Abstract
BACKGROUND
We tested the efficacy of a remote tailored intervention (TeleCARE) compared to a mailed educational brochure for improving colonoscopy uptake among at-risk relatives of colorectal cancer patients and examined subgroup differences based on participant reported cost barriers.
METHODS
Family members of colorectal cancer patients who were not up-to-date with colonoscopy were randomly assigned as family units to TeleCARE (N=232) or an educational brochure (N=249). At the 9-month follow-up, a cost resource letter listing resources for free or reduced-cost colonoscopy was mailed to participants who had reported cost barriers and remained non-adherent. Rates of medically-verified colonoscopy at the 15-month follow-up were compared based on group assignment and within group stratification by cost barriers.
RESULTS
In intent-to-treat analysis, 42.7% of participants in TeleCARE and 24.1% of participants in the educational brochure group had a medically-verified colonoscopy [OR = 2.37; 95% confidence interval (CI) 1.59 to 3.52]. Cost was identified as a barrier in both groups (TeleCARE = 62.5%; educational brochure = 57.0%). When cost was not a barrier, the TeleCARE group was almost four times as likely as the comparison to have a colonoscopy (OR = 3.66; 95% CI= 1.85 to 7.24). The intervention was efficacious among those who reported cost barriers; the TeleCARE group was nearly twice as likely to have a colonoscopy (OR = 1.99; 95% CI = 1.12 to 3.52).
CONCLUSIONS
TeleCARE increased colonoscopy regardless of cost barriers.
IMPACT
Remote interventions may bolster screening colonoscopy regardless of cost barriers and be more efficacious when cost barriers are absent.
Keywords: telehealth, colorectal cancer screening, family history, colonoscopy, cost barriers
INTRODUCTION
Screening is essential for the prevention and control of colorectal cancer (1-3), especially for people who are at increased familial risk of developing the disease (4). Despite an “A” rating from the United States Preventive Services Task Force (5), colonoscopies are underutilized among individuals who are considered at increased risk (6, 7). Approximately 40% of relatives of colorectal cancer patients adhere to screening guidelines (7, 8). Promoting colonoscopy use among family members of colorectal cancer patients is one efficient way to reduce colorectal cancer morbidity (9). However, increasing adherence to risk-based screening guidelines is a complex problem that requires addressing both structural (e.g., cost) and personal (e.g., motivation) barriers to screening (10, 11).
For many people, cost is a major factor in choosing whether or not to obtain a colonoscopy (12-15). According to the National Health Interview Survey (2010), only 21% of uninsured people in the United States are up to date with colorectal cancer screening—a stark contrast to the 59% of insured people who are up to date (8, 15, 16). Although the Patient Protection and Affordable Care Act eliminates screening colonoscopy co-payments for those with private insurance or Medicare, individual states can choose whether to offer no-cost colorectal screening for patients with Medicaid (16). State laws that mandate colorectal cancer screening coverage through insurance vary in the amount of coverage required for different screening services (17). State laws cannot require coverage from insurance plans that are self-funded by the employer and the Affordable Care Act does not apply to health plans that were in place before it was passed (17). Thus, cost is likely to continue to be a barrier to colonoscopy use.
Even when cost is not a barrier, colonoscopy screening uptake remains suboptimal (18), underscoring the need for behavioral interventions. Although behavioral interventions vary in their delivery method and target populations, most are based on assumptions that patients should be educated about colorectal cancer screening, motivated to act, and assisted with overcoming barriers (16, 19-21). Most behavioral interventions have modest effects on colonoscopy uptake; however, few trials have examined how well intervention effects hold across population subgroups such as those with cost barriers (21, 22). The Family CARE trial took a pragmatic approach of targeting relatives of colorectal cancer patients to increase colonoscopy through evidenced-based communications strategies which addressed perception, barriers, motivation, and volition (23). Those in the intervention arm were nearly three times as likely to obtain a colonoscopy at 9-months post-intervention compared to those in the comparison group, with no differences based on rural residency or income (24). Given that cost is a known barrier to colonoscopy use, we sought to determine the intervention effect by the 15-month follow-up when cost barriers to colonoscopy were considered. We also examined the impact of providing resource information for overcoming cost barriers to colonoscopy for those who remained non-adherent at 9 months and identified the time frame in which most participants obtained a colonoscopy after the intervention.
MATERIALS AND METHODS
Trial Design and Oversight
We describe the Family CARE trial in detail elsewhere (23-25). Family CARE was a cluster randomized, two-group trial conducted in the United States that tested the efficacy of a remote, tailored intervention, TeleCARE, to increase colonoscopy uptake among relatives of colorectal cancer patients who were considered to be at increased familial risk of colorectal cancer (ClinicalTrials.gov #: NCT01274143). Random assignment began in 2009 and ended two years later. Primary outcome assessments (colonoscopy within 9 months following the intervention) was completed in September 2012 and 15-month colonoscopy uptake assessment was completed in April 2013 (23-25).
The Institutional Review Boards of participating institutions approved the trial. All participants provided informed consent.
Participant Population
Primarily, population-based cancer registries (California, Colorado, Idaho, New Mexico, and Utah) identified colorectal cancer patients or their next of kin who were then contacted to request information about their relatives. Relatives were contacted about study participation. Relatives resided in 35 states.
Eligibility criteria included: 30 to 74 years of age, considered at increased familial CRC risk [having a first-degree relative diagnosed with colorectal cancer before age 60 years or one first-degree relative diagnosed at age 60 years or older plus an additional first or second-degree biologic relative diagnosed at any age (2, 26, 27)] for whom screening guidelines recommend colonoscopy, no colonoscopy within the last 5 years, a known family history of colorectal cancer, not a member of family with known hereditary cancer syndrome (28) or a candidate for germ line mutation testing, no previous counseling about familial cancer risk or participated in a family cancer trial, and no history of in situ or invasive cancer other than non-melanoma skin cancer. We did not enroll individuals under age 30 to limit the possibility of erroneously enrolling members of families with high penetrance hereditary conditions such as familial adenomatous polyposis or Lynch syndrome. We did not enroll individuals over the age of 74 as the guidelines for screening at the time of the study did not apply to people 75 years of age or older.
Randomization
After completing baseline measures, participants were randomized to study arm using a computer-generated allocation algorithm based on randomized block method (four to eight blocks). Family members were assigned to the same study arm to avoid study contamination. Staff collecting baseline assessments were unaware of the identity of a person's participating relatives to prevent them from predicting group assignment.
Intervention
Educational Brochure
Participants assigned to the educational brochure group received a brochure that described colorectal cancer, the role of family history in determining risk, and the ability of colonoscopy to prevent and detect cancer early. It encouraged participants to discuss colorectal cancer and colonoscopy with healthcare providers. The brochure listed colonoscopy as the recommended screening test for their level of familial risk, but encouraged participants to seek some other form of screening such as stool blood testing if colonoscopy was not feasible. The brochure was designed specifically for the target population by the study investigators using investigator expertise and information gleaned from cognitive interviews conducted with colorectal cancer patients and close relatives representing the study's target population. The brochure was further reviewed by the study's community advisory board for acceptability. At the 9-month follow-up, a cost resource letter listing national and state-specific resources for free or reduced-cost colonoscopy was mailed to all participants who had not yet had a colonoscopy and had indicated that cost was a barrier at any of the assessment points. The educational brochure group received this cost resource letter at the 9-month follow-up as the brochure condition did not include communication with a study genetic counselor and therefore did not include a clinical encounter.
TeleCARE (Tele-Cancer Risk Assessment and Evaluation)
TeleCARE is a multi-faceted, remote risk-communication intervention. Participants assigned to the TeleCARE group received the same educational brochure plus mailed visual aids tailored from their baseline assessment of likelihood to engage in colorectal cancer screening (i.e., risk perception of cancer, self-efficacy for obtaining colonoscopy). TeleCARE participants discussed this information over the phone with one of five genetic counselors who were trained in cancer risk assessment, behavior theory, and motivational interviewing techniques (29, 30). Participants were mailed a tailored letter within one week after the telephone call that summarized the session and the participant's action plan to obtain a colonoscopy. Participants’ healthcare providers were mailed copies of the letter and family history of cancer if participants consented to it. Consistent with usual care, if participants indicated that cost was a barrier during the telephone call, counselors addressed cost barriers and the same type of cost resource letter that was sent to comparison group participants at 9 months was included in the post-phone call letter. Approximately 6 weeks after the intervention, participants received a reminder card with their personalized action plan for colonoscopy. If at 9 months participants remained non-adherent and indicated that cost was a barrier at a previous assessment point, a cost resource letter was sent again. As in the educational brochure group, participants were encouraged to seek some other form of screening if colonoscopy was not feasible.
Outcome Specification and Data Collection
Primary outcome (medically-verified colonoscopy) was assessed at the individual level. Colonoscopies were verified through physician or clinic confirmation if participants provided a medical release. Participants who self-reported a colonoscopy but did not provide a release for medical verification were considered non-adherent to screening. Cancer screening, knowledge, cognitions, and screening barriers were assessed at baseline (pre-randomization), 1 month, and 9 months post-intervention. A 15-month follow-up questionnaire was mailed to participants who reported that they had not had a colonoscopy by the 9-month follow-up or if their screening status was unknown.
Statistical Analysis
Differences in demographic variables between study arms were tested with χ2 tests. Generalized mixed logistic regression models were used to account for the cluster (i.e., nuclear family) effect when evaluating the impact of intervention on colonoscopy uptake by the 15-month assessment. Data were analyzed using participants with known outcomes, negative outcome imputation, and multiple imputation. Family was considered a random effect variable. Negative outcome imputation and multiple imputation were used for intent-to-treat analysis and included all eligible participants who were randomly assigned. Negative outcome imputation assumed that colonoscopy did not occur if there was no documented verification of colonoscopy. Multiple imputation was based on data from age at baseline, sex, household income, and health insurance coverage. Five imputed datasets were used to provide a combined estimate for missing values. Odds ratios (OR) with 95% confidence intervals (CI) compared colonoscopy uptake at 15 months.
To estimate the cumulative incidence of medically-verified colonoscopy following the intervention to 15-month follow-up, curves based on Kaplan-Meier estimates were created along with associated 95% Hall-Werner confidence bands. Cumulative incidences were stratified by study arm. For outcome analysis involving cost as a barrier, stratified logistic regression estimated the intervention effect within those who indicated that cost was a barrier and those who indicated that cost was not a barrier at baseline. Those with missing data on cost as a barrier or colonoscopy use were excluded for this analysis. Family was not included as a random effects variable as clustering could no longer apply when stratifying based on cost as a barrier. The effect of receiving a resource letter describing low-cost options for colonoscopies was examined within the subgroup of people who had not yet received a colonoscopy by 9 months post-intervention. The model controlled for the intervention effect and a potential interaction between intervention and resource letter. Two biostatisticians (K.M.B. and L.M.P.) conducted the analyses using SAS (version 9.3; SAS Institute, Cary, NC).
Sample size and power calculations were based on the trial's primary outcome analysis at 9-month follow-up (24).
RESULTS
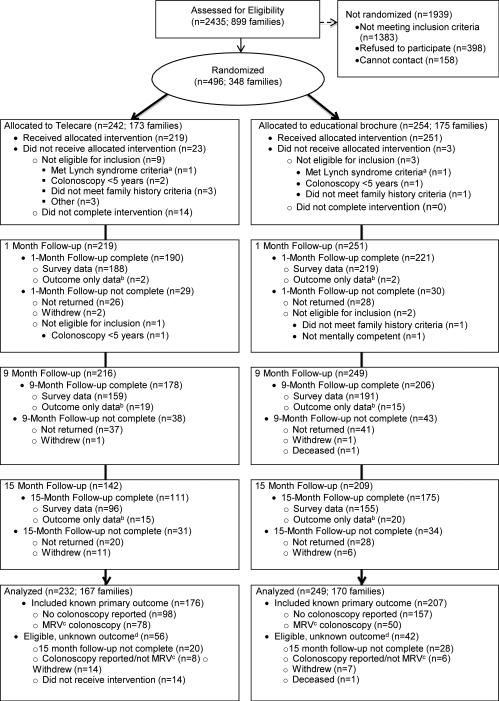
Study enrollment, randomization, and retention data are shown in Figure 1. The TeleCARE group had 167 eligible clusters and the educational brochure group had 170 clusters. The number of family members enrolled per cluster did not differ significantly by treatment arm (24). Demographic characteristics did not differ between the study groups (see Table 1) or when further stratified by participant reported cost barriers to colonoscopy. The participants who received the 15 month assessment did not differ in demographics specified in Table 1 from those who received the 9 month assessment. Twenty-one participants (TeleCARE = 12; Educational Brochure = 9) reported another type of colorectal cancer screening.
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) flowchart.
a Lynch Syndrome Criteria based on work by Tan et al.(28)
b Participants who did not complete a survey were asked to complete a very brief survey containing primary outcome questions only.
c Medical record verified
d Included in imputation analyses.
TeleCARE, Tele-cancer Assessment and Risk Evaluation
Table 1.
Baseline Characteristics by Intervention Group for Intent-to-Treat Analysis
| Characteristic | TeleCARE (n = 232) | Brochure (n = 249) |
|---|---|---|
| Age—mean years (standard deviation) | 49.9 (9.0) | 50.8 (9.0) |
| Sex—no. (%) | ||
| Female | 91 (39.2) | 114 (45.8) |
| Male | 141 (60.8) | 135 (54.2) |
| Race/Ethnicity—no. (%) | ||
| Non-Latino white | 215 (92.2) | 239 (96.0) |
| Other/unreported | 17 (7.8) | 10 (4.0) |
| Marital Status—no. (%) | ||
| Currently married or living as married | 172 (74.1) | 191 (76.7) |
| Not currently married or living as married | 60 (25.9) | 58 (23.3) |
| Educational Level—no. (%) | ||
| High school or less | 51 (22.0) | 42 (16.9) |
| Post-high school | 100 (43.1) | 106 (42.6) |
| Bachelor's | 50 (21.6) | 64 (25.7) |
| Postgraduate | 31 (13.4) | 37 (14.9) |
| Residencea—no. (%) | ||
| Urban | 173 (74.6) | 199 (79.9) |
| Rural | 59 (25.4) | 50 (20.1) |
| Yearly Income ($) —no. (%) | ||
| <$30,000 | 46 (19.8) | 42 (16.8) |
| $30-49,999 | 42 (18.1) | 49 (19.7) |
| ≥$50,000 | 119 (51.3) | 134 (53.9) |
| Missing, refused | 25 (10.8) | 24 (9.6) |
| Employment Status—no. (%) | ||
| Employed | 166 (71.6) | 172 (69.1) |
| Not Employed | 66 (28.5) | 77 (30.9) |
| Health Insurance—no. (%) | ||
| Private | 164 (70.7) | 175 (70.3) |
| Public | 31 (13.4) | 23 (9.2) |
| No coverage | 37 (16.0) | 50 (20.1) |
| Missing | 0 (0.0) | 1 (0.4) |
| Relatives with colorectal cancer—no. (%) | ||
| 1 FDR, 0 SDR | 188 (81.0) | 202 (81.1) |
| ≥2 FDR, 0 SDR | 13 (5.6) | 18 (7.2) |
| 1 FDR, 1 SDR | 30 (12.9) | 22 (8.8) |
| 1 FDR, ≥2 SDR | 0 (0.0) | 6 (2.4) |
| ≥2 FDR, 1 SDR | 1 (0.4) | 1 (0.4) |
| 1 FDR, 0 SDR | 188 (81.0) | 202 (81.1) |
| ≥2 FDR, 0 SDR | 13 (5.6) | 18 (7.2) |
Abbreviations: FDR, first degree relative, SDR, second degree relative, ERS, Economic Research Service; RUCA; rural-urban computing area; Tele-CARE, Tele-Cancer Risk Assessment and Evaluation; USDA, US Department of Agriculture; WWAMI, Washington, Wyoming, Alaska, Montana, and Idaho.
Rural/urban residence was based on RUCA codes at the zip code level. RUCA codes were developed by the University of Washington Rural Health Research Center and the USDA ERS, with the support of the Federal Health Resource and Service Administration's Office of Rural Health Policy and the ERS using standard Census Bureau urbanized area and urban cluster definitions in combination with work commuting data to characterize census tracts and later zip codes.(47) The 10 RUCA categories were aggregated into urban (1-3) and rural (4-10), per the WWAMI Rural Health Research Center.
Colonoscopy Uptake at the 15-Month Follow-up
Overall, 42.7% of those in the TeleCARE group obtained a colonoscopy by 15-months post-intervention compared to 24.1% of those in the educational brochure group (OR = 2.37, 95% CI = 1.59 to 3.52; Table 2). The intervention effect was similar across known outcome and imputed models.
Table 2.
Results for Intervention Effect on Colonoscopy Uptake within 15 months
| Odds of getting Colonoscopy | 95% Confidence Interval | P Value | Medically Verified Colonoscopies | ||
|---|---|---|---|---|---|
| Modela | TeleCARE vs. Education | [Lower, Upper] | TeleCARE % (n) | Education Brochure % (n) | |
| Cases with known outcome | 2.50 | [1.61-3.88] | <0.001 | 44.3% (78/176) | 24.2% (50/207) |
| Negative outcome imputationb | 2.02 | [1.32-3.08] | 0.001 | 33.6% (78/232) | 20.1% (50/249) |
| Multiple imputationc | 2.37 | [1.59-3.52] | <0.001 | 42.7% (99/232) | 24.1% (60/249) |
Each of the 3 separate models represents a different treatment of missing outcomes and included a random effect for family.
Negative outcome imputation treated unknown colonoscopy outcome as no colonoscopy.
Average number of colonoscopies based on 5 imputation sets from the SAS procedure MI
Timing of Intervention Effect
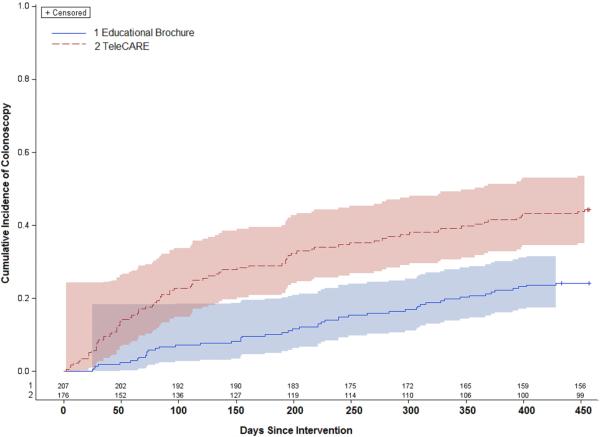
As shown in the cumulative incidence curves (Figure 2), the TeleCARE group had higher cumulative incidence of colonoscopy throughout the 15-month follow-up period. The divergence of cumulative incidence slopes between the TeleCARE and educational brochure group appears primarily within the first nine months of the intervention (i.e., within 276 days after the intervention), suggesting that the intervention effect occurred primarily within those nine months. Thirty-six percent of the TeleCARE participants had a colonoscopy by 9 months and 44.3% by 15 months. Within the educational-brochure group, 16% had a colonoscopy by 9 months and 24.2% by 15 months.
Figure 2.
Cumulative incidence based on Kaplan-Meier estimates of colonoscopy uptake after intervention with number of participants at risk and 95% Hall-Wellner confidence bands.
Impact of Cost as a Barrier and Financial Resource Letter
At 15 months, TeleCARE participants were more likely than those in the educational brochure group to obtain a colonoscopy even when cost had been indicated as a barrier at baseline. Among those who did not identify cost as a barrier [TeleCARE = 66 (37.5%), educational brochure = 89 (43.0%)], 51.5% of the TeleCARE group had a colonoscopy compared to 24.7% of the educational brochure group (OR = 3.66; 95% CI = 1.85 to 7.24; Table 3). Of the 228 people who identified cost as barrier [TeleCARE = 110 (62.5%), educational brochure = 118 (57.0%)], 38.2% of those in the TeleCARE group had a colonoscopy compared to 23.7% of those in the educational brochure group (OR = 1.99; 95% CI = 1.12 to 3.52).
Table 3.
Stratified Analysis of 15-month Intervention Effect Based on Cost as a Barrier
| Odds of getting Colonoscopy | 95% Confidence Interval | P Value | Medically Verified Colonoscopies | ||
|---|---|---|---|---|---|
| Modela | TeleCARE vs. Education | [Lower, Upper] | TeleCARE % (n) | Education Brochure % (n) | |
| Cost not identified as a barrier | 3.66 | [1.85-7.24] | 0.0002 | 51.5% (34/66) | 24.7% (22/89) |
| Cost identified as a barrier | 1.99 | [1.12-3.52] | 0.0189 | 38.2% (42/110) | 23.7% (28/118) |
Each of the 2 separate models represents the subgroup analysis for those who did and did not identify cost as a barrier at baseline. Only those with known barrier information and known outcomes were included.
Two-hundred eighty-seven participants (113 in TeleCARE group, 174 in educational brochure group) reported no colonoscopy by 9 months post-intervention. Colonoscopy uptake by 15 months was not increased by the addition of the cost-resource letter (OR = .80, 95% CI = .52 to 1.23), with no evidence of the effect of the letter differing based on group assignment (p = .50).
DISCUSSION
TeleCARE, a remote, tailored intervention targeted to people at increased familial risk for colorectal cancer, was effective in increasing colonoscopy screening even among those who reported cost barriers. However, the intervention effect was considerably stronger among those who did not report cost as a barrier, a particularly relevant finding in the context of the Affordable Care Act.
It remains to be seen what the full impact of the Affordable Care Act will be on risk-based colorectal cancer screening. Evidence to date suggests that eliminating co-payments has a modest effect on colorectal cancer screening (18, 31, 32). In addition to reducing colorectal cancer incidence and mortality, improving colonoscopy rates among those at increased risk may demonstrate to insurers the value of providing access to cancer screening and prevention services, including remote behavioral interventions. Insurer decisions to uniformly waive colonoscopy co-payments or extend waivers to situations in which a colonoscopy becomes diagnostic (i.e., removal of polyps) will likely be influenced by whether colonoscopies provide cost savings (33). If colonoscopy uptake among those with increased colorectal cancer risk is improved, it may become financially appealing to insurers to waive co-payments for colonoscopy because of the potential to avoid the costs of expensive cancer treatments (18, 33). Interventions such as TeleCARE are one way to promote colonoscopy use among people with higher risk.
The increase in screening rates between 9 months and 15 months was not attributed to the cost-resource letter, which raises questions as to other contributing factors. It is possible that some people needed the additional time to plan and overcome individual barriers to screening such as scheduling and making arrangements for work, childcare, or transportation. It is also possible that participants had a health maintenance visit with their provider which prompted a discussion about and completion of colonoscopy.
We are unable to identify which components of TeleCARE influenced the decision to undergo colonoscopy. A stepped-care approach should be evaluated to determine if the intervention can be streamlined and remain effective (e.g., waiting to send reminders until a person has not obtained screening by 3 months or providing telephone counseling to those who do not have a colonoscopy within 6 months of the intervention). It may be possible to increase the potency of TeleCARE by incorporating patient navigation for those who need it. Navigation services are likely to become more widely available due to the Centers for Medicare and Medicaid Services’ 2014 rule offering state Medicaid agencies the option to reimburse for more community-based preventive services, including those of community health workers (34). Other third party payers are expected to adopt this reimbursement policy. Thus there is potential for community health workers to provide health promotion services, including navigation, to help patients overcome cost and other barriers to cancer prevention (35).
Limitations
Our study was not designed to determine the effect of individual components of TeleCARE's intervention (i.e., telephone call, reminder card). Reminder cards may have some benefit (36), although their stand-alone effectiveness is unknown for people who are ambivalent about having a colonoscopy. TeleCARE is multi-faceted: all participants assigned to TeleCARE received more intervention-related contact than those in the educational brochure (three vs. one, respectively). Therefore, we cannot tease apart the effect of attention (i.e., dose) versus that of the intervention content and delivery (37).
The majority of our study population was non-Latino White, which precluded evaluation of TeleCARE's efficacy among ethnically diverse subgroups. Although motivational interviewing accommodates a wide variety of cultural beliefs, the intervention did not explicitly address cultural beliefs that may conflict with having colonoscopy (38, 39). People with additional sociocultural barriers (e.g. competing life concerns or cultural beliefs such as fatalism) may require patient-navigation interventions beyond the scope of TeleCARE (14, 38, 40). Our study was also not powered to examine differences in uptake by state, which is important as states and communities vary in their capacity for colonoscopy (10, 41).
Finally, it is important to note that although our intervention increased adherence to guideline-concordant colorectal cancer screening from 0% to 42.7%--which is higher than other intervention trials among individuals at increased familial risk (42-44)—the national goal for screening adherence in the general population is 70-80% (45, 46). Given the increased risk in relatives of colorectal cancer patients, screening adherence needs to be higher than what our intervention achieved. As highlighted previously, adherence to colonoscopy is a multi-faceted problem. The efficacy of TeleCARE may be improved by complementing it with an intervention that intervenes on system-level barriers such as provider communication about screening, increasing access to screening patient navigation, direct assistance with coverage for screening, and patient follow up in primary care (12, 16).
Conclusion
Our results suggest that TeleCARE increases colonoscopy screening among relatives of colorectal cancer patients, especially, but not exclusively, when cost is not a barrier. Interventions such as TeleCARE may help maximize the impact of programs and policies that increase access to preventive services by bolstering cancer screening.
Acknowledgments
Authors would like to thank the study's genetic counselors, Kory Jasperson, Amanda Gammon, Anne Naumer, and Lisa Wadge, for providing the intervention.
Funding Sources: Supported by grants from the National Cancer Institute (1R01CA125194-0305; A.Y. Kinney, PI) and the Huntsman Cancer Foundation. The project was also supported by the Shared Resources (P30 CA042014) at Huntsman Cancer Institute (Biostatistics and Research Design, Genetic Counseling, Research Informatics, the Tissue Resource and Applications Core [TRAC], and the Utah Population Database [UPDB]); the Utah Cancer Registry, which is funded by Contract No. HHSN261201000026C from the National Cancer Institute's Surveillance, Epidemiology and End Results (SEER) Program with additional support from the Utah State Department of Health and the University of Utah; the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885, the National Cancer Institute's SEER Program under contract N01PC-2010-00034C awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N01-PC-54404 awarded to the Public Health Institute, and the Centers for Disease Control and Prevention's National Program of Cancer Registries, under agreement U58CCU000807-05 awarded to the Public Health Institute; the Colorado Central Cancer Registry program in the Colorado Department of Public Health and Environment funded by the National Program of Cancer Registries of the Centers for Disease Control and Prevention; the Cancer Data Registry of Idaho supported in part by the National Program of Cancer Registries of the Centers for Disease Control and Prevention; the New Mexico Tumor Registry which is funded by National Cancer Institute Contract No. HHSN261201300010I; the Rocky Mountain Cancer Genetics Network (HHSN261200744000C); the Huntsman Cancer Registry; and the Intermountain Healthcare Oncology Clinical Program and Intermountain Clinical Genetics Institute. This content is solely the responsibility of the authors and does not necessarily reflect the opinions or views of the funding and supporting agencies.
Footnotes
Disclosures: Dr. Burt discloses consultation for Myriad Genetics. No other disclosures were made by authors.
ClinicalTrials.gov identifier #: NCT01274143
References
- 1.Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–73. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levin B, Lieberman DA, McFarland B, Smith RA, Brooks D, Andrews KS, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: A joint guideline from the American Cancer Society, the US Multi-society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58:130–60. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 3.Zauber AG, Winawer SJ, O'Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687–96. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuchs CS, Giovannucci EL, Colditz GA, Hunter DJ, Speizer FE, Willett WC. A prospective study of family history and the risk of colorectal cancer. N Engl J Med. 1994;331:1669–74. doi: 10.1056/NEJM199412223312501. [DOI] [PubMed] [Google Scholar]
- 5.Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627–37. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 6.Jandorf L, Braschi C, Ernstoff E, Wong CR, Thelemaque L, Winkel G, et al. Culturally targeted patient navigation for increasing African Americans' adherence to screening colonoscopy: A randomized clinical trial. Cancer Epidemiol Biomarkers Prev. 2013;22:1577–87. doi: 10.1158/1055-9965.EPI-12-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor DP, Cannon-Albright LA, Sweeney C, Williams MS, Haug PJ, Mitchell JA, et al. Comparison of compliance for colorectal cancer screening and surveillance by colonoscopy based on risk. Genet Med. 2011;13:737–43. doi: 10.1097/GIM.0b013e3182180c71. [DOI] [PubMed] [Google Scholar]
- 8.Lin O, Gluck M, Nguyen M, Koch J, Kozarek R. Screening patterns in patients with a family history of colorectal cancer often do not adhere to national guidelines. Dig Dis Sci. 2013;58:1841–8. doi: 10.1007/s10620-013-2567-3. [DOI] [PubMed] [Google Scholar]
- 9.Ward EM, Fedewa SA, Cokkinides V, Virgo K. The association of insurance and stage at diagnosis among patients aged 55 to 74 years in the national cancer database. The Cancer Journal. 2010;16:614–21. doi: 10.1097/PPO.0b013e3181ff2aec. 10.1097/PPO.0b013e3181ff2aec. [DOI] [PubMed] [Google Scholar]
- 10.Steinwachs D, Allen JD, Barlow WE, Duncan RP, Egede LE, Friedman LS, et al. National Institutes of Health state-of-the-science conference statement: Enhancing use and quality of colorectal cancer screening. Ann Intern Med. 2010;152:663–7. doi: 10.7326/0003-4819-152-10-201005180-00237. [DOI] [PubMed] [Google Scholar]
- 11.Baron RC, Rimer BK, Coates RJ, Kerner J, Kalra GP, Melillo S, et al. Client-directed interventions to increase community access to breast, cervical, and colorectal cancer screening: A systematic review. Am J Prev Med. 2008;35:S56–S66. doi: 10.1016/j.amepre.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Klabunde CN, Lanier D, Breslau ES, Zapka JG, Fletcher RH, Ransohoff DF, et al. Improving colorectal cancer screening in primary care practice: Innovative strategies and future directions. J Gen Intern Med. 2007;22:1195–205. doi: 10.1007/s11606-007-0231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Virgo KS, Burkhardt EA, Cokkinides VE, Ward EM. Impact of health care reform legislation on uninsured and medicaid-insured cancer patients. The Cancer Journal. 2010;16:577–83. doi: 10.1097/PPO.0b013e31820189cb. 10.1097/PPO.0b013e31820189cb. [DOI] [PubMed] [Google Scholar]
- 14.Jones RM, Woolf SH, Cunningham TD, Johnson RE, Krist AH, Rothemich SF, et al. The relative importance of patient-reported barriers to colorectal cancer screening. Am J Prev Med. 2010;38:499–507. doi: 10.1016/j.amepre.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joseph DA, King JB, Miller JW, Richardson LC. Centers for Disease Control, Prevention. Prevalence of colorectal cancer screening among adults--Behavioral Risk Factor Surveillance System, United States, 2010. MMWR. 2012;61(Suppl):51–6. [PubMed] [Google Scholar]
- 16.Gupta S, Sussman DA, Doubeni CA, Anderson DS, Day L, Deshpande AR, et al. Challenges and possible solutions to colorectal cancer screening for the underserved. J Natl Cancer Inst. 2014:106. doi: 10.1093/jnci/dju032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Society AC. [2014 March 20th];Colorectal cancer prevention and early detection. Available from: http://www.cancer.org/cancer/colonandrectumcancer/moreinformation/colonandrectumcancerearlydetection/colorectal-cancer-early-detection-screening-coverage-laws.
- 18.Khatami S, Xuan L, Roman R, Zhang S, McConnel C, Halm EA, et al. Modestly increased use of colonoscopy when copayments are waived. Clin Gastroenterol Hepatol. 2012;10:761–6. e1. doi: 10.1016/j.cgh.2012.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lasser KE, Murillo J, Lisboa S, et al. Colorectal cancer screening among ethnically diverse, low-income patients: A randomized controlled trial. Ann Intern Med. 2011;171:906–12. doi: 10.1001/archinternmed.2011.201. [DOI] [PubMed] [Google Scholar]
- 20.Percac-Lima S, Grant R, Green A, Ashburner J, Gamba G, Oo S, et al. A culturally tailored navigator program for colorectal cancer screening in a community health center: A randomized, controlled trial. J Gen Intern Med. 2009;24:211–7. doi: 10.1007/s11606-008-0864-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rawl SM, Menon U, Burness A, Breslau ES. Interventions to promote colorectal cancer screening: An integrative review. Nurs Outlook. 2012;60:172–81. e13. doi: 10.1016/j.outlook.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lowery JT, Horick N, Kinney AY, Finkelstein DM, Garrett K, Haile RW, et al. A randomized trial to increase colonoscopy screening in members of high-risk families in the colorectal cancer family registry and cancer genetics network. Cancer Epidemiol Biomarkers Prev. 2014;23:601–10. doi: 10.1158/1055-9965.EPI-13-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pengchit W, Walters ST, Simmons RG, Kohlmann W, Burt RW, Schwartz MD, et al. Motivation-based intervention to promote colonoscopy screening: An integration of a fear management model and motivational interviewing. J Health Psychol. 2011;16:1187–97. doi: 10.1177/1359105311402408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinney AY, Boonyasiriwat W, Walters ST, Pappas LM, Stroup AM, Schwartz MD, et al. Telehealth personalized cancer risk communication to motivate colonoscopy in relatives of patients with colorectal cancer: The family care randomized controlled trial. J Clin Oncol. 2014;32:654–62. doi: 10.1200/JCO.2013.51.6765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simmons RG, Lee YC, Stroup AM, Edwards SL, Rogers A, Johnson C, et al. Examining the challenges of family recruitment to behavioral intervention trials: Factors associated with participation and enrollment in a multi-state colonoscopy intervention trial. Trials. 2013;14:116. doi: 10.1186/1745-6215-14-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armelao F, de Pretis G. Familial colorectal cancer: A review. WJG. 2014;20:9292–8. doi: 10.3748/wjg.v20.i28.9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Samadder NJ, Jasperson K, Burt RW. Hereditary and common familial colorectal cancer: Evidence for colorectal screening. Dig Dis Sci. 2015;60:734–47. doi: 10.1007/s10620-014-3465-z. [DOI] [PubMed] [Google Scholar]
- 28.Tan YY, McGaughran J, Ferguson K, Walsh MD, Buchanan DD, Young JP, et al. Improving identification of lynch syndrome patients: A comparison of research data with clinical records. Int J Cancer. 2013;132:2876–83. doi: 10.1002/ijc.27978. [DOI] [PubMed] [Google Scholar]
- 29.Miller W, Rollnick S. Motivational interviewing: Preparing people for change. Guilford Press; New York: 2002. [Google Scholar]
- 30.Rollnick S, Miller WR, Butler C. Motivational interviewing in health care: Helping patients change behavior: Guilford Press. 2008 [Google Scholar]
- 31.Cokkinides V, Bandi P, Shah M, Virgo K, Ward E. The association between state mandates of colorectal cancer screening coverage and colorectal cancer screening utilization among us adults aged 50 to 64 years with health insurance. BMC Health Serv Res. 2011;11:19. doi: 10.1186/1472-6963-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okoro C, Dhingra S, Coates R, Zack M, Simoes E. Effects of Massachusetts health reform on the use of clinical preventive services. J Gen Intern Med. 2014 doi: 10.1007/s11606-014-2865-2. 10.1007/s11606-014-2865-2:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lansdorp-Vogelaar I, van Ballegooijen M, Zauber AG, Habbema JDF, Kuipers EJ. Effect of rising chemotherapy costs on the cost savings of colorectal cancer screening. J Natl Cancer Inst. 2009;101:1412–22. doi: 10.1093/jnci/djp319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Medicaid and children's health insurance programs: Essential health benefits in alternative benefit plans, eligibility notices, fair hearing and appeal processes, and premiums and cost sharing; exchanges: Eligibility and enrollment. 2013 Jul 15; 78 fed. Reg. 42,160, 42,226 to be codified at 42 c.F.R. Pt. 440.130. [PubMed] [Google Scholar]
- 35.Jandorf L, Braschi C, Ernstoff E, Wong CR, Thelemaque L, Winkel G, et al. Culturally targeted patient navigation for increasing African Americans' adherence to screening colonoscopy: A randomized clinical trial. Cancer Epidemiol Biomarkers Prev. 2013;22:1577–87. doi: 10.1158/1055-9965.EPI-12-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milkman KL, Beshears J, Choi JJ, Laibson D, Madrian BC. Planning prompts as a means of increasing preventive screening rates. Prev Med. 2013;56:92–3. doi: 10.1016/j.ypmed.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 37.Mohr DC, Spring B, Freedland KE, Beckner V, Arean P, Hollon SD, et al. The selection and design of control conditions for randomized controlled trials of psychological interventions. Psychother Psychosom. 2009;78:275–84. doi: 10.1159/000228248. [DOI] [PubMed] [Google Scholar]
- 38.Jun J, Oh KM. Asian and Hispanic Americans' cancer fatalism and colon cancer screening. Am J Health Behav. 2013;37:145–54. doi: 10.5993/AJHB.37.2.1. [DOI] [PubMed] [Google Scholar]
- 39.Getrich CM, Sussman AL, Helitzer DL, Hoffman RM, Warner TD, Sánchez V, et al. Expressions of machismo in colorectal cancer screening among New Mexico Hispanic subpopulations. Qual Health Res. 2012;22:546–59. doi: 10.1177/1049732311424509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Percac-Lima S, Lopez L, Ashburner JM, Green AR, Atlas SJ. The longitudinal impact of patient navigation on equity in colorectal cancer screening in a large primary care network. Cancer. 2014;120:2025–31. doi: 10.1002/cncr.28682. [DOI] [PubMed] [Google Scholar]
- 41.Jemal A, Siegel RL, Ma J, Islami F, DeSantis C, Goding Sauer A, et al. Inequalities in premature death from colorectal cancer by state. J Clin Oncol. 2015;33:829–35. doi: 10.1200/JCO.2014.58.7519. [DOI] [PubMed] [Google Scholar]
- 42.Manne SL, Coups EJ, Markowitz A, Meropol NJ, Haller D, Jacobsen PB, et al. A randomized trial of generic versus tailored interventions to increase colorectal cancer screening among intermediate risk siblings. Ann Behav Med. 2009;37:207–17. doi: 10.1007/s12160-009-9103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rawl SM, Champion VL, Scott LL, Zhou H, Monahan P, Ding Y, et al. A randomized trial of two print interventions to increase colon cancer screening among first-degree relatives. Patient Educ Couns. 2008;71:215–27. doi: 10.1016/j.pec.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glanz K, Steffen AD, Taglialatela LA. Effects of colon cancer risk counseling for first-degree relatives. Cancer Epidemiol Biomarkers Prev. 2007;16:1485–91. doi: 10.1158/1055-9965.EPI-06-0914. [DOI] [PubMed] [Google Scholar]
- 45.Paskett ED, Khuri FR. Can we achieve an 80% screening rate for colorectal cancer by 2018 in the United States? Cancer. 2015 doi: 10.1002/cncr.29335. 10.1002/cncr.29335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meester RGS, Doubeni CA, Zauber AG, Goede SL, Levin TR, Corley DA, et al. Public health impact of achieving 80% colorectal cancer screening rates in the United States by 2018. Cancer. 2015 doi: 10.1002/cncr.29336. 10.1002/cncr.29336:n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morrill R, Cromartie J, Hart G. Metropolitan, urban, and rural commuting areas: Toward a better depiction of the United States settlement system. Urban Geography. 1999;20:727–48. [Google Scholar]