Summary
Assays in which the detection of a biological phenomenon is coupled to the production of bioluminescence by luciferase have gained widespread use. As firefly luciferases (FLuc) and kinases share a common substrate (ATP), coupling of a kinase to FLuc allows for the amount of ATP remaining following a kinase reaction to be assessed by quantitating the amount of luminescence produced. Alternatively, the amount of ADP produced by the kinase reaction can be coupled to FLuc through a two-step process. This article describes the bioluminescent assays that were developed for three classes of kinases (lipid, protein and metabolic kinases) and miniaturized to 1536-well format, enabling their use for quantitative high-throughput (qHTS) of small molecule libraries.
Keywords: Quantitative high-throughput screening (qHTS), Yes1, Glucokinase, PI5P4Kα, kinase, bioluminescence, luciferase, ADP-Glo
1. Introduction
Kinases are a diverse class of enzymes that phosphorylate a variety of substrates, including proteins, lipids and metabolites. Dysregulation of phosphorylation leads to diseases such as cancer, inflammation and diabetes (1). Indeed, a variety of drugs that target kinases have been FDA approved but as yet there are many kinases that show promise for the development of a targeted therapy but for which there is no FDA-approved drug (1). Therefore, there is widespread interest in developing inhibitors or activators of kinases as a treatment for disease. Methods to screen kinases in high-throughput formats are important to be able to find leads from chemical libraries and potentially aid the development of kinase-directed therapeutics. For a successful high-throughput screen, both the selected assay methodology and the library design are important. The majority of kinases utilize ATP as the phosphate donor to the substrate and have ADP and the phosphorylated substrate as a product. An important exception is pyruvate kinase which transfers a phosphate from phosphoenolpyruvate (PEP) to ADP yielding ATP as a product (2). Some of the main ways that compounds are assessed for their impact on kinases include quantitating the phosphorylated product with specific antibodies such as HTRF KinEase (Cisbio) (3), competition binding using technologies such as Kinomescan (DiscoveRx) (4), FP assays such as Transcreener ADP Assay (Bellbrooks) (5), and γ-ATP transfer of radiolabel to product (6). More recently, kinase assays have been assessed by coupling the kinase reaction to that of FLuc, which utilizes ATP to generate bioluminescence through the oxidation of luciferin (7).
Luciferase-coupled kinase reactions are homogeneous mix-and-read assays that produce a stable luminescent glow with good signal to background, suitable for high-throughput screening (HTS) applications. A direct coupling of an ATP-dependent kinase to FLuc would involve running the kinase reaction to ~50-80% completion followed by FLuc addition to quantify the remaining substrate ATP in endpoint mode using a reagent such as Kinase-Glo™ (Promega) (8) or EasyLite™ (Perkin Elmer). This high degree of substrate conversion is needed to have sufficient signal to background (S/B) for the development of a robust assay. Ideally, one would like to monitor product formation so that a suitable S/B can be achieved at a much lower level of conversion; generally 5-20% conversion is sufficient, thereby leading to a more sensitive assay design. However, the theoretical shift in IC50 values for an enzyme assay performed at a high percent substrate conversion (e.g. ~80% conversion) is approximately 2-fold, which can be acceptable for screening applications (9). Ideally, initial rate conditions are established in which the enzyme assay has low % conversion to determine accurate enzyme parameters (10). ADP-Glo™ (Promega) (11,12) involves a two-step process for ADP quantification, initially depleting the residual substrate ATP through the action of a soluble adenylyl cyclase in a first step, followed by cyclase inhibition and conversion of the ADP from the kinase reaction into ATP by a nucleotide kinase such as pyruvate kinase with the signal generated once again using a “glow-type” of FLuc (Ultra-Glo™ Recombinant Luciferase; see 15) to quantitate ATP (See Figure 1) (13). For a given kinase reaction, the luminescence signal of the Kinase-Glo™ coupled assay increases as the kinase reaction is inhibited, leaving more substrate ATP while for ADP-Glo™ coupled assay the reverse trend occurs (see Figure 2) (7). As there are compounds that inhibit FLuc (14,15), it is important to run counterassays to assess detection interference following the identification of hits from a luciferase- coupled primary screen (16). For example, GSK has released a Protein Kinase Inhibitor Set (PKIS) developed from published kinases inhibitors that has been profiled against fire fly and renilla reniformas luciferase to aid in the interpretation of assay results derived from the use of this library (17). The availability of libraries targeted toward the kinome developed from so-called ‘privileged structures’ (see 7) can greatly facilitate the identification of chemical scaffolds from which lead optimization can progress.
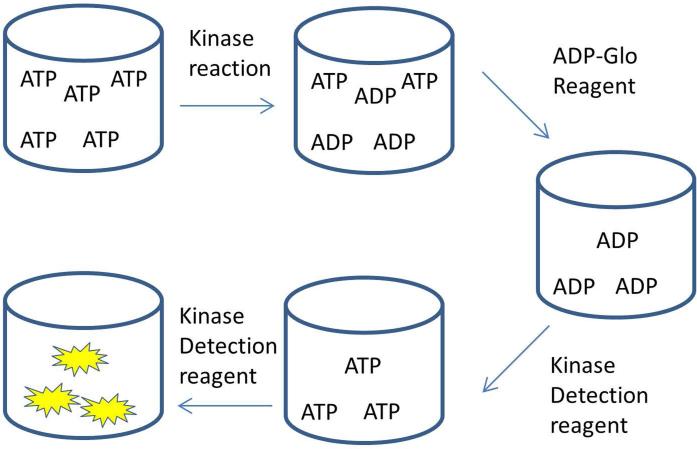
Figure 1. Schematic of the ADP-Glo™ kit detection process.
Following the kinase reaction the remaining ATP is removed by the ADP-Glo™ reagent and then the Kinase Detection reagent generates ATP from the kinase reaction ADP and uses this ATP to generate luminescence.
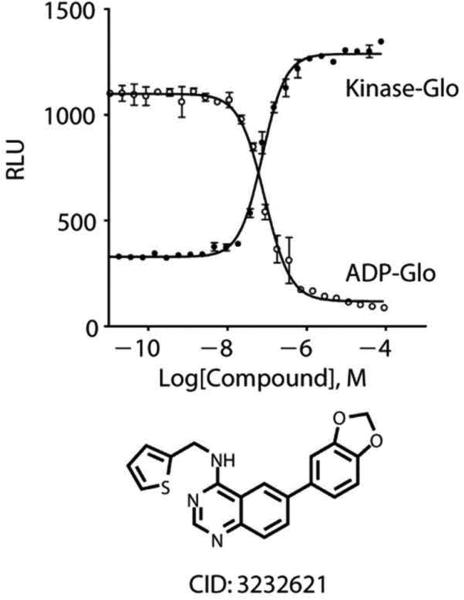
Figure 2. Measurement of ATP or ADP levels following a kinase reaction.
The Raw light Units (RLUs) are plotted for an inhibitor following a CLK4 assay using the Kinase-Glo™ assay (solid circles) and the ADP-Glo™ assay (open circles) detection techniques (7).
The ADP-Glo™ technology was applied to three classes of kinases here indicating the broad utility of this assay methodology: a lipid kinase PI5P4Kα (18), a tyrosine kinase Yes1 (19), and a metabolic kinase Glucokinase (20). The primary goal was to identify inhibitors of PI5P4Kα and Yes1 for the potential use as anticancer agents and activators of glucokinase for the potential treatment of diabetes. Each of these assays was miniaturized to the 1536-well format and used to screen small molecule compound libraries in dose response, a method termed quantitative high-throughput screening (qHTS) (21).
2. Materials
Prepare and store solutions at room-temperature unless otherwise noted. Prepare all solutions using ultrapure water.
2.1 Yes1 tyrosine kinase
Yes1 buffer: 6 nM Yes1 (Millipore), 50 mM Tris pH 7.5, 1 mM DTT, 150 mM NaCl, 0.01% Brij-35, 5% glycerol. Mix gently and store at room-temperature. For continuous dispensing of multiple plates store Yes1 buffer at 4 °C. (see Note 1)
Assay buffer: 50 mM Tris pH 7.5, 1 mM DTT, 150 mM NaCl, 0.01% Brij-35, 5% glycerol. Mix gently and store at room-temperature.
Substrate Poly(E4Y) (Sigma) was dissolved in 50 mM Tris pH 7.5, 150 mM NaCl, 5% glycerol at 10 mg/mL, aliquoted and stored at −80 °C.
Yes1 Substrate buffer: 0.3 mM ATP, 0.3 mM EGTA, 30 mM MgCl2, 0.9 mg/mL poly(E4Y), 50 mM Tris pH 7.5, 1 mM DTT, 150 mM NaCl, 0.01% Brij-35, 5% glycerol. Mix gently and store at room temperature. The Ultrapure ATP from the ADP-Glo™ kit was used. (see Notes 2-3)
ADP-Glo™ Reagent: Thaw at room temperature per manufacturer's protocol.
Kinase Detection Reagent: Thaw at room temperature per manufacturer's protocol. Add kinase detection buffer to powdered kinase detection substrate and swirl gently until fully dissolved.
2.2 Glucokinase (GCK)
Glucokinase buffer: 22.5 nM Glucokinase, 22.5 nM Glucokinase regulatory protein, 3 mM MgCl2, 37.5 mM KCl, 37.5 mM Hepes, 1.5 mM DTT, 0.0375% BSA, 0.015% Tween-20, pH adjusted to 7.1. Proteins were expressed and purified as described in (20). (see Note 1)
No Glucokinase buffer: 22.5 nM Glucokinase regulatory protein, 3 mM MgCl2, 37.5 mM KCl, 37.5 mM Hepes, 1.5 mM DTT, 0.0375% BSA, 0.015% Tween-20, pH adjusted to 7.1.
GCK Substrate Buffer: 1.2 mM ATP and 15 mM Glucose in water. The Ultrapure ATP from the ADP-Glo™ kit was used. (see Notes 2-3)
ADP-Glo™ Reagent: Thaw at room temperature per manufacturer's protocol.
Kinase Detection Reagent: Thaw at room temperature per manufacturer's protocol. Add kinase detection buffer to powdered kinase detection substrate and swirl gently until fully dissolved.
2.3 Lipid Preparation
DPPS (1,2-dipalmitoyl-sn-glycero-3-phosphoserine) (Echelon Biosciences) was suspended in DMSO (333 μL DMSO per 1 mg DPPS), sonicated for 1 minute and mixed by vortexing for 30 seconds, forming a solution. (see Note 4)
PI5P (D-myo-phosphatidylinositol 5 phosphate diC16) from Echelon Biosciences was suspended in DMSO and mixed by vortexing for several minutes (333 μL DMSO per 1 mg PI5P).
Lipid Mix: DPPS:PI5P (2:1 ratio) made by mixing 1000 μL of DPPS to 500 μL of PI5P.
1500 μL of DMSO was added and the resulting lipid mixture was alternately sonicated and vortexed for several minutes. The result is a suspension with no visible particulate matter. (see Notes 5-8)
2.4 PI5P4Kα lipid kinase
PI5P4Kα/PI5P buffer: 15 nM PI5P4Kα, 112.5 μM PI5P (Echelon Biosciences), 225 μM DPPS (Echelon Biosciences), 40 mM Hepes pH 7.4, 0.25 mM EGTA, 0.1% CHAPS. Protein was expressed and purified as described in (18) (see Note 1). To make this reagent, 1259 μL of lipid mix described in 2.3 was added to 315 μL of DMSO. Then 5287 μL of buffer 1 (30 mM Hepes pH 7.4, 1 mM EGTA, 0.1% CHAPS) was added and the mixture was sonicated. Then, 13525 μL of buffer 2 (50 mM Hepes pH 7.4, 0.1% Chaps) was added and the mixture was sonicated. Lastly, enzyme was added and the solution was gently mixed by pipetting. This reagent was stored on wet ice.
No PI5P4Kα buffer: 112.5 μM PI5P (Echelon Biosciences), 225 μM DPPS (Echelon Biosciences), Hepes pH 7.4, EGTA, 0.1% CHAPS.
No PI5P buffer: 15 nM PI5P4Kα, Hepes pH 7.4, EGTA, 0.1% CHAPS.
ATP Buffer: 15 μM ATP, 20 mM Hepes 7.4, 60 mM MgCl2, 0.1% CHAPS. (see Notes 2-3)
ADP-Glo™ Reagent: Thaw at room temperature per manufacturer's protocol. (see Note 9)
Kinase Detection Reagent: Thaw at room temperature per manufacturer's protocol. Add kinase detection buffer to powdered kinase detection reagent and swirl gently until fully dissolved. (see Note 10-12)
3. Methods
3.1 Yes1 tyrosine kinase
Dispense 2 μL of Yes1 enzyme buffer into all but one column of a white 1536-well solid bottom plate (Greiner) with a Flying Reagent Dispenser (Beckman Coulter) leaving a control column that will contain just the buffer components but no Yes1. (see Note 13)
Dispense 2 μL of the assay buffer buffer control in the remaining column as a no enzyme control.
Transfer 23 nL of library compounds dissolved in DMSO from a 1536-well compound plate arrayed in columns 5-48 into the assay plate using a pintool (Kalypsys Systems) (22). (see Note 4)
Transfer 23 nL of control compounds (DMSO used here in column 1, 3-4 and known Yes1 inhibitor dasatinib (23) in 16-pt dose response in column 2) from a 1536-well compound plate arrayed in columns 1-4 into the assay plate using the pintool.
Incubate the enzyme with compound for 15 minutes.
Initiate the enzyme reaction by dispensing 1 μL of Yes1 substrate buffer to all wells of the assay plate.
Cover the plate with a solid lid.
Incubate at room-temperature for 1 hour.
Add 2 μL of ADP-Glo™ reagent. (see Note 14)
Incubate the lidded plate for 40 minutes.
Add 4 μL of Kinase Detection reagent. (see Note 15)
Incubate the lidded plate for 30 minutes. (see Note 16)
Read the luminescence signal with a ViewLux (Perkin-Elmer) with a 1 sec exposure.
Normalize the data to DMSO-treated control columns with (maximum signal) and without (minimum signal) enzyme. (see Note 17)
3.2 Glucokinase (GCK)
The Glucokinase assay was designed to look for inhibitors of the Glucokinase/Glucokinase regulatory protein interaction which would lead to activation of Glucokinase (see Figure 3). This assay could be run with just glucokinase to look for activators or inhibitors. Additional complementary assay formats for interrogating the interaction of Glucokinase with Glucokinase regulatory protein can be found in (20,27).
Dispense 2 μL of Glucokinase/glucokinase regulatory enzyme buffer into all but one column of a white 1536-well solid bottom plate (Greiner) with a Flying Reagent Dispenser (Beckman Coulter) leaving a control column that contains just the buffer components but no glucokinase. (see Note 13)
Dispense 2 μL of no Glucokinase buffer control in the remaining column.
Transfer 23 nL of library compounds dissolved in DMSO from a 1536-well compound plate arrayed in columns 5-48 into the assay plate using a pintool (Kalypsys Systems).
Transfer 23 nL of control compounds (DMSO used here in column 1, 3-4 and known Glucokinase activator cmpd A from EMD Millipore (GKA-EMD) in 16-pt dose response) from a 1536-well compound plate arrayed in columns 1-4 into the assay plate using the pintool.
Incubate the enzyme with compound for 15 minutes.
Initiate the enzyme reaction by dispensing 1 μL of GCK substrate buffer to all wells of the assay plate.
Cover the plate with a solid lid.
Incubate at room-temperature for 1 hour.
Add 2.5 μL of ADP-Glo™ reagent.
Incubate the lidded plate for 40 minutes.
Add 5 μL of Kinase Detection reagent.
Incubate the lidded plate for 30 minutes.
Read the luminescence signal with a ViewLux with a 1 sec exposure.
Normalize the data to DMSO-treated control columns with (maximum signal) and without (minimum signal) enzyme.
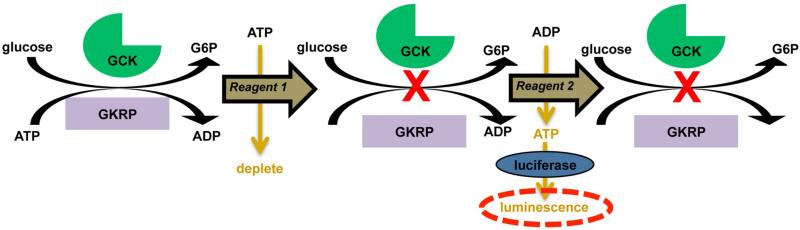
Figure 3. Schematic of the ADP-Glo™ assay for Glucokinase.
This assay monitors Glucokinase activity directly but also indirectly monitors the protein-protein interaction of Glucokinase with Glucokinase regulatory protein. A small molecule that disrupts the interaction of Glucokinase with Glucokinase regulatory protein would lead to an increase in observed luminescence (20).
3.3 PI5P4Kα lipid kinase
Dispense 2 μL of PI5P4Kα/PI5P enzyme buffer into all but two columns of a white 1536-well solid bottom plate (Greiner) with a Flying Reagent Dispenser (Beckman Coulter) leaving a control column that contains just the buffer components but no PI5P4Kα and a control column that contains just the buffer components but no PI5P. (see Note 13 and 18)
Dispense 2 μL of no PI5P4Kα buffer control and no PI5P buffer control in the remaining columns.
Centrifuge for 10 sec at 300×g.
Transfer 23 nL of library compounds dissolved in DMSO from a 1536-well compound plate arrayed in columns 5-48 into the assay plate using a pintool (Kalypsys Systems).
Transfer 23 nL of control compounds (DMSO used here in column 1, 3-4 and known PI5P4Kα inhibitor Tyrphostin AG82 (Cayman Chemical Company) (18) in 16-pt dose response) from a 1536-well compound plate arrayed in columns 1-4 into the assay plate using the pintool.
Incubate the enzyme with compound for 15 minutes.
Initiate the enzyme reaction by dispensing 1 μL of ATP buffer to all wells of the assay plate.
Cover the plate with a solid lid.
Incubate at room-temperature for 1 hour.
Add 2 μL of ADP-Glo™ reagent.
Incubate the lidded plate for 40 minutes.
Add 4 μL of Kinase Detection reagent.
Incubate the lidded plate for 30 minutes.
Read the luminescence signal with a ViewLux (Perkin-Elmer) with a 20 sec exposure. (see Note 19)
Normalize the data to DMSO-treated control columns with (maximum signal) and without (minimum signal) enzyme.
3.4 Data analysis and counter screens
FLuc (Sigma cat # L9506) can be used as a counterscreen (14,16) but for ADP-Glo™ assays it is preferable to use the kit itself and not just FLuc enzyme as the ADP-Glo™ system contains additional proprietary enzyme components. By testing the compound in the presence of the ATP/ADP mix representing the % conversion attained in the assay, it can be determined whether the compound interferes with any of the components of the ADP-Glo™ detection system, including FLuc.
For the Yes1 assay, the average Z’= 0.76, CV= 6.9 and S/B =.23.7 (19). IC50 for control dasatinib = 0.5 nM. Data were deposited in PubChem AID 686947 (primary assay) and 686950 (detection counterassay).
For the Glucokinase assay, the average Z’= 0.7, CV= 4.2% and S/B= 4.2 (20). Data were deposited in PubChem AID 743206.
For the PI5P4Kα assay, the Z’= 0.77, CV= 9.3% and S/B= 12.6 (18). Data were deposited in PubChem AID 652105, 652103, 743286 and 743285 (detection counterassay).
These assay formats can be used to assess mechanism of inhibition by varying the [ATP] relative to the Km as long as the amount of ATP used at each point lies within the linear range of the detection reagent ~ 20 nM to 1 mM as was done previously for PI5P4Kα (18). Competitive inhibitors will show increased IC50 values with increasing concentrations of ATP, noncompetitive inhibitors will show no change and uncompetitive inhibitors will have decreased IC50 values with increasing concentration of ATP.
Acknowledgement
This work was supported by the Molecular Libraries Initiative of the NIH Roadmap for Medical Research. The content of this publication does not necessarily reflect the views of policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
The kinase enzymes and protein substrates are stored at −80 °C and then stored on wet ice until ready to use. The substrates are stored at -20 °C and stored at room-temperature until ready to use.
The reactions described herein were run with ATP near the Km. This allows for maximum sensitivity at identifying inhibitors that are competitive, uncompetitive or noncompetitive with respect to ATP (24). If the Km is unknown for your kinase, the coupled pyruvate kinase/lactate dehydrogenase method can be used to determine it (25).
The presence of contaminating ADP in the ATP used for the kinase reaction will limit the attainable signal to background so the use of Ultrapure ATP, such as the ATP present in the ADP Glo™ kit is recommended.
Test compounds are dissolved in DMSO. The tolerability of the kinase to DMSO should be tested. PI5P4Kα was sensitive to DMSO levels higher than 7.25% (18). The assays described herein use 5% DMSO, which is needed to solubilize the PI5P lipid. The remaining assays, Glucokinase and Yes1 kinase, had <1% DMSO as DMSO was needed only to solubilize the compounds and not the substrates in those assays.
Lipid solutions should be prepared and stored in glass containers to minimize the lipid sticking to plastic.
Lipid prep of PI5P and DPPS in DMSO can be stored at −20 °C and thawed and sonicated prior to use. The PI5P/DPPS mixture is stable to at least six freeze/thaw cycles.
PI5P/DPPS is a suspension and is slightly opaque. The PI5P4Kα/PI5P reagent was stable for at least 16 hours making it amenable to HTS and is a solution with a CV and Z’ across each 1536-well plate indicating that the delivery of the lipid mixture is quite uniform. PI5P alone is minimally soluble in DMSO so the DPPS DMSO solution can be added to the PI5P followed by sonication to assist the formation of the uniform suspension. An alternate assay method for PI5P4Kα using liposomes is described in (26).
While there are shorter chain PI5P lipids available that are more soluble in DMSO, these are not substrates for PI5P4Kα.
ADP-Glo™ reagents can be refrozen and stored at -20 °C. Upon thawing mix gently and check for precipitation. Solution can be warmed at 37 °C for 15 minutes to dissolve precipitate or supernatant can be removed and used.
ADP-Glo™ kit reagents and kinase reaction mixture should be at room temperature prior to the detection steps.
The ADP-Glo™ kit can be used for any enzyme that generates ADP, including ATPases and kinases.
The ADP-Glo™ kit can detect up to 1 mM of ATP/ADP and can also detect as little as 20 nM ADP.
It is important to initially have a control that is all components but no enzyme and a control that is all components but no substrate to assess the level of substrate independent ATPase activity as well as ensure that the substrate does not itself contain a contaminating ATPase.
The ADP-Glo™ kit requires the presence of at least 0.5 mM MgCl2 so if the kinase reaction does not have MgCl2 present it should be added with the ADP-Glo™ reagent. Termination of the kinase reaction is not required with the kit and the use of EDTA to terminate the kinase reaction should be avoided as the Mg2+ is needed for the ADP-Glo™ kit.
The manufacturer's recommendations are to add a 1:1 ratio of ADP-Glo™ reagent to your kinase reaction and then a 1:1 ratio of Kinase Detection reagent to that. The minimum volume that is preferable in a 1536-well plate is 3 μL but the maximum volume the plate can hold is 12 μL with a preference for staying at or below 10.5 μL, so the ADP-Glo™ kit reagents were used at levels below the manufacturer's recommendation. For the assays used herein, this did not adversely affect the assay.
The ADP-Glo™ signal/background is stable for at least 3 hours per the manufacturer's specifications making it amenable to high-throughput screening.
A standard curve can be made using the starting amount of ATP in the kinase reaction and creating admixtures of ATP/ADP representing 0 to 100% conversion.
The bottles used to dispense reagents on the Flying Reagent Dispenser are made of plastic. For the PI5P4Kα/PI5P reagent a glass test tube was placed inside the plastic bottle to minimize sticking of the lipid to plastic.
The exposure time in the ViewLux is significantly longer for the PI5P4Kα assay than the Glucokinase and Yes1 assays.
References
- 1.Cohen P, Alessi DR. Kinase Drug Discovery – What's Next in the Field? ACS Chemical Biology. 2012;8:96–104. doi: 10.1021/cb300610s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walsh MJ, Brimacombe KR, Veith H, Bougie JM, Daniel T, Leister W, Cantley LC, Israelsen WJ, Vander Heiden MG, Shen M, Auld DS, Thomas CJ, Boxer MB. 2-Oxo-N-aryl-1,2,3,4-tetrahydroquinoline-6-sulfonamides as activators of the tumor cell specific M2 isoform of pyruvate kinase. Bioorganic & medicinal chemistry letters. 2011;21:6322–6327. doi: 10.1016/j.bmcl.2011.08.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harbert C, Marshall J, Soh S, Steger K. Development of a HTRF kinase assay for determination of Syk activity. Curr Chem Genomics. 2008;1:20–26. doi: 10.2174/1875397300801010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fabian MA, Biggs WH, 3rd, Treiber DK, Atteridge CE, Azimioara MD, Benedetti MG, Carter TA, Ciceri P, Edeen PT, Floyd M, Ford JM, Galvin M, Gerlach JL, Grotzfeld RM, Herrgard S, Insko DE, Insko MA, Lai AG, Lelias JM, Mehta SA, Milanov ZV, Velasco AM, Wodicka LM, Patel HK, Zarrinkar PP, Lockhart DJ. A small molecule-kinase interaction map for clinical kinase inhibitors. Nat Biotechnol. 2005;23:329–336. doi: 10.1038/nbt1068. [DOI] [PubMed] [Google Scholar]
- 5.Kleman-Leyer KM, Klink TA, Kopp AL, Westermeyer TA, Koeff MD, Larson BR, Worzella TJ, Pinchard CA, van de Kar SA, Zaman GJ, Hornberg JJ, Lowery RG. Characterization and optimization of a red-shifted fluorescence polarization ADP detection assay. Assay and drug development technologies. 2009;7:56–67. doi: 10.1089/adt.2008.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hastie CJ, McLauchlan HJ, Cohen P. Assay of protein kinases using radiolabeled ATP: a protocol. Nat. Protocols. 2006;1:968–971. doi: 10.1038/nprot.2006.149. [DOI] [PubMed] [Google Scholar]
- 7.Tanega C, Shen M, Mott BT, Thomas CJ, MacArthur R, Inglese J, Auld DS. Comparison of Bioluminescent Kinase Assays Using Substrate Depletion and Product Formation. Assay and drug development technologies. 2009;7:606–614. doi: 10.1089/adt.2009.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Somberg R, Goueli SA. Method for Detecting Transferase Enzymatic Activity. In: USPTO, editor. Promega Corporation; USA: 2004. US 2004/0101922 A1. [Google Scholar]
- 9.Wu G, Yuan Y, Hodge CN. Determining appropriate substrate conversion for enzymatic assays in high-throughput screening. J Biomol Screen. 2003;8:694–700. doi: 10.1177/1087057103260050. [DOI] [PubMed] [Google Scholar]
- 10.Segel IH. Enzyme Kinetics: Behavior and Analysis of Rapid Equilibrium and Steady-State Enzyme Systems. John Wiley and Sons; New York: 1993. [Google Scholar]
- 11.Li H, Totoritis RD, Lor LA, Schwartz B, Caprioli P, Jurewicz AJ, Zhang G. Evaluation of an antibody-free ADP detection assay: ADP-Glo. Assay and drug development technologies. 2009;7:598–605. doi: 10.1089/adt.2009.0221. [DOI] [PubMed] [Google Scholar]
- 12.Sanghera J, Li R, Yan J. Comparison of the luminescent ADP-Glo assay to a standard radiometric assay for measurement of protein kinase activity. Assay and drug development technologies. 2009;7:615–622. doi: 10.1089/adt.2009.0237. [DOI] [PubMed] [Google Scholar]
- 13.Zegzouti H, Goueli SA. ADP Detection Based Luminescenct Phosphotransferase or ATP Hydrolase Assay. In: USPTO, editor. Promega Corporation; USA: 2010. US 2010/0075350 A1. [Google Scholar]
- 14.Auld DS, Southall NT, Jadhav A, Johnson RL, Diller DJ, Simeonov A, Austin CP, Inglese J. Characterization of chemical libraries for luciferase inhibitory activity. J Med Chem. 2008;51:2372–2386. doi: 10.1021/jm701302v. [DOI] [PubMed] [Google Scholar]
- 15.Auld DS, Zhang YQ, Southall NT, Rai G, Landsman M, MacLure J, Langevin D, Thomas CJ, Austin CP, Inglese J. A basis for reduced chemical library inhibition of firefly luciferase obtained from directed evolution. J Med Chem. 2009;52:1450–1458. doi: 10.1021/jm8014525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thorne N, Shen M, Lea Wendy A., Simeonov A, Lovell S, Auld Douglas S., Inglese J. Firefly Luciferase in Chemical Biology: A Compendium of Inhibitors, Mechanistic Evaluation of Chemotypes, and Suggested Use As a Reporter. Chemistry & Biology. 2012;19:1060–1072. doi: 10.1016/j.chembiol.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dranchak P, MacArthur R, Guha R, Zuercher WJ, Drewry DH, Auld DS, Inglese J. Profile of the GSK Published Protein Kinase Inhibitor Set Across ATP-Dependent and-Independent Luciferases: Implications for Reporter-Gene Assays. PloS one. 2013;8:e57888. doi: 10.1371/journal.pone.0057888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis MI, Sasaki AT, Shen M, Emerling BM, Thorne N, Michael S, Pragani R, Boxer M, Sumita K, Takeuchi K, Auld DS, Li Z, Cantley LC, Simeonov A. A homogeneous, high-throughput assay for phosphatidylinositol 5-phosphate 4-kinase with a novel, rapid substrate preparation. PloS one. 2013;8:e54127. doi: 10.1371/journal.pone.0054127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel PR, Sun H, Li SQ, Shen M, Khan J, Thomas CJ, Davis MI. Identification of potent Yes1 kinase inhibitors using a library screening approach. Bioorganic & medicinal chemistry letters. 2013;23:4398–4403. doi: 10.1016/j.bmcl.2013.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rees MG, Davis MI, Shen M, Titus S, Raimondo A, Barrett A, Gloyn AL, Collins FS, Simeonov A. A Panel of Diverse Assays to Interrogate the Interaction between Glucokinase and Glucokinase Regulatory Protein, Two Vital Proteins in Human Disease. PloS one. 2014;9:e89335. doi: 10.1371/journal.pone.0089335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inglese J, Auld DS, Jadhav A, Johnson RL, Simeonov A, Yasgar A, Zheng W, Austin CP. Quantitative high-throughput screening: a titration-based approach that efficiently identifies biological activities in large chemical libraries. Proc Natl Acad Sci U S A. 2006;103:11473–11478. doi: 10.1073/pnas.0604348103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yasgar A, Shinn P, Jadhav A, Auld D, Michael S, Zheng W, Austin CP, Inglese J, Simeonov A. Compound Management for Quantitative High-Throughput Screening. JALA Charlottesv Va. 2008;13:79–89. doi: 10.1016/j.jala.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rix U, Hantschel O, Durnberger G, Remsing Rix LL, Planyavsky M, Fernbach NV, Kaupe I, Bennett KL, Valent P, Colinge J, Kocher T, Superti-Furga G. Chemical proteomic profiles of the BCR-ABL inhibitors imatinib, nilotinib, and dasatinib reveal novel kinase and nonkinase targets. Blood. 2007;110:4055–4063. doi: 10.1182/blood-2007-07-102061. [DOI] [PubMed] [Google Scholar]
- 24.Copeland RA. Evaluation of enzyme inhibitors in drug discovery. A guide for medicinal chemists and pharmacologists. Methods Biochem Anal. 2005;46:1–265. [PubMed] [Google Scholar]
- 25.Jenkins WT. The pyruvate kinase-coupled assay for ATPases: a critical analysis. Anal Biochem. 1991;194:136–139. doi: 10.1016/0003-2697(91)90160-u. [DOI] [PubMed] [Google Scholar]
- 26.Demian DJ, Clugston SL, Foster MM, Rameh L, Sarkes D, Townson SA, Yang L, Zhang M, Charlton ME. High-throughput, cell-free, liposome-based approach for assessing in vitro activity of lipid kinases. J Biomol Screen. 2009;14:838–844. doi: 10.1177/1087057109339205. [DOI] [PubMed] [Google Scholar]
- 27.Lloyd DJ, St Jean DJ, Jr., Kurzeja RJ, Wahl RC, Michelsen K, Cupples R, Chen M, Wu J, Sivits G, Helmering J, Komorowski R, Ashton KS, Pennington LD, Fotsch C, Vazir M, Chen K, Chmait S, Zhang J, Liu L, Norman MH, Andrews KL, Bartberger MD, Van G, Galbreath EJ, Vonderfecht SL, Wang M, Jordan SR, Veniant MM, Hale C. Antidiabetic effects of glucokinase regulatory protein small-molecule disruptors. Nature. 2013;504:437–440. doi: 10.1038/nature12724. [DOI] [PubMed] [Google Scholar]