Abstract
Here, we present an uncommon case of inflammatory myofibroblastic tumor (IMT) involving the mesentery. The tumor was composed of loosely arranged round-to-spindle-shaped tumor cells with amphophilic cytoplasm in an inflammatory and myxoid background. The mitotic activity was low (1 per 50 high-power fields) and the tumor cells lacked cellular atypism. Immunohistochemically, the tumor cells demonstrated strong nuclear membranous staining with anaplastic lymphoma kinase (ALK). In situ hybridization for ALK gene rearrangement revealed a splitting apart of the two signals within the tumor cells. Reverse transcription-polymerase chain reaction revealed that the tumor harbored a ran-binding protein 2 (RANBP2)-ALK rearrangement. IMTs are usually characterized by epithelioid-to-round cells featuring increased mitotic activity, occasionally demonstrating unusual tumor cells and more aggressive clinical behavior. To date, 23 IMTs have been reported with RANBP2 and ALK gene rearrangements. However, the present case demonstrated indolent cytological features, leading to a difficulty in differentiating it from desmoid-type fibromatosis.
Keywords: inflammatory myofibroblastic tumor, round cell, anaplastic lymphoma kinase, ran-binding protein 2
Introduction
Inflammatory myofibroblastic tumor (IMT) is a neoplasm composed of myofibroblastic and fibroblastic spindle cells, accompanied by an inflammatory infiltrate containing lymphocytes, plasma cells, eosinophils or neutrophils. It occurs mainly in children and young adults with a slight female predominance. Metastasis occurs in <2% of cases. However, a distinct variant of epithelioid-to-round morphology has been described, associated with a more aggressive clinical course with rapid recurrence and/or mortality compared with conventional IMT, and harboring ran-binding protein 2 (RANBP2) and anaplastic lymphoma kinase (ALK) rearrangement (1). The tumor cells were generally non-cohesive and demonstrated ganglion-like or epithelioid cells distributed in a myxoid stroma. Few binucleate cells were present. Certain tumor cells were tightly packed and arranged in sheets. The mitotic figures ranged from 1 to 4 per 10 high-power fields.
In the present study we present a case of IMT with indolent cytological features mimicking desmoid-type fibromatosis. Written informed consent was obtained from the patient.
Case report
Case presentation
A 35-year-old female presented to the Department of General Surgery in Chi-Mei Medical Center (Tainan, Taiwan) with a loss in body weight and an abdominal mass. During physical examination, a mobile solid tumor was palpated in the right lower quadrant of the patient's abdomen.
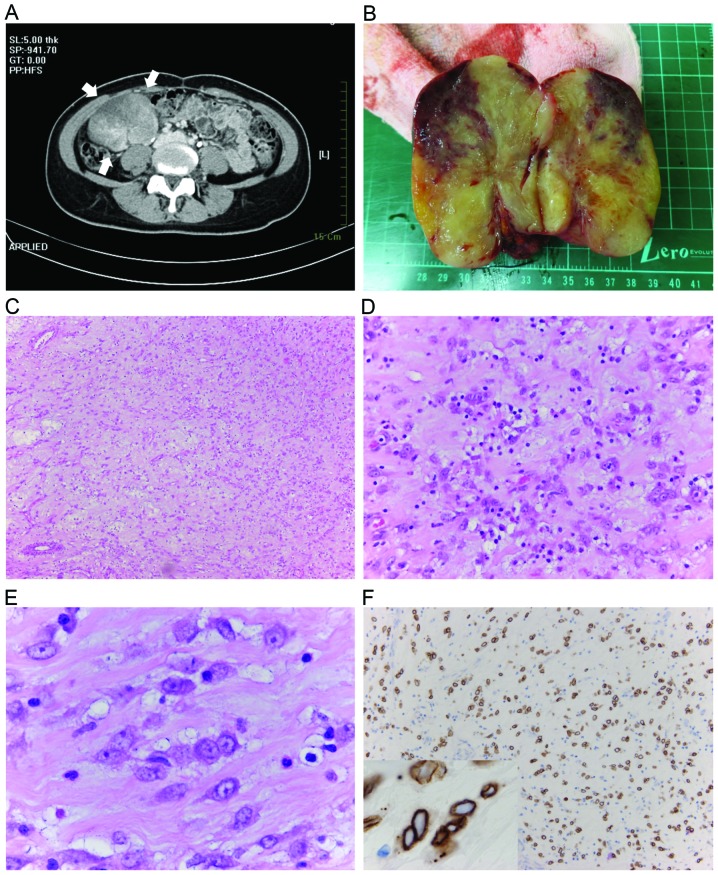
There was no fever and all laboratory examinations including hemograms and biochemistry were within normal limits. Abdominal computed tomography revealed a well-defined mass (8.2 cm in size) with heterogeneous intense enhancement that was located in the right lower quadrant of the abdomen (Fig. 1A). Laparoscopic assisted tumor excision and partial colectomy were performed. The resected solid tumor was 11×9×7 cm in size and contained a yellowish and reddish cut surface with a firm and rubbery texture (Fig. 1B).
Figure 1.
(A) Abdominal computed tomography scan reveals a well-defined mass with heterogeneous intense enhancement in the right lower quadrant of the abdomen (arrows). (B) When resected, the tumor exhibited a yellowish and reddish surface with a firm and rubbery texture. (C) At lower magnification, the tumor is composed of loosely arranged tumor cells in a myxoid background [hematoxylin and eosin (H&E); magnification, ×100]. (D) A prominent lymphoplasmacytic infiltrate, with occasional neutrophils infiltration is also evident (H&E; magnification, ×400). (E) At higher magnification, tumor cells are rounded and epithelioid with slightly amphophilic cytoplasm, vesicular nuclei and prominent nucleoli. Notably, these tumor cells are bland-looking (H&E; magnification, ×1,000). (F) Immunohistochemistry for anaplastic lymphoma kinase reveals strong staining of the nuclear membrane (Anaplastic lymphoma kinase staining, magnification, ×200; inset magnification, ×1,000).
Immunohistochemical analysis
The specimen was routinely processed and embedded in paraffin. Sections of 4 µm thickness were used for hematoxylin and eosin staining and immunohistochemical study with a panel of antibodies, as listed in Table I. The antibodies were obtained from Dako (Glostrup, Denmark), Invitrogen Life Technologies (Carlsbad, CA, USA) and Abcam (Cambridge, UK), as indicated in the table. Polyadenylated RNA isolated from formalin-fixed, paraffin-embedded blocks was used for reverse transcription-polymerase chain reaction (RT-PCR) as previously described (2). Fluorescence in situ hybridization (FISH) analysis was carried out on the 4-µm-thick paraffin sections. The presence of ALK rearrangement was determined using the LSI ALK Dual Color Break Apart Probe (Abbott Molecular/Vysis, Des Plaines, IL, USA) (3).
Table I.
Antibodies used for immunohistochemistry.
| Antigen | Clone | Dilution | Manufacturer | Staining results |
|---|---|---|---|---|
| Cytokeratin | AE1/AE3 | 1:1,500 | Dako | Negative |
| CDK-4 | DCS-31 | 1:4,000 | Invitrogen | Negative |
| CD30 | Ber-H2 | 1:100 | Dako | Positive |
| CD117 | NA | 1:450 | Dako | Negative |
| ALK | 5A4 | 1:50 | Abcam | Positive |
| β-catenin | β-catenin-1 | Ready-to-use | Dako | Negative |
| SMA | 1A4 | 1:200 | Dako | Positive |
All performed with high-voltage microwave boiling in citrate buffer pH 6.0 for 12.5 min for all antibodies as the antigen retrieval method. CDK, cyclin-dependent kinase; ALK, anaplastic lymphoma kinase; SMA, smooth muscle actin; NA, not available.
Diagnosis
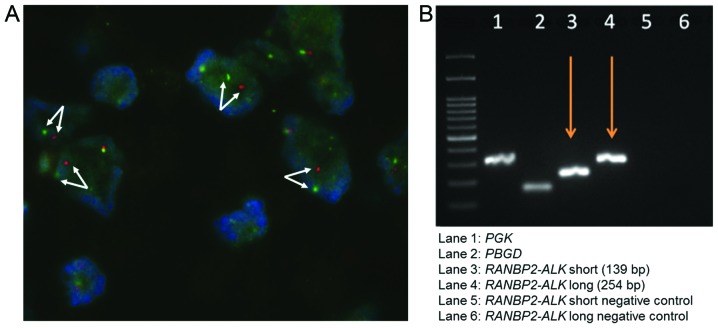
Histologically, the neoplasm was well circumscribed. At low magnification, tumor cells were loosely arranged in an inflammatory and myxoid background (Fig. 1C and D). The tumor cells were spindle-to-epithelioid-shaped and demonstrated slightly amphophilic cytoplasm, vesicular chromatin and prominent nucleoli (Fig. 1E). Mitotic figures were infrequent (average of 1 per 50 high-power fields). The composition of the inflammatory infiltrate included prominent small lymphocytes and plasma cells, admixed with neutrophils. A minor spindle cell component was also present. Immunohistochemically, ALK demonstrated strong staining of the nuclear membrane (Fig. 1F). Smooth muscle actin (SMA) and CD30 were also diffusely expressed. These tumor cells were negative for cytokeratin (CK), CDK-4, CD117 and β-catenin. FISH analysis revealed a splitting apart of the two signals in the tumor nuclei, confirming the presence of ALK rearrangement (Fig. 2A). The expression of RANBP2 was identified by RT-PCR. Thus, a diagnosis of IMT with ALK and RANBP2 rearrangement was confirmed (Fig. 2B).
Figure 2.
(A) Fluorescence in situ hybridization reveals a splitting apart of the two signals (arrows), suggesting the presence of anaplastic lymphoma kinase (ALK) rearrangement (magnification, ×100). (B) Reverse transcription-polymerase chain reaction confirms the presence of ran-binding protein 2-ALK gene fusion..
Discussion
Table II summarizes the clinicopathological features of the 11 previously reported IMTs with RANBP2-ALK gene fusion (IMT-RAs) and the present case (1,2,4–8). Of the 11 previous cases, 10 patients were male and only one was female, and the median age was 27 years (range, 7 months to 57 years). In contrast to conventional IMT, which demonstrates a slight female predominance, IMT-RAs are more prevalent in males than in females. The tumors ranged from 8 to 26 cm (median, 14 cm). Ten tumors were identified in the abdominal cavity, while one was identified in the pleura or chest wall. The histological findings revealed round cells or epithelioid neoplastic cells distributed in a myxoid stroma with inflammatory infiltrates. A spindle cell component was present in three cases studied by Mariño-Enríquez et al (7). Mitotic figures were variable and ranged from 1 to 4 per 10 high-power fields. Cellular atypism was occasionally observed. Unusual tumor cells including hyperchromatic nuclei and multinucleate giant cells were noted by Chen and Lee (2). Binucleate cells were also reported by Li et al (1).
Table II.
Clinical and histological features of 12 cases of inflammatory myofibroblastic tumor with RANBP2-ALK gene fusion.
| Case | Authors (Ref.) | Age | Gender | Site/size (cm) | Treatment | Follow-up | Histopathology |
|---|---|---|---|---|---|---|---|
| 1 | Ma et al (4) | 7 years | Male | Unspecified abdominal mass/NA | SE + CT | Recurred 5 weeks after first resection. Re-excision was performed. Five months later, the tumor recurred again and was re-excised. | Plump neoplastic cells with abundant eosinophilic cytoplasm and large nuclei in a pale-staining, mucoid background containing abundant plasma cells and lymphocytes. |
| 2 | 7 months | Male | Mesentery and omentum/11 | SE | Recurred 8 months after first resection. Re-excision was performed. | Round tumor cells intermixed with with more classic spindle cells in a mucoid background, containing a modest infiltrate of plasma cells, neutrophils and lymphocytes. MF were infrequent. | |
| 3 | Patel et al (5) | 2 years | Male | Retroperitoneal abdominal mass/10 | SE | No evidence of recurrence with 3 years of follow-up. | NA |
| 4 | Chen and Lee (2) | 34 years | Male | Liver/8 | SE | Recurred 5 months after resection. Succumbed to disease ~2 weeks after identification of recurrence. | Round tumor cells with abundant eosinophilic cytoplasm admixed with inflammatory cells in myxomatous background. Unusual tumor cells with hyperchromatic nuclei, ganglion-like cells and multinucleate giant cells. |
| 5 | Butrynski et al (6) | 44 years | Male | Omentum/NA | SE + CT + ALKi | Hepatic, peripancreatic and perirectal masses recurred 1 year after resection. Subsequent exploratory laparotomy with maximal debulking was performed. | Epithelioid cells with amphophilic cytoplasm, embedded in a myxoid stroma with prominent neutrophils. |
| 6 | Mariño-Enríquez et al (7) | 41 years | Male | Omentum/26 | SE + CT + ALKi | Multifocal local recurrence and liver metastases. Alive with no evidence of disease after 40 months. | Sheets of round cells in a myxoid or collagenous background. The inflammatory infiltrate was variable. MF ranged from 1 to 18 per 10 high-power fields (median 4). A minor spindle cell component was present. |
| 7 | 6 years | Male | Omentum and mesentery/14 | SE | NA | NA | |
| 8 | 39 years | Male | Mesentery of the small bowel/14 | SE | NA | NA | |
| 9 | Kozu et al (8) | 57 years | Male | Pleura or chest wall/NA | ALKi | NA | Round tumor cells in a myxoid stroma with infiltrates of histiocytes and lymphocytes. |
| 10 | Li et al (1) | 19 years | Female | Mesentery of the small bowel/19 | SE | Recurred 9 weeks after resection. Succumbed to disease 3 weeks after identification of recurrence. | Ganglion-like tumor cells with slightly amphophilic cytoplasm in a myxoid background, accompanied by infiltrates of neutrophils, eosinophils and lymphoplasma cells. Few binucleate cells were noted. MF ranged from 1 to 4 per 10 high-power fields. Erythrocyte extravasation was also observed. |
| 11 | 39 years | Male | Mesentery of the colon/15 | SE + CT | Recurred 4 months after resection. Alive with disease after 12 months. | ||
| 12 | Present case | 35 years | Female | Mesentery of the colon/11 | SE | No recurrence after 6 months. | Loosely arranged round tumor cells with amphophilic cytoplasm in an inflammatory and myxoid background. Inflammatory cells were composed of predominant lymphocytes admixed with plasma cells and neutrophils. MF were infrequent (1 per 50 high-power fields). |
RANBP2, ran-binding protein 2; ALK, anaplastic lymphoma kinase; SE, surgical excision; CT, chemotherapy; ALKi, ALK inhibitor; NA, data not available; MF, mitotic figures.
Combining the cases from the literature and the case presented here, all 12 cases of IMT-RA were ALK-positive (100%). Nine of the 12 (75%) demonstrated nuclear membrane staining and 3/12 (25%) demonstrated cytoplasmic staining of ALK. Eight out of 9 cases (89%) were desmin-positive, 5/9 (56%) were SMA-positive, 2/9 (22%) were CK-positive, 0/6 (0%) were caldesmin-positive, and 0/3 were epithelial membrane antigen (EMA)-positive. Six of the 8 cases (75%) expressed CD30, which is also observed in anaplastic large cell lymphoma (ALCL). However, nuclear membrane ALK staining has not been observed in ALCL, thereby allowing its distinction from IMT-RA. Furthermore, RANBP2-ALK has not been reported in ALCL (1,7). The correlation between the nuclear membrane staining pattern of ALK and RANBP2-ALK fusion in epithelioid and round cell IMT appears to be consistent (data not shown).
Follow-up information was available for 9/12 cases. The local recurrence rate of IMT-RA was 77% (7/9), with a metastasis rate of 22% (2/9). Two patients succumbed to the disease within 6 months of tumor excision (1,2).
IMT is regarded as a neoplasm of intermediate biological potential, demonstrating myofibroblastic and fibroblastic spindle cells in a background of inflammatory infiltrate, as well as ALK gene rearrangement. Immunoreactivity has been reported for ALK and CD30 (7). The inflammatory pseudotumor was first described in the lung, where it was considered a reparative post-inflammatory condition rather than a neoplastic process (9). The tumors were considered benign until 1991 when Meis and Enzinger reported 38 cases and termed them ‘inflammatory fibrosarcoma’ based on their locally aggressive behavior and recurrent nature and the occurrence of metastases and tumor mortality (10). In 1995, Coffin et al described 84 cases of extrapulmonary inflammatory pseudotumor, coining the term ‘inflammatory myofibroblastic tumor’, which referred to a benign, non-metastasizing proliferation of myofibroblasts with a potential for recurrence and persistent local growth, similar in some respects to fibromatosis (11). The diagnosis of IMT, particularly in cases with round cell or epithelioid morphology and where markedly atypical nuclear features are exhibited (7), is challenging for pathologists, as the tumor requires differential diagnosis from ALCL, high-grade epithelioid leiomyosarcoma, rhabdomyosarcoma, undifferentiated sarcoma, myxoid/round cell liposarcoma, myxofibrosarcoma and dedifferentiated liposarcoma.
IMT is genetically heterogeneous, containing ALK gene (at 2p23) rearrangements with various fusion partner genes, including SEC31L1, ATIC, CARS, PPFIBP1, TPM3, TPM4, CLTC and RANBP2. In addition to ALK gene rearrangement, ROS1 fusions (YWHAE-ROS1 and TFG-ROS1) and a PDGFRβ fusion (NAB2-PDGFRβ) were first described by Lovly et al in 2013 (12).
In 2011, Mariño-Enríquez et al (7) reviewed a series of 11 cases diagnosed with IMT. The tumors were dominated by sheets of epithelioid-to-round cells with a prominent inflammatory infiltrate. ALK was detected by immunohistochemistry in all 11 cases. In 9 cases ALK staining was localized to the nuclear membrane, and in two tumors ALK staining was localized to the cytoplasm with perinuclear accentuation. Follow-up data were available for eight patients, which revealed that all eight developed local recurrences 1–8 months after initial resection, with two patients developing distant metastases and five patients succumbing to the disease after 3–36 months. Therefore, Mariño-Enríquez et al designated these as ‘epithelioid inflammatory myofibroblastic sarcoma’, describing an epithelioid variant of IMT that exhibits a round cell morphology with nuclear membrane or perinuclear ALK staining and pursues an aggressive course with rapid local recurrences. Three of the 11 cases harbored RANBP2-ALK fusion as confirmed by RT-PCR (7).
To our knowledge, 11 cases have been described as IMT-RAs, as confirmed by RT-PCR (Table II). In our case, tumor cells were loosely arranged in an inflammatory and fibromyxoid background, demonstrated a spindle-to-epithelioid morphology, and were devoid of cellular atypism. One characteristic of our case was that mitotic activity was extremely low compared with the 11 cases previously reported. The tumor was composed of tumor cells with a bland-looking spindle shape and lacked cellular atypism or brisk mitotic activity, which could have led to a potential diagnostic error as it was difficult to differentiate it from desmoid-type fibromatosis, which occurs in the abdomen from childhood to adulthood. The immunohistochemical findings in our case matched those described for IMT-RAs, including the expression of ALK assessed by the nuclear membrane staining pattern.
IMT occurs throughout the body, most frequently in the mesentery, omentum, retroperitoneum, pelvis and abdominal soft tissue, which are sites similar to those where desmoid-type fibromatosis occurs. Therefore, IMT with bland cytological features may be histologically indistinguishable from desmoid-type fibromatosis. Desmoid-type fibromatosis is composed of fascicles of spindle-shaped fibroblasts with variable amounts of collagen. In contrast to IMT, desmoid-type fibromatosis exhibits infiltrative borders and expresses immunoreactivity for β-catenin with nuclear labeling. The use of antibodies to β-catenin and ALK may be necessary to achieve a definitive diagnosis.
The RANBP2-ALK fusion gene has been reported to be associated with an uncommon round cell morphology, a unique nuclear membrane ALK staining pattern, and a particularly aggressive clinical course (1). In our case, ALK demonstrated strong staining of the nuclear membrane. FISH was performed to confirm ALK rearrangement. The fusion partner of RANBP2 was identified by RT-PCR. However, the indolent cytology may easily lead to a misdiagnosis.
In conclusion, IMT with round cell or epithelioid morphology is more aggressive than conventional IMT, and is characterized by rapid recurrence and/or mortality. Therefore, patients require a correct and prompt histological diagnosis. The diagnosis of the tumor in our case was extremely challenging due to its spindle-to-epithelioid cell morphology, infrequent mitotic figures and lack of cellular atypism, making it easy to misdiagnose as desmoid-type fibromatosis.
Acknowledgements
The authors are grateful to Professor Hsuan-Ying Huang for his technical support.
References
- 1.Li J, Yin WH, Takeuchi K, et al. Inflammatory myofibroblastic tumor with RANBP2 and ALK gene rearrangement: a report of two cases and literature review. Diagn Pathol. 2013;8:147. doi: 10.1186/1746-1596-8-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen ST, Lee JC. An inflammatory myofibroblastic tumor in liver with ALK and RANBP2 gene rearrangement: combination of distinct morphologic, immunohistochemical, and genetic features. Hum Pathol. 2008;39:1854–1858. doi: 10.1016/j.humpath.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 3.Weremowicz S, Schofield DE. Preparation of cells from formalin-fixed, paraffin-embedded tissue for use in fluorescence in situ hybridization (FISH) experiments. Curr Protoc Hum Genet. 2007;52 doi: 10.1002/0471142905.hg0808s52. Chapter 8, Unit 8.8. [DOI] [PubMed] [Google Scholar]
- 4.Ma Z, Hill DA, Collins MH, et al. Fusion of ALK to the Ran-binding protein 2 (RANBP2) gene in inflammatory myofibroblastic tumor. Genes Chromosomes Cancer. 2003;37:98–105. doi: 10.1002/gcc.10177. [DOI] [PubMed] [Google Scholar]
- 5.Patel AS, Murphy KM, Hawkins AL, et al. RANBP2 and CLTC are involved in ALK rearrangements in inflammatory myofibroblastic tumors. Cancer Genet Cytogenet. 2007;176:107–114. doi: 10.1016/j.cancergencyto.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Butrynski JE, D'Adamo DR, Hornick JL, et al. Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N Engl J Med. 2010;363:1727–1733. doi: 10.1056/NEJMoa1007056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mariño-Enríquez A, Wang WL, Roy A, et al. Epithelioid inflammatory myofibroblastic sarcoma: an aggressive intra-abdominal variant of inflammatory myofibroblastic tumor with nuclear membrane or perinuclear ALK. Am J Surg Pathol. 2011;35:135–144. doi: 10.1097/PAS.0b013e318200cfd5. [DOI] [PubMed] [Google Scholar]
- 8.Kozu Y, Isaka M, Ohde Y, Takeuchi K, Nakajima T. Epithelioid inflammatory myofibroblastic sarcoma arising in the pleural cavity. Gen Thorac Cardiovasc Surg. 2014;62:191–194. doi: 10.1007/s11748-013-0204-x. [DOI] [PubMed] [Google Scholar]
- 9.Gleason BC, Hornick JL. Inflammatory myofibroblastic tumours: where are we now? J Clin Pathol. 2008;61:428–437. doi: 10.1136/jcp.2007.049387. [DOI] [PubMed] [Google Scholar]
- 10.Meis JM, Enzinger FM. Inflammatory fibrosarcoma of the mesentery and retroperitoneum. A tumor closely simulating inflammatory pseudotumor. Am J Surg Pathol. 1991;15:1146–1156. doi: 10.1097/00000478-199112000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Coffin CM, Watterson J, Priest JR, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol. 1995;19:859–872. doi: 10.1097/00000478-199508000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Lovly CM, Lipson D, Otto G, et al. Potentially actionable kinase fusions in inflammatory myofibroblastic tumors. http://meetinglibrary.asco.org/content/115704-132 Presented at ASCO Annual Meeting (abstract 10513) 2013 [Google Scholar]