Abstract
The arteriovenous fistula has been used for more than 50 years to provide vascular access for patients undergoing hemodialysis. More than 1.5 million patients worldwide have end stage renal disease and this population will continue to grow. The arteriovenous fistula is the preferred vascular access for patients, but its patency rate at 1 year is only 60%. The majority of arteriovenous fistulas fail because of intimal hyperplasia. In recent years, there have been many studies investigating the molecular mechanisms responsible for intimal hyperplasia and subsequent thrombosis. These studies have identified common pathways including inflammation, uremia, hypoxia, sheer stress, and increased thrombogenicity. These cellular mechanisms lead to increased proliferation, migration, and eventually stenosis. These pathways work synergistically through shared molecular messengers. In this review, we will examine the literature concerning the molecular basis of hemodialysis vascular access malfunction.
Keywords: arteriovenous fistula, murine model, restenosis, vascular biology, venous neointimal hyperplasia
Arteriovenous fistulas (AVFs) and grafts were introduced over 50 years ago and have been used extensively to provide vascular access for patients requiring hemodialysis (HD).1,2 AVFs have lower rates of infection and complications in comparison to other modes of HD access and are the preferred method of vascular access in dialysis patients.1 The Fistula First Initiative has helped make AVFs the preferred method of HD vascular access.3 As a result, there has been an increase in the number of patients in whom AVFs are placed worldwide, including Europe and Japan.4,5 AVFs have also been recommended in the pediatric population.6 In the United States alone, there are nearly 600,000 patients with end-stage renal disease (ESRD) and approximately 400,000 on HD.7 These numbers are expected to grow in the coming years. Given the magnitude of renal disease, AVFs will continue to be an effective and necessary tool in the coming years.
Although AVFs have proven to be an essential tool, they are by no means without problems. One of the major weaknesses of AVFs is the time it takes for the fistula to mature. This time can be further worsened due to lack of patient education, predialysis planning, and follow-up care. As a result, many patients may need to use tunneled dialysis catheters, which are less favorable due to their associations with bacteremia, resulting in increased morbidity, mortality, and cost.5,8,9
Unfortunately, only 60% of AVFs will be functional at 12 months.10–12 Several studies have shown that patency rates are linked to many variables, ranging from age, presence of diabetes, body mass index, smoking, cytomegalovirus infection, total plasma cholesterol, protein intake, peripheral vascular disease, vessel characteristics, mean arterial pressure, surgical technique, and the use of vascular staples, along with many others.13–17 Despite the heterogeneity of the factors associated with AVF patency, it is suspected that many of them act through pathologically similar molecular mechanisms.
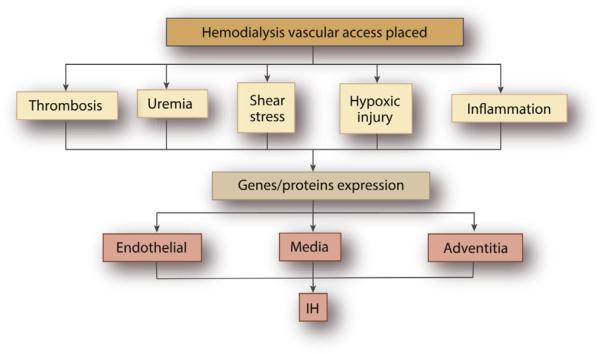
The histology of intimal hyperplasia (IH) is characterized by an abundance of contractile smooth muscle cells, myofibroblasts, fibroblasts, and macrophages, which eventually narrow the venous outflow leading to stenosis and a reduction in blood flow or in many cases thrombosis (Figure 1). There are many studies that have demonstrated that IH occurs because of several vascular biology pathways, including inflammation, uremia, hypoxia, shear-stress, and thrombosis.2,18–22 These mechanisms are thought to work in concert through linked cytokine cascades and possibly epigenetic changes that induce negative remodeling to occur, leading to fistula failure (Figure 2).23 This review will focus on the events triggered by renal failure as well as vascular access (VA) surgery that frequently lead to AVF failure. Because hemodynamic forces play an important role in vascular tone regulation and inflammation, we will review specifically the relation between vascular vessel wall stresses and the molecular mechanisms that are responsible for vessel wall changes and ultimately for AVF dysfunction.23
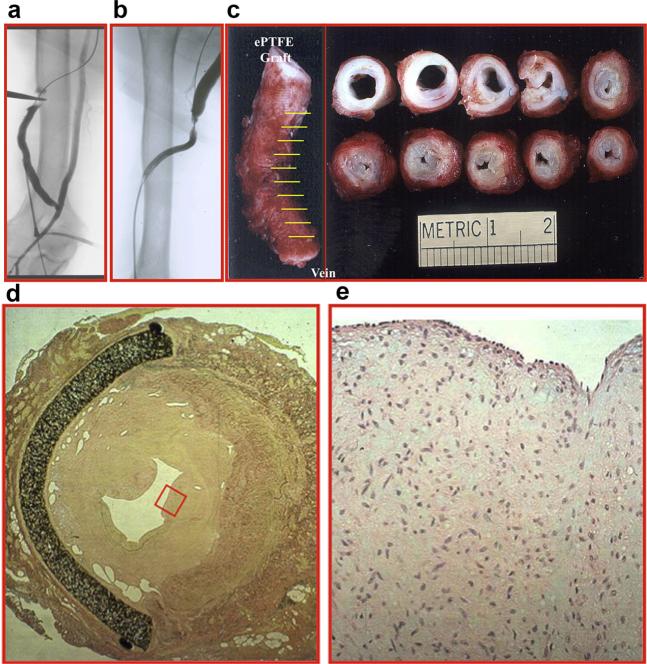
Figure 1.
(a, b) Fistulograms showing a stenosis at the polytetrafluoroethylene graft anastomosis to the basilic vein. (c) A gross specimen from a different patient showing thickening of the vein to graft anastomosis. Hematoxylin and eosin stain (d) at 10× magnification demonstrating a thickened neointima and (e) at 40× magnification (red box in d) showing increased cellular proliferation. ePTFE, expanded polytetrafluoro-ethylene.
Figure 2. Schematic of vascular injuries contributing to stenosis formation in hemodialysis vascular access.
IH, intimal hyperplasia.
Animal models
Given the complex nature of AVF failure in the setting of chronic kidney disease (CKD), several animal models have been established to study this phenomenon. There are many in vitro models utilizing cell culture. Whereas these models are useful for studying isolated phenomenon, they fail to capture all of the factors at play in AVF failure. Initial work in AVF and arteriovenous graft failure utilized carotid artery to jugular vein and also femoral artery to femoral fistulas and grafts. This was performed in pigs, rats, and mice. All of them showed significant IH at varying time points.24–27 However, these did not account for the systemic effects of CKD that play a significant role in AVF failure.28 In order to capture these systemic effects, models with induced kidney failure were developed. These models induced CKD in the animals via complete and/or partial nephrectomy. One kidney would be removed or embolized and/or the upper pole of the remnant kidney would also be ligated or embolized. These one-half or five-sixths nephrectomy models allowed for progression of CKD. Blood urea nitrogen was noted to be elevated for up to 8 weeks after nephrectomy. In the porcine model, stable CKD lasts from 4 to 12 weeks after nephrectomy.29–32 In the murine model, 4 weeks after nephrectomy, the mice underwent placement of an AVF.33 These models provide researchers with comprehensive paradigms to study AVF failure and apply translatable therapies. There are some limitations as there are differences in surgical technique, fistula sites, grafts, effects of HD, repeat cannulation, and ability to undergo angioplasty.33–35 Some of these considerations can be addressed depending on the model used. However, there have been many histological comparisons made among human, porcine, rat, and murine models. These findings show similar cellular phenotypes and staining patterns, and they suggest equivalent molecular mechanisms across all models of AVF failure.20,36,37
Inflammation
Inflammation in the setting of AVF can be divided into 2 parts. First, there is local inflammation caused by the trauma of fistula creation and local hypoxia. Second, there is a systemic rise in inflammation, which occurs because of uremia that is present in CKD patients. The local inflammatory response is characterized by the presence of macrophages (CD68) and infiltrating lymphocytes (CD3). This infiltration is more significant in the setting of CKD.38 CD68- and CD3-positive cells have been found in increased numbers in stenotic vessels. It is hypothesized that this local inflammatory response is caused by the release of macrophage migration inhibitory factor.39,40 Macrophage migration inhibitory factor has been shown to potentiate neointimal thickening by driving inflammatory cells toward the neointima and leading to the proliferation of medial and intimal cells. It has been identified in clinical and experimental models of HD vascular access.41,42 Macrophage migration inhibitory factor acts through the CD74 receptor, chemokine (C-X-C motif) receptor 2, and chemokine (C-X-C motif) receptor 4.40 These in turn act through extracellular signal-regulated and p38 mitogen-activated protein kinase pathways that up-regulates vascular endothelial growth factor (VEGF)-A, interleukin (IL)-8, and monocyte chemotactic protein 1 (MCP-1) (Figure 3). Experimental data using a MCP-1 knockout mouse model show that there is a reduction in IH.43 Moreover, in the murine model of CKD with AVF, there is an increase in gene expression of arginase-1, a marker for proinflammatory macrophages, followed by an increase in inducible nitric oxide, a marker for reparative macrophages in outflow vein samples removed from experimental murine model of AVF with CKD (Figure 4).
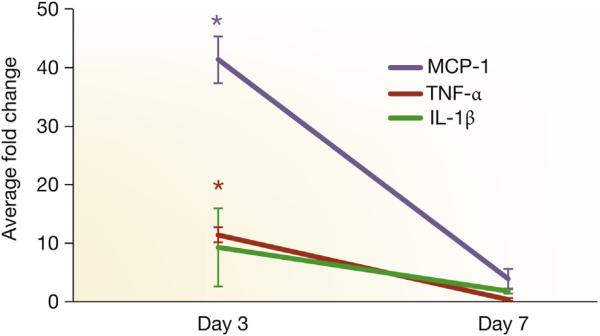
Figure 3. TNF-α, MCP-1, and IL-1β expression by qRT-PCR.
Tissue necrosis factor-alpha (TNF-α), monocyte chemoattractant protein-1 (MCP-1), and interlukin-1 beta (IL-1β) expression by quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) in graft veins and control veins at 3 and 7 days after arteriovenous fistula placement in mice with established chronic kidney disease. There is a significant increase in the mean TNF-α, MCP-1, and IL-1β expression at 3 days in graft veins when compared with control veins (P < 0.05). Each bar shows the mean ± SEM of 3 samples per group. Two-way analysis of variance with Student t test with post hoc Bonferroni correction was performed. *P < 0.05.
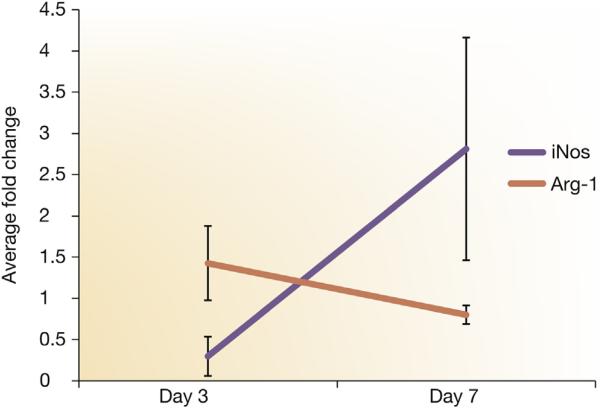
Figure 4. iNOS and Arg-1 expression using qRT-PCR.
Inducible nitric oxide synthase (iNOS) and arginase-1 (Arg-1) expression using quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) in graft veins and control veins at 3 and 7 days after arteriovenous fistula placement in mice with established chronic kidney disease. There is a significant increase in the mean Arg-1 expression with a decrease in iNOS between day 3 and 7. Each bar shows the mean ± SEM of 3 samples per group. Two-way analysis of variance with Student t test with post hoc Bonferroni correction was performed. P < 0.05.
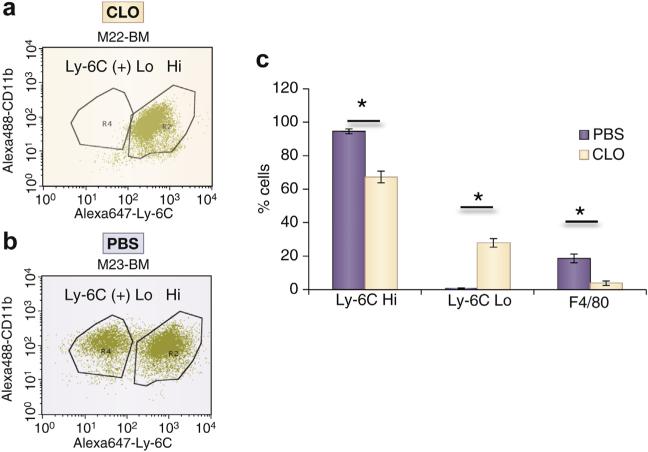
VEGFs have many subtypes that exhibit differential effects that will be discussed later, but in the context of inflammation, they serve as leukocyte chemo attractants, which potentiate local inflammation.44 IL-8 recruits monocytes and neutrophils by acting through chemokine (C-X-C motif) receptors 1 and 2. MCP-1 induces activation and migration of monocytes, memory T lymphocytes, and natural killer cells.45 Of these 3 cellular types, monocytes are likely the most contributory to inducing AVF failure. Experimental data from our laboratory demonstrate that decreasing monocytes and macrophages using clodronate results in a reduction in F4/80 (+) macrophages, reduction in lymphocyte antigen 6 complex (Ly-6C) Hi (+) monocytes, and an increase in Ly-6C Lo (+) monocytes with subsequent reduction in IH (Figures 5 and 6). Ly-6C Hi (+) monocytes contribute to proinflammatory macrophages and Ly-6C Lo (+) monocytes contribute to reparative macrophages.43,46–48 The increase in Ly-6C Lo (+) with a reduction in Ly-6C Hi (+) cells is hypothesized to contribute to the reduction in IH, which is consistent with other vascular injuries.43
Figure 5. Flow cytometry for bone marrow Ly-6C Hi and Ly-6C Lo cells and splenic F4/80(+) cells from animals treated with CLO and PBS at day 14.
(a) Representative flow diagram from a clodronate (CLO)-treated animal. (b) Representative flow diagram from a control–phosphate-buffered saline (PBS)-treated–animal. (c) Pooled data from 2 PBS-treated animals and 3 CLO-treated animals at day 14. Each bar shows the mean ± SEM of 2 to 3 samples per group. Two-way analysis of variance with Student t test with post hoc Bonferroni correction was performed. *P < 0.05.
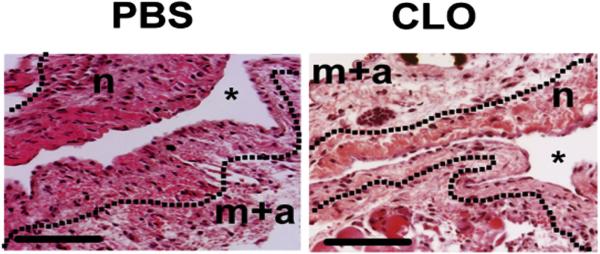
Figure 6. H&E staining of outflow veins removed from animals treated with PBS and CLO at day 14.
Hematoxylin and eosin (H&E) staining of outflow veins removed from PBS-treated and CLO-treated animals at day 14 after arteriovenous fistula placement. There is a reduction in the neointima (n) of the CLO-treated animals when compared with PBS control animals. Asterisk (*) shows the lumen of the vessel. Scale bars = 50 μM. CLO, clodronate; m + a, media/adventitia; PBS, phosphate-buffered saline.
Monocyte infiltration into the AVF is suspected to cause an up-regulation of transforming growth factor beta 1 (TGF-β1) and insulin-like growth factor 1. Both of these cytokines are proatherogenic acting through vascular smooth muscle cells. TGF-β1, despite being classically anti-inflammatory, has been shown to lead to extracellular matrix (ECM) deposition, causing thrombosis.21 Differences in TGF-β1 polymorphisms, result in differing levels of TGF-β1 production, have also been correlated with rates of AVF patency.49
TGF-β1 and its family of receptors have been implicated in many different cellular changes. Recent work has shown that by blocking the downstream core fucosylation of TGF β, one can reduce markers of renal fibrosis.50 Although the mechanisms of renal fibrosis and AVF failure vary, there is a great deal of overlap. Fucosyltransferases have also been shown mainly in cancer research to affect metastasis and cellular adhesion. In addition, changes in glycosylation have been shown to affect cell proliferation, transformation, migration, and apoptosis. Most of this work has taken place in the context of cancer progression.51,52 Examination of these pathways may provide us with a better understanding of HD vascular access failure and thus provide us with targets and therapies.
In addition to TGF-β1, another prominent inflammatory cytokine of interest in IH is tumor necrosis factor alpha (TNF-α). Although historically thought of as an innate immune response mediator, TNF-α can be activated by hypoxia, reactive oxygen species, and local inflammation and through an autocrine pathway.11,14 TNF-α is a 17-kDa protein that acts via a nuclear factor kappa B (NF-κB) of activated B cells receptor pathway for advanced glycation end products and TNF-receptor 1. TNF receptor 1 is the most common receptor in non-lymphoid tissues. However, the receptor pathway for advanced glycation end products has been shown to be unregulated in diabetic models and is likely the driving force for up-regulation of vascular cell adhesion protein 1.53 In smooth muscle cells (SMCs) from veins, TNF-α, induces IL-1β and prostaglandin E2 release. SMCs and endothelial cells (ECs) can also release TNF-α. These responses suggest that SMCs, in addition to ECs in the vessel wall, may serve as local propagators of the immune response.5 TNF-α stimulation of fibroblasts has also been shown to induce proliferation, and these effects are augmented by insulin-like growth factors.54 Additionally, polymorphisms in TNF-α especially a 308 G>A promoter change have been linked to an increased risk of AVF thrombosis.55
Although TNF-α has apoptotic properties, they are masked by the antiapoptotic NF-κB pathway that it activates. Thus, only when the NF-κB pathway and/or protein synthesis are nonfunctional does TNF-α become apoptotic.56 This largely explains why TNF-α appears to be proliferative in the setting of IH. Although many cells experience cellular injury at the time of AVF placement, it is not enough to compromise the antiapoptotic effects of the NF-κB pathway. Thus, TNF-α in the setting of IH contributes synergistically with the proliferative effects of other cytokines released during AVF placement.
Uremia
In addition to local mediators, AVF failure is complicated by systemic changes associated with CKD.28,38,57,58 One of the major effects of ESRD is uremia, which is associated with increased oxidative stress that causes a characteristic change in circulating proteins. This oxidative stress is further complicated by dialysis, which causes activation of phagocytes, release of oxygen radicals, peroxidation of lipids, and ultimately depletion of a patient's antioxidant protectants. Recent work has shown that cytokines implicated in IH formation, which include IL-6, TGF-β1, and TNF-α, are elevated in uremia. Additionally, markers of oxidative damage and lipid peroxidation, 8-hydroxy-2′-deoxyguanosine and 4-hydroxy-2-nonenal, respectively, have also been identified.18 These stressors have been shown to increase the levels of potent mitogens, which include platelet-derived growth factor (PDGF), endothelin 1, and the proliferative stimulator TGF-β, compounded by the local inflammation and hypoxia that take place during AVF creation.59
Uremia has also been shown to affect cellular calcium extrusion, induce fibrosis, increase insulin resistance, and raise calcium phosphate deposition in the vessel wall.60,61 These changes together have been shown to increase vascular calcifications, which have been linked to AVF failure.62–64 Patients with higher serum levels of C-reactive protein and sclerostin, an osteocyte derivative up-regulated in calcified SMCs, have higher rates of independently predicted AVF failure.65 Vascular calcifications have been tied to changes in mineral metabolism, parathyroid hormone, and vitamin D, which in turn are modulated by systemic inflammation. Several studies have shown the effects of parathyroid hormone and vitamin D on vascular SMCs. Although the true mechanism is unknown, it is likely that vitamin D can have differential effects on vascular SMCs depending on confounding cellular signals.36,66
In addition to their direct effects on vascular SMCs, these factors also affect bone metabolism. CKD has been related to abnormal bone pathology. It is unclear how and whether these 2 processes affect one another. Recent work has revealed vitamin K, fibroblast growth factor 23, bone morphogenetic protein-2, osteocalcin, osteopontin, matrix gla, alkaline phosphatase, and others affect bone metabolism, renal activity, and peripheral vasculature.57,67 These factors along with the receptor activator of NF-κB/receptor activator of NF-κB ligand/osteoprotegerin triad may affect osteoclasts both in the bone and in the vessel wall, leading to atherosclerosis. It is likely that the uremia and increased reactive oxygen species in CKD patients may exert differential effects on mesenchymal-derived cells in both the arterial wall and bone.68,69 Studying these mechanisms could provide a stronger basis for many therapies such as phosphate binders, vitamin D, sodium thiosulfate, specific receptor antibodies, and antioxidants.70–72
Hypoxia
Hypoxia is a potent inducer of many genes, which promote angiogenesis and act in concert with inflammatory and shear stress–mediated processes. In the setting of IH, hypoxia deriving from damage to the vasa vasorum of the adventitia layer of AVF primes the vessel for negative remodeling by activating angiogenic and inflammatory genes. Hypoxia exerts its effect on molecular signaling by activation of hypoxia-inducible factors. Of this family of transcription factors, hypoxia-inducible factor 1 α is the best studied and has been found in increased levels in animal and human models of IH.33,73 Hypoxia-inducible factor 1α and its downstream mediators have also been seen in models of venous hypertension. However, these latter models lack the surgical injury of the vasa-vasorum thought to cause hypoxia in IH, suggesting that other mechanisms may also be at work.33,74
Hypoxia-inducible factor 1α expression is thought to be involved in a positive angiogenic feedback loop, influencing a multitude of downstream molecular mechanisms, which results in angiogenesis, inflammation, cell proliferation, and deposition of collagen.75 One of the most important downstream mediators is VEGF. The arterialization of the venous vessel is likely mediated by VEGF-A. It is suspected that overexpression of VEGF-A contributes to negative remodeling and IH.76 A recent study from our laboratory77 demonstrated that reducing VEGFA gene expression at the time of AVF placement reduced IH formation. Polymorphisms in VEGF-A specifically the VEGF-936C/C genotype were found to be 5.54 times more likely to develop and occlude AVF. The polymorphism also affects circulating levels, which were higher than those with the VEGF-936 C/C polymorphism.78
VEGF acts on both receptors in its family VEGFR-1 and VEGFR-2. VEGFR-1 acts in a positive manner through a tyrosine kinase, promoting endothelial growth. VEGFR-1 is also thought to activate macrophages, further increasing the inflammatory setting of the AVF.79 VEGFR-2, also a tyrosine kinase receptor, acts mainly through the phospholipase-cγ protein kinase-c pathway. VEGFR-2 is strongly expressed in vasculature. It promotes EC proliferation and differentiation into vascular cells.80,81 VEGFR-2 is hypothesized to increase SMC proliferation via extracellular signal-regulated and Akt signaling.76 Blockade of the Akt pathway has shown to partially inhibit proliferation of venous SMCs.82 VEGF-A can cause activation of matrix metalloproteinase (MMP)-9, which causes remodeling of the basement membranes and ECM. In addition to VEGF-A, hypoxia-inducible factor 1α also activates MMP-2, which degrades the ECM. ADAMTS-1 is another matrix metalloproteinase that has also been increased in experimental models of AVF and clinical samples and is thought to have a similar role to MMP-2 and MMP-9.33 This remodeling of the ECM works synergistically with the proliferative effects of VEGFA, leading to IH.75 Reducing ADAMTS1 expression using lentivirus short hairpin RNA–mediated technology leads to positive vascular remodeling in experimental animal models.83
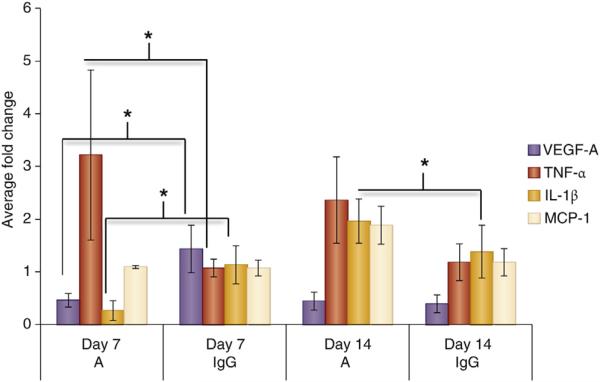
Recent work on VEGF has revealed many subtypes with differing effects. Much of this work has taken place in cancer models and has shown that VEGF isoforms can dictate angiogenesis and in some cases can even be antiangiogenic.84 For example, VEGF-A165b is antiangiogenic and its splice variant VEGF-A165a is notably proangiogenic in the setting of peripheral artery disease.85 Work by Nowak et al.,86 demonstrated that proximal splice site variants generate proangiogenic VEGF isoforms that are activated by TNF-α. TGF-β1 in contrast leads to more distal splice site variants, likely through p38 mitogen-activated phosphatase kinase-Clk/sty kinases.86 In addition, previous work has shown that VEGF and TGF-β1 likely influence each other via a decapentaplegic homolog 3 (SMAD family member 3) pathway. Up-regulation of TGF-β/SMAD family member 3 has been shown to decrease apoptosis and increase secretion of VEGF-A in SMCs. However, this relationship is likely to vary depending on the microenvironment given that both molecules can be proangiogenic or antiangiogenic.87–89 Recent work by Wan et al.,76 has shown that VEGF-121 and VEGF-165 isoforms are expressed as both mRNA and proteins in SMCs exposed to hypoxic conditions. Moreover, reduction in IH using Avastin (Genentech, South San Francisco, CA) experimentally results in an increase in gene expression of TNF-a, IL-1β, and MCP-1 (Figure 7).
Figure 7. Inflammatory cytokines in A-treated versus control animals.
Inflammatory cytokines are elevated in Avastin (A)-treated vessels when compared with control animals (IgG) at days 7 and 14 after arteriovenous fistula placement. Vascular endothelial growth factor-A (VEGF-A), MCP-1, TNF-α, and IL-1β expression by qRT-PCR in graft veins and control veins at 7 and 14 days after arteriovenous fistula placement in mice with established chronic kidney disease. Each bar shows the mean ± SEM of 4 to 6 samples per group. Two-way analysis of variance with Student t test with post hoc Bonferroni correction was performed. *P < 0.05. IL-1β, interlukin-1 beta; MCP-1, monocyte chemoattractant protein-1; qRT-PCR, reverse transcriptase polymerase chain reaction; TNF-α, tissue necrosis factor-alpha.
In addition to VEGF-A, another major peptide activated by hypoxia is PDGF. PDGF acts on phosphorylated platelet-derived growth factor β receptor to increase myofibroblast expression mediated through the Akt and extracellular signal-regulated pathways.22 Several studies have demonstrated the efficacy of blocking many of these pathways through receptor antagonists, gene knockouts, and environmental changes in reducing IH.76,90
Hemodynamics
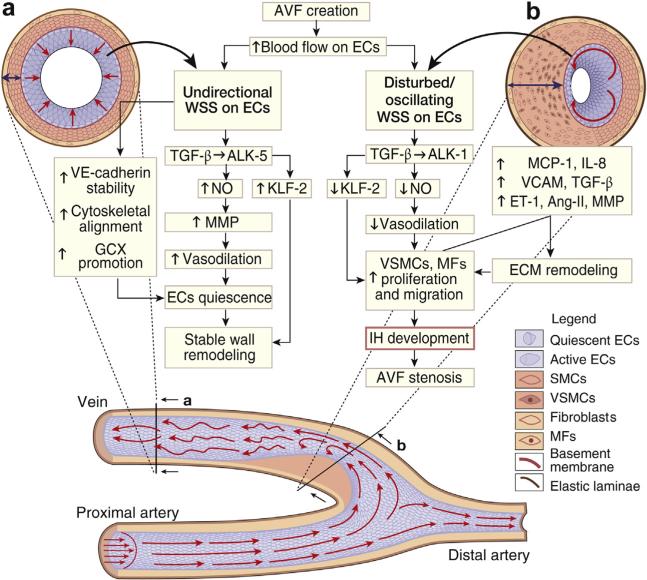
AVF stenosis occurs predominantly on the venous side of the anastomotic region where ECs experience high, non-physiological blood flow gradients with flow instability (Figure 8).91,92 The hemodynamic changes following AVF creation result in short-term and long-term vascular remodeling with an increase in vessel caliber and thickening of venous wall (i.e., venous arterialization).93 The hemodynamic features causing vascular wall remodeling are fluid wall shear stress (WSS, the frictional force exerted by blood flow on vessel wall luminal surface) and intramural tensile strain caused by elevated blood pressure.94–97
Figure 8. Schematic representation of molecular mechanism of IH development in native AVF.
Section of a side-to-end arteriovenous fistula (AVF). Laminar blood flow coming from the proximal artery stimulates the endothelial cells (ECs) with unidirectional wall shear stress (WSS) until the anastomosis level where the blood flow splits in 2 directions. At the vein curvature, the blood flow becomes unstable with disturbed and oscillating WSS and reverse flows at the inner curvature of the anastomosis. After the curvature, blood flow oscillations decrease and WSS returns to almost unidirectional. The different WSS patterns generated on the endothelium lead wall remodeling. At (a), the unidirectional WSS maintains vessel patency while at (b) oscillating and reversing WSS impair ECs quiescence leading to intimal hyperplasia (IH). ALK-5, activin receptor-like kinases 1/5; Ang-II, angiotensin II; ECM, extracellular matrix; ET-1, endothelin 1; GCX, glycocalyx; IL-8, interleukin 8; KLF-2, Krüppel-like factor 2; MCP-1 monocytes chemoattractant protein 1; MFs, myofibroblasts; MMP, metalloproteinase; NO, nitric oxide; SMCs, smooth muscle cells; TGF-β, transforming growth factor β; VCAM, vascular cell adhesion protein; VE, vascular endothelial; VSMC, vascular smooth muscle cells.
After AVF creation, there are important increases in WSS and tensile strain caused by increased blood flow and pressure, which result in arterial and venous remodeling. AVF maturation induces an increase in vessel wall diameter that reestablishes physiological stress levels within the maturation period of 4 to 6 weeks.98 This vascular remodeling is called outward remodeling and likely derives from the well-known effect of laminar shear stress increase on ECs. We have previously shown that a mechanism of outward remodeling increases vessel lumen after AVF surgery.99 The preoperative peak value of shear stress predicted with a very good agreement the dimensional and functional changes observed after AVF maturation.100 However, whereas this phenomenon occurs in the venous side of the AVF, near the anastomosis, ECs are locally exposed to large and fast gradients of WSS in space and time. This is accompanied with reverse blood flow, which can also develop with fast velocity fluctuations (Figure 8), as was recently shown.101 Computational studies of hemodynamics in AVF reported complex flow patterns in areas that are more prone to development of IH, as the inner side of the venous segment of AVF.91 ECs are essential regulators of WSS-induced vessel remodeling.102–104 Numerous studies have addressed the effects of normal and pathological WSS on vascular biology and especially on the endothelium. EC morphology, gene and protein expression, as well as protein release are all strongly affected by different WSS patterns.105,106
It has been demonstrated, both in vivo and in vitro, that ECs exposed to unidirectional and relatively high WSS show a quiescent phenotype induced by the sustained expression of several molecules. One of the most important is the transcription factor Krüppel-like factor 2.107 Krüppel-like factor 2 induces several downstream events that cause ECs to assume an antioxidant state with a reduced mitotic and apoptotic rate.108 It induces also a down-regulation of proinflammatory paracrine signals such as MCP-1 and IL-8.109 The mechanotransduction of physiological patterns of WSS has profound effects on EC morphology, causing cell elongation, which aligns toward the blood flow direction because of a cytoskeletal rearrangement. In vitro, static EC monolayers can develop dense actin peripheral bands with a few randomly orientated f-actin fibers crossing the cell body. Physiological flows induce the disassembly of actin peripheral bands and the formation of long, parallel actin stress fibers aligned to flow direction.110,111 WSS also induces adherens junction stability and promotes glycocalyx formation.93,112,113 These phenomena regulate EC permeability of the endothelium and seem to maintain a quiescent EC state.82,114 Finally, unidirectional WSS can increase endothelial nitric oxide (NO) release115 that modulates the activation of MMP-2 and MMP-9. The MMP-induced degradation of elastin fibers is necessary in permanent vessel wall remodeling.111
Reduced endothelial permeability and unidirectional WSS downregulate the production of chemoattractant signals such as MCP-1 and IL-8 by ECs. WSS also serves to abrogate inflammation caused by AVF placement. Along with beneficial effects in terms of reendothelialization and inflammation, unidirectional/high WSS is also involved in vessel outward remodeling. Studies performed both in vivo and in vitro showed that hemodynamics influence the balance between NO and reactive oxygen species production. For example, unidirectional WSS promotes the constitutive and the inducible forms of NO synthase, the precursors of NO.
In contrast to unidirectional flow, oscillating flow adjacent to the vessel wall can locally develop in AVFs (Figure 8). These vacillating forces have been suggested to cause activation of a series of pathways that induce IH.116–119 Oscillating WSS directly affects EC gene expression causing an increase in the autocrine EC proliferation pathway that ultimately leads to up-regulation of mitogen-activated protein kinases, NF-κB nuclear translocation,110,120 and a down-regulation of Krüppel-like factor 2 expression.105 The overall effect is a paracrine proliferative (i.e., increased level of IL-8, MCP-1 production), proinflammatory, prooxidant EC state and an impaired vascular tone regulation due to the reduction of NO synthesis and the increase of endothelin 1 and angiotensin-2 levels.105,117 EC structure and function are also deeply affected by mechanotransduction of flow oscillations. Oscillating WSS affects adherens junction stability by inducing altered endothelial permeability and preventing favorable cytoskeletal rearrangement.93,121 Adherens junction instability causes impairment of contact inhibition of EC proliferation due the switch of Akt-induced EC survival signaling to the extracellular signal-regulated /mitogen-activated protein kinase proliferative pathway.99 Another important effect of oscillating WSS is the alteration of EC surface glycocalyx. This has a detrimental effect on EC permeability and function.113 In vitro knockout of Syndecan-1 and Glypican-1, two of the core proteins of EC glycocalyx, abolishes WSS-induced cytoskeletal remodeling and NO production, respectively.122 Oscillating WSS also causes an increase in surface adhesion molecules (vascular cell adhesion protein 1), inducing an increased EC surface reactivity to circulating molecules.106 Finally, oscillating WSS leads to an increase of cytokines as TNF-α123 and interferon gamma124 that induce MMP-2 and MMP-9 production and unbalance matrix deposition and degradation, affecting vascular wall remodeling.125 Thus, oscillating WSS that may develop in specific sites of AVF has important effects on nuclear and protein EC regulation, potentially related to formation of neointimal layer.119
Beyond NO and reactive oxygen species stimulation, a recent investigation has shown that WSS-induced vessel remodeling is related to heme oxygenase (HO) production. Inducible (HO-1) and constitutive (HO-2) forms are heme-degrading enzymes that have been related to AVF patency and survival.126–129 HO acts as a vasorelaxant, antioxidant, and anti-inflammatory agent by generating carbon monoxide and biliverdin and releasing ferrous iron from heme.130 It has been recently reported that HO-1 is produced in varying responses to both high and low WSS as the result of different pathways and to different extents.131 High flow-induced HO-1 is generated downstream to NO and mitochondria-derived hydrogen peroxide. Low flow induces lower levels of HO-1 that lead to macrophage infiltration and superoxide production within the vessel wall. A recent study performed in a carotid ligation mice model showed that HO-1 induced by vascular damage, probably via NF-κB activation, is vasoprotective, resulting in vasodilation.128 The same study showed that HO-2 deficiency is related to an increase in development of IH. These in vivo studies suggest a fundamental role for HO enzymes in promoting outward remodeling and preventing IH. However, to define the precise and univocal relationship between WSS and HO induction in AVF further studies are required.
Biology of AVF failure
All the mechanisms described importantly influence the biology of vascular tissue responsible for AVF failure. Histology of stenotic AVF tissues reveals that an aggressive IH is composed mainly of smooth muscle alpha-actin-positive, vimentin-positive, and desmin-negative myofibroblasts (MFs) that migrate towards the intima of the injured vessel.37 In this intimal layer, increased levels of endothelin 1, TGF-β, MCP-1, IL-6, and PDGF that colocalize with markers of oxidative stress are present.21,59,108,126
As mentioned previously, oscillating WSS in time and direction in the venous side of AVF anastomosis affects endothelium integrity; sustaining EC paracrine signaling while it impairs direct NO-induced vasodilation and inhibition of SMC migration, leading to AVF stenosis.37,115 A recent study showed the serum of patients with matured AVFs had significantly higher levels of matrix tissue inhibitor of metalloproteinase compared with the serum of those with ailed accesses.132 This suggests an imbalance of MMP inhibition after initial wound recovery activation, leading to sustained ECM remodeling and cell migration.
The previously mentioned functional changes in vascular cells within the neointimal layer are highly dependent on the activation of TGF-β superfamily signaling.133-135 The effects of TGF-β on EC depend on context. TGF-β can bind distinct receptor complexes, in which the subunits forming the receptors determine signaling specificity. Depending on the activin receptor-like type of the receptor, TGF-β signaling can inhibit endothelial cell proliferation (activin receptor-like 5) or elicit an opposite response (activin receptor-like 1).
The effect of fluid WSS on TGF-β signaling in EC is a matter of interest. Recent reports show that steady flows induce SMAD family member 7 up-regulation that causes inhibition of TGF-β signaling in mouse embryo mesenchymal progenitor cells while oscillating flows induce phosphorylation of SMAD family members 1/5, which stimulates EC migration and proliferation.136,137,139 Finally TGF-β binding to activin receptor-like 5 is required for WSS-induced expression of Krüppel-like factor 2 in embryonic EC.138 Finally, it has been very recently reported that WSS modules the endothelial-to-mesenchymal transition.139
TGF-β1 is the main activator of fibroblast transition to the MF phenotype. MFs are the main cellular component of AVF stenosis. MFs directly produce ECM proteins and MMP, participating in ECM deposition and degradation, which are required for MF migration to intima, during vessel healing. The fate of MFs at the end of the healing process may determine positive or negative vessel remodeling.37 To end physiological tissue repair, a massive MF apoptosis is induced by reduced growth factors, NO generation, and intact ECM.54,140 A sustained TGF-β expression, as well as the persistence of ECM remodeling, abolishes MF disappearance leading to IH development.141,142 Besides the intimal layer in AVF, the adventitial layer is also importantly affected by wall remodeling induced by AVF creation.99 Thus, the adventitia may be the site of fibrosis and of major vascular reactive oxygen species production that directly inactivates NO, causing impairment of vessel tone regulation.22,143 This evidence suggests that the adventitial layer plays an important role in wound healing and may importantly affect vascular remodeling.
The surgical injury related to AVF creation itself may cause EC denudation and exposure of collagen type I, leading to platelet adhesion, PDGF release, and tissue factor activation. In addition to activation of the coagulation cascade, the inflammatory cascade may be activated, with up-regulation of NF-κB and the related activation of mRNA transcription. Thus, this injury may recruit leukocytes, induce EC release of heparanases (with effect on EC proliferation), and extracellular proteases (MMPs) that affect ECM remodeling and vascular SMC migration.144 Despite the fact that vascular injury induced by surgical procedure potentially causes activation of several pathways related to IH, the fact that vessel stenosis and VA early failure develop only in a fraction of surgical procedures suggests the coexistence of other factors in the development of IH.
Thrombosis
Thrombosis occurs in the setting of AVF secondary to inflammation, WSS, and disturbed flow of AVFs, which can initiate and work synergistically to enhance the thrombotic cascade. It is hypothesized that uremia and endothelial damage can up-regulate thrombotic antibodies, platelet factors, and EC receptors. This prothrombotic state is then worsened by shear stress and the hemodynamic changes in an AVF ultimately leading to thrombosis.145–152 Several studies have found increased levels of inflammatory cytokines, high-sensitivity C-reactive protein, D-dimer, soluble IL-2 receptor, and IL-6, among others. In addition, CKD patients have higher levels of von Willebrand factor and p selectin, both proteins that can cause platelet activation. Thrombinantithrombin III has also been found to be elevated, and although the difference in levels is significant, its efficacy is questionable due to its lagging nature and lack of specificity, along with D-dimer.150,153 This cascade is enhanced by thromboxanes and factors released from the platelets themselves.154–156
In addition to these factors, rates of thrombosis are increased by some underlying hypercoagulable states such as G20210A, but not other blood groups.157–161 There is some conflicting evidence regarding factor V Leiden, as one study demonstrated an increased risk, but another found no difference after regression analysis.158,162 Given the importance of factor V Leiden, it likely plays a significant role in inducing thrombosis. One study found the single-nucleotide polymorphism rs6019 in the factor V Leiden to be an independent risk factor for AVF thrombosis. This polymorphism increases the half-life of factor V Leiden, by increasing its resistance to protein C.163
Serum low-density lipoprotein has been associated with AVF thrombosis, but anticardiolipin antibodies, triglycerides, total cholesterol, and high-density lipoprotein have not.164 This is despite the fact that these latter 4 factors have been implicated in atherosclerotic vessel damage and that high titers of anticardiolipin antibodies have been found in ESRD patients.159 The rs1466535 single-nucleotide polymorphism in the low-density lipoprotein receptor–related protein 1 gene has also been significantly associated with AVF failure. Low-density lipoprotein receptor–related protein 1 is hypothesized to act on MMP-9 and controlled by a PDGF/Smad signaling.162
Additionally, HD has been shown to increase several thrombotic and inflammatory proteins, despite heparin therapy. The study found significant increases in p selectin, CD40L, stimulated platelet fibrinogen, Co TAT, D-dimer, von Willebrand factor, and high-sensitivity C-reactive protein after HD. This suggests that shifting levels of uremia even if corrective may lead to hypercoagulability.153,165,166
High levels of antiphospholipid antibody IgA b-glycoprotein I antibodies have been associated with AVF and are independent risk factors for CVD in ESRD patients. Antiphospholipid antibodies are more prevalent in patients with ESRD, likely due to uremic changes and endothelial injury. This hypercoagulable state, along with the up-regulation of endothelial receptors, promotes AVF thrombosis.148,149 In addition to finding IgA anti-β2GPI, a recent study also identified the human platelet antigen 3aa genotype as an independent risk factor for AVF failure. Human platelet antigen 3aa maps to a portion of glycoprotein IIb, with no known hemostatic function, but it has been associated with both venous and arterial thrombosis.167 In a study examining Chinese Han patients, a glycoprotein IIb human platelet antigen 3 a/b polymorphism, specifically the b allele was linked to native AVF thrombosis.168 These studies show that changes in the vessel wall lead to increased thrombogenicity, which is further compounded by systemic uremia.
Therapeutic strategies and future perspectives
Pharmacological treatments are potential strategies to improve clinical outcomes of AVF. For example, statin treatment has been previously adopted with the aim to protect against vascular injury and prolong VA patency. However, in patients treated with statins, VA survival was not ameliorated169 despite the promising results obtained in a murine model.170 There are potentially several explanations of this observation including the patients treated with statin were older, with a higher percentage of diabetics and more were female. Additionally, the type and amount of statin that was used was not defined. For example, atorvastatin is more anti-inflammatory than simvastatin, and high-dose statins reduce VEGFA and MMP expression that has been associated with HD vascular access failure. In a different study, statin use had improved outcomes; however, it was not statistically significant. In this study as well, statin patients were older and a higher percentage were diabetic compared with those not on statins.171 As suggested by Birch and Florescu,172 the anti-inflammatory effects of statins are likely to be insufficient in AVF remodeling due the underlying ESRD context.
To use new therapeutic strategies, the biological mechanisms responsible for AVF failure must be identified more precisely. Despite the important discoveries of the last 20 years in this field, there are still important open questions. To improve our knowledge, it is important to study dynamics of IH formation in robust animal models. In the patients, new noninvasive imaging modalities may help to investigate the dynamic response of the vessel wall after AVF creation. An additional important aspect of this research is the possibility to study individual patient responses and to relate them with pathological conditions and genetic factors. This may allow clinicians to identify and treat patients at an increased risk of AVF failure.
Because of the previously discussed role of hemodynamic factors in development of IH, another strategy we can employ to improve AVF outcomes is computer-assisted surgical planning. This software can be used to optimize AVF geometry and reduce blood velocity oscillations, flow, and shear stress instability.34,173 In addition, we also suggest that patient-specific identification of AVFs exposed to important flow oscillations may allow clinicians to identify patients at increased risk of AVF failure and follow them more closely. These evaluations could improve AVF maturation and long-term patency rates.
CONCLUSIONS
AVF complications are the main cause of hospitalization of dialysis patients with very high social and economical costs. IH is the principal cause of arteriovenous fistula failure. Currently available strategies to minimize IH growth and consequent AVF failure are largely ineffective. Mechanical and pharmacological treatments of AVF stenosis present high rates of restenosis and failure.3,174 There has been a great deal of effort made to understand the basis of this failure with the aim to correct it. These studies have revealed several major intertwined pathways such as inflammation, uremia, hypoxia, and shear stress leading to IH and propagating thrombosis. However, the precise mechanisms involved and their interplay are not completely understood at the moment and the ongoing research may improve this knowledge in the near future. Future work will allow for development of surgical strategies, interventions, and medical management to significantly improve the clinical outcomes of these life-saving procedures.
Because of the importance of hemodynamic factors on AVF outcome, we suggest that computer-assisted surgical planning could allow optimizing AVF geometry with the aim to reduce blood velocity oscillations and flow instability.34,173 In addition, identification of AVF exposed to important flow oscillations may allow us to more intensively follow AVF with high risk of failure. These evaluations could improve AVF maturation and long-term patency, limiting uncontrolled IH growth.
ACKNOWLEDGMENTS
This work was funded by grant HL098967 (SM) from the National Heart, Lung, and Blood Institute. M.F. is recipient of a fellowship from Fondazione Aiuti per la Ricerca sulle Malattie Rare, ARMR, Bergamo, Italy.
Footnotes
DISCLOSURE
All the authors declared no competing interests.
REFERENCES
- 1.Sands JJ. Increasing AV fistulas: revisiting a time-tested solution. Semin Dial. 2000;13:351–353. doi: 10.1046/j.1525-139x.2000.00098.x. [DOI] [PubMed] [Google Scholar]
- 2.Roy-Chaudhury P, Lee TC. Vascular stenosis: biology and interventions. Curr Opin Nephrol Hypertens. 2007;16:516–522. doi: 10.1097/MNH.0b013e3282efa57f. [DOI] [PubMed] [Google Scholar]
- 3.Schinstock CA, Albright RC, Williams AW, et al. Outcomes of arteriovenous fistula creation after the Fistula First Initiative. Clin J Am Soc Nephrol. 2011;6:1996–2002. doi: 10.2215/CJN.11251210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rayner HC, Besarab A, Brown WW, et al. Vascular access results from the Dialysis Outcomes and Practice Patterns Study (DOPPS): performance against Kidney Disease Outcomes Quality Initiative (K/DOQI) Clinical Practice Guidelines. Am J Kidney Dis. 2004;44(suppl 2):22–26. doi: 10.1053/j.ajkd.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Vassalotti JA, Jennings WC, Beathard GA, et al. for the Fistula First Breakthrough Initiative Community Education Committee Fistula first breakthrough initiative: targeting catheter last in fistula first. Semin Dial. 2012;25:303–310. doi: 10.1111/j.1525-139X.2012.01069.x. [DOI] [PubMed] [Google Scholar]
- 6.Manook M, Calder F. Practical aspects of arteriovenous fistula formation in the pediatric population. Pediatr Nephrol. 2013;28:885–893. doi: 10.1007/s00467-012-2328-0. [DOI] [PubMed] [Google Scholar]
- 7.Zachara BA. Selenium and selenium-dependent antioxidants in chronic kidney disease. Adv Clin Chem. 2015;68:131–151. doi: 10.1016/bs.acc.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Rayner HC, Pisoni RL. The increasing use of hemodialysis catheters: evidence from the DOPPS on its significance and ways to reverse it. Semin Dial. 2010;23:6–10. doi: 10.1111/j.1525-139X.2009.00675.x. [DOI] [PubMed] [Google Scholar]
- 9.Lopez-Vargas PA, Craig JC, Gallagher MP, et al. Barriers to timely arteriovenous fistula creation: a study of providers and patients. Am J Kidney Dis. 2011;57:873–882. doi: 10.1053/j.ajkd.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 10.Rooijens PP, Tordoir JH, Stijnen T, et al. Radiocephalic wrist arteriovenous fistula for hemodialysis: meta-analysis indicates a high primary failure rate. Eur J Vasc Endovasc Surg. 2004;28:583–589. doi: 10.1016/j.ejvs.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 11.Huijbregts HJT, Bots ML, Wittens CHA, et al. for the CIMINO Study Group Hemodialysis arteriovenous fistula patency revisited: results of a prospective, multicenter initiative. Clin J Am Soc Nephrol. 2008;3:714–719. doi: 10.2215/CJN.02950707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Jaishi AA, Oliver MJ, Thomas SM, et al. Patency rates of the arteriovenous fistula for hemodialysis: a systematic review and meta-analysis. Am J Kidney Dis. 2014;63:464–478. doi: 10.1053/j.ajkd.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 13.Wong V, Ward R, Taylor J, et al. Factors associated with early failure of arteriovenous fistulae for haemodialysis access. Eur J Vasc Endovascr Surg. 1996;12:207–213. doi: 10.1016/s1078-5884(96)80108-0. [DOI] [PubMed] [Google Scholar]
- 14.Feldman HI, Joffe M, Rosas SE, et al. Predictors of successful arteriovenous fistula maturation. AmJ Kidney Dis. 2003;42:1000–1012. doi: 10.1016/j.ajkd.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Aitken E, Jackson A, Kong C, et al. Renal function, uraemia and early arteriovenous fistula failure. BMC Nephrol. 2014;15:179. doi: 10.1186/1471-2369-15-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gagliardi GM, Rossi S, Condino F, et al. Malnutrition, infection and arteriovenous fistula failure: is there a link? J Vasc Access. 2011;12:57–62. doi: 10.5301/jva.2010.5831. [DOI] [PubMed] [Google Scholar]
- 17.Smith GE, Gohil R, Chetter IC. Factors affecting the patency of arteriovenous fistulas for dialysis access. J Vasc Surg. 2012;55:849–855. doi: 10.1016/j.jvs.2011.07.095. [DOI] [PubMed] [Google Scholar]
- 18.Wasse H, Huang R, Naqvi N, et al. Inflammation, oxidation and venous neointimal hyperplasia precede vascular injury from AVF creation in CKD patients. J Vasc Access. 2012;13:168–174. doi: 10.5301/jva.5000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roy-Chaudhury P, Wang Y, Krishnamoorthy M, et al. Cellular phenotypes in human stenotic lesions from haemodialysis vascular access. Nephrol Dial Transplant. 2009;24:2786–2791. doi: 10.1093/ndt/gfn708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roy-Chaudhury P, Arend L, Zhang J, et al. Neointimal hyperplasia in early arteriovenous fistula failure. Am J Kidney Dis. 2007;50:782–790. doi: 10.1053/j.ajkd.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 21.Stracke S, Konner K, Kostlin I, et al. Increased expression of TGF-beta1 and IGF-I in inflammatory stenotic lesions of hemodialysis fistulas. Kidney Int. 2002;61:1011–1019. doi: 10.1046/j.1523-1755.2002.00191.x. [DOI] [PubMed] [Google Scholar]
- 22.Simone S, Loverre A, Cariello M, et al. Arteriovenous fistula stenosis in hemodialysis patients is characterized by an increased adventitial fibrosis. J Nephrol. 2014;27:555–562. doi: 10.1007/s40620-014-0050-7. [DOI] [PubMed] [Google Scholar]
- 23.Campos B, Lee T, Roy-Chaudhury P. Arteriovenous fistula failure: is there a role for epigenetic regulation? Semin Nephrol. 2013;33:400–406. doi: 10.1016/j.semnephrol.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 24.Rotmans JI, Pattynama PM, Verhagen HJ, et al. Sirolimus-eluting stents to abolish intimal hyperplasia and improve flow in porcine arteriovenous grafts: a 4-week follow-up study. Circulation. 2005;111:1537–1542. doi: 10.1161/01.CIR.0000159332.18585.B5. [DOI] [PubMed] [Google Scholar]
- 25.Rotmans JI, Verhagen HJ, Velema E, et al. Local overexpression of C-type natriuretic peptide ameliorates vascular adaptation of porcine hemodialysis grafts. Kidney Int. 2004;65:1897–1905. doi: 10.1111/j.1523-1755.2004.00598.x. [DOI] [PubMed] [Google Scholar]
- 26.Misra S, Fu AA, Anderson JL, et al. The rat femoral arteriovenous fistula model: increased expression of matrix metalloproteinase-2 and -9 at the venous stenosis. J Vasc Interv Radiol. 2008;19:587–594. doi: 10.1016/j.jvir.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Yang B, Shergill U, Fu AA, et al. The mouse arteriovenous fistula model. J Vasc Interv Radiol. 2009;20:946–950. doi: 10.1016/j.jvir.2009.03.044. [DOI] [PubMed] [Google Scholar]
- 28.Lee T, Chauhan V, Krishnamoorthy M, et al. Severe venous neointimal hyperplasia prior to dialysis access surgery. Nephrol Dial Transplant. 2011;26:2264–2270. doi: 10.1093/ndt/gfq733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Misra S, Gordon JD, Fu AA, et al. The porcine remnant kidney model of chronic renal insufficiency. J Surg Res. 2006;135:370–379. doi: 10.1016/j.jss.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Hughes D, Fu AA, Puggioni A, et al. Adventitial transplantation of blood outgrowth endothelial cells in porcine haemodialysis grafts alleviates hypoxia and decreases neointimal proliferation through a matrix metalloproteinase-9-mediated pathway–a pilot study. Nephrol Dials Transplant. 2008;24:85–96. doi: 10.1093/ndt/gfn433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Misra S, Fu AA, Anderson JL, et al. Fetuin-A expression in early venous stenosis formation in a porcine model of hemodialysis graft failure. J Vasc Interv Radiol. 2008;19:1477–1482. doi: 10.1016/j.jvir.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chamberlain RM, Shirley DG. Time course of the renal functional response to partial nephrectomy: measurements in conscious rats. Exp Physiol. 2007;92:251–262. doi: 10.1113/expphysiol.2006.034751. [DOI] [PubMed] [Google Scholar]
- 33.Misra S, Shergill U, Yang B, et al. Increased expression of HIF-1alpha, VEGF-A and its receptors, MMP-2, TIMP-1, and ADAMTS-1 at the venous stenosis of arteriovenous fistula in a mouse model with renal insufficiency. J Vasc Interv Radiol. 2010;21:1255–1261. doi: 10.1016/j.jvir.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ene-Iordache B, Cattaneo L, Dubini G, Remuzzi A. Effect of anastomosis angle on the localization of disturbed flow in ’side-to-end’ fistulae for haemodialysis access. Nephrol Dial Transplant. 2013;28:997–1005. doi: 10.1093/ndt/gfs298. [DOI] [PubMed] [Google Scholar]
- 35.Ene-Iordache B, Remuzzi A. Disturbed flow in radial-cephalic arteriovenous fistulae for haemodialysis: low and oscillating shear stress locates the sites of stenosis. Nephrol Dial Transplant. 2012;27:358–368. doi: 10.1093/ndt/gfr342. [DOI] [PubMed] [Google Scholar]
- 36.Brahmbhatt A, NievesTorres E, Yang B, et al. The Role of Iex-1 in the pathogenesis of venous neointimal hyperplasia associated with hemodialysis arteriovenous fistula. PLoS One. 2014;9:e102542. doi: 10.1371/journal.pone.0102542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riella MC, Roy-Chaudhury P. Vascular access in haemodialysis: strengthening the Achilles’ heel. Nat Rev Nephrol. 2013;9:348–357. doi: 10.1038/nrneph.2013.76. [DOI] [PubMed] [Google Scholar]
- 38.Liang A, Wang Y, Han G, et al. Chronic kidney disease accelerates endothelial barrier dysfunction in a mouse model of an arteriovenous fistula. Am J Physiol Renal Physiol. 2013;304:F1413–F1420. doi: 10.1152/ajprenal.00585.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Misra S, Fu AA, Rajan DK, et al. Expression of hypoxia inducible factor-1 alpha, macrophage migration inhibition factor, matrix metalloproteinase-2 and -9, and their inhibitors in hemodialysis grafts and arteriovenous fistulas. J Vasc Interv Radiol. 2008;19:252–259. doi: 10.1016/j.jvir.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 40.Asare Y, Schmitt M, Bernhagen J. The vascular biology of macrophage migration inhibitory factor (MIF): expression and effects in inflammation, atherogenesis and angiogenesis. Thromb Haemost. 2013;109:391–398. doi: 10.1160/TH12-11-0831. [DOI] [PubMed] [Google Scholar]
- 41.Stangenberg S, Nguyen LT, Chen H, et al. Oxidative stress, mitochondrial perturbations and fetal programming of renal disease induced by maternal smoking. Int J Biochem Cell Biol. 2015;64:81–90. doi: 10.1016/j.biocel.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 42.van der Weerd NC, Grooteman MP, Nube MJ, et al. Hepcidin in chronic kidney disease: not an anaemia management tool, but promising as a cardiovascular biomarker. Neth J Med. 2015;73:108–118. [PubMed] [Google Scholar]
- 43.Virzì GM, Clementi A, de Cal M, et al. Oxidative stress: dual pathway induction in cardiorenal syndrome type 1 pathogenesis. Oxid Med Cell Longev. 2015;2015:391790. doi: 10.1155/2015/391790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Veillat V, Carli C, Metz CN, et al. Macrophage migration inhibitory factor elicits an angiogenic phenotype in human ectopic endometrial cells and triggers the production of major angiogenic factors via CD44, CD74, and MAPK signaling pathways. J Clin Endocrinol Metab. 2010;95:E403–412. doi: 10.1210/jc.2010-0417. [DOI] [PubMed] [Google Scholar]
- 45.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carbo C, Arderiu G, Escolar G, et al. Differential expression of proteins from cultured endothelial cells exposed to uremic versus normal serum. Am J Kidney Dis. 2008;51:603–612. doi: 10.1053/j.ajkd.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 47.Martin-Rodriguez S, Caballo C, Gutierrez G, et al. TLR4 and NALP3 inflammasome in the development of endothelial dysfunction in uraemia. Eur J Clin Invest. 2015;45:160–169. doi: 10.1111/eci.12392. [DOI] [PubMed] [Google Scholar]
- 48.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 49.Heine GH, Ulrich C, Sester U, et al. Transforming growth factor beta1 genotype polymorphisms determine AV fistula patency in hemodialysis patients. Kidney Int. 2003;64:1101–1107. doi: 10.1046/j.1523-1755.2003.00176.x. [DOI] [PubMed] [Google Scholar]
- 50.Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126:855–867. doi: 10.1016/j.cell.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 51.Zhao YY, Takahashi M, Gu JG, et al. Functional roles of N-glycans in cell signaling and cell adhesion in cancer. Cancer Sci. 2008;99:1304–1310. doi: 10.1111/j.1349-7006.2008.00839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shen N, Lin H, Wu T, et al. Inhibition of TGF-β1-receptor posttranslational core fucosylation attenuates rat renal interstitial fibrosis. Kidney Int. 2013;84:64–77. doi: 10.1038/ki.2013.82. [DOI] [PubMed] [Google Scholar]
- 53.Takeda R, Suzuki E, Satonaka H, et al. Blockade of endogenous cytokines mitigates neointimal formation in obese Zucker rats. Circulation. 2005;111:1398–1406. doi: 10.1161/01.CIR.0000158482.83179.DB. [DOI] [PubMed] [Google Scholar]
- 54.Dixon BS. Why don't fistulas mature? Kidney Int. 2006;70:1413–1422. doi: 10.1038/sj.ki.5001747. [DOI] [PubMed] [Google Scholar]
- 55.Sener EF, Taheri S, Korkmaz K, et al. Association of TNF-α -308 G > A and ACE I/D gene polymorphisms in hemodialysis patients with arteriovenous fistula thrombosis. Int Urol Nephrol. 2014;46:1419–1425. doi: 10.1007/s11255-013-0580-2. [DOI] [PubMed] [Google Scholar]
- 56.Sidawy AN, Gray R, Besarab A, et al. Recommended standards for reports dealing with arteriovenous hemodialysis accesses. J Vasc Surg. 2002;35:603–610. doi: 10.1067/mva.2002.122025. [DOI] [PubMed] [Google Scholar]
- 57.Kokubo T, Ishikawa N, Uchida H, et al. CKD accelerates development of neointimal hyperplasia in arteriovenous fistulas. J Am Soc Nephrol. 2009;20:1236–1245. doi: 10.1681/ASN.2007121312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang B, Vohra PK, Janardhanan R, et al. Expression of profibrotic genes in a murine remnant kidney model. J Vasc Interv Radiol. 2011;22:1765–1772. e1761. doi: 10.1016/j.jvir.2011.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weiss MF, Scivittaro V, Anderson JM. Oxidative stress and increased expression of growth factors in lesions of failed hemodialysis access. Am J Kidney Dis. 2001;37:970–980. doi: 10.1016/s0272-6386(05)80013-7. [DOI] [PubMed] [Google Scholar]
- 60.Jankovic A, Damjanovic T, Djuric Z, et al. Impact of vascular calcifications on arteriovenous fistula survival in hemodialysis patients: a five-year follow-up. Nephron. 2015;129:247–252. doi: 10.1159/000380823. [DOI] [PubMed] [Google Scholar]
- 61.DeFronzo RA, Alvestrand A, Smith D, et al. Insulin resistance in uremia. J Clin Invest. 1981;67:563–568. doi: 10.1172/JCI110067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rostand SG, Drueke TB. Parathyroid hormone, vitamin D, and cardiovascular disease in chronic renal failure. Kidney Int. 1999;56:383–392. doi: 10.1046/j.1523-1755.1999.00575.x. [DOI] [PubMed] [Google Scholar]
- 63.Buzello M, Tornig J, Faulhaber J, et al. The apolipoprotein e knockout mouse: a model documenting accelerated atherogenesis in uremia. J Am Soc Nephrol. 2003;14:311–316. doi: 10.1097/01.asn.0000045048.71975.fc. [DOI] [PubMed] [Google Scholar]
- 64.Georgiadis GS, Georgakarakos EI, Antoniou GA, et al. Correlation of preexisting radial artery macrocalcifications with late patency of primary radiocephalic fistulas in diabetic hemodialysis patients. J Vasc Surg. 2014;60:462–470. doi: 10.1016/j.jvs.2014.02.042. [DOI] [PubMed] [Google Scholar]
- 65.Balci M, Kirkpantur A, Turkvatan A, et al. Sclerostin as a new key player in arteriovenous fistula calcification. Herz. 2015;40:289–297. doi: 10.1007/s00059-013-3992-y. [DOI] [PubMed] [Google Scholar]
- 66.Mitsuhashi T, Morris RC, Ives HE. 1,25-dihydroxyvitamin D3 modulates growth of vascular smooth muscle cells. J Clin Invest. 1991;87:1889–1895. doi: 10.1172/JCI115213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Danziger J. Vitamin K-dependent proteins, warfarin, and vascular calcification. Clin J Am Soc Nephrol. 2008;3:1504–1510. doi: 10.2215/CJN.00770208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hruska KA, Mathew S, Memon I, et al. The pathogenesis of vascular calcification in the chronic kidney disease mineral bone disorder: the links between bone and the vasculature. Semin Nephrol. 2009;29:156–165. doi: 10.1016/j.semnephrol.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yamanouchi D, Takei Y, Komori K. Balanced mineralization in the arterial system: possible role of osteoclastogenesis/osteoblastogenesis in abdominal aortic aneurysm and stenotic disease. Circ J. 2012;76:2732–2737. doi: 10.1253/circj.cj-12-1240. [DOI] [PubMed] [Google Scholar]
- 70.Lu L, Erhard P, Salomon RG, Weiss MF. Serum vitamin E and oxidative protein modification in hemodialysis: a randomized clinical trial. Am J Kidney Dis. 2007;50:305–313. doi: 10.1053/j.ajkd.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 71.Wu M, Rementer C, Giachelli CM. Vascular calcification: an update on mechanisms and challenges in treatment. Calcif Tissue Int. 2013;93:365–373. doi: 10.1007/s00223-013-9712-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moe SM, Reslerova M, Ketteler M, et al. Role of calcification inhibitors in the pathogenesis of vascular calcification in chronic kidney disease (CKD). Kidney Int. 2005;67:2295–2304. doi: 10.1111/j.1523-1755.2005.00333.x. [DOI] [PubMed] [Google Scholar]
- 73.Misra S, Fu AA, Puggioni A, et al. Increased expression of hypoxiainducible factor-1 alpha in venous stenosis of arteriovenous polytetrafluoroethylene grafts in a chronic renal insufficiency porcine model. J Vasc Interv Radiol. 2008;19:260–265. doi: 10.1016/j.jvir.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 74.Zhu Y, Lawton MT, Du R, et al. Expression of hypoxia-inducible factor-1 and vascular endothelial growth factor in response to venous hypertension. Neurosurgery. 2006;59:687–696. doi: 10.1227/01.NEU.0000228962.68204.CF. [discussion: 687-696] [DOI] [PubMed] [Google Scholar]
- 75.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 76.Wan J, Lata C, Santilli A, et al. Supplemental oxygen reverses hypoxia induced smooth muscle cell proliferation by modulating HIF-alpha and VEGF levels in a rabbit arteriovenous fistula model. Ann Vasc Surg. 2014;28:725–736. doi: 10.1016/j.avsg.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang B, Janardhanan R, Vohra P, et al. Adventitial transduction of lentivirus-shRNA-VEGF-A in arteriovenous fistula reduces venous stenosis formation. Kidney Int. 2014;85:289–306. doi: 10.1038/ki.2013.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Candan F, Yildiz G, Kayatas M. Role of the VEGF 936 gene polymorphism and VEGF-A levels in the late-term arteriovenous fistula thrombosis in patients undergoing hemodialysis. Int Urol Nephrol. 2014;46:1815–1823. doi: 10.1007/s11255-014-0711-4. [DOI] [PubMed] [Google Scholar]
- 79.Ohtani K, Egashira K, Hiasa K, et al. Blockade of vascular endothelial growth factor suppresses experimental restenosis after intraluminal injury by inhibiting recruitment of monocyte lineage cells. Circulation. 2004;110:2444–2452. doi: 10.1161/01.CIR.0000145123.85083.66. [DOI] [PubMed] [Google Scholar]
- 80.Shibuya M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J Biochem Mol Biol. 2006;39:469–478. doi: 10.5483/bmbrep.2006.39.5.469. [DOI] [PubMed] [Google Scholar]
- 81.Huusko J, Merentie M, Dijkstra MH, et al. The effects of VEGF-R1 and VEGF-R2 ligands on angiogenic responses and left ventricular function in mice. Cardiovasc Res. 2010;86:122–130. doi: 10.1093/cvr/cvp382. [DOI] [PubMed] [Google Scholar]
- 82.Janda K, Krzanowski M, Gajda M, et al. Cardiovascular risk in chronic kidney disease patients: intima-media thickness predicts the incidence and severity of histologically assessed medial calcification in radial arteries. BMC Nephrol. 2015;16:78. doi: 10.1186/s12882-015-0067-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fragoso A, Silva AP, Gundlach K, et al. Magnesium and FGF-23 are independent predictors of pulse pressure in pre-dialysis diabetic chronic kidney disease patients. Clinical Kidney J. 2014;7:161–166. doi: 10.1093/ckj/sfu003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vempati P, Popel AS, Mac Gabhann F. Extracellular regulation of VEGF: isoforms, proteolysis, and vascular patterning. Cytokine Growth Factor Rev. 2014;25:1–19. doi: 10.1016/j.cytogfr.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kikuchi R, Nakamura K, MacLauchlan S, et al. An antiangiogenic isoform of VEGF-A contributes to impaired vascularization in peripheral artery disease. Nat Med. 2014;20:1464–1471. doi: 10.1038/nm.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nowak DG, Woolard J, Amin EM, et al. Expression of pro- and anti-angiogenic isoforms of VEGF is differentially regulated by splicing and growth factors. J Cell Sci. 2008;121:3487–3495. doi: 10.1242/jcs.016410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Geng L, Chaudhuri A, Talmon G, et al. TGF-Beta suppresses VEGFA-mediated angiogenesis in colon cancer metastasis. PLoS One. 2013;8:e59918. doi: 10.1371/journal.pone.0059918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nakagawa T, Li JH, Garcia G, et al. TGF-beta induces proangiogenic and antiangiogenic factors via parallel but distinct Smad pathways. Kidney Int. 2004;66:605–613. doi: 10.1111/j.1523-1755.2004.00780.x. [DOI] [PubMed] [Google Scholar]
- 89.Shi X, Guo LW, Seedial SM, et al. TGF-β/Smad3 inhibit vascular smooth muscle cell apoptosis through an autocrine signaling mechanism involving VEGF-A. Cell Death Dis. 2014;5:e1317. doi: 10.1038/cddis.2014.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lata C, Green D, Wan J, et al. The role of short-term oxygen administration in the prevention of intimal hyperplasia. J Vasc Surg. 2013;58:452–459. doi: 10.1016/j.jvs.2012.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Modaresi A, Nafar M, Sahraei Z. Oxidative stress in chronic kidney disease. Iranian J Kidney Dis. 2015;9:165–179. [PubMed] [Google Scholar]
- 92.Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002;62:1524–1538. doi: 10.1046/j.1523-1755.2002.00600.x. [DOI] [PubMed] [Google Scholar]
- 93.Puchades MJ, Saez G, Munoz MC, et al. Study of oxidative stress in patients with advanced renal disease and undergoing either hemodialysis or peritoneal dialysis. Clin Nephrol. 2013;80:177–186. doi: 10.5414/CN107639. [DOI] [PubMed] [Google Scholar]
- 94.Lehoux S. Redox signalling in vascular responses to shear and stretch. Cardiovasc Res. 2006;71:269–279. doi: 10.1016/j.cardiores.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 95.Sakoh T, Nakayama M, Tanaka S, et al. Association of serum total bilirubin with renal outcome in Japanese patients with stages 3-5 chronic kidney disease. Metabolism. 2015;69:1096–1102. doi: 10.1016/j.metabol.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 96.Dursun B, Dursun E, Suleymanlar G, et al. Carotid artery intima-media thickness correlates with oxidative stress in chronic haemodialysis patients with accelerated atherosclerosis. Nephrol Dial Transplant. 2008;23:1697–1703. doi: 10.1093/ndt/gfm906. [DOI] [PubMed] [Google Scholar]
- 97.Byon CH, Chen Y. Molecular mechanisms of vascular calcification in chronic kidney disease: the link between bone and the vasculature. Curr Osteoporos Rep. 2015;13:206–215. doi: 10.1007/s11914-015-0270-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ene-Iordache B, Mosconi L, Antiga L, et al. Radial artery remodeling in response to shear stress increase within arteriovenous fistula for hemodialysis access. Endothelium. 2003;10:95–102. doi: 10.1080/10623320303365. [DOI] [PubMed] [Google Scholar]
- 99.Tucker PS, Scanlan AT, Dalbo VJ. Chronic kidney disease influences multiple systems: describing the relationship between oxidative stress, inflammation, kidney damage, and concomitant disease. Oxid Med Cell Longev. 2015;2015:806358. doi: 10.1155/2015/806358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Manini S, Passera K, Huberts W, et al. Computational model for simulation of vascular adaptation following vascular access surgery in haemodialysis patients. Comput Methods Biomech Biomed Engin. 2014;17:1358–1367. doi: 10.1080/10255842.2012.745857. [DOI] [PubMed] [Google Scholar]
- 101.Ene-Iordache B, Semperboni C, Dubini G, Remuzzi A. Disturbed flow in a patient-specific arteriovenous fistula for hemodialysis: multidirectional and reciprocating near-wall flow patterns. J Biomech. 2015;48:2195–2200. doi: 10.1016/j.jbiomech.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 102.Nishikawa M, Ishimori N, Takada S, et al. AST-120 ameliorates lowered exercise capacity and mitochondrial biogenesis in the skeletal muscle from mice with chronic kidney disease via reducing oxidative stress. Nephrol Dial Transplant. 2015;30:934–942. doi: 10.1093/ndt/gfv103. [DOI] [PubMed] [Google Scholar]
- 103.Guijarro C, Egido J. Transcription factor-kappa B (NF-kappa B) and renal disease. Kidney Int. 2001;59:415–424. doi: 10.1046/j.1523-1755.2001.059002415.x. [DOI] [PubMed] [Google Scholar]
- 104.Assis RP, Castro JF, Gutierres VO, et al. Effects of uremic solutes on reactive oxygen species in vitro model systems as a possibility of support the renal function management. BMC Nephrol. 2015;16:50. doi: 10.1186/s12882-015-0029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chevalier RL. Congenital urinary tract obstruction: the long view. Adv Chronic Kidney Dis. 2015;22:312–319. doi: 10.1053/j.ackd.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ruiz S, Pergola PE, Zager RA, Vaziri ND. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int. 2013;83:1029–1041. doi: 10.1038/ki.2012.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zaniew M, Zachwieja J, Warzywoda A, et al. [Influence of vitamin E and N-acetylcysteine on intracellular oxidative stress in T lymphocytes in children treated with dialysis]. Wiad Lek. 2005;58(suppl 1):58–65. in Polish. [PubMed] [Google Scholar]
- 108.Zhang L, Coombes J, Pascoe EM, et al. for the HERO Study Collaborative Group The effect of pentoxifylline on oxidative stress in chronic kidney disease patientswith erythropoiesis-stimulating agent hyporesponsiveness: sub-study of the HERO trial [e-pub ahead of print]. Redox Rep. doi: 10.1179/1351000215Y.0000000022. doi: 10.1179/ 1351000215Y.0000000022, accessed December 22, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Locatelli F, Canaud B, Eckardt KU, et al. Oxidative stress in end-stage renal disease: an emerging threat to patient outcome. Nephrol Dial Transplant. 2003;18:1272–1280. doi: 10.1093/ndt/gfg074. [DOI] [PubMed] [Google Scholar]
- 110.Cachofeiro V, Goicochea M, de Vinuesa SG, et al. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int Suppl. 2008;111:S4–S9. doi: 10.1038/ki.2008.516. [DOI] [PubMed] [Google Scholar]
- 111.Fassett RG, Robertson IK, Ball MJ, et al. Effects of atorvastatin on oxidative stress in chronic kidney disease. Nephrology (Carlton) 2015;20:697–705. doi: 10.1111/nep.12502. [DOI] [PubMed] [Google Scholar]
- 112.Vaziri ND, Liu S, Farzaneh SH, et al. Dose-dependent deleterious and salutary actions of the Nrf2 inducer dh404 in chronic kidney disease. Free Radic Biol Med. 2015;86:374–381. doi: 10.1016/j.freeradbiomed.2015.04.022. [DOI] [PubMed] [Google Scholar]
- 113.Vaziri ND, Zhao YY, Pahl MV. Altered intestinal microbial flora and impaired epithelial barrier structure and function in CKD: the nature, mechanisms, consequences and potential treatment [e-pub ahead of print]. Nephrol Dial Transplant. doi: 10.1093/ndt/gfv095. doi: 10.1093/ndt/gfv095, accessed December 22, 2015. [DOI] [PubMed] [Google Scholar]
- 114.Huang TH, Chen YT, Sung PH, et al. Peripheral blood-derived endothelial progenitor cell therapy prevented deterioration of chronic kidney disease in rats. Am J Transl Res. 2015;7:804–824. [PMC free article] [PubMed] [Google Scholar]
- 115.Schelling JR. Tubular atrophy in the pathogenesis of chronic kidney disease progression. Pediatr Nephrol. 2015:1–4. doi: 10.1007/s00467-015-3169-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zafrani L, Ince C. Microcirculation in acute and chronic kidney diseases. Am J Kidney Dis. 2015;66:1083–1094. doi: 10.1053/j.ajkd.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 117.Granata S, Zaza G, Simone S, et al. Mitochondrial dysregulation and oxidative stress in patients with chronic kidney disease. BMC Genomics. 2009;10:388. doi: 10.1186/1471-2164-10-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91:327–387. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sellin L, Kielstein JT, de Groot K. [Hyperuricemia—more than gout: impact on cardiovascular risk and renal insufficiency]. Z Rheumatol. 2015;74:322–328. doi: 10.1007/s00393-014-1481-1. in German. [DOI] [PubMed] [Google Scholar]
- 120.Gross P, Massy ZA, Henaut L, et al. Para-cresyl sulfate acutely impairs vascular reactivity and induces vascular remodeling. J Cell Physiol. 2015;230:2927–2935. doi: 10.1002/jcp.25018. [DOI] [PubMed] [Google Scholar]
- 121.Tschopp J. Mitochondria: sovereign of inflammation? Eur J Immunol. 2011;41:1196–1202. doi: 10.1002/eji.201141436. [DOI] [PubMed] [Google Scholar]
- 122.Oberg BP, McMenamin E, Lucas FL, et al. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int. 2004;65:1009–1016. doi: 10.1111/j.1523-1755.2004.00465.x. [DOI] [PubMed] [Google Scholar]
- 123.Ma Y, Zhou L, Dong J, et al. Arterial stiffness and increased cardiovascular risk in chronic kidney disease. Int Urol Nephrol. 2015;47:1157–1164. doi: 10.1007/s11255-015-1009-x. [DOI] [PubMed] [Google Scholar]
- 124.Ali BH, Adham SA, Al Za'abi M, et al. Ameliorative effect of chrysin on adenine-induced chronic kidney disease in rats. PLoS One. 2015;10:e0125285. doi: 10.1371/journal.pone.0125285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Roy-Chaudhury P, Spergel LM, Besarab A, et al. Biology of arteriovenous fistula failure. J Nephrol. 2007;20:150–163. [PubMed] [Google Scholar]
- 126.Juncos JP, Grande JP, Kang L, et al. MCP-1 contributes to arteriovenous fistula failure. J Am Soc Nephrol. 2011;22:43–48. doi: 10.1681/ASN.2010040373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Juncos JP, Tracz MJ, Croatt AJ, et al. Genetic deficiency of heme oxygenase-1 impairs functionality and form of an arteriovenous fistula in the mouse. Kidney Int. 2008;74:47–51. doi: 10.1038/ki.2008.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kang L, Grande JP, Farrugia G, et al. Functioning of an arteriovenous fistula requires heme oxygenase-2. Am J Physiol Renal Physiol. 2013;305:F545–F552. doi: 10.1152/ajprenal.00234.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lin CC, Yang WC, Lin SJ, et al. Length polymorphism in heme oxygenase-1 is associated with arteriovenous fistula patency in hemodialysis patients. Kidney Int. 2006;69:165–172. doi: 10.1038/sj.ki.5000019. [DOI] [PubMed] [Google Scholar]
- 130.Zhou Q, Liao JK. Pleiotropic effects of statins: basic research and clinical perspectives. Circ J. 2010;74:818–826. doi: 10.1253/circj.cj-10-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Freidja ML, Toutain B, Caillon A, et al. Heme oxygenase 1 is differentially involved in blood flow-dependent arterial remodeling: role of inflammation, oxidative stress, and nitric oxide. Hypertension. 2011;58:225–231. doi: 10.1161/HYPERTENSIONAHA.111.170266. [DOI] [PubMed] [Google Scholar]
- 132.Machowska A, Carrero JJ, Lindholm B, Stenvinkel P. Therapeutics targeting persistent inflammation in chronic kidney disease. Transl Res. 2016;167:204–213. doi: 10.1016/j.trsl.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 133.Zhang MH, Pan MM, Ni HF, et al. [Effect of Cordyceps sinensis powder on renal oxidative stress and mitochondria functions in 5/6 nephrectomized rats]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2015;35:443–449. in Chinese. [PubMed] [Google Scholar]
- 134.Chokhandre MK, Mahmoud MI, Hakami T, et al. Vitamin D & its analogues in type 2 diabetic nephropathy: a systematic review. J Diabetes Metab Disord. 2015;14:58. doi: 10.1186/s40200-015-0186-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liu MC, Lee YW, Lee PT, et al. Cyclophilin A is associated with peripheral artery disease and chronic kidney disease in geriatrics: the Tianliao Old People (TOP) study. Sci Rep. 2015;5:9937. doi: 10.1038/srep09937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yazdi PG, Moradi H, Yang JY, et al. Skeletal muscle mitochondrial depletion and dysfunction in chronic kidney disease. Int J Clin Exp Med. 2013;6:532539. [PMC free article] [PubMed] [Google Scholar]
- 137.Uribarri J, He JC. The low AGE diet: a neglected aspect of clinical nephrology practice? Nephron. 2015;130:48–53. doi: 10.1159/000381315. [DOI] [PubMed] [Google Scholar]
- 138.Egorova AD, Van der Heiden K, Van de Pas S, et al. Tgfβ/Alk5 signaling is required for shear stress induced klf2 expression in embryonic endothelial cells. Dev Dyn. 2011;240:1670–1680. doi: 10.1002/dvdy.22660. [DOI] [PubMed] [Google Scholar]
- 139.Moonen JA, Lee ES, Schmidt M, et al. Endothelial-to-mesenchymal transition contributes to fibro-proliferative vascular disease and is modulated by fluid shear stress. Cardiovasc Res. 2015;108:377–386. doi: 10.1093/cvr/cvv175. [DOI] [PubMed] [Google Scholar]
- 140.Barros AF, Borges NA, Ferreira DC, et al. Is there interaction between gut microbial profile and cardiovascular risk in chronic kidney disease patients? Future Microbiol. 2015;10:517–526. doi: 10.2217/fmb.14.140. [DOI] [PubMed] [Google Scholar]
- 141.Textor SC, Lerman LO. Paradigm shifts in atherosclerotic renovascular disease: where are we now? J Am Soc Nephrol. 2015;26:2074–2080. doi: 10.1681/ASN.2014121274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chen NX, O'Neill KD, Allen MR, et al. Low bone turnover in chronic kidney disease is associated with decreased VEGF-A expression and osteoblast differentiation. Am J Nephrol. 2015;41:464–473. doi: 10.1159/000438461. [DOI] [PubMed] [Google Scholar]
- 143.Wu X, Guan Y, Yan J, et al. ShenKang injection suppresses kidney fibrosis and oxidative stress via transforming growth factor-β/Smad3 signalling pathway in vivo and in vitro. J Pharm Pharmacol. 2015;67:1054–1065. doi: 10.1111/jphp.12412. [DOI] [PubMed] [Google Scholar]
- 144.van Hinsbergh VW. Endothelium—role in regulation of coagulation and inflammation. Semin Immunopathol. 2012;34:93–106. doi: 10.1007/s00281-011-0285-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rios DR, Carvalho M, Lwaleed BA, et al. Hemostatic changes in patients with end stage renal disease undergoing hemodialysis. Clin Chim Acta. 2010;411:135–139. doi: 10.1016/j.cca.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 146.Turitto VT, Hall CL. Mechanical factors affecting hemostasis and thrombosis. Thromb Res. 1998;92(suppl 2):S25–S31. doi: 10.1016/s0049-3848(98)00157-1. [DOI] [PubMed] [Google Scholar]
- 147.Corti R, Hutter R, Badimon JJ, Fuster V. Evolving concepts in the triad of atherosclerosis, inflammation and thrombosis. J Thromb Thrombolysis. 2004;17:35–44. doi: 10.1023/B:THRO.0000036027.39353.70. [DOI] [PubMed] [Google Scholar]
- 148.Serrano A, Garcia F, Serrano M, et al. IgA antibodies against β2 glycoprotein I in hemodialysis patients are an independent risk factor for mortality. Kidney Int. 2012;81:1239–1244. doi: 10.1038/ki.2011.477. [DOI] [PubMed] [Google Scholar]
- 149.Fischer MJ, Rauch J, Levine JS. The antiphospholipid syndrome. Semin Nephrol. 2007;27:35–46. doi: 10.1016/j.semnephrol.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Costa E, Rocha S, Rocha-Pereira P, et al. Cross-talk between inflammation, coagulation/fibrinolysis and vascular access in hemodialysis patients. J Vasc Access. 2008;9:248–253. [PubMed] [Google Scholar]
- 151.Erdem Y, Haznedaroglu IC, Celik I, et al. Coagulation, fibrinolysis and fibrinolysis inhibitors in haemodialysis patients: contribution of arteriovenous fistula. Nephrol Dial Transplant. 1996;11:1299–1305. [PubMed] [Google Scholar]
- 152.Milburn JA, Ford I, Cassar K, et al. Platelet activation, coagulation activation and C-reactive protein in simultaneous samples from the vascular access and peripheral veins of haemodialysis patients. Int J Lab Hematol. 2012;34:52–58. doi: 10.1111/j.1751-553X.2011.01356.x. [DOI] [PubMed] [Google Scholar]
- 153.Milburn JA, Ford I, Mutch NJ, et al. Thrombin-Anti-thrombin levels and patency of arterio-venous fistula in patients undergoing haemodialysis compared to healthy volunteers: a prospective analysis. PLoS One. 2013;8:e67799. doi: 10.1371/journal.pone.0067799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Smits JH, van der Linden J, Blankestijn PJ, Rabelink TJ. Coagulation and haemodialysis access thrombosis. Nephrol Dial Transplant. 2000;15:1755–1760. doi: 10.1093/ndt/15.11.1755. [DOI] [PubMed] [Google Scholar]
- 155.Chien CT, Fan SC, Lin SC, et al. Glucagon-like peptide-1 receptor agonist activation ameliorates venous thrombosis-induced arteriovenous fistula failure in chronic kidney disease. Thromb Haemost. 2014;112:1051–1064. doi: 10.1160/TH14-03-0258. [DOI] [PubMed] [Google Scholar]
- 156.Martinovic Z, Basic-Jukic N, Pavlovic DB, Kes P. Importance of platelet aggregation in patients with end-stage renal disease. Acta Clin Croat. 2013;52:472–477. [PubMed] [Google Scholar]
- 157.Klamroth R, Orlovic M, Fritsche I, et al. The influence of thrombophilic risk factors on vascular access survival in chronic dialysis patients in a retrospective evaluation. Vasa. 2013;42:32–39. doi: 10.1024/0301-1526/a000245. [DOI] [PubMed] [Google Scholar]
- 158.Rios DR, Fernandes AP, Carvalho MG, et al. Hemodialysis vascular access thrombosis: the role of factor V Leiden, prothrombin gene mutation and ABO blood groups. Clin Chim Acta. 2011;412:425–429. doi: 10.1016/j.cca.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 159.Jamshid R, Reza SA, Abbas G, Raha A. Incidence of arteriovenous thrombosis and the role of anticardiolipin antibodies in hemodialysis patients. Int Urol Nephrol. 2003;35:275–282. doi: 10.1023/b:urol.0000020354.61227.40. [DOI] [PubMed] [Google Scholar]
- 160.Salmela B, Hartman J, Peltonen S, et al. Thrombophilia and arteriovenous fistula survival in ESRD. Clin J Am Soc Nephrol. 2013;8:962–968. doi: 10.2215/CJN.03860412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Knoll GA, Wells PS, Young D, et al. Thrombophilia and the risk for hemodialysis vascular access thrombosis. J Am Soc Nephrol. 2005;16:1108–1114. doi: 10.1681/ASN.2004110999. [DOI] [PubMed] [Google Scholar]
- 162.Verschuren JJW, Ocak G, Dekker FW, et al. Candidate gene analysis of arteriovenous fistula failure in hemodialysis patients. Clin J Am Soc Nephrol. 2013;8:1358–1366. doi: 10.2215/CJN.11091012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Allon M, Zhang L, Maya ID, et al. Association of factor V Leiden gene polymorphism with arteriovenous graft failure. Am J Kidney Dis. 2012;59:682–688. doi: 10.1053/j.ajkd.2011.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Serati AR, Roozbeh J, Sagheb MM. Serum LDL levels are a major prognostic factor for arteriovenous fistula thrombosis (AVFT) in hemodialysis patients. J Vasc Access. 2007;8:109–114. [PubMed] [Google Scholar]
- 165.Milburn JA, Cassar K, Ford I, et al. Prothrombotic changes in platelet, endothelial and coagulation function following hemodialysis. Int J Artif Organs. 2011;34:280–287. doi: 10.5301/ijao.2011.6469. [DOI] [PubMed] [Google Scholar]
- 166.Bartels PC, Schoorl M, Schoorl M, et al. Activation of coagulation during treatment with haemodialysis. Scand J Clin Lab Invest. 2000;60:283–290. doi: 10.1080/003655100750046440. [DOI] [PubMed] [Google Scholar]
- 167.Hadhri S, Rejeb MB, Belarbia A, et al. Hemodialysis duration, human platelet antigen HPA-3 and IgA isotype of anti-β2glycoprotein I antibodies are associated with native arteriovenous fistula failure in Tunisian hemodialysis patients. Thromb Res. 2013;131:e202–e209. doi: 10.1016/j.thromres.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 168.Wu JH, Zhang DW, Cheng XL, et al. Platelet glycoprotein IIb HPA-3 a/b polymorphism is associated with native arteriovenous fistula thrombosis in chronic hemodialysis patients. Ren Fail. 2012;34:960–963. doi: 10.3109/0886022X.2012.706865. [DOI] [PubMed] [Google Scholar]
- 169.Pisoni R, Barker-Finkel J, Allo M. Statin therapy is not associated with improved vascular access outcomes. Clin J Am Soc Nephrol. 2010;5:1447–1450. doi: 10.2215/CJN.02740310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Janardhanan R, Yang B, Vohra P, et al. Simvastatin reduces venous stenosis formation in a murine hemodialysis vascular access model. Kidney Int. 2013;84:338–352. doi: 10.1038/ki.2013.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Birch N, Fillaus J, Florescu MC. The effect of statin therapy on the formation of arteriovenous fistula stenoses and the rate of reoccurrence of previously treated stenoses. Hemodial Int. 2013;17:586–593. doi: 10.1111/j.1542-4758.2012.00762.x. [DOI] [PubMed] [Google Scholar]
- 172.Florescu MC, Birch N. Statin therapy and hemodialysis vascular access—were we bringing a knife to a gunfight and were hoping to win? Semin Dial. 2012;25:700–702. doi: 10.1111/j.1525-139X.2012.01059.x. [DOI] [PubMed] [Google Scholar]
- 173.Sigovan M, Rayz V, Gasper W, et al. Vascular remodeling in autogenous arterio-venous fistulas by MRI and CFD. Ann Biomed Eng. 2013;41:657–668. doi: 10.1007/s10439-012-0703-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kwon H, Choi JY, Ko HK, et al. Comparison of surgical and endovascular salvage procedures for juxta-anastomotic stenosis in autogenous wrist radiocephalic arteriovenous fistula. Ann Vasc Surg. 2014;28:1840–1846. doi: 10.1016/j.avsg.2014.06.060. [DOI] [PubMed] [Google Scholar]