Abstract
This study aims to explore the aphicidal activity and underlying mechanism of Illicium verum Hook. f. that is used as both food and medicine. The contact toxicity of the extracts from I. verum fruit with methyl alcohol (MA), ethyl acetate (EA), and petroleum ether (PE) against Myzus persicae (Sulzer), and the activities of acetylcholinesterase (AChE) and glutathione S-transferases (GSTs) of M. persicae after contact treatment were tested. The results showed that MA, EA, and PE extracts of 1.000 mg/l caused, respectively, M. persicae mortalities of 68.93%, 89.95% and 74.46%, and the LC50 of MA, EA, and PE extracts were 0.31, 0.14 and 0.27 mg/l at 72 h after treatment, respectively; the activities of AChE and GSTs in M. persicae were obviously inhibited by the three extracts, as compared with the control, with strong dose and time-dependent effects, the inhibition rates on the whole reached more than 50.00% at the concentration of 1.000 mg/l at 72 h after treatment. The inhibition of the extracts on AChE and GSTs activities (EA extract > PE extract > MA extract) were correlated with theirs contact toxic effects, so it is inferred that the decline of the metabolic enzymes activities may be one of important reasons of M. persicae death. The study results suggested that I. verum extracts have potential as a eco-friendly biopesticide in integrated pest management against M. persicae.
Keywords: botanical insecticide, contact toxcity, insecticidal mechanism, acetylcholinesterase, glutathione S-transferase
Introduction
Myzus persicae (Sulzer) (Hemiptera: Aphididae), which is also known as the green peach or peach-potato aphid, is an economically significant pest found in a wide range of field crops and ornamentals worldwide. M. persicae is highly polyphagous and attacks hundreds of species from more than fifty plant families, including agroindustrial crops (potato, sugar beets, and tobacco), horticultural crops (plants of Brassicaceae, Solanaceae, and Cucurbitaceae families), and stone fruits (peach, apricot, and cherry). M. persicae also transmits more than a hundred viral diseases to more than 400 host plants (Gaspari et al. 2007, Kasprowicz et al. 2008), these viral diseases are responsible for direct and indirect damages that cause significant economic losses.
Control measures against M. persicae populations around the world heavily rely on the continued and repeated use of synthetic insecticides (Liu et al. 2007, Rattan 2010). Neonicotinoids, such as imidacloprid, clothianidin, and thiamethoxam, are currently the main class of insecticides used for M. persicae control. However, studies have reported varying levels of resistance against imidacloprid in M. persicae from Europe, USA, Japan, and China (Nauen and Denholm 2005, Margaritopoulos et al. 2007, Foster et al. 2008), which presents a threat to the long-term efficacy of this insecticide class. Insecticides that can be used specifically against M. persicae control are limited, and the frequent use of systemic insecticides to manage insect pests has led to the destabilization of the ecosystem. Thus, alternatives are clearly needed.
Botanicals containing active insecticidal phytochemicals provide an alternative to synthetic pesticides because of their generally low environmental pollution, low toxicity to humans, and other advantages, such as high selectivity, little or no harmful effect on nontarget organisms, rapid degradation, low residual, and minimal cross-resistance because of their natural complex agents and novel modes of action against insects (Isman 2006, Siskos et al. 2009, Mann et al. 2012, Chae et al. 2014). Furthermore, an increasing number of reports on the negative environmental and health impact of synthetic insecticides and the increasingly stringent environmental regulation of pesticides have resulted in renewed interest in the development and use of natural products from plants, which are rich sources of bioactive chemicals (Nukenine et al. 2010, Huang et al. 2011, Isman, 2014a,b).
Illicium verum Hook. f. (Austrobaileyales: Schisandraceae), which is more commonly known as star anise, is a medium-sized evergreen tree native to southwest China. I. verum is widely cultivated in the tropical and subtropical areas of Asia and is used as both food and medicine according to a publication made by the Ministry of Health of the People’s Republic of China on March 2002, thereby implying the low or nontoxicity of I. verum to humans (Li et al. 2013). Previous studies on I. verum have mainly focused on its applications in food and medicine (Ohira et al. 2009, Yang et al. 2010), but a few studies have demonstrated that the essential oil of I. verum is biologically active and can be used to control Sitophilus zeamais (Ho et al. 1995), Blattella germanica (Chang and Ahn 2002), Lasioderma serricorne, Callosobruchus chinensis (Kim et al. 2003), Aedes aegypti (Dana and Wej 2006), and Culex pipiens (Kimbaris et al. 2012).
An important aspect of insecticide toxicology is the elucidation of insecticidal mechanisms by determining the activities of detoxifying enzymes after insecticides have entered the target insects (Vanhaelen et al. 2001, Francis et al. 2005). Acetylcholinesterase (AChE; EC 3.1.1.7) is widely found in insect nervous systems and is the target of organophosphate and carbamate insecticides. AChE is an important enzyme during the excitation phase of nerve conduction in the insect body. Target pests die when AChE activity in vivo is inhibited to a particular extent (Ramsey et al. 2010, Li et al. 2013). Glutathione S-transferases (GSTs; EC 2.5.1.18) has an important function in the detoxification of many endogenous and exogenous toxic substances, including allelochemicals from plants. GSTs also catalyze the conjugation reaction of endogenous glutathione (GSH) and electrophilic compounds (Francis et al. 2005). AChE and GSTs generally have key functions in the interaction between insects and host plants or chemical pesticides. Studies on enzyme inhibition significantly enhance the current understanding of enzyme specificity and the mechanisms by which enzymes and toxic agents work (Li et al. 2007, Zhang et al. 2012).
At present, chemical composition and biological activity of I. verum fruit extracts against S. zeamais adults have been studied (Li et al. 2014). In the present study, the contact toxicity of I. verum fruit extracts with different solvents against adult M. persicae and their effects on the activities of two important enzymes (AChE and GSTs) were tested to explore the aphicidal potential and underlying mechanism of the extracts. The results of this study would be useful for future applications of the indigenous plant source I. verum to control aphid pest.
Materials and Methods
Aphids
Experiments were conducted using clonal lineages of M. persicae maintained in a greenhouse at the School of Plant Protection, Anhui Agricultural University (No.130 West Changjiang Rd, Hefei, Anhui, China). Aphids were reared on greenhouse potted cabbage Brassica oleracea var. (Brassicaceae) placed inside vented Perspex cages (45 × 45 × 50 cm3) maintained at a controlled temperature (22 ± 2°C) under light and dark (LD) photoperiod of 16 and 8 h, respectively, with 50 ± 5% relative humidity (RH). Identical-sized wingless adult aphids used in the experiments were collected from the cabbage on April 2013.
Plant Material
The I. verum fruits from Guangxi (China) were purchased from a local supermarket in Hefei, China.
Extract Preparation
I. verum fruits were dried in an oven (GRX–9071B; Yiheng Scientific Instruments Co. Ltd., Shanghai, China) at 40°C for 2 d, ground into powder by using an electric grinding mill (DD–120B; Linda Machinery Co. Ltd., Zhejiang, China), and sifted through a 40 mesh sieve. The dry powder (150 g) was placed into a 1.0 liter round-bottomed flask. Methyl alcohol (MA; polarity, 5.1; highly polar), ethyl acetate (EA; polarity, 4.4; weakly polar), and petroleum ether (PE; boiling point range, 60–90°C; polarity, 0.0; nonpolar) were sequentially added at a ratio of 1:5 (w/v) at room temperature (25°C). The mixture was incubated in the dark for 48 h and then filtered (Whatman No. 2, Whatman Inc., Clifton, NJ, USA). The samples were leached twice via the above same procedure. The final filtrates were collected from each solvent to obtain the crude extracts. The combined filtrate was dried and concentrated using a vacuum rotary evaporator (Buchi rotavapor R–124; Flawil, Switzerland) then weighed using an electronic balance (FA2104; Hangping Co., Shanghai, China). All samples were stored in air-tight brown bottles at 4°C in a refrigerator until needed.
Contact Toxicity Assay
The slide-dip method was utilized to evaluate the contact toxicity of the extracts against aphids (Wang and Shen 2007). Approximately 30 newly adult aphids were attached on their backs to a 2-cm wide two-sided adhesive plaster on glass slides. Range-finding tests were conducted to determine the appropriate testing concentrations of the extracts. The MA, EA, and PE extracts of I. verum were serially diluted into five appropriate concentrations (1.000, 0.500, 0.250, 0.125, and 0.063 mg/l) by using 1:4 (v:v) aqueous solution of acetone as the solvent based on the results of preliminary experiments. Glass slides with aphids were immersed in the dilutions for 5 s, and the remaining solution on the slides was absorbed with bibulous paper. The slides were placed on 12-cm diameter glass petri dishes and maintained in an artificial climate chamber at 25 ± 1°C, 75 ± 5% RH, and an LD photoperiod of 14:10. The mortality in each treatment was recorded at 24, 48, and 72 h after treatment. A test aphid was considered dead if it did not move its legs when its abdomen was probed with a soft brush. The control sample was treated with 1:4 (v:v) of acetone aqueous solution only. All treatments and control experiments were replicated six times.
Determination of Enzyme Activity
Based on the method used to perform the contact toxicity assay, approximately 300 aptery adult aphids were collected from the cabbage leaves and placed on a nylon yarn net. The nylon yarn nets with aphids were then immersed in different dilutions with concentrations of 0.063, 0.125, 0.250, 0.500, and 1.000 mg/l for 5 s and sampled 24 h after treatment to create the first set of enzyme extracts. Subsequently, another set of test insects was treated with the respective lethal concentration LC50 after 72 h of treatment (the LC50 values of the MA, EA, and PE extracts were 0.31, 0.14, and 0.27 mg/l, respectively) and sampled at 12, 24, 48, 60, and 72 h after treatment to create the second set of enzyme extracts.
All test insects were accurately weighed and rinsed twice or thrice with physiological saline. The insects were then placed in physiological saline with a mass-to-volume ratio of 10% and then homogenated. The supernatant was separated from the homogenate by using a refrigerated centrifuge (TGL–18R, Hema Medical Instruments Co., Ltd., Zhuhai, China) at 4°C and 3500 rpm for 10 min. The supernatant was used in the subsequent enzyme assays. The enzyme samples were stored at –70°C in an ultra-low temperature freezer (Sanyo, Tokyo, Japan). The enzyme extraction step was performed in an ice bath, and the physiological saline and homogenizer were precooled before each experiment to prevent enzyme inactivation.
The total protein content was determined using Coomassie brilliant blue. The activities of AChE and GSTs were measured according to the instructions of the AChE and GSTs detection kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) with a DU730 spectrophotometer (Beckman Coulter, Brea, CA, USA). Each treatment was performed thrice.
Statistical Analysis
Treatment mortality rates were corrected using the mortality rate of the control sample (Abbott 1925). The mean values and standard errors of enzyme activities were counted using the DPS software (Tang and Feng 2007), and the means of corrected mortalities and of enzyme activities were compared using Duncan’s new multiple range test with a significance level of 0.05. The corrected data of mortalities were transformed into their arcsine square-root values for the analysis of variance (ANOVA). The relationship between mortality and concentration was modeled using the DPS program (Tang and Feng 2007). Untransformed data were presented as mean ± SE.
Results
Contact Toxicity
The results of the contact toxicity experiment indicated that the I. verum fruit extract in different solvents were highly toxic to M. persicae adults (Table 1). The contact toxicity effects of the extract in three solvents were distinctly enhanced by increasing concentrations. The MA, EA, and PE extracts at the highest concentration 1.000 mg/l caused M. Persicae mortalities of 68.93%, 89.95%, and 74.46% at 72 h after treatment, respectively.
Table 1.
Contact activity between I. verum fruit extracts and M. persicae (mean values ± SE)a
| Treatment | Concentration of extract (mg/l) | Corrected morality (%) |
||
|---|---|---|---|---|
| 24 h | 48 h | 72 h | ||
| MA extract | 1.000 | 51.11 ± 0.64 a | 57.78 ± 0.64 a | 68.93 ± 1.39 a |
| 0.500 | 31.04 ± 1.81 b | 41.00 ± 3.60 b | 54.49 ± 4.21 b | |
| 0.250 | 22.15 ± 1.51 c | 37.77 ± 0.65 bc | 48.88 ± 1.69 bc | |
| 0.125 | 15.52 ± 0.89 d | 27.68 ± 1.87 c | 38.88 ± 0.65 c | |
| 0.063 | 8.82 ± 1.52 e | 15.31 ± 2.41 d | 24.43 ± 0.74 d | |
| EA extract | 1.000 | 57.94 ± 3.65a | 62.32 ± 2.40 a | 89.95 ± 3.26 a |
| 0.500 | 41.11 ± 0.65 b | 54.55 ± 3.61 a | 70.04 ± 1.20 b | |
| 0.250 | 34.44 ± 0.67 bc | 42.22 ± 0.65 b | 55.60 ± 2.32 bc | |
| 0.125 | 27.77 ± 0.71 c | 35.55 ± 0.67 bc | 47.78 ± 0.64 bc | |
| 0.063 | 17.37 ± 3.17 d | 28.88 ± 0.70 c | 37.77 ± 0.65 c | |
| PE extract | 1.000 | 42.18 ± 2.32 a | 51.11 ± 0.64 a | 74.46 ± 0.73 a |
| 0.500 | 27.77 ± 0.71 b | 38.88 ± 0.65 b | 61.16 ± 1.74 b | |
| 0.250 | 22.20 ± 0.77 bc | 28.84 ± 1.38 c | 44.43 ± 1.69 c | |
| 0.125 | 16.67 ± 0.10 c | 25.49 ± 1.44 c | 34.41 ± 1.35 d | |
| 0.063 | 10.89 ± 2.14 d | 18.86 ± 0.82 d | 28.84 ± 1.38 d | |
aThe mortalities of the control samples were less than 5%. Data followed by different letters in the same column show significant differences by ANOVA followed by Tukey’s test at 5% level of significance.
The regression equations and LC50 for the contact toxicity of the three extracts against M. persicae adults were obtained via linear regression analysis of the relationship between the treatment concentrations and the arcsine square-root values of the mortalities. The LC50 values of the MA, EA, and PE extracts were 0.31, 0.14, and 0.27 mg/l, respectively, 72 h after treatment (Table 2). Therefore, the highest contact toxicity was demonstrated by the EA extract, followed by the PE extract, and the MA extract.
Table 2.
Regression analysis on the contact toxicity of I. verum fruit extracts to M. persicae (72 h)
| Extracts | Regression equation of toxicity | LC50 of extract (mg/l) | Relative coefficient (r) | 95% confidence interval (mg/l) | Chi square (χ2) |
|---|---|---|---|---|---|
| MA extract | y = 0.91x + 5.47 | 0.31 | 0.9885 | 0.23–0.43 | 1.84 |
| EA extract | y = 1.08x + 5.91 | 0.14 | 0.9806 | 0.10–0.19 | 0.58 |
| PE extract | y = 1.03x + 5.59 | 0.27 | 0.9832 | 0.21–0.31 | 1.26 |
Effects of Different Extracts on AChE Activity in M. persicae Adults
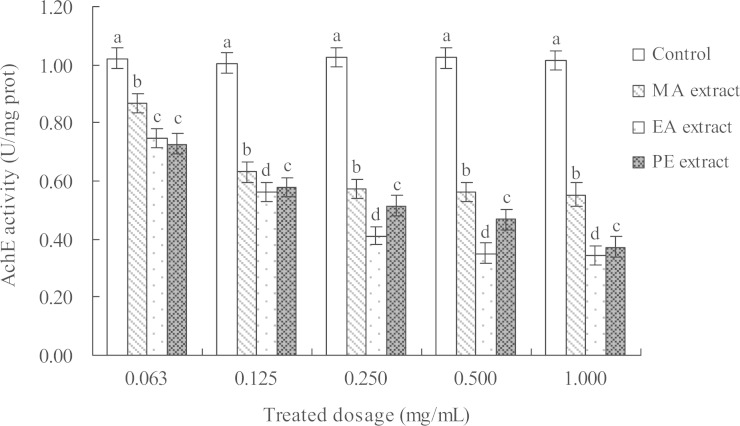
The activity of AChE in M. persicae adults was measured 24 h after treatment with five concentrations of the three extracts. The results (Fig. 1) indicated that these extracts had different inhibition effects on AChE activity. The AChE inhibition rates of the three extracts were in the following order: EA extract > PE extract > MA extract. The inhibition became more intense with increasing extract concentrations. AChE activity was notably inhibited by the three extracts compared with the control sample. The inhibitions of the MA, EA, and PE extracts at 1.000 mg/l reached as high as 45.62%, 62.75%, and 63.32%, respectively.
Fig. 1.
Effect of the different concentrations of I. verum fruit extracts on the AChE activity in adult M. persicae at 24 h. Columns indicate mean ± SE. Varying letters at the top refer to the mean significant difference of different extracts at the 0.05 level (Tukey’s test). The same below.
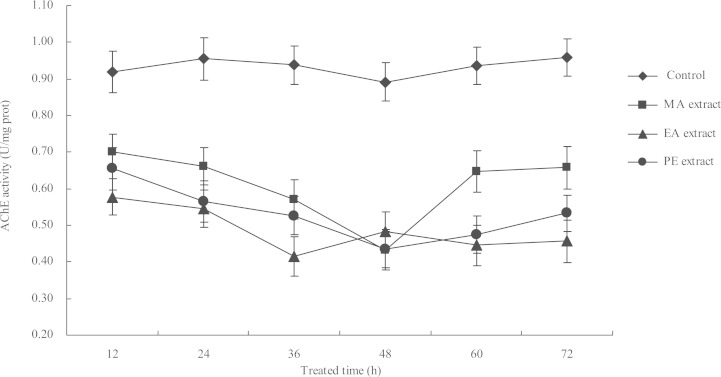
The activity of AChE in M. persicae adults treated with LC50 concentrations of the three extracts (72 h after treatment; MA, 0.31 mg/l; EA, 0.14 mg/l; PE, 0.27 mg/l) were tested at 12, 24, 36, 48, 60, and 72 h after treatment. The results showed that AChE activity was significantly inhibited compared with that in the control sample (Fig. 2). The effects of the three extracts on AChE activity are time-dependent, wherein the AChE activity was initially inhibited (from 12 h to 48 h for the MA and EA extracts; from 12 h to 36 h for the PE extract) but was slightly restored when the treatment time was prolonged. The highest inhibition ratios reached 55.68% and 51.23% at 48 h after treatment with the MA and PE extracts, respectively, and 55.76% at 36 h after treatment with the EA extract.
Fig. 2.
Effect of LC50 (at 72 h) concentrations of I. verum fruit extracts on the AChE activity in adult M. persicae at different exposure times.
Effect of Different Extracts on GST Activities in M. persicae Adults
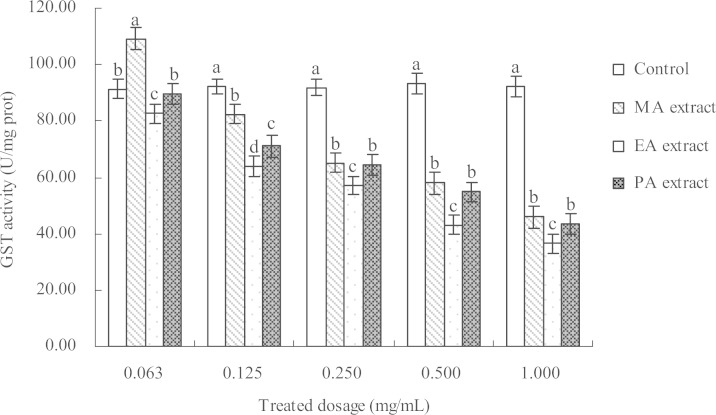
The activity of GSTs in M. persicae adults treated with concentrations of 0.06, 0.13, 0.25, 0.50, and 1.000 mg/l for each extract were tested 24 h after treatment. The results indicated that the PE, EA, and MA extracts had distinct inhibitory effects on GSTs activity (Fig. 3). The low concentration (0.06 mg/l) of the MA extract induced GSTs, and the activity of GSTs increased by 19.56% compared with that in the control sample. Other concentrations also inhibited the activity of GSTs, but the activity improved when the concentration was increased. The GSTs activity levels in the treated insects were significantly lower than in the control sample, and different inhibitory effects on GSTs activity were observed for the different extracts. Moreover, the inhibition of the EA extract was notably higher than that of the other two extracts. The GSTs activity was more significantly inhibited with increasing extract concentration. The inhibition of the three extracts on the GSTs activity was in the following order: EA extract > PE extract > MA extract. The activity of GSTs was notably inhibited by the three extracts compared with the control sample. The inhibition of the MA, EA, and PE extracts reached as high as 50.13%, 60.34%, and 52.76%, respectively, at a concentration of 1.000 mg/l.
Fig. 3.
Effect of different concentrations of I. verum fruit extracts on the GSTs activity in adult M. persicae 24 h after treatment.
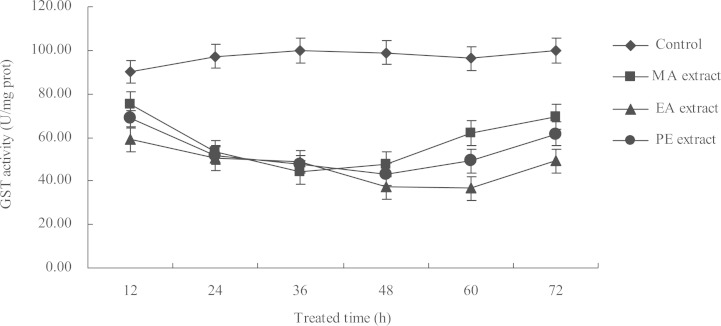
The activity of GSTs in M. persicae adults treated with the LC50 concentration (at 72 h) of the three extracts were tested 12, 24, 36, 48, 60, and 72 h after treatment. The results showed that the GSTs activity in M. persicae after treatment with the three extracts were clearly inhibited as compared with the control sample (Fig. 4). The effect of the three extracts on GTSs activity are time-dependent, wherein the activity was initially inhibited (within 36 h for the MA extract; within 48 h for the EA and PE extracts) but was slightly restored with longer treatment time. The highest inhibition ratios of the MA, EA, and PE extracts to GSTs activity were 55.87% (after 36 h of treatment), 60.02% (after 60 h of treatment), and 56.58% (after 48 h of treatment), respectively.
Fig. 4.
Effect of LC50 concentrations (at 72 h) of I. verum fruit extracts on the GSTs activity in adult M. persicae at different exposure times.
Discussion
The aphicidal activity and influence of the MA, EA, and PE extracts of I. verum fruit on the activities of AchE and GSTs in M. persicae were studied. The results of this study confirmed that the MA, EA, and PE extracts of I. verum fruit possessed significant contact activity against M. persicae adults. Based on the observed mortality rates and LC50 values, the contact toxicity of the three extracts against M. persicae is in the following order: EA extract > PE extract > MA extract. This result is consistent with the experimental result against S. zeamais (Li et al. 2013).
A number of studies have shown that trans-anethole had insecticidal activity against Blattella germanica (Chang and Ahn 2002), Euproctis chrysorrhoea (Erler and Cetin 2008), Ceratitis capitata, Bactrocera dorsalis, and Bactrocera cucurbitae (Chang et al. 2009), Aedes aegypti (Waliwitiya et al. 2009), Liposcelis bostrychophila (Zhao et al. 2012), Spodoptera littoralis (Pavela 2014a), and Culex quinquefasciatus (Pavela 2014b). Also, other studies have indicated that hexadecanoic acid had larvicidal activity to Aedes aegypti (Ozek et al. 2014), larvicidal and pupicidal activity to Anopheles stephensi, Culex quinquefasciatus, and Aedes aegypti (Ragavendran and Natarajan 2015); and Benzyl alcohol had pediculicidal activity to Pediculus humanus capitis (Yang et al. 2005). Our previous GC-MS analysis result (Wei et al. 2014) have highlighted that the most abundant component was trans-anethole, whose percentage was 41.14%, 52.54%, and 72.25% in the MA, EA, and PE extracts, respectively; that of hexadecanoic acid was 0, 4.07%, and 2.15%, respectively; that of benzyl alcohol was 0, 4.04%, and 2.80%, respectively. That is, among the three kinds of extracts, EA extract contains more trans-anethole than MA extract, and the most hexadecanoic acid and the most benzyl alcohol; percentage of trans-anethole is lowest, and no hexadecanoic acid and benzyl alcohol in the MA extract. PE extract contains the most trans-anethole, but lower hexadecanoic acid and lower benzyl alcohol than EA extract. The results suggest the major insecticidal active compound in I. verum fruit is trans-anethole, but hexadecanoic acid and benzyl alcohol also may play a role in insect pest control. The kinds and percent contents of insecticidal active components in the three kinds of extracts were different, so the three kinds of extracts had different control effects against M. persicae.
Many secondary metabolites from deleterious plants have different biological effects, including inhibition and induction of several important enzymes. Determining the inhibitory abilities of exogenous compounds on the activities of enzymes in the insect body is an important method for evaluating insecticidal activities (Li et al. 2007, Tatun et al. 2014a,b). The enzyme AchE, which quickly hydrolyzes the neurotransmitter acetylcholine (ACh) in the synaptic cleft to terminate the conduction of nerve impulses, is one of the most important enzymes that influence the nervous system (Philippou et al. 2010). AChE is the target site of the two major classes of insecticides, namely, organophosphates and carbamates, which irreversibly inhibit AChE and cause the death of insects (Fournier 2005). The experimental results showed that the I. verum extracts significantly inhibited the AChE activity in M. persicae adults. Therefore, the extracts probably have neurotoxic effects on M. persicae.
The GSTs family is one of the major detoxifying enzyme families found in metazoans (Araujo et al. 2008). GSTs are involved in the detoxification of various plant xenobiotics (Francis et al. 2005) and usually catalyze the conjugation of the thiol group of reduced glutathione to electrophilic toxic xenobiotics and endogenously activated compounds molecules, thereby increasing their solubility and promoting rapid excretion or facilitating degradation (Enayati et al. 2005, Li et al. 2007, Ramsey et al. 2010). GSTs are potential drug targets. The inhibitory properties of plant extracts against GSTs may have been the pharmacological basis of their efficacy (Kolawole et al. 2011). Plant extracts enter tissues and organs of target insects and affect the activity of various detoxifying enzymes. Several secondary plant metabolites may inhibit GSTs activity, whereas others can activate GSTs activity (Vanhaelen et al. 2001, Francis et al. 2005, Matthews et al. 2010). The lethal effects of secondary plant metabolites on insects are related to the induction or inhibition of GSTs (Ramsey et al. 2010).
All three extracts showed an obvious concentration effect on the activity of AChE and GSTs in M. persicae. The low concentration (0.06 mg/l) of the MA extract induced GSTs, which is probably the stress response of insects. This response enhanced the detoxification metabolism of substances into secondary plant substances. In other treatments with higher concentrations, these two enzymes were characterized by an inhibitory effect, which became more apparent with increasing concentration. At the LC50 concentration of each extract, the overall performance of the activities of these two enzymes were first inhibited but was then restored with prolonged processing time.
In addition, all three extracts showed a strong time effect on the activity of AchE and GSTs in the body of M. persicae. The activities of the two enzymes were first inhibited and was then induced after treatment with LC50s of the three extracts. This result may be a self-adjusting reaction of an insect to plant xenobiotics, which partially reactivated the two enzymes. As such, the effect of the extracts was continuously eliminated. The effects of the three extracts were slightly different in terms of inhibition speed and extent. Therefore, these extracts may be effective inhibitors of AchE and GSTs in vivo. The important metabolic enzymes in the body of the aphid under normal physiological function were strongly inhibited by the extracts from I. verum.
The present study showed that the toxic effects of the three extracts from I. verum fruit against M. persicae adults were distinctly correlated with the activities of the detoxifying enzymes in M. persicae. The extract with a higher contact activity has more inhibitory effects on the GSTs in vivo. The results implied that reduced activities of AChE and GSTs in M. persicae accounted for the death of pests after treatment with the extracts. Secondary metabolites present in plants apparently function as a defense mechanism and inhibit different physiological and biochemical processes. The phytochemical biomolecules could be used to maximize the effectiveness and specificity of future insecticide designs with specific or multiple target sites, while ensuring economic and ecological sustainability (Rattan 2010). The yield of Chinese star anise accounts for more than 80% of the global output (Li et al. 2013). Given that star anise fruits are readily available in China, we suggest that I. verum extracts be explored as new natural aphicidal agent against M. persicae aphids in alternative management programs.
Acknowledgments
The work was supported by the Key project of Natural Science Foundation of Education Department of Anhui Province (KJ2015A099), the National Natural Science Foundation of China (31401734), the Key Project of China Tobacco Corporation (110201202003), the Key Project of Anhui Province Tobacco Company (20150551007), and the discipline backbone cultivation project of Anhui Agricultural University (2014XKPY-08).
References Cited
- Abbott W. S. 1925. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 18: 265–267. [Google Scholar]
- Araujo R. A., Guedes R. N. C., Oliveira M. G. A., Ferreira G. H. 2008. Enhanced activity of carbohydrate and lipid metaboliaing enzymes insecticide-resistant populations of the maize weevil, Sitophilus zeamais. B. Entomol. Res. 98: 417–424. [DOI] [PubMed] [Google Scholar]
- Chae S. H., Kim S. I., Yeon S. H., Perumalsamy H., Ahn Y. J. 2014. Fumigant toxicity of Summer Savory and Lemon Balm oil constituents and efficacy of spray formulations containing the oils to B- and neonicotinoid-resistant Q-biotypes of Bemisia tabaci (Homoptera: Aleyrodidae). J. Econ. Entomol. 107: 286–292. [DOI] [PubMed] [Google Scholar]
- Chang C. L., Cho I. K., Li Q. X. 2009. Insecticidal activity of basil oil, trans-Anethole, estragole, and linalool to adult fruit flies of Ceratitis capitata, Bactrocera dorsalis, and Bactrocera cucurbitae. J. Econ. Entomol. 102: 203–209. [DOI] [PubMed] [Google Scholar]
- Chang K. S., Ahn Y. J. 2002. Fumigant activity of (E)-anethole identified in Illicium verum fruit against Blattella germanica. Pest Manag. Sci. 58: 161–166. [DOI] [PubMed] [Google Scholar]
- Dana C., Wej C. 2006. Essential oils as potential adulticides against two populations of Aedes aegypti, the laboratory and natural field strains, in Chiang Mai province, northern Thailand. Parasitol Res. 99: 715–721. [DOI] [PubMed] [Google Scholar]
- Enayati A. A., Ranson H., Hemingway J. 2005. Insect glutathione transferases and insecticide resistance. Insect Mol. Biol. 14: 3–8. [DOI] [PubMed] [Google Scholar]
- Erler F., Cetin H. 2008. Larvicidal activity of essential oil from Pimpinella anisum and its major component, trans-anethole, against Euproctis chrysorrhoea. Fresen. Environ. Bull. 17: 2170–2174. [Google Scholar]
- Foster S. P., Cox D., Oliphant L., Mitchinson S., Denholm I. 2008. Correlated responses to neonicotinoid insecticides in clones of the peach-potato aphid, Myzus persicae(Hemiptera: Aphididae). Pest Manag. Sci. 64: 1111–1114. [DOI] [PubMed] [Google Scholar]
- Francis F., Vanhaelen N., Haubruge E. 2005. Glutathione S-transferases in the adaptation to plant secondary metabolites in the Myzus persicae aphid. Arch. Insect Biochem. Physiol. 58: 166–174. [DOI] [PubMed] [Google Scholar]
- Fournier D. 2005. Mutations of acetylcholinesterase which confer insecticide resistance in insect populations. Chem-Biol. Interact. 157–158: 257–261. [DOI] [PubMed] [Google Scholar]
- Gaspari M., Lykouressis D., Perdikis D., Polissiou M. 2007. Nettle extract effects on the aphid Myzus persicae and its natural enemy, the predator Macrolophus pygmaeus (Hemiptera: Miridae). J. Appl. Entomol. 131: 652–657. [Google Scholar]
- Ho S. H., Ma Y., Goh P. M., Sim K. Y. 1995. Star anise, Illicium verum Hook. f. as a potential grain protectant against Tribolium castaneum (Herbst) and Sitophilus zeamais Motsch. Postharvest Biol. Technol. 6: 341–347. [Google Scholar]
- Huang Y. Z., Hua H. X., Li S. G., Yang C. J. 2011. Contact and fumigant toxicities of calamusenone isolated from Acorus gramineus rhizome against adults of Sitophilus zeamais and Rhizopertha dominica. Insect Sci. 18: 181–188. [Google Scholar]
- Isman M. B. 2006. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 51: 45–66. [DOI] [PubMed] [Google Scholar]
- Isman M. B., Grieneisen M. L. 2014a. Botanical insecticides inspired by plant–herbivore chemical interactions. Trends Plant Sci. 19: 29–35. [DOI] [PubMed] [Google Scholar]
- Isman M. B., Grieneisen M. L. 2014b. Botanical insecticide research: many publications, limited useful data. Trends Plant Sci. 19: 140–145. [DOI] [PubMed] [Google Scholar]
- Kasprowicz L., Malloch G., Pickup J., Fenton B. 2008. Spatial and temporal dynamics of Myzus persicae clones in fields and suction traps. Agr. Forest Entomol. 10: 91–100. [Google Scholar]
- Kim S. I., Roh J. Y., Kim D. H., Lee H. S., Ahn Y. J. 2003. Insecticidal activities of aromatic plant extracts and essential oils against Sitophilus oryzae and Callosobruchus chinensis. J. Stored Prod. Res. 39: 293–303. [Google Scholar]
- Kimbaris A., Koliopoulos G., Michaelakis A., Konstantopoulou M. 2012. Bioactivity of Dianthus caryophyllus, Lepidium sativum, Pimpinella anisum, and Illicium verum essential oils and their major components against the West Nile vector Culex pipiens. Parasitol. Res. 111: 2403–2410. [DOI] [PubMed] [Google Scholar]
- Kolawole A. O., Okonji R. E., Ajele J. O. 2011. Tithonia diversifolia, Cyperus rotundus and Hyptis suaveloensis ethanol extracts combinatorially and competitively inhibit affinity purified cowpea storage bruchid (Callosobrochus maculatus) glutathione S-transferase. Arthropod-Plant Interact. 5: 175–184. [Google Scholar]
- Li S. G., Li M. Y., Huang Y. Z., Hua R. M., Lin H. F., He Y. J., Wei L. L., Liu Z. Q. 2013. Fumigant activity of Illicium verum fruit extracts and their effects on the acetylcholinesterase and glutathione S-transferase activities in adult Sitophilus zeamais. J. Pest Sci. 86: 677–683. [Google Scholar]
- Li X., Schuler M. A., Berenbaum M. R. 2007. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu. Rev. Entomol. 52: 231–253. [DOI] [PubMed] [Google Scholar]
- Liu Z. L., Goh S. H., Ho S. H. 2007. Screening of Chinese medicinal herbs for bioactivity against Sitophilus zeamais Motschulsky and Tribolium castaneum (Herbst). J. Stored Prod. Res. 43: 290–296. [Google Scholar]
- Mann R. S., Tiwari S., Smoot J. M., Rouseff R. L., Stelinski L. L. 2012. Repellency and toxicity of plant-based essential oils and their constituents against Diaphorina citri Kuwayama (Hemiptera: Psyllidae). J. Appl. Entomol. 136: 87–96. [Google Scholar]
- Margaritopoulos J. T., Skouras P. J., Nikolaidou P., Manolikaki J., Maritsa K. 2007. Insecticide resistance status of Myzus persicae (Hemiptera: Aphididae) populations from peach and tobacco in mainland Greece. Pest Manag. Sci. 63: 821–829. [DOI] [PubMed] [Google Scholar]
- Matthews H. J., Down R. E., Audsley N. 2010. Effects of manduca sexta allatostatin and an analogue on the peach-potato aphid Myzus persicae (Hemiptera: Aphididae) and degradation by enzymes in the aphid gut. Arch. Insect Biochem. Physiol. 75: 139–157. [DOI] [PubMed] [Google Scholar]
- Nauen R., Denholm I. 2005. Resistance of insect pests to neonicotinoid insecticides: Current status and future prospects. Arch. Insect Biochem. Physiol. 58: 200–215. [DOI] [PubMed] [Google Scholar]
- Nukenine E. N., Adler C., Reichmuth C. 2010. Efficacy of Clausena anisata and Plectranthus glandulosus leaf powder against Prostephanus truncatus (Coleoptera: Bostrichidae) and two strains of Sitophilus zeamais (Coleoptera: Curculionidae) on maize. J. Pest Sci. 83: 181–190. [Google Scholar]
- Ohira H., Torii N., Aida T. M., Watanabe M., Smith R. L. J. 2009. Rapid separation of shikimic acid from Chinese star anise (Illicium verum Hook. f.) with hot water extraction. Sep. Purif. Technol. 69: 102–108. [Google Scholar]
- Ozek G., Suleimen Y., Tabanca N., Doudkin R., Gorovoy P. G., Goger F., Wedge D. E., Ali A., Khan I. A., Baser K. H. C. 2014. Chemical diversity and biological activity of the volatiles of five Artemisia species from far east Russia. Rec. Nat. Prod. 8: 242–261. [Google Scholar]
- Pavela R. 2014a. Acute, synergistic and antagonistic effects of some aromatic compounds on the Spodoptera littoralis Boisd. (Lep., Noctuidae) larvae. Ind. Crop Prod. 60: 247–258. [Google Scholar]
- Pavela R. 2014b. Insecticidal properties of Pimpinella anisum essential oils against the Culex quinquefasciatus and the non-target organism Daphnia magna. J. Asia-Pac. Entomol. 17: 287–293. [Google Scholar]
- Philippou D., Field L., Moores G. 2010. Metabolic enzyme (s) confer imidacloprid resistance in a clone of Myzus persicae (Sulzer) (Hemiptera: Aphididae) from Greece. Pest Manag. Sci. 66: 390–395. [DOI] [PubMed] [Google Scholar]
- Ragavendran C., Natarajan D. 2015. Insecticidal potency of Aspergillus terreus against larvae and pupae of three mosquito species Anopheles stephensi, Culex quinquefasciatus, and Aedes aegypti. Environ. Sci. Pollut. R. 22: 17224–17237. [DOI] [PubMed] [Google Scholar]
- Ramsey J. S., Rider D. S., Walsh T. K., De Vos M., Gordon K. H. J., Ponnala L., Macmil S. L., Roe B. A., Jander G. 2010. Comparative analysis of detoxification enzymes in Acyrthosiphon pisum and Myzus persicae. Insect Mol. Biol. 19: 155–164. [DOI] [PubMed] [Google Scholar]
- Rattan R. S. 2010. Mechanism of action of insecticidal secondary metabolites of plant origin. Crop Prot. 29: 913–920. [Google Scholar]
- Siskos E. P., Konstantopoulou M. A., Mazomenos B. E. 2009. Insecticidal activity of Citrus aurantium peel extract against Bactrocera oleae and Ceratitis capitata adults (Diptera: Tephritidae). J. Appl. Entomol. 133: 108–116. [Google Scholar]
- Tang Q. Y., Feng M. F. 2007. DPS date processing system: experimental design, statistical analysis and data mining. Science Press, Beijing: [in Chinese]. [DOI] [PubMed] [Google Scholar]
- Tatun N., Vajarasathira B., Tungjitwitayakul J., Sakurai S. 2014a. Inhibitory effects of plant extracts on growth, development and alpha-amylase activity in the red flour beetle Tribolium castaneum (Coleoptera: Tenebrionidae). Eur. J. Entomol. 111: 181–188. [Google Scholar]
- Tatun N., Vajarasathira B., Tungjitwitayakul J., Sakurai S. 2014b. Inhibitory effects of plant latex on trehalase activity and trehalase gene expression in the red flour beetle, Tribolium castaneum (Coleoptera: Tenebrionidae). Eur. J. Entomol. 111: 11–18. [Google Scholar]
- Vanhaelen N., Haubruge E., Francis F. 2001. Hoverfly glutathione S-transferases and effect of Brassicaceae secondary metabolites. Pest Biochem. Physiol. 71: 170–177. [Google Scholar]
- Waliwitiya R., Kennedy C. J., Lowenberger C. A. 2009. Larvicidal and oviposition-altering activity of monoterpenoids, trans-anethole and rosemary oil to the yellow fever mosquito Aedes aegypti (Diptera: Culicidae). Pest Manag. Sci. 65: 241–248. [DOI] [PubMed] [Google Scholar]
- Wang X. Y., Shen Z. R. 2007. Potency of some novel insecticides at various environmental temperatures on Myzus persicae. Phytoparasitica 35: 414–422. [Google Scholar]
- Wei L. L., Hua R. M., Li M. Y., Li S. G., Huang Y. Z., He Y. J., Shen Z. H. 2014. Chemical composition and biological activity of star anise Illicium verum extracts against maize weevil, Sitophilus zeamais adults. J. Insect Sci. 14: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F. L., Zhu F., Lei C.L. 2010. Garlic essential oil and its major component as fumigants for controlling Tribolium castaneum (Herbst) in chambers filled with stored grain. J. Pest Sci. 83: 311–317. [Google Scholar]
- Yang Y. C., Lee H. S., Lee S. H., Clark J. M., Ahn Y. J. 2005. Ovicidal and adulticidal activities of Cinnamomum zeylanicum bark essential oil compounds and related compounds against Pediculus humanus capitis (Anoplura : Pediculicidae). Int. J. Parasitol. 14: 1595–1600. [DOI] [PubMed] [Google Scholar]
- Yeom H. J., Kang J. S., Kim G. H., Park I. K. 2012. Insecticidal and acetylcholine esterase inhibition activity of Apiaceae plant essential oils and their constituents against adults of German cockroach (Blattella germanica). J. Agr. Food Chem. 60: 7194–7203. [DOI] [PubMed] [Google Scholar]
- Zhang Y. E., Ma H. J., Feng D. D., Lai X. F., Chen Z. M., Xu M. Y., Yu Q. Y., Zhang Z. 2012. Induction of detoxification enzymes by quercetin in the silkworm. J. Econ. Entomol. 105: 1034–1042. [DOI] [PubMed] [Google Scholar]
- Zhao N. N., Zhou L. G., Liu Z. L., Du S. S., Deng Z. W. 2012. Evaluation of the toxicity of the essential oils of some common Chinese spices against Liposcelis bostrychophila. Food Control 26: 486–490. [Google Scholar]