000;41:2514–2522. [PubMed] [Google Scholar]
- 38.Lee CM, Jung WK, Na G, Lee DS, Park SG, Seo SK, Yang JW, Yea SS, Lee YM, Park WS, Choi IW. Inhibitory effects of the platelet-activating factor receptor antagonists, CV-3988 and Ginkgolide B, on alkali burn-induced corneal neovascularization. Cutan Ocul Toxicol. 2015;34:53–60. doi: 10.3109/15569527.2014.903573. [DOI] [PubMed] [Google Scholar]