Abstract
Azadirachta indica, also known as neem, is commonly found in many semi-tropical and tropical countries including India, Pakistan, and Bangladesh. The components extracted from neem plant have been used in traditional medicine for the cure of multiple diseases including cancer for centuries. The extracts of seeds, leaves, flowers, and fruits of neem have consistently shown chemopreventive and antitumor effects in different types of cancer. Azadirachtin and nimbolide are among the few bioactive components in neem that have been studied extensively, but research on a great number of additional bioactive components is warranted. The key anticancer effects of neem components on malignant cells include inhibition of cell proliferation, induction of cell death, suppression of cancer angiogenesis, restoration of cellular reduction/oxidation (redox) balance, and enhancement of the host immune responses against tumor cells. While the underlying mechanisms of these effects are mostly unclear, the suppression of NF-κB signaling pathway is, at least partially, involved in the anticancer functions of neem components. Importantly, the anti-proliferative and apoptosis-inducing effects of neem components are tumor selective as the effects on normal cells are significantly weaker. In addition, neem extracts sensitize cancer cells to immunotherapy and radiotherapy, and enhance the efficacy of certain cancer chemotherapeutic agents. This review summarizes the current updates on the anticancer effects of neem components and their possible impact on managing cancer incidence and treatment.
Keywords: Neem, Mitochondria and apoptosis, Cancer cell death and proliferation, Tumor microenvironment and metabolism, Angiogenesis, Azadirachtin and nimbolide
1. Introduction
Neem (Azadirachta indica) is a fast-growing evergreen tree, and is resistant to drought and high temperature. It is native to semi-tropical and tropical climates and found in countries such as India, Pakistan, and Bangladesh [1–3]. It is consumed as a vegetable in some parts of the Asian subcontinent but mostly used as traditional medicine for centuries to cure multiple human diseases and illnesses [4,5]. For example, neem components have been shown to have antifungal, anthelmintic, antibacterial, antiviral, anti-diabetic, contraceptive, and sedative effects [4,5]. Apart from aforementioned properties, findings from laboratory research suggest that the components of neem possess potent anticancer effects [3,6]. Studies of extracts from all major parts of neem plant including leaves, flowers, fruits, and seeds, have shown promising chemopreventive and therapeutic effects in pre-clinical research [3]. The underlying mechanisms of such anticancer effects of neem have begun to unravel with accumulating studies.
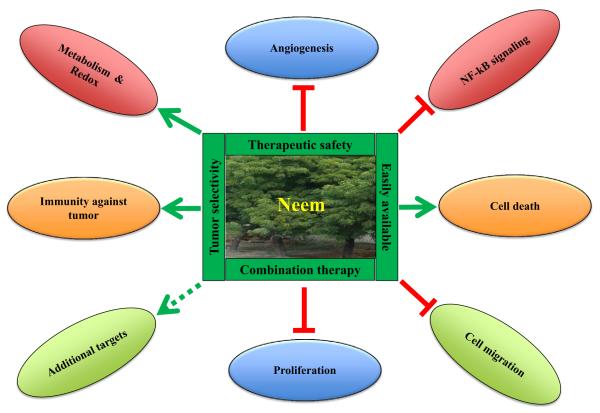
Cancer cells are characterized by a number of hallmarks, including excessive cell growth, reprogramming of energy metabolism that supports the uncontrolled proliferation, immortality, resistance to cell death, induction of angiogenesis, the ability to invade and metastasize to distant sites, and suppression of immune response against tumor cells [7–9]. As illustrated in Fig. 1, preclinical studies have shown compelling evidence suggesting that the anticancer effects of neem are mediated through modulation of multiple cellular processes [3]. Neem components inhibit proliferation, induce apoptosis and other forms of cell death, and reduce cellular oxidative stress (Fig. 1). The expression of genes regulating multiple cellular processes is altered in response to neem leaf extract (NLE) in carcinogen-induced hamster buccal pouch (HBP) model [10]. Tumor microenvironment plays an essential role in angiogenesis and metastasis. Tumor cells possess the ability to modulate their surrounding environment (or microenvironment), which stimulates inflammation, facilitates cell invasion, and induces angiogenesis [8,11,12]. Therefore, tumor microenvironment plays essential roles in the onset and progression of tumors. Interestingly, neem components appear to modulate tumor microenvironment via a number of mechanisms including attenuation of angiogenesis and enhanced cytotoxicity of the immune system. For example, in vitro study suggests that proliferation and migration of human endothelial cells were inhibited by NLE, which results in attenuated angiogenesis in the tumor microenvironment [13]. In addition, neem extracts show selective cytotoxicity towards cancer cells compared to normal cells, which has significance in reducing toxicity during cancer therapy [14–16].
Fig. 1.
The spectrum of neem (Azadirachta indica) components as anti-cancer agents. Several beneficial properties of neem components including easy availability, tumor selectivity, therapeutic safety, and use in combination with other anticancer drugs are implicated in cancer therapy. Neem extracts deliver their anticancer efficacy by suppression of angiogenesis, cell migration and proliferation, and NF-κB signaling. The neem extracts enhance cell death, immunity against tumor, cellular metabolism including modulation of redox potentials.
Neem extracts are prepared using a variety of different solvents including ether, petrol ether, ethyl acetate, and diluted alcohol. Therefore, the spectrum of bioactive components and the percentage of individual components in the extract vary depending on the process of extraction. This variance is likely to affect the accuracy of data interpretation and comparisons across multiple studies using neem extract. Since most researchers use such solvent-extracted mixture of neem components to study its effects on cancer, the involvement of individual components and their respective functions are not well understood. Few bioactive components have been studied extensively, which include azadirachtin and nimbolide. Azadirachtin is a secondary metabolite of neem and mainly concentrated in neem seeds [4,17]. Due to the complexity of its structure, the first synthesis of azadirachtin was not accomplished until 22 after its discovery [17]. Nimbolide was first isolated from the leaves and flowers of neem, but later it was also found in the other parts of the tree [6]. Understanding the effects of individual components will facilitate the development of therapies involving single agent or combinatorial treatment regimens. The anticancer actions of neem extracts and individual components are summarized in Table 1.
Table 1.
Key neem components and their potential targets.
| S.N. | Components | Functions | Target | Model system | Ref |
|---|---|---|---|---|---|
| 1. | Neem leaf extract (NLE) | Proliferation-inhibitory effect | Multiple cell cycle molecules | Prostate cancer cells | [18,19] |
| Induces apoptosis | Multiple apoptosis-modulating molecules |
Prostate cancer cells, primary chronic lymphocytic leukemia cells |
[19,30] | ||
| In vivo: proliferation inhibition |
Proliferating cell nuclear antigen (PCNA) and cytokeratin |
DMBA-induced HBP mouse model | [10,29] | ||
| In vivo: apoptosis induction | Bim, Bax, Apaf-1, caspases, and Bcl-2 |
Breast cancer tissue, prostate cancer xenografts, and DMBA-induced HBP oral carcinogenesis model |
[10,18,29,45–47] | ||
| Enhances immunity | Peripheral blood mononuclear cells (PBMCs), macrophages, natural killer (NK) cells, CD40–CD40L, interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α) |
Murine Ehrlich carcinoma and B16 melanoma |
[50,51] | ||
| Enhances immunity | Spleen and peripheral blood: macrophages, cytokines, and immune cells |
Ehrlich’s carcinoma cells, B16 melanoma, lung sarcoma and lymphosarcoma in the liver in Balb/c mouse model. |
[49,52–54] | ||
| Increases the immunogenicity of vaccinations |
Surface antigen of B16 melanoma cell (B16MelSAg), breast tumor associated antigen (BTAA), and carcinoembryonic antigen (CEA) |
B16 melanoma tumor, breast cancer cells, and CEA + colorectal cancer cells |
[53,55,56] | ||
| Alleviates mutagenicity of carcinogens |
Likely drug metabolizing enzymes | In vivo bone marrow micronuclei test | [55,68,71] | ||
| Maintain cellular redox balance |
Antioxidant phase II enzymes, glutathione level, protein oxidation, and lipid oxidation and peroxidation |
Benzo(a)pyrene-induced stomach tumor model and DMBA-induced skin papilloma model, DMBA-induced rat mammary carcinogenesis model, MNNG-induced carcinogenesis model, and DMBA-induced HBP oral carcinogenesis model |
[72–76,85,87] | ||
| Attenuates angiogenesis | Human umbilical vein endothelial cells (HUVECs), vascular endothelial growth factor (VEGF) |
DMBA-induced HBP carcinogenesis model, chemical carcinogen-induced mammary tumorigenesis |
[13,73,89] | ||
| 2. | Azadirachtin | Induces apoptosis | Bcl-2 family proteins, survivin and caspase-3, -8, and -9 |
Cervical cancer cells | [23] |
| Cell cycle arrest | p53, p21, cyclin B, cyclin D1, and PCNA |
Human cervical cancer (HeLa) cells | [23] | ||
| 3. | Nimbolide | Cell cycle arrest | Cyclins, CDKs, and CKIs, cell cycle checkpoint proteins are CHK2 and Rad17 |
Colon carcinoma cells | [24,25] |
| Induces apoptosis | Bcl-2 family proteins, survivin, and caspase-3, -8, and -9. |
Breast, prostate, hepatocarcinoma, cervical, choriocarcinoma, colon, lymphoma, leukemia, and melanoma |
[20,23,26,27] | ||
| Disrupts cell cycle progression | Unclear | Breast, cervical, choriocarcinoma, lymphoma, leukemia cells HL-60, THP1, and melanoma cells |
[20,23,26,27] | ||
| Retards tumor cell migration, invasion, and angiogenesis |
Metalloproteinase-2/9 (MMP-2/9), VEGF, ERK1/2, NF-κB |
Colon cancer cells | [25] | ||
| Inhibits cell growth | Unclear | Breast cancer cells | [20] | ||
| 4. | Gedunin | Inhibits cell proliferation | Bioinformatic analysis identifies 52 genes involved |
Ovarian cancer cells | [28] |
| 5. | Neem leaf glycoprotein (NLGP) |
Increases host immunity | Various immune cells in favor for type 1 immunity, maturation of dendritic cells |
PBMC derived from HNSCC patients, myeloid derived dendritic cells |
[57–61] |
| Relieves tumor immune suppression |
Regulatory T cells (Tregs) | Mouse tumor model | [64] | ||
| Restores the impaired chemotactic activity of PBMC |
CXCR3-mediated axis, CCR5-mediated axis, CXCR4-mediated axis |
PBMC derived from HNSCC patients | [66] | ||
| Maturation of DCs and increased immunity against CEA |
Maturation of dendritic cells, various immune cells |
Swiss mice, peripheral blood from HNSCC patients |
[58,59,67–69] | ||
| 6. | Mixture of neem limonoids and other components |
Induces apoptosis | Intrinsic: cytochrome c, Bcl-2 family proteins Extrinsic: death receptors |
Leukemia, prostate, cervical, colon, stomach, breast, choriocarcinoma, and hepatocarcinoma |
[14,16,18,20,23,30–32,102] |
| Induces caspase-independent cell death |
Apoptosis-inducing factor (AIF) | Prostate | [32] | ||
| Induces autophagy | Unclear | Prostate and colon | [32] | ||
| Alleviates mutagenicity of carcinogens |
Likely drug metabolizing enzymes | In vitro Ames test | [71] | ||
| Maintain cellular redox balance |
Phase I reactions and phase II enzyme GST |
Rat | [84,86] |
2. Neem components inhibit cancer cell proliferation
Uncontrolled cancer cell growth and proliferation are one of the fundamental hallmarks in cancer and play important role in the development of tumor and cancer metastasis [7,8]. Therefore, inhibiting the growth of tumor cells is a common feature of many chemopreventive and therapeutic agents. Extracts of neem suppress the proliferation and growth of tumor cells through disruption of cell cycle progression. For example, neem seed oil inhibits growth of HeLa cervical cancer cells [14], and NLE shows proliferation-inhibitory effects in prostate cancer cells [18,19]. Interestingly, the androgen-dependency status fails to modulate anti-proliferative effects of NLE in prostate cancer cells. For example, neem extract disrupts proliferation of both androgen-dependent and -independent prostate cancer cells [18,19]. Since androgen-refractory prostate cancer cells are more resistant to apoptosis and lead to prostate cancer recurrence, treatment with active components of neem may provide therapeutic benefits to patients with recurrent prostate cancer. Similar to lack of androgen dependency, the anti-proliferative effects of neem are consistent in both estrogen-dependent and -independent breast cancer cells [20].
Cell cycle progression is tightly controlled by a complex network of regulatory proteins including cyclins, cyclin-dependent kinases (CDKs), CDK inhibitors (CKIs), cell cycle checkpoint proteins, and transcription factors such as E2F [8,21,22]. Studies on the effects of neem or its components on cell cycle and proliferation of tumor cells have identified multiple target proteins. For example, treatment of HeLa cells with azadirachtin decreases the levels of cyclin B and cyclin D1, and induces the expression of CKI p21, which collectively led to G0/G1 cell cycle arrest [23]. Analysis of cell cycle distribution in nimbolide-treated colon carcinoma cells revealed that this active neem component induces both G0/G1 and G2/M arrest accompanied by alterations in cyclins, CDKs and CKIs [24,25]. Additional nimbolide targets for G2/M cell cycle checkpoint proteins are CHK2 and Rad17 [24]. Although detailed mechanisms are unknown, nimbolide disrupts cell cycle progression, and thus inhibits proliferation of HeLa [23], breast cancer [20], choriocarcinoma [26], lymphoma [27], leukemia and melanoma cells [27]. Additional neem components that have been characterized show similar suppressive effects on the growth and proliferation of tumor cells. For example, treatment with NLE or neem-derived gedunin decreases proliferation of pancreatic or ovarian cancer cells, respectively [28]. The subsets of differentially regulated genes induced by gedunin, identified by bioinformatics analysis, encode proteins involved in cell cycle control as well as other cellular processes. Interestingly, the combination of gedunin and cisplatin further decreases the proliferation of treated ovarian cancer cells by almost 50% compared to the cells treated with cisplatin alone [28]. These findings suggest the possibility that gedunin and other potential neem components could enhance the efficacy of chemotherapeutic agents, and such combinatorial therapy may offer additional benefits.
In vivo studies of neem extracts or components show significant anticancer properties, confirming the clinical relevance of the in vitro findings. NLE inhibits the process of carcinogenesis in carcinogen 7,12-dimethylbenz[a]anthracene (DMBA)-induced HBP mouse model, which is accompanied by decreased expression of proliferating cell nuclear antigen (PCNA) and upregulation of cytokeratin, suggesting that neem components suppress proliferation and induce differentiation, respectively [10,29].
3. The effects of neem components on cancer cell death
Besides inhibiting cancer cell proliferation, neem components exert anticancer effect by induction of apoptosis as well as other forms of cell death including autophagy. Extracts from seeds and leaves of neem induce apoptosis in different types of cancer cells such as leukemia [16, 30], prostate [18,31,32], cervical [23], colon [32], stomach [33], and breast [20,32] as well as choriocarcinoma [26], and hepatocarcinoma cells [26]. These findings suggest a proapoptotic effect of neem extracts on a broad spectrum of cancer cell types. Similarly, the administration of individual neem components also induces cancer cell death. For example, nimbolide induces apoptosis in breast cancer [20], prostate cancer [34], hepatocarcinoma [26], cervical cancer [23], choriocarcinoma [26], colon cancer [25], lymphoma, leukemia, and melanoma cells [27]. Azadirachtin shows similar effect in cervical cancer cells [23]. An increasing number of less-characterized limonoids that have been recently isolated from different parts of neem also exhibited proapoptotic effects in leukemia and stomach cancer cells [16,33]. Consistent with the anti-proliferative effects of neem, its proapoptotic potentials are not affected by the hormone-dependent status in prostate cancer [19] and breast cancer cells [20].
Apoptosis occurs through the intrinsic mitochondrial pathway or the extrinsic pathway mediated by membrane-associated death receptors [35–37]. Neem limonoids induce apoptosis through the intrinsic pathway in prostate cancer and cervical cancer cells, accompanied by increased release of cytochrome c from mitochondria [23,32]. The mitochondrial release of cytochrome c is one of the initiating events during apoptosis via intrinsic apoptotic pathway [37,38]. This cytochrome c release is regulated by proapoptotic members (e.g. Bax and Bad) and antiapoptotic members of the Bcl-2 family (e.g. Bcl-2 and Bcl-xL). Thus Bcl-2 family proteins are important targets for exerting anticancer effects of neem in cancer cells. For example, neem-induced apoptosis in prostate cancer cells is mediated by the concurrent decrease of Bcl-2 and increase of Bax levels [31]. In addition, treatment with individual component nimbolide induces expression of Bad and Bax in breast cancer cells, while decreases the levels of Bcl-2 and Bcl-xL [20]. Similar pattern of modulation of Bcl-2 family proteins has also been observed upon nimbolide or azadirachtin exposure to cervical cancer cells [23], and in nimbolide treated choriocarcinoma cells [26]. Nimbolide and azadirachtin also induce expression of caspases while suppress antiapoptotic protein survivin in cervical cancer cells [23]. In addition to the well-characterized neem components, a newly-isolated neem limonoid, 2,3-dihydro-3α-methoxynimbolide, shows proapoptotic effects in stomach cancer cells through modulation of caspase activities accompanied by modulation of the ratio of Bax/Bcl-2 protein levels [33]. These findings demonstrate that neem components exert anticancer effects by modulating Bcl-2 family proteins, caspases, and additional regulatory proteins. Apoptosis or cell death is a very complex process involving multiple groups of protein, thus targeting multiple components in the apoptotic pathway is likely to improve the anticancer efficacy of neem components or extracts. Interestingly, neem-induced apoptosis occurs through a p53-independent mechanism in colon cancer cells, as loss of p53 fails to prevent neem-induced apoptosis [32]. Since loss of p53 function is a common deficiency in majority of cancers, neem may provide unique clinical benefits compared to most chemotherapeutic agents that rely on p53 function to induce apoptosis in tumor cells. Ionizing radiation-induced apoptosis is further enhanced by concurrent treatment of neuroblastoma cells with NLE [39]. This is likely due to the fact that the combination of neem extract and ionizing radiation enhances transcription of genes encoding proapoptotic proteins including Bak, Bax, and caspases to a greater extent compared to radiation alone. Neem limonoids also induce apoptosis through activation of the extrinsic apoptotic pathways in breast, colon, prostate, stomach, and leukemic cancer cells [16,20,32,33]. The extrinsic apoptotic pathway initiates with the binding of death receptors to cognate ligands and the recruitment of adaptor proteins, and converges with the intrinsic pathway at the activation of downstream executioner caspases [35,36]. Nevertheless, the modulatory effects of neem components on Bcl-2 family proteins are likely to further enhance death receptor-mediated apoptosis induced by neem components.
The general modulatory effects of neem extracts on the elimination of cancer cells are also highlighted by their capability to induce other forms of cell death, such as caspase-independent apoptosis and autophagy. The release of apoptosis-inducing factor (AIF) from mitochondria translocates to cell nucleus, and subsequently, induces DNA fragmentation and chromatin condensation causing caspase-independent apoptosis [40,41]. Neem oil limonoids promote the release of mitochondrial AIF in addition to cytochrome c, and thus, also induces caspase-independent cell death in prostate cancer cells [32]. Autophagy is another form of programmed cell death that plays important roles in normal cell growth [42]. Emerging evidence suggests that deregulation of autophagy may also regulate tumorigenesis [43]. Reduced levels of autophagic activity are observed in transformed cells than normal cells, and autophagy may account for the cell death induced by certain therapeutic agents [42,44]. Our findings in prostate and colon cancer cells indicate that neem oil induces concomitant autophagy and apoptosis, both of which do not require p53 activity [32]. In addition, the two forms of cell death appeared to crosstalk through unknown mechanism(s) and inhibition of autophagy enhances elimination of cancer cells via apoptotic pathway [32]. Our studies also show increased expression of autophagy marker upon neem oil exposure in the presence of caspase inhibitor [32], suggesting that autophagy may also contribute to cell death during absence or inhibition of apoptosis. The close interconnection between apoptosis and autophagy is also supported by the findings that other natural compounds such as resveratrol enhances apoptosis upon autophagy inhibition [45].
The in vitro apoptosis-inducing property of neem in tumor cells has also been validated by in vivo studies. For example, treatment with NLE increases the rate of apoptosis in cancer tissues using 4T1 breast cancer mouse model [46]. In addition, NLE-induced inhibition of prostate cancer xenografts in nude mice is associated with the induction of tumor cell apoptosis, and reduction of prostate-specific antigen and tumor growth [18,47]. Studies of DMBA-induced HBP oral carcinogenesis model revealed that neem components or extracts induce a number of molecular events that are associated with the induction of apoptosis, including increased expression of Bim, Bax, Apaf-1, caspase 8 and caspase 3, inhibition of Bcl-2 expression, and increased PARP cleavage [10,29,48].
Among the rich body of literature exploring the apoptosis-inducing effects of neem, a study published recently shows that one patient with chronic lymphocytic leukemia (CLL) demonstrated disease regression following oral administration of NLE [30]. To investigate the underlying mechanism of clinical efficacy, the authors studied the effects of NLE on the primary CLL cells derived from 41 patients. Treatment with NLE decreased the viability of CLL cells in a dose-dependent manner through inducing multiple forms of cell death, demonstrated by cleavage of PARP and caspases, inhibition of Bcl-2 and p53 expression, loss of mitochondrial membrane permeability, nuclear translocation of AIF as well as increased LC3-II levels [30].
4. Neem components modulate tumor immune environment
Among the several components of tumor microenvironment, immune system shows a potential monitoring role. Although immunomodulating effects of neem have been studied extensively using NLE, the effects of other parts of the plant including seeds and flowers on immune system have been modestly explored. NLE shows no cytotoxicity against Ehrlich carcinoma cells. Treatment with NLE prior to cancer cell inoculation in mice reduces tumor burden and improves survival [49,50]. Further studies elucidated that NLE suppresses tumor growth through augmentation of immune activities rather than direct effects on tumor cells. NLE not only restricts the proliferation of tumor cells but also induces apoptosis through stimulation of peripheral blood mononuclear cells (PBMCs) [51,52]. The profile of surface markers in the PBMC derived from both healthy individuals and patients with head and neck cancer are altered by NLE favoring active immune response [51]. Upon treatment with NLE, the monocytes in PBMC show increased expression of cluster of differentiation-40 (CD40), while CD56+ lymphocytes show increased expression of CD40 ligand (CD40L) [51]. The upregulation of CD40–CD40L stimulates macrophages to release higher level of interleukin-12 (IL-12). The increased expression of IL-2 leads to activation of natural killer (NK) cells by inducing perforin–granzyme B system in NK cells. NLE also stimulates the secretion of cytotoxic cytokines interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α) from PBMC [52]. The supernatant derived from these cells reduces the level of Bcl-2 but increases the level of caspase 3 in tumor cells causing higher apoptosis [52]. The growth inhibition of NLE-treated tumor cells also seems inhibited due to the downregulation of cyclin D1, through a mechanism independent of increased cytokine release [52].
The spleen is one of the most pivotal organs involved in immune regulation. It has been reported that the administration of NLE in mice not only increases the weight of the spleen but also enhances the activity of spleen macrophages evidenced by increased expression of activation marker CD44 [53]. The spleen cells derived from NLE-treated mice secrete higher levels of IFN-γ and TNF-α [49,54]. The number of NK cells, NK-T cells, CD4+ helper T cells, CD8+ cytotoxic T cells, and monocytes in the peripheral blood and spleen are also significantly elevated upon NLE exposure [49,50,53]. The increased expression of activation marker CD25 is induced in T cells and the expression of MAC-3 is elevated in the monocytes indicating macrophage differentiation due to NLE exposure [55]. In addition, PBMC and spleen mononuclear cells derived from these NLE-treated mice show greater cytotoxicity against Ehrlich’s carcinoma cells when co-incubated in vitro [49]. The tumor nodules of lung sarcoma and lymphosarcoma in the liver are reduced by NLE treatment in Balb/c mouse model, which is most likely attributed to the cytotoxic effects of NLE-enhanced immune responses in these mice [55].
NLE also improves the immunogenicity of vaccinations and may serve as vaccine adjuvant [54,56]. The surface antigen of B16 melanoma cell (B16MelSAg) is poorly immunogenic, and thus, not preferred as potential option for vaccination. In vivo studies have revealed that the addition of NLE to B16MelSAg vaccine stimulates the generation of B16MelSAg-targeting antibody and induces antibody-dependent cellular cytotoxicity (ADCC) against tumor cells presenting B16MelSAg [54]. Such ADCC effects have been consistent between in vitro and in vivo studies. Enhanced immunogenecity induced by vaccine–NLE combination inhibits growth of B16 melanoma tumor in mice to a greater extent compared to vaccine alone [54]. Similar to B16MelSAg, breast tumor associated antigen (BTAA), a surface antigen with low immunogenicity, is detected specifically in breast cancer cells and tumors. BTAA-immunized mice and rats both had increased production of IgG and decreased IgM antibodies upon the administration of NLE [56]. The antibodies generated in these animals induce ADCC and cytotoxic T cell response against BTAA-positive MCF-7 cells, while the antibodies generated in the animals immunized with BTAA alone showed little cytotoxic effects [56]. Co-immunization with BTAA and NLE also induced IFN-γ secretion but decreased IL-10 release from spleen cells, which indicates the induction of a T-helper (Th) 1 response [56]. Interestingly, the sera of mice treated with NLE show significant reactivity with carcinoembryonic antigen (CEA), likely due to the presence of proteins in NLE that shares homology with CEA molecule [57]. NLE treated mice sera recognize CEA expressed on human colorectal cancer tissues as effectively as CEA monoclonal antibody [57].
Studies suggest that one of the effective components in NLE responsible for its immune activating effects is a glycoprotein [58]. Neem leaf glycoprotein (NLGP) mitigates deficiencies of the immune system in tumor-bearing hosts through a complex network of actions. Consistent with the effects of NLE, the administration of NLGP in mice and rats increases the count of CD4+ helper T cells, CD8+ cytotoxic T cells, and monocytes [59]. In addition, DX5+ NK cells, CD11b+ macrophages, and CD11c+ dendritic cells (DCs) are also increased in these animals [59]. Generation of IgG in vivo, IgG2a in particular, is also enhanced by NLGP [59]. NLGP also induces maturation of DCs as effectively as well-known DC maturation inducer lipopolysaccharides (LPS) as evidenced by comparable upregulation of CD83, CD80, CD86, CD40 and major histocompatibility complexes (MHCs) [60]. NLGP-matured DCs subsequently induce the expression of CD28 and CD40 on T cells, thus promoting the interaction between DCs and T cells [60]. NLGP also induces the expression of early activation marker CD69 on lymphocytes, monocytes, and DCs [61]. An increase in CD45RO+ memory T cells accompanied by a reduction of CD45RA+ CD62L+ naive T cells has also been observed in response to NLGP exposure [61]. Activated T cells release elevated level of signature Th1 cytokine IFN-γ but lower level of Th2 cytokine IL-4, resulting in a type 1 (Th1) immune microenvironment [59–61]. DCs and monocytes are similarly biased towards Th1 immunity, with induction of IL-12 and TNF α, and suppression of IL-10 expression [61]. Transcription factor T-bet-associated with Th1 immune responses is also induced by NLGP in peripheral immune cells [61]. In vitro treatment of matured DCs isolated from patients with stage IIIB cervical cancer (CaCx IIIB) with NLGP demonstrates cytokine release profile biased towards Th1 immune response and activated cytotoxic T lymphocytes [62]. The consistency between the in vivo immune activating actions of NLGP and NLE thus confirms that NLGP is, at least partially, responsible for the immunomodulating functions of NLE.
Regulatory T cells (Tregs) are inherited subpopulation of T cells that suppress the immune system in order to prevent excessive immune reactions and autoimmunity. However, the development of tumors involves marked increase of Tregs as a mechanism to suppress the immune activity against tumor cells in the host and is associated with worse prognosis in cancer patients [63]. While there is a report showing no effect of NLGP on the number of CD4+CD25+Foxp3+ Tregs [59], downregulation of FoxP3+ Tregs has been observed upon NLGP treatment in another study [61]. On the other hand, NLGP impairs the functions of Tregs through modulation of key proteins without direct cytotoxic effects as elaborated below [64]. Tregs isolated from CaCx IIIB patients stimulate the overexpression of immunosuppressive protein indoleamine 2,3 dioxygenase (IDO) by the DCs from the same host [65]. Even in the presence of LPS, Tregs prevent tolerogenic DCs overexpressing IDO from maturation [65]. Interestingly, the addition of NLGP in the co-culture of DCs and Tregs derived from CaCx IIIB patients suppresses the expression of cytotoxic T-lymphocyte antigen 4 (CTLA4) on Tregs [65]. Thus NLGP-induced modulation of Tregs associates with inhibition of IDO overexpression and induction of optimal maturation of DCs [65]. NLGP also down-regulates the expression of transcription factors FoxP3, CTLA4 and TNF receptor GITR that play important roles in immune suppression by Tregs [64]. The interactions between transcription factors FoxP3, p-NFATc3, and p-Smad2/3 were also inhibited following NLGP exposure [64]. IFN-γ secretion, cellular proliferation, and tumor cell cytotoxicity of effector T cells are enhanced by NLGP, thus restoring the functions of effector T cells that were impaired by Tregs [64]. NLGP also increases the levels of IFN-γ and IL-12 and causes decreased expression of IL-10, transforming growth factor (TGF-β), vascular endothelial growth factor (VEGF) and IDO, creating a hostile microenvironment for the tumor [64].
Deregulations in signaling pathway mediated by chemokines and their receptors, which mitigated the migration of PBMC, have been observed in head and neck carcinomas [66,67]. In vitro NLGP treatment of PBMC isolated from these patients upregulates CXCR3A, a marker of innate immune cells, and suppresses CXCR3B, thus rectifying the deregulation of CXCR3 expression [66]. The secretion of CXCL10, the CXCR3 ligand, by these PBMCs is also been increased by NLGP [66]. Consistent with the involvement of CXCR3A–CXCL10 axis in the migration of PBMC, NLGP enhances the migration of these cells towards chemoattractants [66]. The growth of head and neck carcinoma in mice is restricted by NLGP through immunomodulation [66]. In addition, the expression of CCR5 and secretion of its ligands, including regulated on activation normal T cell expressed and secreted (RANTES) and macrophage inflammatory proteins (MIP-1α and MIP-1β) from macrophages/monocytes in the peripheral blood from the patients with head and neck carcinomas are restored following treatment with NLGP [67]. The levels of these proteins are also increased by NLGP treatment in the macrophages/monocytes derived from healthy individuals. Therefore, the migration of these macrophages/monocytes towards standard chemo attractant, which depends on CCR5 chemotaxis, is enhanced by NLGP [67]. CCR5+ monocytes also express greater level of molecules stimulatory to T cell activation, including class 1 human leukocyte antigen (HLA) and T-cell surface receptor ligand CD80 [67]. In vitro experiment suggests that interaction with these CCR5+ monocytes improved the cytotoxic efficacy of effector T cells against cancer cells [67]. However, NLGP shows little effect on the expression of CCR5 and ligands (RANTES, MIP-1α and MIP-1β) in oral cancer cells [67]. In contrast, NLGP down-regulates the expression of CXCR4 and its ligand CCL22, which attenuates the migration of Tregs at the site of tumor [64].
NLGP-matured DCs show both humoral and cellular immune responses against CEA following immunization [68]. NLGP-induced maturation of DCs improves the uptake and processing of CEA by DCs and enhances antigen presentation to B and T cells. Therefore, the generation of CEA specific antibody, mainly IgG2a, is effectively stimulated [68]. T cell proliferation, IFN-γ secretion, and cytotoxicity against CEA+ colon tumor cells are also enhanced by NLGP [68]. Adoptive cell transfer in mice prevents the formation of tumors following two subsequent tumor inoculations, attributing to strong antibody response and CEA-specific T cell cytotoxicity [68]. Generation of effector memory T cells in these mice, evidenced by CD44 upregulation and CD62L reduction on lymphocytes is likely involved in protecting the animals from the second tumor inoculation [68]. In addition, CEA immunization plus NLGP treatment similarly activates macrophages and stimulates Th1 immune response, consistent with the observations in DCs [69,70]. The expression levels of CD19 on B cell surface and CD11b on macrophages are significantly increased by CEA immunization with NLGP adjuvant both in vitro and in mice, which may associate with enhanced immunity [69]. Interestingly, these macrophages show increased production of nitric oxide, which turns out to be interdependent with induced Th1 immunity in these cells [70].
5. The effects of neem components on metabolizing enzymes
The metabolism of xenobiotics including carcinogens, and maintenance of cellular reduction/oxidation (redox) are carried out by drug metabolizing enzymes. Due to these properties, their expression and activities are effective targets for therapeutic agents [71]. Phase I reaction often transforms xenobiotics into reactive intermediates, and these intermediate products are further modified by phase II metabolic enzymes. Both phase I and II metabolizing enzymes could be modulated by neem extracts, leading to decreased carcinogen genotoxicity, and balanced redox level. In vitro Ames test showed that neem oil alleviates the mutagenicity of carcinogens DMBA and mitomycin [72]. The formation of bone marrow micronuclei induced by DMBA or N-methyl-N’-nitro-N-nitrosoguanidine (MNNG) is decreased by NLE [55,68]. Consistent with its effect on reducing genotoxicity, NLE decreases the incidence of tumors in animals exposed to different carcinogens [73–77].
Cellular reactive oxygen species (ROS), produced mainly through mitochondrial respiratory chain reactions, serve as an important signaling molecule in modulation of cell proliferation and survival [78–81]. However, excessive cellular ROS damage the cellular macromolecules such as protein, lipid, and DNA. Increased ROS production beyond physiological level can be caused by deregulated metabolism and mitochondrial dysfunction, and is associated with aging and chronic diseases like cancer [78–82]. Therefore, control of cellular ROS levels and redox balance effectively prevents oxidative stress-induced damage and decreases cancer incidence [83,84]. Dietary supplement of neem flowers in rats represses most phase I reactions, especially those involved in the metabolic activation of carcinogens, and increases the activity of phase II enzyme glutathione-S-transferase (GST) [85]. GST is one of the most important anti-oxidant phase II enzyme families, and it conjugates the substrate with reductive glutathione and reduces the cellular oxidative levels. In contrast to neem flowers, NLE shows no significant effect on phase I enzymes but only induces a number of antioxidant phase II enzymes in treated mice [86]. In vitro treatment with neem extract induces the expression of glutathione S-transferase-pi (GSTPi) in tumor cell lines as well as in human peripheral blood lymphocytes [87]. The levels of glutathione in liver and extrahepatic organs have been found elevated by NLE treatment [88]. The incidence of benzo(a)pyrene-induced stomach tumors and DMBA-induced skin papillomas are both significantly reduced in these NLE-treated mice. Tumor burden is also significantly decreased in both mouse models [88]. Consistent modulations on metabolic enzymes and cellular redox level are also observed in DMBA-induced rat mammary carcinogenesis model [73], MNNG-induced carcinogenesis model [76], and DMBA-induced HBP oral carcinogenesis model [74–76]. Protein oxidation, and lipid oxidation and peroxidation as indicators of cellular oxidative status are decreased by NLE in the liver, blood circulation, and tumor tissues in mice [73–77,88]. Interestingly, different fractions of NLE show differing antioxidant potential and free radical scavenging activities, likely due to the difference in bioactive components in each fraction. The ethyl acetate fraction of neem leaf shows the highest potential, followed by the methanolic extract, and the crude ethanolic extract showed the least potential [74].
It is noteworthy that the antioxidant effects of neem may be context dependent and there are discrepancies between different studies that need to be resolved. In vitro studies showed that treatment with azadirachtin or nimbolide increases the total cellular ROS level in human choriocarcinoma and cervical cancer cells [23,26]. Since extremely high level of cellular ROS causes excessive cell damage, resulting in the elimination of defective cells through apoptosis, it is possible that neem-induced ROS generation in these cells is associated with their increased apoptosis rate [23,26]. Intake of NLE in rats also stimulates the production of ROS in oocytes, which is associated with increased apoptosis in these cells [89].
6. Neem components inhibit tumor invasion and angiogenesis
Tumor creates a favorable microenvironment that induces angiogenesis and facilitates cell invasion in order to promote continued tumor growth and metastasis [8,11,12]. Therefore, targeting these events will impede the progression of tumorigenesis. Although detailed mechanisms remain unclear, findings from the studies on DMBA-induced HBP carcinogenesis model suggest that NLE inhibits angiogenesis [74]. Other studies shed light on the potential anti-angiogenic mechanisms of neem components. For example, studies in rat model show that ethanolic fraction of neem leaf downregulates angiogenic protein VEGF-A, thus causing inhibition of angiogenesis in chemical carcinogen-induced mammary tumorigenesis [90]. NLE inhibits migration of human endothelial cells resulting in attenuation of angiogenesis [13]. An in vitro study showed that nimbolide reduces angiogenesis through repressing the promoter activity and expression of VEGF [25]. The migration and invasion of colon cancer cells are attenuated through inhibition of metalloproteinase-2/9 (MMP-2/9) expression at mRNA and protein levels [25].
Consistent with the notion that different subgroups of neem components are likely to have distinct anticancer actions, the ethyl acetate fraction (EAF) of neem leaf shows greater anti-angiogenic effects compared to the methanolic extract of neem leaf, reflecting the unique composition and bioactivity of each fraction [74]. Similarly, comparison of azadirachtin and nimbolide showed that both agents prevent DMBA-induced HBP carcinogenesis through similar mechanisms including inhibition of angiogenesis, suppression of proliferation, induction of apoptosis, and reduction of oxidative stress. However, nimbolide appears to be more effective in modulating these molecular events and shows higher chemopreventive potency compared to azadirachtin [91]. Therefore, understanding the anticancer actions of individual neem bioactive components is necessary for the development of more effective agents for chemopreventive dietary supplements and therapeutic advantage.
7. Additional anticancer mechanisms of neem components
Neem extracts or components improve the genome stability and reduce accumulation of carcinogenic DNA mutations. Neem extract increases the cellular level of O6-methylguanine-DNA methyltransferase (MGMT) as well as its demethylation activity in peripheral blood lymphocytes and cancer cells [87]. Since MGMT is responsible for the repair of O6-alkylguanines, DNA lesions that occur naturally or induced by alkylating agents enhance the function of MGMT, which likely reduces carcinogenic mutations and maintains genome stability.
Interestingly, NLE also shows cancer type specific anticancer activities. A recent study found that specific extract of neem leaf suppressed hormone-induced androgen receptor and prostate specific antigen (PSA) in prostate cancer cells [47]. They further showed that oral administration of this extract decreases the level of PSA in mice and significantly reduces the growth of prostate tumor xenograft. In parallel to the findings in prostate cancer, immunohistochemical analysis of DMBA-induced rat mammary gland tumors showed that NLE treatment down-regulates the levels of estradiol and estrogen receptors, indicating an anticancer mechanism specific for breast cancer [73]. Consistent with the observations in other carcinogenesis animal models as described above, neem extract also shows general modulatory effects on drug metabolizing enzymes and cellular redox balance in these rats [73]. NLE generated using ethanol, methanol or ethyl acetate differs in their chemopreventive potency, likely due to the different profile of bioactive compounds in each fraction [73].
Comparison of healthy individuals with colon cancer patients suggests that the cancer patient sera possess increased level of total sialic acid (TSA) normalized to total proteins [92]. Consistent with the correlation between TSA level and colon cancer, subcutaneous injection of dimethylhydrazine (DMH) in rats results in 100% incidence of colon cancer, accompanied by significantly elevated level of TSA in the blood [93]. The administration of NLE to these rats effectively reduces the TSA level as well as cancer incidence [93].
Azadirachtin and nimbolide prevent DMBA-induced HBP carcinogenesis similarly through suppression of proliferation, induction of apoptosis, decrease of oxidative stress, and inhibition of angiogenesis. However, nimbolide seems more effective in modulating these molecular events and shows higher chemopreventive potency compared to azadirachtin [91]. Therefore, knowledge of individual bioactive component in NLE is necessary for the development of more effective agents for chemopreventive dietary supplements and therapeutic advantage.
8. The effects of neem components on NF-κB signaling
Nuclear Factor-κB (NF-κB) is one of the most studied transcription factor families, which regulates the transcription of the genes involved in proliferation, survival, migration, inflammation, angiogenesis, and virtually every cellular process [94]. Increased activity or constitutive activation of NF-κB signaling is observed in most types of cancer and plays an important role in carcinogenesis. Therefore, inhibition of NF-κB activity is a common anticancer mechanism of many therapeutic agents and natural compounds. Studies have revealed that neem extracts or components suppress NF-κB signaling, thus potentially contributing to its chemopreventive and therapeutic effects. NF-κB is constitutively expressed and restrained in the cytosol by inhibitor of NF-κB (IκB) through direct binding. When IκB kinase (IKK) is activated by upstream signals, it phosphorylates IκB, resulting in the degradation of IκB and release of NF-κB to translocate into the nucleus. Studies found that the expression and the activity of these major regulators in NF-κB pathway and NF-κB itself can be modulated by neem extract and suppress NF-κB signaling [10,23,95–97]. These in vitro observations have also been validated by in vivo study, in which the expression of NF-κB, IκB and IKK is differentially modulated by NLE and led to the abrogation of NF-κB signaling [10].
Mechanistic study demonstrates that nimbolide suppresses the activity of NF-κB at the levels of nuclear translocation of p65/p50 heterodimer and DNA binding affinity in colon cancer cells [25]. Nimbolide-mediated NF-κB suppression could also occur through direct inhibition of IKK, which appears to require cysteine-179 of IKK-β [86,97]. Since NF-κB is a positive regulator of tumorigenic protein expression, NF-κB suppression is associated with decreased transcription of Bcl-2, Bcl-xL, IAP-1, IAP-2, survivin, Mcl-1, cyclin D1, c-Myc, MMP 9, ICAM-1, CXCR4 and VEGF [25,86,97]. Collectively, this mediates the effects of nimbolide on cellular functions such as proliferation, apoptosis, cell migration, and angiogenesis. Consistent with these molecular events, intraperitoneal injection of nimbolide decreases the growth of inoculated colon cancer xenografts [86].
9. Combination of neem components with other therapies
Due to the complex nature of cancer, single agent regimens have significant limitations, and the clinical therapeutic efficacy can be improved by combining of multiple agents. Interestingly, neem has been shown to improve the efficacy of other anticancer drugs besides its anticancer functions as a single agent [97–99]. The combination of neem-derived gedunin and cisplatin further decreases the proliferation of treated ovarian cancer cells by almost 50% compared to the cells treated with only cisplatin [28]. The combination of sub-lethal dose of ethanolic NLE and cisplatin also provides synergistic effects in decreasing the viability of breast and cervical cancer cells compared to individual compound alone [100].
Bone marrow suppression, i.e. the occurrence of leukopenia and neutropenia, has been a life threatening complication of chemotherapy. Currently available solutions include the administration of colony stimulating factor (CSF), granulocyte colony stimulating factor (GCSF), and granulocyte macrophage colony stimulating factor (GMCSF). Unfortunately, these adjuvants have the adverse effects on stimulating tumor growth. Consistent with the immune stimulating functions of neem, treatment with NLE alleviates cyclophosphamide-caused leukopenia and neutropenia in both healthy mice and the mice bearing tumors [101]. In vitro treatment with NLE also enhances the tumor cell cytotoxicity of peripheral blood monocytes derived from these mice [101]. Consistent with in vitro findings, concurrent application of NLE increases the tumor-suppressing efficacy of cyclophosphamide in mice, further inhibits the growth of tumor and improves the survival of the host [101]. In addition, NLE shows comparable effects as GCSF in prevention of leukocyte apoptosis caused by treatment with cisplatin plus 5-fluorouracil (5-FU) in mice [102]. As a substitute for GCSF, NLE could alleviate leukopenia caused by cisplatin plus 5-FU and enhance the cytotoxicity of peripheral blood cells through increasing the number of cytotoxic T cells and NK cells [102].
Recombinant TNF-related apoptosis-inducing ligand (TRAIL) protein and TRAIL receptor agonistic antibodies have shown promising anticancer efficacy with mild side effects in clinical trials [103]. However, tumors frequently acquire resistance to TRAIL therapy, which can be overcome by sensitizing agents [103]. Interestingly, the addition of nimbolide sensitizes colon cancer cells to TRAIL-induced apoptosis, which is dependent on nimbolide-induced upregulation of p53 and Bax [98]. Nimbolide also down-regulates a number of survival proteins, increases ROS production, activates extracellular signal-regulated kinases (ERK) and mitogen activated protein kinases (MAPK), and upregulates the death receptors (DR) that interact with TRAIL, i.e. DR4 and DR5. These actions of nimbolide collectively enhance the apoptotic effects of TRAIL therapy [98].
In addition, nimbolide potentiates the apoptosis induced by inflammatory cytokine TNF-α and chemotherapeutic agents 5-FU and thalidomide in blood cancer cells [97]. Such sensitization of tumor cells to cytotoxic agents by nimbolide occurs through decreasing NF-κB activity, consistent with its suppressive effects on NF-κB signaling. NF-κB signaling pathway is also one of the prosurvival pathways activated by ionizing radiation, which protects cancer cells from radiation-induced apoptosis [104]. Therefore, neem-induced suppression of NF-κB activation is likely to increase the radiosensitivity of tumor cells, which could potentially improve the clinical effectiveness of radiation therapy. Studies in pancreatic cancer cells show that treatment with NLE suppresses the radiation-induced activation of NF-κB signaling, resulting in increased caspase activity and cell death compared to the cells receiving radiation alone [39]. In addition, radiation-induced apoptosis of neuroblastoma cells is enhanced by the concurrent treatment with NLE through increasing the expression of proapoptotic proteins [39]. However, it is unclear whether suppression of NF-κB signaling mediates this effect or other signaling also contributes to increased apoptosis [39]. Further investigation is warranted to determine whether neem could sensitize cancer cells to the cytotoxic effects of other therapeutic regimens and whether combination therapy could offer potential clinical benefits.
10. Therapeutic safety and tumor selective properties of neem components
Therapeutic safety and tumor selectivity are two key features for potential anti-tumor therapeutic regimens. Neem seed oil shows no mutagenic effects based on the results of in vitro Ames test and in vivo micronucleus assay [72]. The in vivo therapeutic safety of neem in mice and rats is further supported by the findings that treatment with NLGP shows no changes in the organ microstructure and hematological system, including immune cells of animals [59]. Overall, no behavioral changes or increased death associates with the administration of NLGP [59].
Since high tumor selectivity is crucial to minimize the side effects of anticancer drugs, it is an important concern for any anticancer therapeutic agent to differentiate normal cells from tumor cells. Compared to healthy cells that are equipped with a balanced machinery of cellular homeostasis, tumor cells have transformed cell structures and functions, which potentially make them vulnerable to toxic effects of anticancer agents [7,105,106]. Several studies suggest that anti-proliferative and proapoptotic actions of neem are selective for tumor cells. For example, methanolic extraction (MEX) of neem seed oil free of terpenoid/limonoid shows significantly stronger inhibitory effects on the viability of HeLa cells compared to normal murine fibroblasts [14]. Comparison of the oxidative stress level in the two types of cells demonstrates that MEX mixture specifically induces the intracellular level of malonaldialdehyde (MDA) in HeLa cells but not in murine fibroblasts. Such increased cellular oxidative stress is likely to contribute to the observed cell membrane damages that lead to apoptotic cell death and decreased cellular viability. The susceptibility of tumor cells to MEX may be associated with the modification of the plasma membrane lipids during transformation of normal cells to malignant cells. The ethanolic neem leaf extract also specifically targets the breast as well as cervical cancer cells, without showing significant effects on the viability of primary lymphocytes [100].
Since there are fundamental differences in cell structures and functions of normal cells with malignant cells from different origins, a direct comparison between tumor cells and their corresponding normal cells will define the underlying mechanisms of differential neem extract cytotoxicity. Comparison of limonoid cytotoxicity on leukemia cells and normal lymphocyte cells demonstrates significant tumor selectivity, and all three neem compounds tested show greater tumor specificity than cisplatin [16]. In vivo and in vitro models on the exploration of therapeutic efficacy of neem components also reveal that these components boost the host immunity against tumor cells. For example, a study related to the therapeutic efficacy of NLGP on mouse sarcoma demonstrates enhanced potential of effector T-cells host immune response [107]. Neem components also reported to induce upregulation of CD40 and CD40L ligands, maturation of DCs, CD83, CD80 and CD86, and downregulation of Treg cells that create an anticancer environment [5,61].
Comparison of the effects of tamoxifen and different formulations of neem extracts on multiple cell lines demonstrates that neem components show greater tumor selectivity than tamoxifen [108]. Therefore, thorough studies are warranted to explore the different spectrums of tumor selectivity using various types of cancer cells and understand the underlying mechanism of such tumor-specificity. The targets of neem components include molecules involved in cell cycle, cell death, host immune system, and cellular redox maintenance. Therefore, further studies targeting these candidate pathways may explain the tumor specificity of neem components.
11. Conclusions and future perspectives
As a natural resource, neem extract has the advantages of easy availability, low cost and safety to humans, which collectively make neem-derived compounds valuable candidates for anticancer therapy. Preclinical studies have primarily established neem as a potential preventive and therapeutic agent against various types of cancer. The anticancer actions of neem extract are associated with modulation of major hallmark events in tumor cells including inhibition of excessive proliferation, induction of cell death, suppression of angiogenesis, restoration of cellular redox balance, and enhancement of immune response against tumor cells (Table 1). With the profile of bioactive components in neem not completely clear, identification of effective anticancer components and study of individual components require further research. Although the preclinical findings and limited in vivo observations so far are encouraging, more extensive studies in animal models are needed to validate current knowledge on the functions of neem and explore the effects of neem on the other properties of tumor cells, such as increased cell mobility and metastasis. Full understanding of the underlying anticancer mechanisms of neem is required before neem can be tested for its chemopreventive and therapeutic efficacy in clinical settings. Identification and characterization of individual anticancer components of neem are also prerequisites for the development of neem-based therapeutic regimens. The potential of using neem to enhance the efficacy of other chemotherapeutic agents or as adjuvant in immunotherapy and radiotherapy is also worth further exploration. Taken together, current studies suggest that neem components have high potential for consideration as effective anticancer agents with minimal or no side effects during therapy.
Acknowledgements
This work was supported in part by the American Cancer Society Research Scholar Grant RSG-12-214-01 – CCG, and the U.S. Department of Defense under Award Number W81XWH-14-1-0013 to DC; and the National Cancer Institute Center Support Grant P30 CA016056 to the Roswell Park Cancer Institute. We apologize to those colleagues whose publications inadvertently could not be cited or cited incorrectly.
References
- [1].Radwanski SA, Wickens GE. Vegetative fallows and potential value of the neem tree (Azadirachta indica) in the tropics. Econ. Bot. 1981;35:398–414. [Google Scholar]
- [2].Schmutterer H. Properties and potential of natural pesticides from the neem tree, Azadirachta indica. Annu. Rev. Entomol. 1990;35:271–297. doi: 10.1146/annurev.en.35.010190.001415. [DOI] [PubMed] [Google Scholar]
- [3].Paul R, Prasad M, Sah NK. Anticancer biology of Azadirachta indica L (neem): a mini review. Free Radic. Res. 2011;12:467–476. doi: 10.4161/cbt.12.6.16850. [DOI] [PubMed] [Google Scholar]
- [4].Veitch GE, Boyer A, Ley SV. The azadirachtin story. Angew. Chem. Int. Ed. Engl. 2008;47:9402–9429. doi: 10.1002/anie.200802675. [DOI] [PubMed] [Google Scholar]
- [5].Puri HS. Neem: The Divine Tree Azadirachta indica. 1st edition CRC Press; 1999. [Google Scholar]
- [6].Bodduluru LN, Kasala ER, Thota N, Barua CC, Sistla R. Chemopreventive and therapeutic effects of nimbolide in cancer: the underlying mechanisms. Toxicol. In Vitro. 2014;28:1026–1035. doi: 10.1016/j.tiv.2014.04.011. [DOI] [PubMed] [Google Scholar]
- [7].Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- [8].Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- [9].Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- [10].Manikandan P, Ramalingam SM, Vinothini G, Ramamurthi VP, Singh IP, Anandan R, Gopalakrishnan M, Nagini S. Investigation of the chemopreventive potential of neem leaf subfractions in the hamster buccal pouch model and phytochemical characterization. Eur. J. Med. Chem. 2012;56:271–281. doi: 10.1016/j.ejmech.2012.08.008. [DOI] [PubMed] [Google Scholar]
- [11].Spano D, Zollo M. Tumor microenvironment: a main actor in the metastasis process. Clin. Exp. Metastasis. 2012;29:381–395. doi: 10.1007/s10585-012-9457-5. [DOI] [PubMed] [Google Scholar]
- [12].Gao F, Liang B, Reddy ST, Farias-Eisner R, Su X. Role of inflammation-associated microenvironment in tumorigenesis and metastasis. Curr. Cancer Drug Targets. 2014;14:30–45. doi: 10.2174/15680096113136660107. [DOI] [PubMed] [Google Scholar]
- [13].Mahapatra S, Young CY, Kohli M, Karnes RJ, Klee EW, Holmes MW, Tindall DJ, Donkena KV. Antiangiogenic effects and therapeutic targets of Azadirachta indica leaf extract in endothelial cells. Evid. Based Complement. Altern. Med. 2012;2012:303019. doi: 10.1155/2012/303019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ricci F, Berardi V, Risuleo G. Differential cytotoxicity of MEX: a component of neem oil whose action is exerted at the cell membrane level. Molecules. 2008;14:122–132. doi: 10.3390/molecules14010122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Di Ilio V, Pasquariello N, van der Esch AS, Cristofaro M, Scarsella G, Risuleo G. Cytotoxic and antiproliferative effects induced by a non terpenoid polar extract of A. indica seeds on 3T6 murine fibroblasts in culture. Mol. Cell. Biochem. 2006;287:69–77. doi: 10.1007/s11010-005-9062-x. [DOI] [PubMed] [Google Scholar]
- [16].Kikuchi T, Ishii K, Noto T, Takahashi A, Tabata K, Suzuki T, Akihisa T. Cytotoxic and apoptosis-inducing activities of limonoids from the seeds of Azadirachta indica (neem) J. Nat. Prod. 2011;74:866. doi: 10.1021/np100783k. [DOI] [PubMed] [Google Scholar]
- [17].Morgan ED. Azadirachtin, a scientific gold mine. Bioorg. Med. Chem. 2009;17:4096–4105. doi: 10.1016/j.bmc.2008.11.081. [DOI] [PubMed] [Google Scholar]
- [18].Mahapatra S, Karnes RJ, Holmes MW, Young CY, Cheville JC, Kohli M, Klee EW, Tindall DJ, Donkena KV. Novel molecular targets of Azadirachta indica associated with inhibition of tumor growth in prostate cancer. AAPS J. 2011;13:365–377. doi: 10.1208/s12248-011-9279-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gunadharini DN, Elumalai P, Arunkumar R, Senthilkumar K, Arunakaran J. Induction of apoptosis and inhibition of PI3K/Akt pathway in PC-3 and LNCaP prostate cancer cells by ethanolic neem leaf extract. J. Ethnopharmacol. 2011;134:644–650. doi: 10.1016/j.jep.2011.01.015. [DOI] [PubMed] [Google Scholar]
- [20].Elumalai P, Gunadharini DN, Senthilkumar K, Banudevi S, Arunkumar R, Benson CS, Sharmila G, Arunakaran J. Induction of apoptosis in human breast cancer cells by nimbolide through extrinsic and intrinsic pathway. Toxicol. Lett. 2012;215:131–142. doi: 10.1016/j.toxlet.2012.10.008. [DOI] [PubMed] [Google Scholar]
- [21].Olashaw N, Pledger WJ. Paradigms of growth control: relation to Cdk activation. Sci. STKE. 2002;2002:re7. doi: 10.1126/stke.2002.134.re7. [DOI] [PubMed] [Google Scholar]
- [22].Johnson DG, Degregori J. Putting the oncogenic and tumor suppressive activities of E2F into context. Curr. Mol. Med. 2006;6:731–738. doi: 10.2174/1566524010606070731. [DOI] [PubMed] [Google Scholar]
- [23].Priyadarsini RV, Murugan RS, Sripriya P, Karunagaran D, Nagini S. The neem limonoids azadirachtin and nimbolide induce cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells. Free Radic. Res. 2010;44:624–634. doi: 10.3109/10715761003692503. [DOI] [PubMed] [Google Scholar]
- [24].Roy MK, Kobori M, Takenaka M, Nakahara K, Shinmoto H, Tsushida T. Inhibition of colon cancer (HT-29) cell proliferation by a triterpenoid isolated from Azadirachta indica is accompanied by cell cycle arrest and up-regulation of p21. Planta Med. 2006;72:917–923. doi: 10.1055/s-2006-946694. [DOI] [PubMed] [Google Scholar]
- [25].Babykutty S, Priya PS, Nandini RJ, Kumar MAS, Nair MS, Srinivas P, Gopala S. Nimbolide retards tumor cell migration, invasion, and angiogenesis by downregulating MMP-2/9 expression via inhibiting ERK1/2 and reducing DNA-binding activity of NF-kappaB in colon cancer cells. Mol. Carcinog. 2012;51:475–490. doi: 10.1002/mc.20812. [DOI] [PubMed] [Google Scholar]
- [26].Harish Kumar G, Chandra Mohan KV, Jagannadha Rao A, Nagini S. Nimbolide a limonoid from Azadirachta indica inhibits proliferation and induces apoptosis of human choriocarcinoma (BeWo) cells. Investig. New Drugs. 2009;27:246–252. doi: 10.1007/s10637-008-9170-z. [DOI] [PubMed] [Google Scholar]
- [27].Roy MK, Kobori M, Takenaka M, Nakahara K, Shinmoto H, Isobe S, Tsushida T. Antiproliferative effect on human cancer cell lines after treatment with nimbolide extracted from an edible part of the neem tree (Azadirachta indica) Phytother. Res. 2007;21:245–250. doi: 10.1002/ptr.2058. [DOI] [PubMed] [Google Scholar]
- [28].Kamath SG, Chen N, Xiong Y, Wenham R, Apte S, Humphrey M, Cragun J, Lancaster JM. Gedunin, a novel natural substance, inhibits ovarian cancer cell proliferation. Int. J. Gynecol. Cancer. 2009;19:1564–1569. doi: 10.1111/IGC.0b013e3181a83135. [DOI] [PubMed] [Google Scholar]
- [29].Subapriya R, Kumaraguruparan R, Nagini S. Expression of PCNA, cytokeratin, Bcl-2 and p53 during chemoprevention of hamster buccal pouch carcinogenesis by ethanolic neem (Azadirachta indica) leaf extract. Clin. Biochem. 2006;39:1080–1087. doi: 10.1016/j.clinbiochem.2006.06.013. [DOI] [PubMed] [Google Scholar]
- [30].Chitta KS, Khan AN, Ersing N, Swaika A, Masood A, Paulus A, Qadeer A, Advani P, Sher T, Miller KC, Lee K, Chanan-Khan AA. Neem leaf extract induces cell death by apoptosis and autophagy in B-chronic lymphocytic leukemia cells. Leuk. Lymphoma. 2014;55:652–661. doi: 10.3109/10428194.2013.807927. [DOI] [PubMed] [Google Scholar]
- [31].Kumar S, Suresh PK, Vijayababu MR, Arunkumar A, Arunakaran J. Anticancer effects of ethanolic neem leaf extract on prostate cancer cell line (PC-3) J. Ethnopharmacol. 2006;105:246–250. doi: 10.1016/j.jep.2005.11.006. [DOI] [PubMed] [Google Scholar]
- [32].Srivastava P, Yadav N, Lella R, Schneider A, Jones A, Marlowe T, Lovett G, O’Loughlin K, Minderman H, Gogada R, Chandra D. Neem oil limonoids induces p53-independent apoptosis and autophagy. Carcinogenesis. 2012;33:2199–2207. doi: 10.1093/carcin/bgs269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Manosroi A, Kitdamrongtham W, Ishii K, Shinozaki T, Tachi Y, Takagi M, Ebina K, Zhang J, Manosroi J, Akihisa R, Akihisa T. Limonoids from Azadirachta indica var. siamensis extracts and their cytotoxic and melanogenesis-inhibitory activities. Chem. Biodivers. 2014;11:505–531. doi: 10.1002/cbdv.201300406. [DOI] [PubMed] [Google Scholar]
- [34].Raja Singh P, Arunkumar R, Sivakamasundari V, Sharmila G, Elumalai P, Suganthapriya E, Brindha Mercy A, Senthilkumar K, Arunakaran J. Anti-proliferative and apoptosis inducing effect of nimbolide by altering molecules involved in apoptosis and IGF signalling via PI3K/Akt in prostate cancer (PC-3) cell line. Cell Biochem. Funct. 2014;32:217–228. doi: 10.1002/cbf.2993. [DOI] [PubMed] [Google Scholar]
- [35].Galluzzi L, Kepp O, Kroemer G. Mitochondria: master regulators of danger signalling. Nat. Rev. Mol. Cell Biol. 2012;13:780–788. doi: 10.1038/nrm3479. [DOI] [PubMed] [Google Scholar]
- [36].Tait SW, Green DR. Mitochondria and cell signalling. J. Cell Sci. 2012;125:807–815. doi: 10.1242/jcs.099234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yadav N, Chandra D. Mitochondrial and postmitochondrial survival signaling in cancer. Mitochondrion. 2014;16:18–25. doi: 10.1016/j.mito.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–2933. [PubMed] [Google Scholar]
- [39].Veeraraghavan J, Aravindan S, Natarajan M, Awasthi V, Herman TS, Aravindan N. Neem leaf extract induces radiosensitization in human neuroblastoma xenograft through modulation of apoptotic pathway. Anticancer Res. 2011;31:161–170. [PubMed] [Google Scholar]
- [40].Joza N, Susin SA, Daugas E, Stanford WL, Cho SK, Li CY, Sasaki T, Elia AJ, Cheng H-YM, Ravagnan L. Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature. 2001;410:549–554. doi: 10.1038/35069004. [DOI] [PubMed] [Google Scholar]
- [41].Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- [42].Gozuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene. 2004;23:2891–2906. doi: 10.1038/sj.onc.1207521. [DOI] [PubMed] [Google Scholar]
- [43].Liu H, He Z, von Rutte T, Yousefi S, Hunger RE, Simon HU. Down-regulation of autophagy-related protein 5 (ATG5) contributes to the pathogenesis of early-stage cutaneous melanoma. Sci. Transl. Med. 2013;5:202ra123. doi: 10.1126/scitranslmed.3005864. [DOI] [PubMed] [Google Scholar]
- [44].White E. Deconvoluting the context-dependent role for autophagy in cancer. Nat. Rev. Cancer. 2012;12:401–410. doi: 10.1038/nrc3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Prabhu V, Srivastava P, Yadav N, Amadori M, Schneider A, Seshadri A, Pitarresi J, Scott R, Zhang H, Koochekpour S, Gogada R, Chandra D. Resveratrol depletes mitochondrial DNA and inhibition of autophagy enhances resveratrol-induced caspase activation. Mitochondrion. 2013;13:493–499. doi: 10.1016/j.mito.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Othman F, Motalleb G, Lam Tsuey Peng S, Rahmat A, Fakurazi S, Pei Pei C. Extract of Azadirachta indica (neem) leaf induces apoptosis in 4T1 breast cancer BALB/c mice. Cell J. 2011;13:107–116. [PMC free article] [PubMed] [Google Scholar]
- [47].Wu Q, Kohli M, Bergen HR, III, Cheville JC, Karnes RJ, Cao H, Young CY, Tindall DJ, McNiven MA, Donkena KV. Preclinical evaluation of the super-critical extract of Azadirachta indica (neem) leaves in vitro and in vivo on inhibition of prostate cancer tumor growth. Mol. Cancer Ther. 2014;13:1067–1077. doi: 10.1158/1535-7163.MCT-13-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Subapriya R, Bhuvaneswari V, Nagini S. Ethanolic neem (Azadirachta indica) leaf extract induces apoptosis in the hamster buccal pouch carcinogenesis model by modulation of Bcl-2, Bim, caspase 8 and caspase 3. Asian Pac. J. Cancer Prev. 2005;6:515–520. [PubMed] [Google Scholar]
- [49].Haque E, Baral R. Neem (Azadirachta indica) leaf preparation induces prophylactic growth inhibition of murine Ehrlich carcinoma in Swiss and C57BL/6 mice by activation of NK cells and NK-T cells. Immunobiology. 2006;211:721–731. doi: 10.1016/j.imbio.2006.02.005. [DOI] [PubMed] [Google Scholar]
- [50].Baral R, Chattopadhyay U. Neem (Azadirachta indica) leaf mediated immune activation causes prophylactic growth inhibition of murine Ehrlich carcinoma and B16 melanoma. Int. Immunopharmacol. 2004;4:355–366. doi: 10.1016/j.intimp.2003.09.006. [DOI] [PubMed] [Google Scholar]
- [51].Bose A, Baral R. Natural killer cell mediated cytotoxicity of tumor cells initiated by neem leaf preparation is associated with CD40–CD40L-mediated endogenous production of interleukin-12. Hum. Immunol. 2007;68:823–831. doi: 10.1016/j.humimm.2007.08.002. [DOI] [PubMed] [Google Scholar]
- [52].Bose A, Haque E, Baral R. Neem leaf preparation induces apoptosis of tumor cells by releasing cytotoxic cytokines from human peripheral blood mononuclear cells. Phytother. Res. 2007;21:914–920. doi: 10.1002/ptr.2185. [DOI] [PubMed] [Google Scholar]
- [53].Beuth J, Schneider H, Ko HL. Enhancement of immune responses to neem leaf extract (Azadirachta indica) correlates with antineoplastic activity in BALB/c-mice. In Vivo. 2006;20:247–251. [PubMed] [Google Scholar]
- [54].Baral R, Mandal I, Chattopadhyay U. Immunostimulatory neem leaf preparation acts as an adjuvant to enhance the efficacy of poorly immunogenic B16 melanoma surface antigen vaccine. Int. Immunopharmacol. 2005;5:1343–1352. doi: 10.1016/j.intimp.2005.03.008. [DOI] [PubMed] [Google Scholar]
- [55].Beuth J, Schneider H, Ko HL. Enhancement of immune responses to neem leaf extract (Azadirachta indica) correlates with antineoplastic activity in BALB/c-mice. 2006;20:247–251. [PubMed] [Google Scholar]
- [56].Mandal-Ghosh I, Chattopadhyay U, Baral R. Neem leaf preparation enhances Th1 type immune response and anti-tumor immunity against breast tumor associated antigen. Cancer Immun. 2007;7:8. [PMC free article] [PubMed] [Google Scholar]
- [57].Sarkar K, Bose A, Laskar S, Choudhuri SK, Dey S, Roychowdhury PK, Baral R. Antibody response against neem leaf preparation recognizes carcinoembryonic antigen. Int. Immunopharmacol. 2007;7:306–312. doi: 10.1016/j.intimp.2006.10.014. [DOI] [PubMed] [Google Scholar]
- [58].Goswami KK, Barik S, Sarkar M, Bhowmick A, Biswas J, Bose A, Baral R. Targeting STAT3 phosphorylation by neem leaf glycoprotein prevents immune evasion exerted by supraglottic laryngeal tumor induced M2 macrophages. Mol. Immunol. 2014;59:119–127. doi: 10.1016/j.molimm.2014.01.015. [DOI] [PubMed] [Google Scholar]
- [59].Mallick A, Ghosh S, Banerjee S, Majumder S, Das A, Mondal B, Barik S, Goswami KK, Pal S, Laskar S, Sarkar K, Bose A, Baral R. Neem leaf glycoprotein is nontoxic to physiological functions of Swiss mice and Sprague Dawley rats: histological, biochemical and immunological perspectives. Int. Immunopharmacol. 2013;15:73–83. doi: 10.1016/j.intimp.2012.11.006. [DOI] [PubMed] [Google Scholar]
- [60].Goswami S, Bose A, Sarkar K, Roy S, Chakraborty T, Sanyal U, Baral R. Neem leaf glycoprotein matures myeloid derived dendritic cells and optimizes anti-tumor T cell functions. Vaccine. 2010;28:1241–1252. doi: 10.1016/j.vaccine.2009.11.018. [DOI] [PubMed] [Google Scholar]
- [61].Bose A, Chakraborty K, Sarkar K, Goswami S, Haque E, Chakraborty T, Ghosh D, Roy S, Laskar S, Baral R. Neem leaf glycoprotein directs T-bet-associated type 1 immune commitment. Hum. Immunol. 2009;70:6–15. doi: 10.1016/j.humimm.2008.09.004. [DOI] [PubMed] [Google Scholar]
- [62].Roy S, Goswami S, Bose A, Chakraborty K, Pal S, Haldar A, Basu P, Biswas J, Baral R. Neem leaf glycoprotein partially rectifies suppressed dendritic cell functions and associated T cell efficacy in patients with stage IIIB cervical cancer. Clin. Vaccine Immunol. 2011;18:571–579. doi: 10.1128/CVI.00499-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Elkord E, Alcantar-Orozco EM, Dovedi SJ, Tran DQ, Hawkins RE, Gilham DE. T regulatory cells in cancer: recent advances and therapeutic potential. Expert. Opin. Biol. Ther. 2010;10:1573–1586. doi: 10.1517/14712598.2010.529126. [DOI] [PubMed] [Google Scholar]
- [64].Chakraborty T, Bose A, Barik S, Goswami KK, Banerjee S, Goswami S, Ghosh D, Roy S, Chakraborty K, Sarkar K, Baral R. Neem leaf glycoprotein inhibits CD4+CD25+Foxp3+ Tregs to restrict murine tumor growth. Immunotherapy. 2011;3:949–969. doi: 10.2217/imt.11.81. [DOI] [PubMed] [Google Scholar]
- [65].Roy S, Barik S, Banerjee S, Bhuniya A, Pal S, Basu P, Biswas J, Goswami S, Chakraborty T, Bose A, Baral R. Neem leaf glycoprotein overcomes indoleamine 2,3 dioxygenase mediated tolerance in dendritic cells by attenuating hyperactive regulatory T cells in cervical cancer stage IIIB patients. Hum. Immunol. 2013;74:1015–1023. doi: 10.1016/j.humimm.2013.04.022. [DOI] [PubMed] [Google Scholar]
- [66].Chakraborty K, Bose A, Pal S, Sarkar K, Goswami S, Ghosh D, Laskar S, Chattopadhyay U, Baral R. Neem leaf glycoprotein restores the impaired chemotactic activity of peripheral blood mononuclear cells from head and neck squamous cell carcinoma patients by maintaining CXCR3/CXCL10 balance. Int. Immunopharmacol. 2008;8:330–340. doi: 10.1016/j.intimp.2007.10.015. [DOI] [PubMed] [Google Scholar]
- [67].Chakraborty K, Bose A, Chakraborty T, Sarkar K, Goswami S, Pal S, Baral R. Restoration of dysregulated CC chemokine signaling for monocyte/macrophage chemotaxis in head and neck squamous cell carcinoma patients by neem leaf glycoprotein maximizes tumor cell cytotoxicity. Cell Mol. Immunol. 2010;7:396–408. doi: 10.1038/cmi.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Sarkar K, Goswami S, Roy S, Mallick A, Chakraborty K, Bose A, Baral R. Neem leaf glycoprotein enhances carcinoembryonic antigen presentation of dendritic cells to T and B cells for induction of anti-tumor immunity by allowing generation of immune effector/memory response. Int. Immunopharmacol. 2010;10:865–874. doi: 10.1016/j.intimp.2010.04.024. [DOI] [PubMed] [Google Scholar]
- [69].Sarkar K, Bose A, Chakraborty K, Haque E, Ghosh D, Goswami S, Chakraborty T, Laskar S, Baral R. Neem leaf glycoprotein helps to generate carcinoembryonic antigen specific anti-tumor immune responses utilizing macrophage-mediated antigen presentation. Vaccine. 2008;26:4352–4362. doi: 10.1016/j.vaccine.2008.06.048. [DOI] [PubMed] [Google Scholar]
- [70].Sarkar K, Bose A, Haque E, Chakraborty K, Chakraborty T, Goswami S, Ghosh D, Baral R. Induction of type 1 cytokines during neem leaf glycoprotein assisted carcinoembryonic antigen vaccination is associated with nitric oxide production. Int. Immunopharmacol. 2009;9:753–760. doi: 10.1016/j.intimp.2009.02.016. [DOI] [PubMed] [Google Scholar]
- [71].Sheweita SA. Drug-metabolizing enzymes: mechanisms and functions. Curr. Drug Metab. 2000;1:107–132. doi: 10.2174/1389200003339117. [DOI] [PubMed] [Google Scholar]
- [72].Vinod V, Tiwari PK, Meshram GP. Evaluation of mutagenic and antimutagenic activities of neem (Azadirachta indica) seed oil in the in vitro Ames Salmonella/microsome assay and in vivo mouse bone marrow micronucleus test. J. Ethnopharmacol. 2011;134:931–937. doi: 10.1016/j.jep.2011.02.003. [DOI] [PubMed] [Google Scholar]
- [73].Vinothini G, Manikandan P, Anandan R, Nagini S. Chemoprevention of rat mam-mary carcinogenesis by Azadirachta indica leaf fractions: modulation of hormone status, xenobiotic-metabolizing enzymes, oxidative stress, cell proliferation and apoptosis. Food Chem. Toxicol. 2009;47:1852–1863. doi: 10.1016/j.fct.2009.04.045. [DOI] [PubMed] [Google Scholar]
- [74].Manikandan P, Letchoumy PV, Gopalakrishnan M, Nagini S. Evaluation of Azadirachta indica leaf fractions for in vitro antioxidant potential and in vivo modulation of biomarkers of chemoprevention in the hamster buccal pouch carcinogenesis model. Food Chem. Toxicol. 2008;46:2332–2343. doi: 10.1016/j.fct.2008.03.013. [DOI] [PubMed] [Google Scholar]
- [75].Subapriya R, Bhuvaneswari V, Ramesh V, Nagini S. Ethanolic leaf extract of neem (Azadirachta indica) inhibits buccal pouch carcinogenesis in hamsters. Cell Biochem. Funct. 2005;23:229–238. doi: 10.1002/cbf.1143. [DOI] [PubMed] [Google Scholar]
- [76].Subapriya R, Kumaraguruparan R, Abraham SK, Nagini S. Protective effects of ethanolic neem leaf extract on N-methyl-N’-nitro-N-nitrosoguanidine-induced genotoxicity and oxidative stress in mice. Drug Chem. Toxicol. 2004;27:15–26. doi: 10.1081/dct-120027894. [DOI] [PubMed] [Google Scholar]
- [77].Balasenthil S, Arivazhagan S, Ramachandran CR, Ramachandran V, Nagini S. Chemopreventive potential of neem (Azadirachta indica) on 7,12-dimethylbenz[a]anthracene (DMBA) induced hamster buccal pouch carcinogenesis. J. Ethnopharmacol. 1999;67:189–195. doi: 10.1016/s0378-8741(99)00015-x. [DOI] [PubMed] [Google Scholar]
- [78].Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell. 2012;48:158–167. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Fruehauf JP, Meyskens FL., Jr. Reactive oxygen species: a breath of life or death? Clin. Cancer Res. 2007;13:789–794. doi: 10.1158/1078-0432.CCR-06-2082. [DOI] [PubMed] [Google Scholar]
- [80].Cui X. Reactive oxygen species: the Achilles’ heel of cancer cells? Antioxid. Redox Signal. 2012;16:1212–1214. doi: 10.1089/ars.2012.4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Nogueira V, Hay N. Molecular pathways: reactive oxygen species homeostasis in cancer cells and implications for cancer therapy. Clin. Cancer Res. 2013;19:4309–4314. doi: 10.1158/1078-0432.CCR-12-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Wallace DC. Mitochondria and cancer. Nat. Rev. Cancer. 2012;12:685–698. doi: 10.1038/nrc3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Lenaz G. Mitochondria and reactive oxygen species. Which role in physiology and pathology? Adv. Exp. Med. Biol. 2012;942:93–136. doi: 10.1007/978-94-007-2869-1_5. [DOI] [PubMed] [Google Scholar]
- [85].Kusamran WR, Ratanavila A, Tepsuwan A. Effects of neem flowers, Thai and Chinese bitter gourd fruits and sweet basil leaves on hepatic monooxygenases and glutathione S-transferase activities, and in vitro metabolic activation of chemical carcinogens in rats. Food Chem. Toxicol. 1998;36:475–484. doi: 10.1016/s0278-6915(98)00011-8. [DOI] [PubMed] [Google Scholar]
- [86].Dasgupta T, Banerjee S, Yadava PK, Rao AR. Chemopreventive potential of Azadirachta indica (neem) leaf extract in murine carcinogenesis model systems. J. Ethnopharmacol. 2004;92:23–36. doi: 10.1016/j.jep.2003.12.004. [DOI] [PubMed] [Google Scholar]
- [87].Niture SK, Rao US, Srivenugopal KS. Chemopreventative strategies targeting the MGMT repair protein: augmented expression in human lymphocytes and tumor cells by ethanolic and aqueous extracts of several Indian medicinal plants. Int. J. Oncol. 2006;29:1269–1278. [PubMed] [Google Scholar]
- [88].Subapriya R, Kumaraguruparan R, Abraham SK, Nagini S. Protective effects of ethanolic neem leaf extract on DMBA-induced genotoxicity and oxidative stress in mice. J. Herb. Pharmacother. 2005;5:39–50. [PubMed] [Google Scholar]
- [89].Tripathi A, Shrivastav TG, Chaube SK. Aqueous extract of Azadirachta indica (neem) leaf induces generation of reactive oxygen species and mitochondria-mediated apoptosis in rat oocytes. J. Assist. Reprod. Genet. 2012;29:15–23. doi: 10.1007/s10815-011-9671-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Arumugam A, Agullo P, Boopalan T, Nandy S, Lopez R, Gutierrez C, Narayan M, Rajkumar L. Neem leaf extract inhibits mammary carcinogenesis by altering cell proliferation, apoptosis, and angiogenesis. Free Radic. Res. 2014;15:26–34. doi: 10.4161/cbt.26604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Priyadarsini RV, Manikandan P, Kumar GH, Nagini S. The neem limonoids azadirachtin and nimbolide inhibit hamster cheek pouch carcinogenesis by modulating xenobiotic-metabolizing enzymes, DNA damage, antioxidants, invasion and angiogenesis. Free Radic. Res. 2009;43:492–504. doi: 10.1080/10715760902870637. [DOI] [PubMed] [Google Scholar]
- [92].Feijoo C, Paez de la Cadena M, Rodriguez-Berrocal FJ, Martinez-Zorzano VS. Sialic acid levels in serum and tissue from colorectal cancer patients. Cancer Lett. 1997;112:155–160. doi: 10.1016/s0304-3835(96)04564-8. [DOI] [PubMed] [Google Scholar]
- [93].Ramzanighara A, Ezzatighadi F, Rai DV, Dhawan DK. Effect of neem (Azadirchta indica) on serum glycoprotein contents of rats administered 1,2 dimethylhydrazine. Toxicol. Mech. Methods. 2009;19:298–301. doi: 10.1080/15376510802646523. [DOI] [PubMed] [Google Scholar]
- [94].Van Waes C. Nuclear factor-kappaB in development, prevention, and therapy of cancer. Clin. Cancer Res. 2007;13:1076–1082. doi: 10.1158/1078-0432.CCR-06-2221. [DOI] [PubMed] [Google Scholar]
- [95].Kavitha K, Vidya Priyadarsini R, Anitha P, Ramalingam K, Sakthivel R, Purushothaman G, Singh AK, Karunagaran D, Nagini S. Nimbolide, a neem limonoid abrogates canonical NF-kappaB and Wnt signaling to induce caspase-dependent apoptosis in human hepatocarcinoma (HepG2) cells. Eur. J. Pharmacol. 2012;681:6–14. doi: 10.1016/j.ejphar.2012.01.024. [DOI] [PubMed] [Google Scholar]
- [96].Schumacher M, Cerella C, Reuter S, Dicato M, Diederich M. Anti-inflammatory, pro-apoptotic, and anti-proliferative effects of a methanolic neem (Azadirachta indica) leaf extract are mediated via modulation of the nuclear factor-κB pathway. Genes Nutr. 2011;6:149–160. doi: 10.1007/s12263-010-0194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Gupta SC, Prasad S, Reuter S, Kannappan R, Yadav VR, Ravindran J, Hema PS, Chaturvedi MM, Nair M, Aggarwal BB. Modification of cysteine 179 of IkappaBalpha kinase by nimbolide leads to down-regulation of NF-kappaB-regulated cell survival and proliferative proteins and sensitization of tumor cells to chemotherapeutic agents. J. Biol. Chem. 2010;285:35406–35417. doi: 10.1074/jbc.M110.161984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Gupta SC, Reuter S, Phromnoi K, Park B, Hema PS, Nair M, Aggarwal BB. Nimbolide sensitizes human colon cancer cells to TRAIL through reactive oxygen species- and ERK-dependent up-regulation of death receptors, p53, and Bax. J. Biol. Chem. 2011;286:1134–1146. doi: 10.1074/jbc.M110.191379. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [99].Bodduluru LN, Kasala ER, Thota N, Barua CC, Sistla R. Chemopreventive and therapeutic effects of nimbolide in cancer: the underlying mechanisms. Toxicol. In Vitro. 2014;28:1026–1035. doi: 10.1016/j.tiv.2014.04.011. [DOI] [PubMed] [Google Scholar]
- [100].Sharma C, Vas AJ, Goala P, Gheewala TM, Rizvi TA, Hussain A. Ethanolic neem (Azadirachta indica) leaf extract prevents growth of MCF-7 and HeLa cells and potentiates the therapeutic index of cisplatin. J. Oncol. 2014;2014:321754. doi: 10.1155/2014/321754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Ghosh D, Bose A, Haque E, Baral R. Pretreatment with neem (Azadirachta indica) leaf preparation in Swiss mice diminishes leukopenia and enhances the antitumor activity of cyclophosphamide. Phytother. Res. 2006;20:814–818. doi: 10.1002/ptr.1948. [DOI] [PubMed] [Google Scholar]
- [102].Ghosh D, Bose A, Haque E, Baral R. Neem (Azadirachta indica) leaf preparation prevents leukocyte apoptosis mediated by cisplatin plus 5-fluorouracil treatment in Swiss mice. Chemotherapy. 2009;55:137–144. doi: 10.1159/000211558. [DOI] [PubMed] [Google Scholar]
- [103].Kruyt FA. TRAIL and cancer therapy. Cancer Lett. 2008;263:14–25. doi: 10.1016/j.canlet.2008.02.003. [DOI] [PubMed] [Google Scholar]
- [104].Deorukhkar A, Krishnan S. Targeting inflammatory pathways for tumor radiosensitization. Biochem. Pharmacol. 2010;80:1904–1914. doi: 10.1016/j.bcp.2010.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Zwang Y, Oren M, Yarden Y. Consistency test of the cell cycle: roles for p53 and EGR1. Cancer Res. 2012;72:1051–1054. doi: 10.1158/0008-5472.CAN-11-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Blagosklonny MV, Pardee AB. Exploiting cancer cell cycling for selective protection of normal cells. Cancer Res. 2001;61:4301–4305. [PubMed] [Google Scholar]
- [107].Stetler-Stevenson WG, Gavil NV. Normalization of the tumor microenvironment: evidence for tissue inhibitor of metalloproteinase-2 as a cancer therapeutic. Connect. Tissue Res. 2014;55:13–19. doi: 10.3109/03008207.2013.867339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Jafari S, Saeidnia S, Hajimehdipoor H, Ardekani MR, Faramarzi MA, Hadjiakhoondi A, Khanavi M. Cytotoxic evaluation of Melia azedarach in comparison with, Azadirachta indica and its phytochemical investigation. Daru. 2013;21:37. doi: 10.1186/2008-2231-21-37. [DOI] [PMC free article] [PubMed] [Google Scholar]