Abstract
ErbB2 is known to upregulate glycolysis in breast cancer, however, the precise mechanisms remain unclear. In the present study, ErbB2 upregulated Hexokinase II (HK II) activity by increasing the binding of HK II to the mitochondrial outer membrane. Dysregulated glucose metabolism in high ErbB2-expressing breast cancer cells induces susceptibility to glucose starvation and glycolysis inhibition. Additionally, HK II has a tendency to dissociate from the mitochondria outer membrane in ErbB2-overexpressing cells following treatment with the HK II inhibitor, 3-BrPA. Furthermore, 3-BrPA treatment results in decreased mitochondria membrane potential and release of cytochrome c into cytoplasm in ErbB2-overexpressing cells, leading to activation of the mitochondrial apoptotic signaling pathway. In summary, the results demonstrate a novel mechanism for ErbB2-activated glycolysis and reveal that 3-BrPA is effective in reducing ErbB2-positive breast cancer cell viability by targeting HK II in vitro and in vivo.
Keywords: ErbB2, 3-BrPA, hexokinase II
Introduction
Cancer cells differ from untransformed cells by a large number of bioenergetic and metabolic changes, which sustain their high rate of growth and proliferation (1–3). According to the Warburg effect, the bioenergetic switch from a high-energy yielding process (TCA cycle) to a low energy yielding process (glycolysis) is a hallmark of most cancer cells. Therefore, targeting of metabolic adaptations that inhibit glycolysis has been widely studied, as part of the search for novel therapies for cancer cells (4,5). Currently, inhibitors of glycolysis and agents that impair mitochondrial oxidative phosphorylation (OXPHOS) have been exploited to target malignant cancer cells by inducing apoptosis or autophagic cell death (4,5).
ErbB2 (Her2/neu) is an oncogene that is overexpressed in ~30% of breast cancers, and its expression is correlated with a poor prognosis (6). The overexpression of ErbB2 increases the transformation and/or metastatic potential of breast cancer cells (7). Additionally, it has been reported that overexpression of ErbB2 leads to increased glucose uptake, lactate production and decreased oxygen consumption in multiple human breast cancer cell lines (8). In a recent study, a novel mitochondrial localization of ErbB2 that negatively regulates mitochondrial respiration in breast cancer cells was reported (9). Furthermore, breast cancer cells with high levels of mitochondrial ErbB2 were more resistant to the ErbB2-targeting antibody trastuzumab (9).
Hexokinase (HK) is a key enzyme that catalyzes the first step in the glycolysis pathway. This enzyme transfers a phosphate group from ATP to glucose, forming glucose-6-phosphate. In human cells, there are four isoforms of HK (I–IV). Regulation of HK II is involved in glucose or lactate metabolism, and blocking its activity is an effective way of inhibiting glycolysis (10). Glycolysis of cancer cell metabolism is associated with the binding of HK II to the outer mitochondrial membrane protein voltage-dependent anion channel, and this association appears important for mitochondrial homeostasis (10,11). HK II association with the mitochondrial outer membrane is involved in mitochondria-mediated apoptosis and increased resistance of cancer cells to chemotherapeutic drugs (11,12). 3-Bromopyruvate (3-BrPA) is a halogenated and alkylating analog of pyruvic acid, and is able to inhibit glycolysis through the dissociation of HK II from mitochondria (13). Previous studies demonstrated that 3-BrPA can induce cell death through the mitochondria-mediated intrinsic apoptotic pathway, which is coupled with the release of cytochrome c from mitochondria to cytoplasm leading to activation of the caspase cascade (14). The aim of this study was to examine whether inhibition of glucose metabolism pathway may specifically inhibit the ErbB2-overexpressing cells. Our study may provide novel perspectives for clinical applications of cancer cell treatment by targeting on the ErbB2-promoted glycolysis.
Materials and methods
Cell lines and culture conditions
The MCF7, MDA-MB-231 and BT474 human breast cancer cell lines were purchased from the American Type Culture Collection (Manassas, VA, USA). The cells were cultured in Dulbecco's modified Eagle's medium (DMEM)/F-12 (Mediatech Inc., Manassas, VA, USA) with 10% fetal bovine serum (FBS) at 37°C, in a 5% CO2 humidified incubator.
Western blotting and antibodies
The cells were harvested and lysed in a buffer containing 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 2 mM EDTA, 1% Triton, 1 mM PMSF and Protease Inhibitor Cocktail (Sigma, St. Louis, MO, USA) for 20 min on ice. Lysates were cleared by centrifugation at 14,000 rpm at 4°C for 10 min. Supernatants were collected and protein concentrations were determined by the Bradford assay (Bio-Rad, Hercules, CA, USA). The proteins were then separated with a SDS/polyacrylamide gel and transferred to a nitrocellulose membrane (Bio-Rad). After blocking in phosphate-buffered saline (PBS) with 5% non-fat dry milk for 1 h, the membranes were incubated overnight at 4–8°C with the primary antibodies in PBS with 5% non-fat dry milk. The following antibodies were utilized: anti-ErbB2 mouse antibody (1:1000, OP15, Calbiochem, National Harbor, MD, USA), anti-HK II mouse antibody (1:1000, no. 8169, Cell Signaling Technology Inc., Beverly, MA, USA), anti-prohibitin rabbit antibody (1:1000, no. 2426, Cell Signaling Technology Inc.), anti-α-Tubulin rabbit antibody (1:1000, no. 2125, Cell Signaling Technology Inc.), anti-mtHSP70 mouse antibody (1:1000, MA3-028, Thermo Scientific, Carlsbad, CA, USA), anti-cytochrome C rabbit antibody (1:1000, no. 11940, Cell Signaling Technology Inc.), anti-PARP rabbit antibody (1:1000, no. 9542, Cell Signaling Technology Inc.), anti-AIF rabbit antibody (1:1000, no. 4642, Cell Signaling Technology Inc.) and anti-β-actin rabbit antibody (1:2000, no. 4970, Cell Signaling Technology Inc.). Membranes were extensively washed with PBS and incubated with horseradish peroxidase-conjugated secondary anti-mouse antibody or anti-rabbit antibody (1:2,000, Bio-Rad). After additional washes with PBS, antigen-antibody complexes were visualized with the enhanced chemiluminescence kit (Pierce, Rockford, IL, USA).
Cell viability assay
The cancer cells were treated with 3-BrPA (Sigma Aldrich, St. Louis, MO, USA) with the indicated concentrations for 24 h. The cells were seeded in a 96-well plate, at a density of 3×103 cells/well in 0.2 ml DMEM containing 10% FBS. After overnight incubation under the same cultivating conditions, each well was refreshed with 0.2 ml serum-free medium (SFM) for another day. The cells were then treated with 0.2 ml SFM containing various concentrations of 3-BrPA. The drug-containing SFM was refreshed after 2 days, and incubated under the same conditions for another 2 days. Cell viability was accessed with an MTT reagent (Sigma Diagnostics, Inc., St. Louis, MO, USA), and by measuring the absorbance at 590 nm using a SpectraMax microplate reader (340PC384; Molecular Devices, Sunnyvale, CA, USA). Relative viability was obtained from the absorbance at 590 nm (A590 nm) of drug-treated cells/A590 nm of untreated cells. The experiment was repeated three times.
Small interfering (si)RNA and plasmid DNA transfection
Specific siRNA for the knockdown of ErbB2 was purchased from Sigma-Aldrich (MISSION® siRNA SIHK0723) and a plasmid vector containing wild-type Myc-DDK-tagged ErbB2 (cat no. RC212583) was purchased from OriGene Technologies, Inc., Rockville, MD, USA. Transfection was performed using the Oligofectamine™ transfection reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) according to the manufacturer's instructions. Forty-eight hours after transfection, whole-cell lysates were prepared for further analysis by Western blot and cytotoxicity assay.
Isolation of mitochondria
High-purity, intact mitochondria were isolated using the Qproteome® Mitochondria Isolation kit (cat no. 37612, Qiagen GmbH, Hilden, Germany) according to the manufacturer's instructions.
Detection of mitochondrial membrane potential
Mitochondrial membrane potential was detected using a kit from BD™ MitoScreen (JC-1; BD Pharmingen, San Diego, CA, USA) according to the manufacturer's instructions.
HK activity
HK activity was measured using the Hexokinase colorimetric assay kit (Sigma-Aldrich) according to the manufacturer's instructions. Absorbance was measured at 563 nm using a SpectraMax M5 plate reader (Molecular Devices). One unit of HK is the amount of enzyme that will generate 1.0 mM of NADH per min at pH 8.0 and room temperature. The results were normalized to the amount of total protein compared to the control cells.
Xenograft experiments
Female athymic nude mice were used in the present study. Nude mice were subcutaneously injected into the right flank with 2×106 MDA-MB-231 empty vector cells (231V) or MDA-MB-231 ErbB2 cells (231 ErbB2). When the tumors reached >150 mm3 in size, the mice were randomly divided into four groups (8 mice per group) as follows: i) 231V cells treated with PBS (control) and 3-BrPA (10 mg/kg intraperitoneal, twice/week for 3 weeks); ii) 231 ErbB2 cells treated with PBS-treated control and 3-BrPA. Mice were weighed weekly and tumor diameters were measured with calipers twice per week for >5 weeks. The tumor volumes were calculated using the formula: volume (mm3) = (W × L)/2, where W and L are the minor and major diameters (in mm), respectively. All of the experiments involving mouse models complied with Chinese laws and the guidelines of the Ethics Committee of Jilin University, Changchun, China.
Statistical analysis
The unpaired Student's t-test was used for data analysis. Data were shown as mean ± standard error (SE). P<0.05 was considered to represent a statistically significant difference.
Results
ErbB2-positive breast cancer cells are more sensitive to glucose starvation
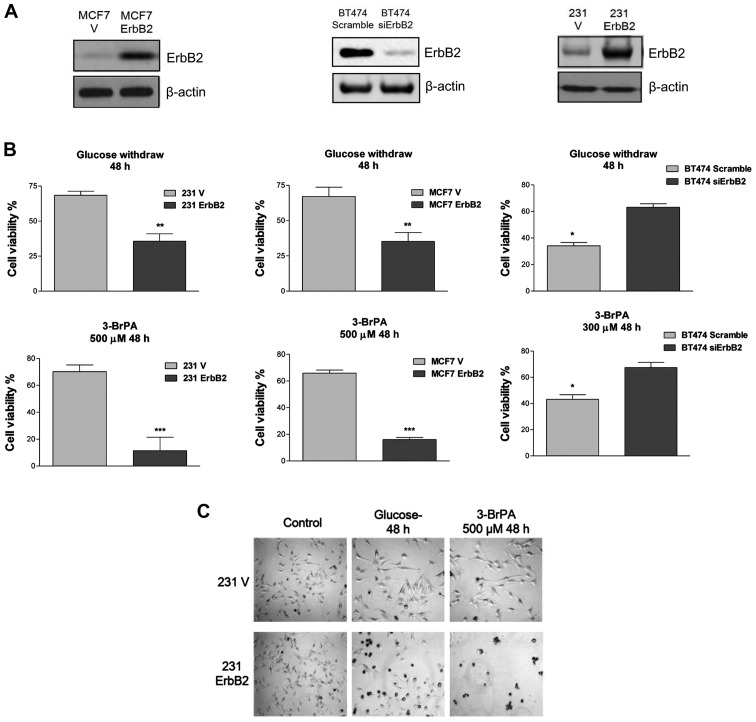
A previous study demonstrated that ErbB2 promotes glycolysis through the activation of LDHA in breast cancer cells (8). To evaluate the ability of ErbB2 to modulate sensitivity of breast cancer cells to glucose starvation, an overexpressed vector containing wild-type ErbB2 was transfected into two ErbB2-negative cell lines, MDA-MB-231 and MCF7. Additionally, ErbB2 expression was knocked down by siRNA in BT474 cells, which normally express high levels of ErbB2. The enforced expression and knockdown of ErbB2 protein expression was confirmed by western blot analysis (Fig. 1A). To investigate whether ErbB2 signaling is involved in rapid cell death following glucose withdrawal, the present study examined the response of these cell lines to the withdrawal of glucose and the glycolytic product pyruvate, an alternate substrate for the TCA cycle. Within 48 h of glucose and pyruvate withdrawal, cells with a high ErbB2 expression (231ErbB2, MCF7ErbB2 and BT474) exhibited rapid cell death, whereas cells that expressed low levels of ErbB2 exhibited only a minor (25%) loss of viability (Fig. 1B). These results indicated that overexpression of ErbB2 in breast cancer cells enhanced their sensitivity to nutrition depletion. Following this, the viability of these cells in response to treatment with 3-BrPA, a glycolysis inhibitor that specifically targets HK II, was determined. The 231ErbB2 and MCF7ErbB2 cells exhibited a large decrease in the viability ratio compared with control cells, and knockdown of ErbB2 in BT474 cells revealed resistance to 3-BrPA treatment (Fig. 1C). This indicated that breast cancer cells overexpressing ErbB2 were more sensitive to the glycolysis inhibitor, the latter being a potential therapeutic target for the treatment of ErbB2-positive breast cancer patients.
Figure 1.
Breast cancer cells with a high expression of ErbB2 are more sensitive to glucose depletion than ErbB2-negative cells. (A) Overexpression of ErbB2 in MCF7 and MDA-MB-231 breast cancer cells, and siRNA knockdown of ErbB2 in BT474 breast cancer cells. Cells were transfected with vector containing wild-type ErbB2 or siErbB2. Whole cell lysates were subjected to western blot analysis, with β-actin used as a loading control. (B) 231V, 231ErbB2; MCF7, MCF7ErbB2 and BT474, BT474 siErbB2 cells were cultured in low glucose conditions for 48 h. ErbB2-overexpressing cells exhibited lower viability in low glucose conditions compared with control cells, which had significantly higher cell viability (top panels). The 231, MCF7 and BT474 cells were treated with 3-BrPA for 48 h at the indicated concentrations. Following this, cell viability was assayed (lower panels). (C) 231V and 231ErB2 cells were treated with 3-BrPA and cultured under low glucose condition for 48 h. The cell morphology and apoptosis were then examined by microscopy. Data are the mean values of three independent experiments; bars, SE. *P<0.05; **P<0.01; ***P<0.001.
ErbB2 enhances mitochondrial HK II distribution and increases HK activity
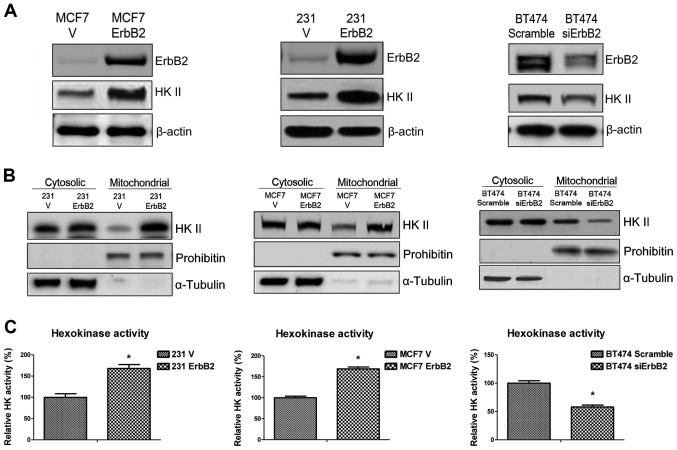
The putative mechanisms underlying ErbB2-mediated sensitivity to glucose starvation were investigated. Since the major function of the 3-BrPA glycolysis inhibitor is to dissociate HK II from mitochondrial outer membrane, the expression of HK II in ErbB2-overexpressing cells was examined. Western blot analysis revealed that ErbB2 expression upregulated HK II expression in 231ErbB2 and MCF7ErbB2 cells, and HK II expression was significantly decreased when ErbB2 was knocked down in BT474 cells (Fig. 2A). HK II associates with the outer surface of the external mitochondrial membrane through specific binding to voltage-dependent anion channels, where it catalyzes the first step of glycolysis through the phosphorylation of glucose. Therefore, we hypothesized that ErbB2 may regulate the association of HK II with the mitochondrial outer membrane. As demonstrated in Fig. 2B, the overexpression of ErbB2 increased mitochondrial HK II distribution, but did not regulate cytosolic HK II in 231ErbB2 and MCF7ErbB2 cells. Similar results were obtained in cells with ErbB2 knockdown (Fig. 2B). HK activity was then measured in the MCF7, 231 and BT474 cells. In concordance with the earlier results, ErbB2 increased the activity of HK (Fig. 2C). Thus, the results demonstrated that ErbB2 promotes mitochondrial HK II expression and activity in the three breast cancer cell lines.
Figure 2.
Overexpression of ErbB2 in breast cancer cells increases mitochondrial localization and activity of HK II. (A) Overexpression of ErbB2 upregulated the expression of HK II. Whole cell lysates were collected and for western blotting analysis, and β-actin was used as a loading control. (B) Overexpression of ErbB2 did not alter the expression of HK II in cytoplasm but increased the distribution of HK II in mitochondria. The cytosolic and mitochondrial fractions of cells were isolated. Prohibitin was used as a mitochondria marker and loading control. α-tubulin was used as a cytoplasm marker and loading control. (C) The activity of HK was measured in the breast cancer cell lines. The results were normalized to the amount of total protein compared with the control cells. Data are the mean values of three independent experiments; bars, SE. *P<0.05.
ErbB2 promotes the dissociation of HKII from mitochondria in response to 3-BrPA treatment
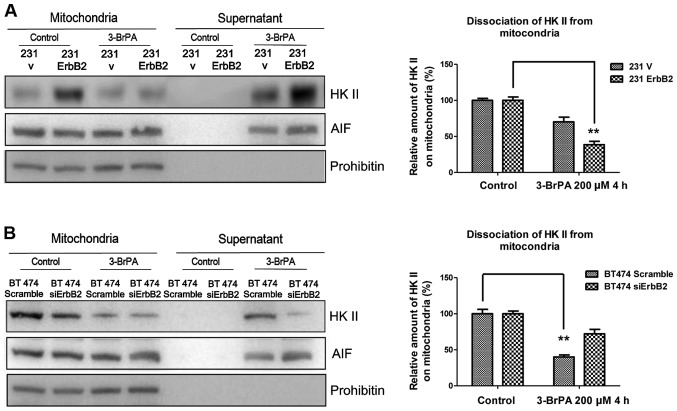
A previous study has demonstrated that mitochondria-associated HK II is involved in the regulation of apoptosis (12). To investigate the putative mechanism, the detachment of HK II from mitochondria under the 3-BrPA treatment was investigated. The rationale of these experiments was that proteins released from the isolated mitochondria would be present in the supernatant and could therefore be separated from the mitochondrial-associated proteins by centrifugation. The mitochondrial fractions were isolated from cells and treated with 3-BrPA for 2 h. The supernatant and mitochondria pellets were separated by centrifugation and immunoblotted for HK II and control markers. As expected, the overexpression of ErbB2 significantly promoted the dissociation of HK II from mitochondria following 3-BrPA treatment (Fig. 3A). By contrast, BT474 ErbB2 knockdown cells had less HK II dissociation (Fig. 3B). The results indicate that the high ErbB2-expressing cells are more susceptible to losing the association between HK II and the mitochondrial outer membrane when treated with the specific HK II inhibitor, 3-BrPA.
Figure 3.
HK II dissociation from mitochondria is increased in ErbB2-overexpressing breast cancer cells. (A) The intact mitochondrial fractions from 231V and 231ErbB2 cells and (B) BT474 scramble and BT474 siErbB2 cells were isolated and treated with 3-BrPA for 2 h. The fractions were then centrifuged to separate the supernatant and mitochondria. The amount of HK II in the supernatant was an indicator of the dissociation of mitochondria by 3-BrPA (left) and the western blots were quantified (right). AIF was used as a positive control and prohibitin as a negative control. Data are the mean values of three independent experiments; bars, SE. **P<0.01.
The dissociation of HK II from mitochondria leads to cytochrome c release from mitochondria to the cytoplasm
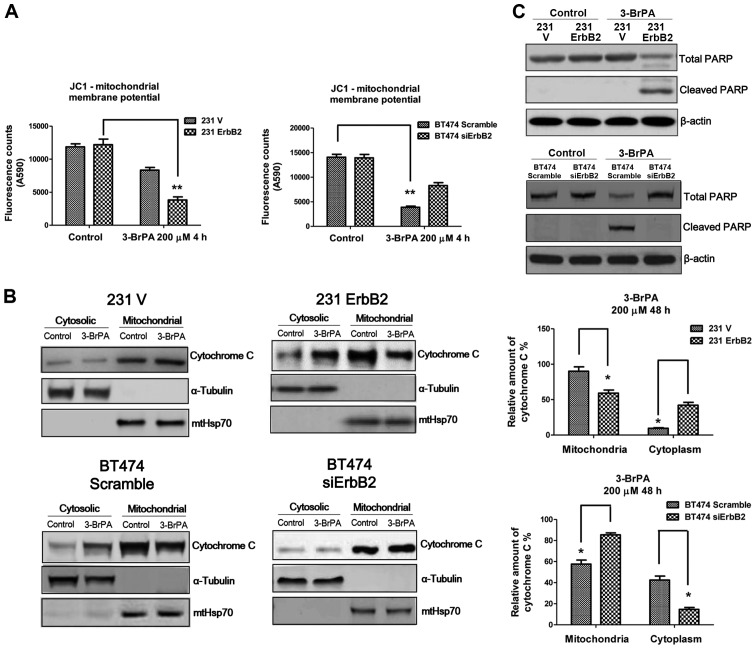
Previous studies demonstrated that 3-BrPA can induce cell death through the mitochondria-mediated intrinsic apoptotic pathway, which is coupled to the release of cytochrome c from mitochondria to the cytosol, activating the caspase cascade (12). The role of ErbB2 in altering mitochondrial membrane potential in response to 3-BrPA treatment was investigated using the JC1 assay. Fig. 4A demonstrates significant changes in mitochondrial membrane potential in the ErbB2-overexpressing and knockdown cells following 3-BrPA treatment, compared to the control cells. The 231ErbB2 cells exhibited increased cytochrome c release from mitochondria to the cytoplasm compared to the control cells, and knockdown of ErbB2 in BT474 cells reduced the release of cytochrome c into the cytoplasm (Fig. 4B). Levels of cleaved PARP in ErbB2-overexpressing cells under 3-BrPA treatments were then compared. Consistently, the results demonstrated increased levels of cleaved PARP in the high ErbB2-expressing cells, following 3-BrPA treatment (Fig. 4C). In general, breast cancer cells with overexpression of ErbB2 were more sensitive to 3-BrPA through the dissociation of HK II from mitochondria, leading to the release of cytochrome c from mitochondria to the cytosol and activation of the mitochondrial apoptosis pathway.
Figure 4.
Overexpression of ErbB2 decreased the mitochondria membrane potential following 3-BrPA treatment, inducing apoptosis. (A) The mitochondria membrane potential (MMP) was measured in 231V, 231ErbB2, BT474 scramble and BT474 siErbB2 cells following treatment with 3-BrPA at 200 µM for 4 h. (B) The cells groups were treated with 3-BrPA at 300 µM for 16 h, and the cytosolic and mitochondrial fractions were then isolated for western blot analysis. The high ErbB2-expressing cells exhibited more release of cytochrome C from mitochondria to cytoplasm under 3-BrPA treatments (left). The quantitation of the western blot analysis was quantified (right). (C) Whole cell lysates were isolated from the above 3-BrPA treated cells and subjected to western blot analysis for the detection of PARP cleavage. The high ErbB2-expressing cells exhibited increased levels of cleaved PARP following 3-BrPA treatment. Plotted data are mean values of three independent experiments; bars, SE. *P<0.05; **P<0.01.
3-BrPA exhibits higher therapeutic efficiency in ErbB2-overexpressing cancer cells in vivo
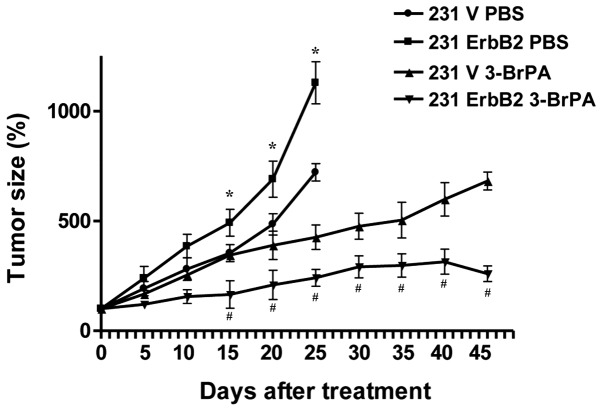
To confirm our in vitro results in vivo, a xenograft nude mouse model was used to further investigate 3-BrPA treatment. The 231V, 231ErbB2, MCF7V and MCF7ErbB2 cells were inoculated into the mammary fat pads of 6-week-old nude mice. Following tumor formation, tumor xenografts were treated with 3-BrPA. Forty-five days following injection, mice were sacrificed by exsanguination under general anesthesia. As expected, 3-BrPA significantly inhibited 231ErbB2-derived tumor growth compared with 231V-derived tumors (Fig. 5; P<0.05). These in vivo data support our in vitro results demonstrating that ErbB2 promoted the dissociation of HKII from mitochondria, resulting in increased sensitivity to 3-BrPA treatment.
Figure 5.
3-BrPA has a greater inhibitory effect in ErbB2-overexpressing breast cancer cells than control cells in nude mice. Pre-established 231V or 231ErbB2 tumor xenografts were treated with control (phosphate-buffered saline), 3-BrPA (10 mg/kg, twice/week for 3 weeks). Data are presented as the mean ± standard error of the mean of three independent experiments. *P<0.05, 231 ErbB2 PBS group vs. 231 ErbB2 3-BrPA group; #P<0.05, 231 V 3-BrPA group vs. 231 ErbB2 3-BrPA group.
Discussion
In the 1920s, Warburg hypothesized that cancer cells possess a unique energy metabolism, and suggested this was due to a shift in energy production from mitochondrial OXPHOS to aerobic glycolysis (1). Studies of the changes of cancer cell metabolism predicted by Warburg, may provide a rationale and an insight for anticancer therapies. Thus, regulation of the enzymes involved in glucose or lactate production, such as HK II, glucose transporter 1 and lactate dehydrogenase A (LDHA), may be an effective means of inhibiting cancer cells (15). Multiple oncogenes and tumor suppressors have been reported to reprogram glucose metabolism in cancer cells, including Ras (16), ErbB2 (9), epidermal growth factor receptor (17), protein kinase B (also known as Akt) (18), Src (19), phosphatase and tensin homolog (20) and p53 (21). For example, oncogenic signaling through the PI3K/Akt pathway, commonly upregulated in cancer, promotes glycolysis through multiple mechanisms. Akt signaling increases the expression and membrane localization of glucose transporters and increases the activity of glycolytic enzymes, such as phosphofructokinase and HK II (18). Furthermore, the p53 tumor suppressor negatively regulates the expression of the glycolytic protein phosphoglycerate mutase-2 (21). Thus, oncogene signaling, not only activates cancer cell mitogenic pathways that drive unchecked growth of cancer, but also promotes a coordinated metabolic transformation of cancer cells by activating metabolic pathways and transcriptionally regulating metabolic enzymes. As a well-studied oncogene, ErbB2 promotes cancer cell glycolysis and is important in the regulation of the anti-apoptosis pathway during cell stress. In breast cancer cells, the ErbB2 oncogene activates signaling pathways that regulates the proliferation, metastasis, invasion and resistance to chemotherapeutic drugs of cancer cells. Additionally, ErbB2 signaling has been demonstrated to transcriptionally upregulate the glycolytic enzyme LDHA (8). To the best of our knowledge, the present study, demonstrates for the first time that increased mitochondrial localization of HK II is regulated by ErbB2. Overexpression of ErbB2 in breast cancer cells upregulated HK II expression and promoted the localization of HK II on the mitochondrial outer membrane. The results of the present study demonstrate a novel mechanism for ErbB2-stimulated glycolysis via the activation of HK II.
Cancer cells expressing higher levels of ErbB2 are highly dependent on glucose supply, therefore, we investigated whether cancer cells expressing high levels of ErbB2 were more sensitive to the depletion of glucose than ErbB2 negative cells. The present study demonstrates that ErbB2-overexpressing breast cancer cells exhibit a higher apoptosis tendency under glucose withdrawal conditions, compared to ErbB2-negative breast cancer cells. This result indicates a dual role of ErbB2 in the regulation of cancer cell apoptosis. It has been reported previously that c-Myc enhances glycolysis by increasing glucose uptake, upregulating the expression of LDH, and favoring the production of the M2 isoform of pyruvate kinase 2 a key regulator of glycolytic flux (22). An additional study demonstrated that glucose deprivation induces the apoptosis of c-Myc-transformed cancer cells. Furthermore, exogenously enhanced glycolysis by overexpression of LDHA sensitizes cells to glucose deprivation-induced apoptosis, suggesting that cells with oncogene-activated upregulation of glycolysis are more susceptible to glucose depletion. Similar results were observed in the present study.
ErbB2-overexpressing cancer cells are more susceptible to apoptosis when treated with other glycolysis inhibitors such as 2-DG and Oxamate (8). In the present study, cancer cells expressing higher levels of ErbB2 underwent more apoptosis in glucose starvation conditions, thus, we investigated whether inhibition of glucose metabolism pathway could specifically inhibit ErbB2-overexpressing cells. 3-BrPA is a specific inhibitor of HK II, through the induction of apoptosis. ErbB2-overexpressing breast cancer cells were observed to be more sensitive to 3-BrPA treatment through the dissociation of HK II from the mitochondrial outer membrane, suggesting 3-BrPA may be a therapeutic anti-cancer drug for the specific inhibition of ErbB2 high-expressing cancer cells. The increased dissociation of HK II from the mitochondrial membrane induced mitochondria-mediated apoptosis. However, the detailed mechanisms for the ErbB2-induced dissociation of HK II remain unclear. The anti-tumor efficacy of 3-BrPA in vivo was evaluated by measuring tumor volumes. The xenograft model experiment in the present study demonstrated 3-BrPA treatments were more effect in ErbB2-overexpressing breast cancer cells, supporting our in vitro results.
In conclusion, we suggest a novel mechanism for ErbB2-activated glycolysis in breast cancer cells in vitro and in vivo, and this may contribute to the development of clinical therapies for the treatment of breast cancer patients.
Acknowledgements
The present authors thank Dr Shengnan Ren for critically reviewing and revising the manuscript, and Mr. Zhuo Liu for assistance with data analysis. We also thank Dr Xuebo Chen for help with animal experiments. This study was supported by a Pre-doctoral Fellowship (grant no: 201201037), awarded to Ms. Sujie Gao by Jilin Provincial Science & Technology Department, Jilin University.
References
- 1.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. [PubMed] [Google Scholar]
- 2.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect, The metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferreira LM. Cancer metabolism: the Warburg effect today. Exp Mol Pathol. 2010;89:372–380. doi: 10.1016/j.yexmp.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Zhao Y, Butler EB, Tan M. Targeting cellular metabolism to improve cancer therapeutics. Cell Death Dis. 2013;4:e532. doi: 10.1038/cddis.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vander Heiden. MG:T argeting cancer metabolism: A therapeutic window opens. Nat Rev Drug Discov. 2011;10:671–684. doi: 10.1038/nrd3504. [DOI] [PubMed] [Google Scholar]
- 6.Gutierrez C, Schiff R. HER2: Biology detection, and clinical implications. Arch Pathol Lab Med. 2011;135:55–62. doi: 10.1043/2010-0454-RAR.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angelini PD, Fluck Zacarias MF, Pedersen K, Parra-Palau JL, Guiu M, et al. Constitutive HER2 signaling promotes breast cancer metastasis through cellular senescence. Cancer Res. 2013;73:450–458. doi: 10.1158/0008-5472.CAN-12-2301. [DOI] [PubMed] [Google Scholar]
- 8.Zhao YH, Zhou M, Liu H, Ding Y, Khong HT, Yu D, Fodstad O, Tan M. Upregulation of lactate dehydrogenase A by ErbB2 through heat shock factor 1 promotes breast cancer cell glycolysis and growth. Oncogene. 2009;28:3689–3701. doi: 10.1038/onc.2009.229. [DOI] [PubMed] [Google Scholar]
- 9.Patel NI. Barrientos A and Landgraf R: The growth factor receptor ERBB2 regulates mitochondrial activity on a signaling time scale. J Biol Chem. 2013;288:35253–35265. doi: 10.1074/jbc.M113.478271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolf A, Agnihotri S, Micallef J, Mukherjee J, Sabha N, Cairns R, Hawkins C, Guha A. Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J Exp Med. 2011;208:313–326. doi: 10.1084/jem.20101470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gershon TR, Crowther AJ, Tikunov A, Garcia I, Annis R, Yuan H, Miller CR, Macdonald J, Olson J, Deshmukh M. Hexokinase-2-mediated aerobic glycolysis is integral to cerebellar neurogenesis and pathogenesis of medulloblastoma. Cancer Metab. 2013;1:2. doi: 10.1186/2049-3002-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shulga N, Wilson-Smith R, Pastorino JG. Hexokinase II detachment from the mitochondria potentiates cisplatin induced cytotoxicity through a caspase-2 dependent mechanism. Cell Cycle. 2009;8:3355–3364. doi: 10.4161/cc.8.20.9853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ganapathy-Kanniappan S, Geschwind JF, Kunjithapatham R, Buijs M, et al. 3-Bromopyruvate induces endoplasmic reticulum stress, overcomes autophagy and causes apoptosis in human HCC cell lines. Anticancer Res. 2010;30:923–935. [PubMed] [Google Scholar]
- 14.Chen Z, Zhang H, Lu W, Huang P. Role of mitochondria-associated hexokinase II in cancer cell death induced by 3-bromopyruvate. Biochim Biophys Acta. 2009;87:553–560. doi: 10.1016/j.bbabio.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teicher BA, Linehan WM, Helman LJ. Targeting cancer metabolism. Clin Cancer Res. 2012;18:5537–5545. doi: 10.1158/1078-0432.CCR-12-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaglio D, Metallo CM, Gameiro PA, Hiller K, et al. Oncogenic K-Ras decouples glucose and glutamine metabolism to support cancer cell growth. Mol Syst Biol. 2011;7:523. doi: 10.1038/msb.2011.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huber SM, Misovic M, Mayer C, Rodemann HP, Dittmann K. EGFR-mediated stimulation of sodium/glucose cotransport promotes survival of irradiated human A549 lung adenocarcinoma cells. Radiother Oncol. 2012;103:373–379. doi: 10.1016/j.radonc.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Elstrom RL, Bauer DE, Buzzai M, Karnauskas R, Harris MH, Plas DR, et al. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 2004;64:3892–3899. doi: 10.1158/0008-5472.CAN-03-2904. [DOI] [PubMed] [Google Scholar]
- 19.Valle-Casuso JC, González-Sánchez A, Medina JM, Tabernero A. HIF-1 and c-Src mediate increased glucose uptake induced by endothelin-1 and connexin43 in astrocytes. PLoS One. 2012;7:e32448. doi: 10.1371/journal.pone.0032448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blouin MJ, Zhao Y, Zakikhani M, Algire C, Piura E, Pollak M. Loss of function of PTEN alters the relationship between glucose concentration and cell proliferation, increases glycolysis, and sensitizes cells to 2-deoxyglucose. Cancer Lett. 2010;289:246–253. doi: 10.1016/j.canlet.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 21.Cheung EC, Vousden KH. The role of p53 in glucose metabolism. Curr Opin Cell Biol. 2010;2:186–191. doi: 10.1016/j.ceb.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 22.David CJ, Chen M, Assanah M, Canoll P, Manley JL. HnRNP proteins controlled by c-Myc deregulate pyruvate kinase mRNA splicing in cancer. Nature. 2010;7279:364–368. doi: 10.1038/nature08697. [DOI] [PMC free article] [PubMed] [Google Scholar]