Abstract
Purpose of Review
Uterine fibroids are extremely common, and can cause significant morbidity, yet the exact etiology of these tumors remains elusive and there are currently no long-term treatments available. In this review we aim to provide an overview of steroid hormones, genetic abnormalities, and stem cells in the pathogenesis of uterine fibroids.
Recent Findings
A universal feature of fibroids is responsiveness to estrogen and progesterone, and most of the currently available therapies exploit this characteristic. Ulipristal acetate has recently shown particular promise for providing long-term relief from uterine fibroids. Additionally, fibroid stem cells were isolated and appear to be necessary for growth. The recent discovery of somatic mutations involving MED12 or HMGA2 in the majority of fibroids and the links to their pathophysiology were also significant advances.
Summary
The recent shift in focus from hormones to fibroid stem cells and genetic aberrations should lead not only to a deeper understanding of the specific etiology of fibroids, but also to the discovery of new therapeutic targets. Targeting the products of genetic mutations or fibroid stem cells has the potential to achieve both better control of current tumors and the prevention of new fibroids.
Keywords: Fibroids, steroid hormones, stem cells, genetics
Introduction
Uterine fibroids occur in up to 80% of reproductive-age women, causing significant morbidity in up to 30% of women[1-4]. In the United States, more than 200,000 surgical procedures are performed for the treatment of fibroids, with yearly cost estimates of $5.9-34.4 billion[5]. Despite this impressive prevalence, the exact etiology of uterine fibroids remains elusive and there are currently no long-term treatments available. Studies have suggested that fibroids are monoclonal tumors developed from a single myocyte[6, 7], but the inciting event for neoplastic transformation of a myocyte is currently unknown. Tumor growth is characterized by slow proliferation with concurrent deposition of abundant extracellular matrix (figure 1), usually in a steroid-hormone dependent manner[8, 9]. This review provides an overview of the current state of knowledge on the role of steroid hormones in fibroid development, treatments targeting steroid hormone action, and the more recent discoveries regarding genetic abnormalities and stem cells in the pathogenesis of uterine fibroids.
Figure 1.
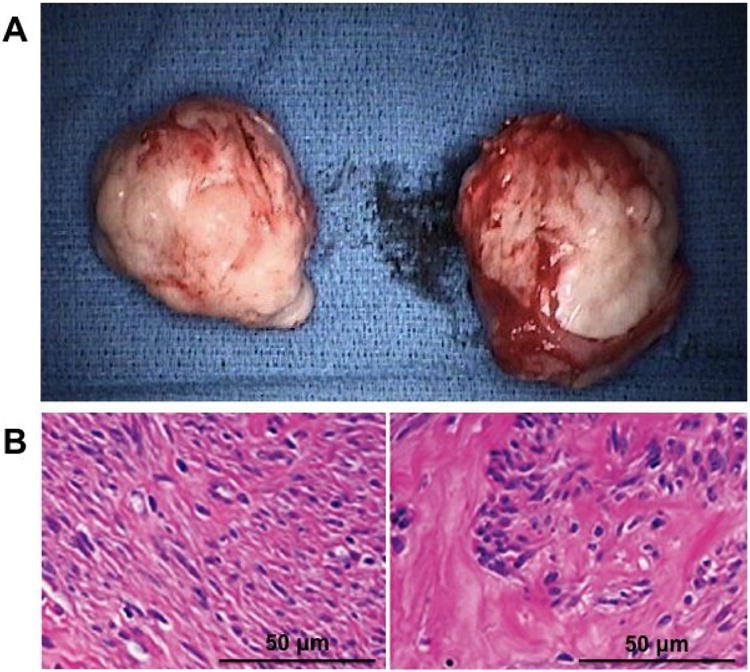
(A) Gross fibroid specimen after surgical removal; (B) Representative hematoxylin and eosin stain of myometrium (left), with organized, normal-appearing smooth muscle cells, and fibroid tissue (right), with whorls of acellular extracellular matrix surrounding small clusters of disorganized smooth muscle cells.
Steroid Hormones
A universal feature of fibroids is responsiveness to estrogen and progesterone, and most of the currently available therapies exploit this characteristic.
Estrogen and Aromatase
Estrogen upregulates gene expression of multiple growth factors, collagens, and the estrogen and progesterone receptors (ER, PR), all thought to play a role in fibroid pathogenesis[10-12]. Estrogen action is mediated through its nuclear receptors, ERα and ERβ, expressed in both myometrial and fibroid tissue[13-15]. ERα is a more potent activator of transcription and is thought to be regulated by ERβ, although much remains unknown about the exact roles of the two receptors and their interactions[16]. Additionally, there have now been several studies that have reported specific ERα polymorphisms that increase susceptibility to uterine fibroids[17, 18].
Fibroids respond to estrogen in the bloodstream as a result of ovarian steroidogenesis, and also produce estrogen in situ through local conversion of androgens by aromatase[19]. Fibroids have been shown to have higher estrogen levels then adjacent myometrium, and correspondingly increased aromatase and 17β-HSD type 1 levels[19-22]. Interestingly, aromatase RNA is not found in the myometrium of women without fibroids[19]. The addition of androstenedione alone to cultured fibroid cells leads to estradiol production, with resultant cellular proliferation comparable to that caused by the addition of estradiol alone, suggesting that fibroids are capable of producing sufficient estrogen to sustain their own growth[21]. The addition of aromatase inhibitors to fibroid cell culture reverses this effect[21].
Progesterone
In addition to estrogen and aromatase, there is accumulating evidence that progesterone plays a critical role in uterine fibroid expansion[23] and is essential for estrogen-related fibroid growth[24-28]. Progesterone acts through two isoforms of PR, PR-A and PR-B, both of which exhibit higher expression in fibroids compared with adjacent myometrium[29-31]. Similar to ER, relatively little is known about the specific roles and interplay of PR-A and PR-B in fibroids.
In support of a key role for progesterone, markers of proliferation and mitotic counts are highest in fibroid tissue during the luteal phase[25, 28] and fibroid proliferative activity in postmenopausal women has been shown to increase significantly with combined estrogen and progestin replacement but not with estrogen replacement alone[25]. In a xenograft mouse model, Ishikawa et al. showed that estrogen regulates expression of PR via ERα, and progesterone directly stimulates fibroid growth[26]. In this model, estrogen with progesterone stimulated both fibroid cell proliferation and extracellular matrix formation, and these effects were abolished by co-treatment with a progesterone receptor antagonist[26]. These findings suggest a more permissive role for estrogen, allowing fibroid responsiveness to progesterone via induction of PR[4, 26].
Recently, in a xenograft model, Qiang, et al. (2014) demonstrated that treatment with estrogen and progesterone resulted in the formation of extracellular matrix production via downregulation of miR-29b[32*]. Gene expression of miR-29b has been consistently shown to be lower in fibroid tissues compared with adjacent normal myometrium tissues, both in vitro and in vivo[32*-34] and increasing mir-29b levels in fibroid cells with mir-29b lentivirus decreased levels of collagen 1a1[32*]. Lastly, estrogen with progesterone, but not estrogen alone, decreased miR-29b expression, suggesting a role for progesterone in promoting uterine fibroid growth via miR29n downregulation[32*].
Medical Treatments
While the mainstay of fibroid treatment has traditionally been surgical, much recent research has focused on less invasive medical therapies. Historically, GnRH agonists were first-line therapy for fibroids, but they can cause severe menopausal symptoms, and cannot be used long-term. A number of reviews are available on non-surgical management of fibroids[35,36*,37-39], so the topic will not be reviewed in depth here. Currently available therapies are summarized in table 1. As proof of principle of the above-mentioned hormonal aspects, we will briefly review aromatase inhibitors and selective progesterone receptor modulators (SPRMs), highlighting the exiting recent progress with ulipristal acetate.
Table 1.
| Treatment Option | Route of Administration |
Mechanism of Action |
Potential Side Effects |
Pregnancy Category |
FDA Approved | Decreases Bleeding |
Decreases Tumor Size |
Additional Comments |
|---|---|---|---|---|---|---|---|---|
| GnRH agonists | Intramuscular, subcutaneous, or nasal spray | Abolishes GnRHpulsatility | Severe hypoestrogenemia: hot flashes, vaginal dryness, bone loss | X | Preoperative correction of anemia from fibroids | Yes | Yes | Initial flare effect; Requires add-back therapy after 6 months |
| GnRH Antagonists | Subcutaneous (Ganirelix), Oral (Elagolix) | Competitive inhibition of GnRH | Severe hypoestrogenemia: hot flashes, vaginal dryness, bone loss | X | No | Yes | Yes | Avoids flare effect of GnRH agonists |
| Selective Progesterone Receptor Modulators | Oral | Varied progesterone antagonism | Endometrial thickening/hyperplasia | X | No | Yes | Yes | Ulipristal approved for fibroid treatment in Europe and Canada |
| Aromatase Inhibitors | Oral | Competitive inhibition of aromatase | Bone loss | D | No | Yes | Yes | Can cause follicular stimulation |
| Oral Contraceptives | Oral | Stabilizes endometrium | Venous thromboembolism | X | Heavy menstrual bleeding | Yes | No | |
| Levonorgestrel-releasing IUD | Intrauterine | Induces endometrial atrophy | Breakthrough spotting, expulsion | X | Heavy menstrual bleeding | Yes | Conflicting data | Should not be used with intracavitary fibroids |
| Tranexamic acid | Oral | Inhibits Fibrinolysis | Fibroid infarction | B | Heavy menstrual bleeding from fibroids | Yes | No | Can be used for acute bleeding |
| Danazol | Oral | Synthetic androgen, inhibits steroidogenesis | Androgenic: voice changes, acne, hirsutism | X | No | Yes | No | High risk of side effects, use is generally discouraged |
Aromatase Inhibitors
Because aromatase is thought to play such a critical role in estrogen production in fibroids, aromatase inhibitors are a logical treatment choice. Non-steroidal aromatase inhibitors reversibly bind the aromatase enzyme, decreasing binding by androstenedione or testosterone and thus decreasing conversion to estradiol[40, 41]. While the original aromatase inhibitors were relatively nonselective and fraught with side effects, third generation aromatase inhibitors are more selective and have superior bioavailability and side effect profiles. Anastrozole and letrozole are able to inhibit >98% of aromatase activity[40, 42], and have been shown to result in significant reduction of fibroid volume and improvement in symptoms in multiple clinical trials[43-46]. Moreover, aromatase inhibitors avoid the side effects of the severe hypoestrogenism caused by GnRH agonists, particularly hot flushes[44].
While most women tolerate aromatase inhibitors relatively well, there are potential side effects. The most commonly reported side effects include hot flashes and musculoskeletal pain. Importantly, aromatase inhibitors are often used off-label in the follicular phase for ovulation induction or controlled ovarian stimulation, necessitating contraception in women not desiring conception[47]. Although there have been conflicting results regarding the potential for systemic hypoestrogenism with prolonged aromatase inhibitor use[44, 48], there is concern for both increased bone loss and cardiovascular risk with long-term aromatase inhibitor use, particularly in younger patients[42]. The breast cancer literature has also brought some questions as to the utility of aromatase inhibitors, reporting both decreased effectiveness in overweight and obese women and the development of resistance over time[40, 42]. Additionally, the effects of aromatase inhibitors are only temporary, and fibroids regrow with cessation of treatment, albeit to smaller volumes[44]. Taken together, the current evidence suggests that aromatase inhibitors are, at best, a short-term solution in select populations of women.
Selective Progesterone Receptor Modulators
All of the SPRMs that have been studied in clinical trials—mifepristone (RU486), asoprisnil (J867), ulipristal acetate (CDB2914), and telapristone acetate (CDB4124)—have been shown to reduce fibroid size and improve quality of life[49-51]. In vitro, fibroid cells treated with ulipristal acetate, telapristone acetate, or asoprisnil exhibit decreased cell proliferation and increased apoptosis[52-56], Moreover, asoprisnil and ulipristal both decrease extracellular matrix formation[55, 57, 58]. These effects are not seen with treatment of myometrial cells, suggesting tissue-specificity of these drugs. Both asoprisnil and ulipristal acetate also have high affinity for PR[59, 60], suggesting that the genome-wide binding status of PR liganded with ulipristal or asoprisnil should be further investigated.
Most recent research has focused on ulipristal acetate. Although the Food and Drug Administration has not yet approved ulipristal acetate for indications beyond contraception in the US, it has been approved in both Canada and Europe for the treatment of fibroids. Clinical trials have shown that, while GnRH agonist causes greater overall reduction in fibroid volume, ulipristal acetate has longer-lasting effects after cessation of treatment[8, 50, 61, 62]. Additionally, ulipristal acetate results in a lower incidence of hot flashes, impact on bone density, and suppression of E2 levels when compared to GnRH agonist[62, 63]. Moreover, Donnez et al. recently reported that repeated 3-month courses of ulipristal resulted in amenorrhea in almost 90% of women and was well tolerated[64*, 65**]. This exciting study suggests that ulipristal could be the first long-term treatment for uterine fibroids.
Because SPRMs block progesterone action in the endometrium, concern has been raised that they may result in endometrial thickening and premalignant or malignant transformation. There are now studies showing that treatment with SPRMs does not appear to result in increased mitosis or atypia; however, asymmetry of stromal and epithelial growth and cystic, dilated glands have been reported, and are now classified as progesterone receptor modulator-associated endometrial change (PAEC)[49, 66, 67]. Encouragingly, one study observed reversal of these changes and return to normal endometrial histology six months after ulipristal acetate discontinuation[67], and the study of repeated courses of ulipristal did not report any increase in PAEC or other histological changes[65**], but longer term studies are needed to definitively understand the risks and side effects of SPRMs.
Somatic Stem Cells
Somatic stem cells were first discovered in myometrial tissues, where they are capable of both self-renewal and the production of tissue-specific daughter cells under the influence of estrogen and progesterone[68-70]. More recently, small populations of fibroid cells consistent with somatic stem cells have also been isolated[71, 72]. Despite the fact that fibroids contain lesser stem cells than the myometrium[73], there is evidence that the fibroid stem cell population is essential for steroid hormone-dependent fibroid growth[71, 72]. In a mouse xenograft model, injected cell suspensions containing fibroid stem cells mixed with myometrial cells grew into substantially bigger tumors and had higher proliferation indices under the influence of estrogen and progesterone than injected suspensions containing only differentiated fibroid cells with myometrial cells[72]. Perhaps most interestingly, fibroid stem cells have minimal to no ER and PR expression, yet respond to estrogen and progesterone stimulation with tumor expansion. Additionally, fibroid stem cells cannot induce proliferation or tumor growth without the presence of differentiated fibroid or myometrial cells. These observations have led us to hypothesize that fibroid stem cells rely on paracrine signaling from surrounding mature myometrial and fibroid cells to facilitate estrogen and progesterone action[72].
The wingless-type (WNT)/β-catenin pathway was recently proposed by Ono et al.[74*] as a possible mechanism for paracrine interaction between fibroid stem and differentiated cells. In that study, mature myometrial cells secreted WNT ligands in response to estrogen and progesterone treatment, resulting in nuclear translocation of β-catenin in proximal fibroid stem cells. Intranuclear β-catenin increased expression of genes involved in growth and proliferation. Additionally, inhibiting WNT binding or β-catenin in fibroid stem cells resulted in significantly decreased tumor growth—an effect not seen in mature fibroid cells[74*].
Much remains to be explored in fibroid stem cells. Originally, fibroid stem cells were isolated using the Hoechst dye exclusion technique for side populations (SP)[75, 76], however, the SP technique is expensive, exhibits significant sensitivity to minor staining variations, and is detrimental to cell survival, making further study of fibroid cells difficult[77]. As a solution to these pitfalls, we recently reported a novel way of isolating fibroid stem cells using cell surface markers CD34 and CD49b[78**]. Cell sorting using antibodies to these cell surface proteins revealed 3 distinct cell populations: CD34+/CD49b+, CD34+/CD49b-, and CD34-/CD49b- cells (figure 2). CD34+/CD49b+ cells were highly enriched with stem cells whereas the other two groups did not contain any stem cells. Moreover, genes specific to stem cells, such as KLF4, NANOG, OCT4 were overexpressed in the CD34+/CD49b+ cells further suggesting that these cells are indeed stem cells[78**]. Interestingly, CD34+/CD49b- cells had intermediate levels of these stem cell factors compared to CD34-/CD49b- cells. Additionally, ER-alpha and PR were significantly underexpressed in CD34+/CD49b+ cells, consistent with prior studies on SP, and CD34+/CD49b- cells again showed intermediate expression levels between CD34+/CD49b+ and CD34-/CD49b- cells[78**]. Taken together, these results led us to hypothesize that CD34+/CD49b+ cells are largely fibroid somatic stem cells, capable of asymmetric division allowing both self-renewal and the production of intermediary daughter cells, or CD34+/CD49b-cells, which ultimately develop into fully differentiated fibroid cells, or CD34-/CD49b- cells. An unbiased genome-wide investigation to better characterize the three populations on a molecular level is currently underway and will hopefully lead to new therapeutic targets.
Figure 2.
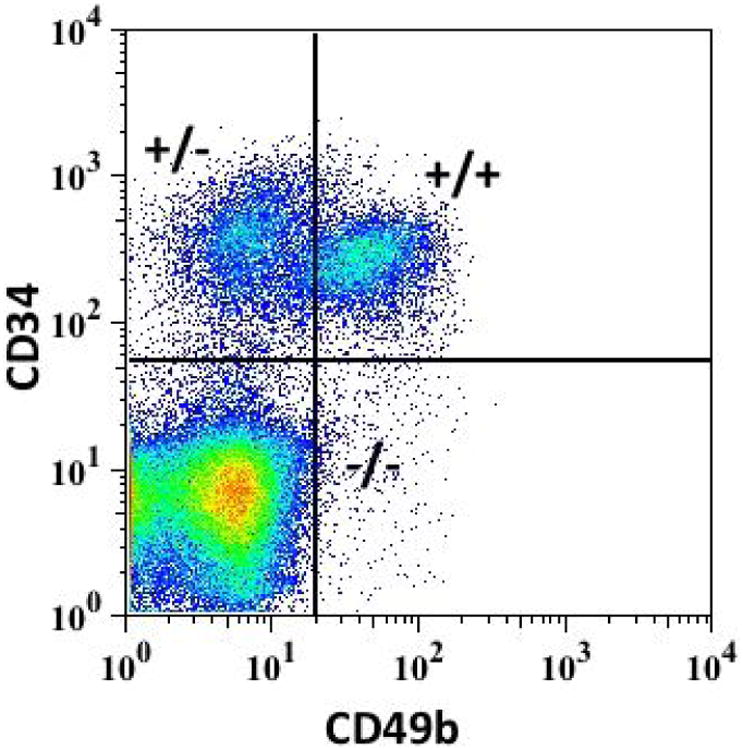
Cell sorting by flow cytometry using antibodies to CD34 and CD49b revealed 3 distinct populations in fibroid cells: CD34+/CD34+ (+/+), CD34+/CD49b- (+/-), and CD34-/CD49b- (-/-). +/+ cells had characteristics of somatic stem cells, whereas -/- cells had a well-differentiated phenotype. We hypothesize that +/- are an intermediate cell type between +/+ and -/- cells.
Genetic Abnormalities
Recent research suggests that most fibroids fall into one of four categories of mutations: MED12 mutations, FH inactivation, COL4A6-COL4A5 deletions, or HMGA2 overexpression[79, 80*]. In one study of HMGA2 and MED12 mutations in fibroids, the two mutations appear to be mutually exclusive, raising the possibility that different genetic abnormalities in fibroids actually represent separate pathophysiology[81]. In support of this hypothesis, HMGA2 aberrations are highly correlated with big fibroid tumors, whereas tumors with MED12 mutations tend to be smaller[82, 83]. Because of their possible role in stem cell action, we will focus on HMGA2 and MED12 mutations in this review.
HMGA2
Mutations involving HMGA2 are found in approximately 7.5% of fibroid tumors and HMGA2 overexpression is due to rearrangements involving chromosome 12q14-15[83]. In mouse neural stem cells, HMGA2 expression inhibits senescence by downregulating p16INK4a, a suppressor of stem cell self-renewal[84]. Similarly, HMGA2 has been shown to downregulate p14Arf, also a negative regulator of self-renewal, in fibroid cells[85]. Finally, uterine fibroids exhibit underexpression of Let-7, which is known to suppress HMGA2[86]. These findings have led us to hypothesize that the Let7-HMGA2-p14Arf pathway may play a significant role in fibroid stem cells when altered, resulting in increased self-renewal and decreased senescence.
MED12
In the largest study of MED12 mutations in fibroids, specific MED12 mutations were found in 70% of fibroids, although smaller studies have reported a prevalence anywhere from 48% to 92%[80, 87]. It has been shown that stem cells from fibroid tissue, but not from myometrial tissue, carry MED12 mutations, supporting our hypothesis that a genetic hit may explain the transformation of a myometrial stem cell to a fibroid stem cell[72]. MED12 regulates Wnt signaling by binding to β-catenin, making it possible that absence of or defects in MED12 in fibroid stem cells could lead to unregulated Wnt/β-catenin pathway-stimulated tumor growth[4, 88]. Moreover, MED12 deficiency, possibly in somatic stem cells, releases negative regulation of TGFβ signaling, resulting in increased proliferation in cancer cells[89, 90]. Taken together, this evidence suggests that MED12 deficiency could lead to activation of the Wnt/β-catenin and TGFβ pathway, thereby supporting stem cell renewal, proliferation, and fibrosis in uterine fibroids[4, 90-92].
Conclusions
Historically, the vast majority of fibroid research has focused on the role of steroid hormones in fibroid pathogenesis. The result of this work has been the development of medical treatment options targeting steroid hormones, such as GnRH agonists, aromatase inhibitors and anti-progestins. To date, we have not found a medical treatment for uterine fibroids that results in permanent tumor shrinkage or eradication, or that can be used long-term with minimal side effects, although the data on ulipristal acetate look promising. Finding an effective, long-term treatment for fibroids could have great public health implications, given their high prevalence and associated medical costs. The recent shift in focus from hormones to fibroid stem cells and genetic aberrations should lead not only to a deeper understanding of the specific etiology of fibroids, but also to the discovery of new therapeutic targets. Targeting the products of genetic mutations or fibroid stem cells has the potential to achieve both better control of current tumors and the prevention of the development of new fibroids.
Key Points.
- A universal feature of fibroids is responsiveness to estrogen and progesterone.
- Ulipristal could be the first long-term treatment for uterine fibroids.
- Fibroids contain somatic stem cells that are necessary for growth, but require paracrine signals from surrounding matures cells.
- Genetic mutations, particularly those affecting MED12 and HMGA2, likely explain some of fibroid pathogenesis.
- Targeting the products of genetic mutations or fibroid stem cells has the potential to achieve both better control of current tumors and the prevention of the development of new fibroids.
Acknowledgments
SEB received funding from National Institutes of Health Grant NIH/NICHD P01-HD0578.
Funding: National Institutes of Health Grant NIH/NICHD P01-HD0578
Footnotes
Conflicts of interest: The authors have no conflicts of interest to declare.
References
- 1.Parker WH. Etiology, symptomatology, and diagnosis of uterine myomas. Fertility and sterility. 2007;87:725–736. doi: 10.1016/j.fertnstert.2007.01.093. [DOI] [PubMed] [Google Scholar]
- 2.Myers ER, Barber MD, Gustilo-Ashby T, et al. Management of uterine leiomyomata: what do we really know? Obstetrics and gynecology. 2002;100:8–17. doi: 10.1016/s0029-7844(02)02019-7. [DOI] [PubMed] [Google Scholar]
- 3.Cramer SF, Patel A. The frequency of uterine leiomyomas. American journal of clinical pathology. 1990;94:435–438. doi: 10.1093/ajcp/94.4.435. [DOI] [PubMed] [Google Scholar]
- 4.Bulun SE. Uterine fibroids. The New England journal of medicine. 2013;369:1344–1355. doi: 10.1056/NEJMra1209993. [DOI] [PubMed] [Google Scholar]
- 5.Cardozo ER, Clark AD, Banks NK, et al. The estimated annual cost of uterine leiomyomata in the United States. American journal of obstetrics and gynecology. 2012;206:211, e211–219. doi: 10.1016/j.ajog.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Townsend DE, Sparkes RS, Baluda MC, McClelland G. Unicellular histogenesis of uterine leiomyomas as determined by electrophoresis by glucose-6-phosphate dehydrogenase. American journal of obstetrics and gynecology. 1970;107:1168–1173. doi: 10.1016/s0002-9378(15)30365-3. [DOI] [PubMed] [Google Scholar]
- 7.Linder D, Gartler SM. Glucose-6-phosphate dehydrogenase mosaicism: utilization as a cell marker in the study of leiomyomas. Science. 1965;150:67–69. doi: 10.1126/science.150.3692.67. [DOI] [PubMed] [Google Scholar]
- 8.Kim JJ, Kurita T, Bulun SE. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocrine reviews. 2013;34:130–162. doi: 10.1210/er.2012-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flake GP, Moore AB, Sutton D, et al. The natural history of uterine leiomyomas: light and electron microscopic studies of fibroid phases, interstitial ischemia, inanosis, and reclamation. Obstetrics and gynecology international. 2013;2013:528376. doi: 10.1155/2013/528376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andersen J, DyReyes VM, Barbieri RL, et al. Leiomyoma primary cultures have elevated transcriptional response to estrogen compared with autologous myometrial cultures. Journal of the Society for Gynecologic Investigation. 1995;2:542–551. doi: 10.1016/1071-5576(94)00053-4. [DOI] [PubMed] [Google Scholar]
- 11.Maruo T, Ohara N, Wang J, Matsuo H. Sex steroidal regulation of uterine leiomyoma growth and apoptosis. Human reproduction update. 2004;10:207–220. doi: 10.1093/humupd/dmh019. [DOI] [PubMed] [Google Scholar]
- 12.Li S, McLachlan JA. Estrogen-associated genes in uterine leiomyoma. Annals of the New York Academy of Sciences. 2001;948:112–120. doi: 10.1111/j.1749-6632.2001.tb03992.x. [DOI] [PubMed] [Google Scholar]
- 13.Pedeutour F, Quade BJ, Weremowicz S, et al. Localization and expression of the human estrogen receptor beta gene in uterine leiomyomata. Genes, chromosomes & cancer. 1998;23:361–366. doi: 10.1002/(sici)1098-2264(199812)23:4<361::aid-gcc12>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 14.Benassayag C, Leroy MJ, Rigourd V, et al. Estrogen receptors (ERalpha/ERbeta) in normal and pathological growth of the human myometrium: pregnancy and leiomyoma. The American journal of physiology. 1999;276:E1112–1118. doi: 10.1152/ajpendo.1999.276.6.E1112. [DOI] [PubMed] [Google Scholar]
- 15.Andersen J, Barbieri RL. Abnormal gene expression in uterine leiomyomas. Journal of the Society for Gynecologic Investigation. 1995;2:663–672. doi: 10.1016/1071-5576(95)00021-6. [DOI] [PubMed] [Google Scholar]
- 16.Jakimiuk AJ, Bogusiewicz M, Tarkowski R, et al. Estrogen receptor alpha and beta expression in uterine leiomyomas from premenopausal women. Fertility and sterility. 2004;82(Suppl 3):1244–1249. doi: 10.1016/j.fertnstert.2004.02.130. [DOI] [PubMed] [Google Scholar]
- 17.Al-Hendy A, Salama SA. Ethnic distribution of estrogen receptor-alpha polymorphism is associated with a higher prevalence of uterine leiomyomas in black Americans. Fertility and sterility. 2006;86:686–693. doi: 10.1016/j.fertnstert.2006.01.052. [DOI] [PubMed] [Google Scholar]
- 18.Feng Y, Lin X, Zhou S, et al. The associations between the polymorphisms of the ER-alpha gene and the risk of uterine leiomyoma (ULM) Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2013;34:3077–3082. doi: 10.1007/s13277-013-0874-0. [DOI] [PubMed] [Google Scholar]
- 19.Bulun SE, Simpson ER, Word RA. Expression of the CYP19 gene and its product aromatase cytochrome P450 in human uterine leiomyoma tissues and cells in culture. The Journal of clinical endocrinology and metabolism. 1994;78:736–743. doi: 10.1210/jcem.78.3.8126151. [DOI] [PubMed] [Google Scholar]
- 20.Folkerd EJ, Newton CJ, Davidson K, et al. Aromatase activity in uterine leiomyomata. Journal of steroid biochemistry. 1984;20:1195–1200. doi: 10.1016/0022-4731(84)90366-2. [DOI] [PubMed] [Google Scholar]
- 21.Sumitani H, Shozu M, Segawa T, et al. In situ estrogen synthesized by aromatase P450 in uterine leiomyoma cells promotes cell growth probably via an autocrine/intracrine mechanism. Endocrinology. 2000;141:3852–3861. doi: 10.1210/endo.141.10.7719. [DOI] [PubMed] [Google Scholar]
- 22.Shozu M, Murakami K, Inoue M. Aromatase and leiomyoma of the uterus. Seminars in reproductive medicine. 2004;22:51–60. doi: 10.1055/s-2004-823027. [DOI] [PubMed] [Google Scholar]
- 23.Cermik D, Arici A, Taylor HS. Coordinated regulation of HOX gene expression in myometrium and uterine leiomyoma. Fertility and sterility. 2002;78:979–984. doi: 10.1016/s0015-0282(02)03366-6. [DOI] [PubMed] [Google Scholar]
- 24.Ishikawa H, Fenkci V, Marsh EE, et al. CCAAT/enhancer binding protein beta regulates aromatase expression via multiple and novel cis-regulatory sequences in uterine leiomyoma. The Journal of clinical endocrinology and metabolism. 2008;93:981–991. doi: 10.1210/jc.2007-2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lamminen S, Rantala I, Helin H, et al. Proliferative activity of human uterine leiomyoma cells as measured by automatic image analysis. Gynecologic and obstetric investigation. 1992;34:111–114. doi: 10.1159/000292738. [DOI] [PubMed] [Google Scholar]
- 26.Ishikawa H, Ishi K, Serna VA, et al. Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology. 2010;151:2433–2442. doi: 10.1210/en.2009-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hassan MH, Salama SA, Arafa HM, et al. Adenovirus-mediated delivery of a dominant-negative estrogen receptor gene in uterine leiomyoma cells abrogates estrogen- and progesterone-regulated gene expression. The Journal of clinical endocrinology and metabolism. 2007;92:3949–3957. doi: 10.1210/jc.2007-0823. [DOI] [PubMed] [Google Scholar]
- 28.Kawaguchi K, Fujii S, Konishi I, et al. Mitotic activity in uterine leiomyomas during the menstrual cycle. American journal of obstetrics and gynecology. 1989;160:637–641. doi: 10.1016/s0002-9378(89)80046-8. [DOI] [PubMed] [Google Scholar]
- 29.Brandon DD, Bethea CL, Strawn EY, et al. Progesterone receptor messenger ribonucleic acid and protein are overexpressed in human uterine leiomyomas. Am J Obstet Gynecol. 1993;169:78–85. doi: 10.1016/0002-9378(93)90135-6. [DOI] [PubMed] [Google Scholar]
- 30.Englund K, Blanck A, Gustavsson I, et al. Sex steroid receptors in human myometrium and fibroids: changes during the menstrual cycle and gonadotropin-releasing hormone treatment. The Journal of clinical endocrinology and metabolism. 1998;83:4092–4096. doi: 10.1210/jcem.83.11.5287. [DOI] [PubMed] [Google Scholar]
- 31.Nisolle M, Gillerot S, Casanas-Roux F, et al. Immunohistochemical study of the proliferation index, oestrogen receptors and progesterone receptors A and B in leiomyomata and normal myometrium during the menstrual cycle and under gonadotrophin-releasing hormone agonist therapy. Human reproduction. 1999;14:2844–2850. doi: 10.1093/humrep/14.11.2844. [DOI] [PubMed] [Google Scholar]
- 32*.Qiang W, Liu Z, Serna VA, et al. Downregulation of miR-29b is essential for pathogenesis of uterine leiomyoma. Endocrinology. 2014:en20131763. doi: 10.1210/en.2013-1763. This study showed that progesterone-induced downregulation of miR-29b is necessary for tumor growth and expansion of extracellular matrix, suggesting that restoring miR-29b action could potentially treat uterine fibroids. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marsh EE, Lin Z, Yin P, et al. Differential expression of microRNA species in human uterine leiomyoma versus normal myometrium. Fertility and sterility. 2008;89:1771–1776. doi: 10.1016/j.fertnstert.2007.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang T, Zhang X, Obijuru L, et al. A micro-RNA signature associated with race, tumor size, and target gene activity in human uterine leiomyomas. Genes, chromosomes & cancer. 2007;46:336–347. doi: 10.1002/gcc.20415. [DOI] [PubMed] [Google Scholar]
- 35.Chabbert-Buffet N, Esber N, Bouchard P. Fibroid growth and medical options for treatment. Fertility and sterility. 2014;102:630–639. doi: 10.1016/j.fertnstert.2014.07.1238. [DOI] [PubMed] [Google Scholar]
- 36*.Doherty L, Mutlu L, Sinclair D, Taylor H. Uterine fibroids: clinical manifestations and contemporary management. Reproductive sciences. 2014;21:1067–1092. doi: 10.1177/1933719114533728. A very comprehensive review of all available treatments for fibroids, including those still being investigated in vitro, that should be useful for both clinicians and researchers. [DOI] [PubMed] [Google Scholar]
- 37.Sabry M, Al-Hendy A. Medical treatment of uterine leiomyoma. Reproductive sciences. 2012;19:339–353. doi: 10.1177/1933719111432867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moravek MB, Yin P, Ono M, et al. Ovarian steroids, stem cells and uterine leiomyoma: therapeutic implications. Human reproduction update. 2015;21:1–12. doi: 10.1093/humupd/dmu048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh SS, Belland L. Contemporary management of uterine fibroids: focus on emerging medical treatments. Current medical research and opinion. 2015;31:1–12. doi: 10.1185/03007995.2014.982246. [DOI] [PubMed] [Google Scholar]
- 40.Chumsri S, Howes T, Bao T, et al. Aromatase, aromatase inhibitors, and breast cancer. The Journal of steroid biochemistry and molecular biology. 2011;125:13–22. doi: 10.1016/j.jsbmb.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Michaud LB, Buzdar AU. Risks and benefits of aromatase inhibitors in postmenopausal breast cancer. Drug safety : an international journal of medical toxicology and drug experience. 1999;21:297–309. doi: 10.2165/00002018-199921040-00005. [DOI] [PubMed] [Google Scholar]
- 42.Lonning PE, Eikesdal HP. Aromatase inhibition 2013: clinical state of the art and questions that remain to be solved. Endocrine-related cancer. 2013;20:R183–201. doi: 10.1530/ERC-13-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parsanezhad ME, Azmoon M, Alborzi S, et al. A randomized, controlled clinical trial comparing the effects of aromatase inhibitor (letrozole) and gonadotropin-releasing hormone agonist (triptorelin) on uterine leiomyoma volume and hormonal status. Fertility and sterility. 2010;93:192–198. doi: 10.1016/j.fertnstert.2008.09.064. [DOI] [PubMed] [Google Scholar]
- 44.Duhan N, Madaan S, Sen J. Role of the aromatase inhibitor letrozole in the management of uterine leiomyomas in premenopausal women. European journal of obstetrics, gynecology, and reproductive biology. 2013;171:329–332. doi: 10.1016/j.ejogrb.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 45.Hilario SG, Bozzini N, Borsari R, Baracat EC. Action of aromatase inhibitor for treatment of uterine leiomyoma in perimenopausal patients. Fertility and sterility. 2009;91:240–243. doi: 10.1016/j.fertnstert.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 46.Varelas FK, Papanicolaou AN, Vavatsi-Christaki N, et al. The effect of anastrazole on symptomatic uterine leiomyomata. Obstetrics and gynecology. 2007;110:643–649. doi: 10.1097/01.AOG.0000279151.20878.60. [DOI] [PubMed] [Google Scholar]
- 47.Pavone ME, Bulun SE. Clinical review: The use of aromatase inhibitors for ovulation induction and superovulation. The Journal of clinical endocrinology and metabolism. 2013;98:1838–1844. doi: 10.1210/jc.2013-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shozu M, Murakami K, Segawa T, et al. Successful treatment of a symptomatic uterine leiomyoma in a perimenopausal woman with a nonsteroidal aromatase inhibitor. Fertility and sterility. 2003;79:628–631. doi: 10.1016/s0015-0282(02)04761-1. [DOI] [PubMed] [Google Scholar]
- 49.Spitz IM. Clinical utility of progesterone receptor modulators and their effect on the endometrium. Curr Opin Obstet Gynecol. 2009;21:318–324. doi: 10.1097/GCO.0b013e32832e07e8. [DOI] [PubMed] [Google Scholar]
- 50.Islam MS, Protic O, Giannubilo SR, et al. Uterine leiomyoma: available medical treatments and new possible therapeutic options. The Journal of clinical endocrinology and metabolism. 2013;98:921–934. doi: 10.1210/jc.2012-3237. [DOI] [PubMed] [Google Scholar]
- 51.Bouchard P, Chabbert-Buffet N, Fauser BC. Selective progesterone receptor modulators in reproductive medicine: pharmacology, clinical efficacy and safety. Fertility and sterility. 2011;96:1175–1189. doi: 10.1016/j.fertnstert.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 52.Chen W, Ohara N, Wang J, et al. A novel selective progesterone receptor modulator asoprisnil (J867) inhibits proliferation and induces apoptosis in cultured human uterine leiomyoma cells in the absence of comparable effects on myometrial cells. The Journal of clinical endocrinology and metabolism. 2006;91:1296–1304. doi: 10.1210/jc.2005-2379. [DOI] [PubMed] [Google Scholar]
- 53.Luo X, Yin P, Coon VJ, et al. The selective progesterone receptor modulator CDB4124 inhibits proliferation and induces apoptosis in uterine leiomyoma cells. Fertility and sterility. 2010;93:2668–2673. doi: 10.1016/j.fertnstert.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu Q, Takekida S, Ohara N, et al. Progesterone receptor modulator CDB-2914 down-regulates proliferative cell nuclear antigen and Bcl-2 protein expression and up-regulates caspase-3 and poly(adenosine 5′-diphosphate-ribose) polymerase expression in cultured human uterine leiomyoma cells. The Journal of clinical endocrinology and metabolism. 2005;90:953–961. doi: 10.1210/jc.2004-1569. [DOI] [PubMed] [Google Scholar]
- 55.Yoshida S, Ohara N, Xu Q, et al. Cell-type specific actions of progesterone receptor modulators in the regulation of uterine leiomyoma growth. Seminars in reproductive medicine. 2010;28:260–273. doi: 10.1055/s-0030-1251483. [DOI] [PubMed] [Google Scholar]
- 56.Sasaki H, Ohara N, Xu Q, et al. A novel selective progesterone receptor modulator asoprisnil activates tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated signaling pathway in cultured human uterine leiomyoma cells in the absence of comparable effects on myometrial cells. The Journal of clinical endocrinology and metabolism. 2007;92:616–623. doi: 10.1210/jc.2006-0898. [DOI] [PubMed] [Google Scholar]
- 57.Morikawa A, Ohara N, Xu Q, et al. Selective progesterone receptor modulator asoprisnil down-regulates collagen synthesis in cultured human uterine leiomyoma cells through up-regulating extracellular matrix metalloproteinase inducer. Human reproduction. 2008;23:944–951. doi: 10.1093/humrep/den025. [DOI] [PubMed] [Google Scholar]
- 58.Xu Q, Ohara N, Liu J, et al. Progesterone receptor modulator CDB-2914 induces extracellular matrix metalloproteinase inducer in cultured human uterine leiomyoma cells. Molecular human reproduction. 2008;14:181–191. doi: 10.1093/molehr/gan004. [DOI] [PubMed] [Google Scholar]
- 59.DeManno D, Elger W, Garg R, et al. Asoprisnil (J867): a selective progesterone receptor modulator for gynecological therapy. Steroids. 2003;68:1019–1032. doi: 10.1016/j.steroids.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 60.Blithe DL, Nieman LK, Blye RP, et al. Development of the selective progesterone receptor modulator CDB-2914 for clinical indications. Steroids. 2003;68:1013–1017. doi: 10.1016/s0039-128x(03)00118-1. [DOI] [PubMed] [Google Scholar]
- 61.Talaulikar VS, Manyonda IT. Ulipristal acetate: a novel option for the medical management of symptomatic uterine fibroids. Advances in therapy. 2012;29:655–663. doi: 10.1007/s12325-012-0042-8. [DOI] [PubMed] [Google Scholar]
- 62.Donnez J, Tomaszewski J, Vazquez F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. The New England journal of medicine. 2012;366:421–432. doi: 10.1056/NEJMoa1103180. [DOI] [PubMed] [Google Scholar]
- 63.Donnez J, Tatarchuk TF, Bouchard P, et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. The New England journal of medicine. 2012;366:409–420. doi: 10.1056/NEJMoa1103182. [DOI] [PubMed] [Google Scholar]
- 64*.Donnez J, Hudecek R, Donnez O, et al. Efficacy and safety of repeated use of ulipristal acetate in uterine fibroids. Fertility and sterility. 2015;103:519–527. e513. doi: 10.1016/j.fertnstert.2014.10.038. A randomized controlled trial showing equivalent efficacy between 5mg and 10 mg doses of ulipristal acetate in decreasing fibroid size, bleeding and pain, and increasing quality of life. [DOI] [PubMed] [Google Scholar]
- 65**.Donnez J, Vazquez F, Tomaszewski J, et al. Long-term treatment of uterine fibroids with ulipristal acetate. Fertility and sterility. 2014;101:1565–1573. e1561–1518. doi: 10.1016/j.fertnstert.2014.02.008. The first clinical trial of repeated courses of ulipristal acetate, up to four 3-month courses. Showed that ulipristal acetate continues to be just as effective over time, and is tolerated well by patients. This study raises the possibility that ulipristal could be the first long-term treatment of uterine fibroids. [DOI] [PubMed] [Google Scholar]
- 66.Mutter GL, Bergeron C, Deligdisch L, et al. The spectrum of endometrial pathology induced by progesterone receptor modulators. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2008;21:591–598. doi: 10.1038/modpathol.2008.19. [DOI] [PubMed] [Google Scholar]
- 67.Williams AR, Bergeron C, Barlow DH, Ferenczy A. Endometrial morphology after treatment of uterine fibroids with the selective progesterone receptor modulator, ulipristal acetate. International journal of gynecological pathology : official journal of the International Society of Gynecological Pathologists. 2012;31:556–569. doi: 10.1097/PGP.0b013e318251035b. [DOI] [PubMed] [Google Scholar]
- 68.Szotek PP, Chang HL, Zhang L, et al. Adult mouse myometrial label-retaining cells divide in response to gonadotropin stimulation. Stem cells. 2007;25:1317–1325. doi: 10.1634/stemcells.2006-0204. [DOI] [PubMed] [Google Scholar]
- 69.Arango NA, Szotek PP, Manganaro TF, et al. Conditional deletion of beta-catenin in the mesenchyme of the developing mouse uterus results in a switch to adipogenesis in the myometrium. Developmental biology. 2005;288:276–283. doi: 10.1016/j.ydbio.2005.09.045. [DOI] [PubMed] [Google Scholar]
- 70.Ono M, Maruyama T, Masuda H, et al. Side population in human uterine myometrium displays phenotypic and functional characteristics of myometrial stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:18700–18705. doi: 10.1073/pnas.0704472104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mas A, Cervello I, Gil-Sanchis C, et al. Identification and characterization of the human leiomyoma side population as putative tumor-initiating cells. Fertility and sterility. 2012;98:741–751. e746. doi: 10.1016/j.fertnstert.2012.04.044. [DOI] [PubMed] [Google Scholar]
- 72.Ono M, Qiang W, Serna VA, et al. Role of stem cells in human uterine leiomyoma growth. PloS one. 2012;7:e36935. doi: 10.1371/journal.pone.0036935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chang HL, Senaratne TN, Zhang L, et al. Uterine leiomyomas exhibit fewer stem/progenitor cell characteristics when compared with corresponding normal myometrium. Reproductive sciences. 2010;17:158–167. doi: 10.1177/1933719109348924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ono M, Yin P, Navarro A, et al. Paracrine activation of WNT/beta-catenin pathway in uterine leiomyoma stem cells promotes tumor growth. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:17053–17058. doi: 10.1073/pnas.1313650110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jordan CT, Guzman ML, Noble M. Cancer stem cells. The New England journal of medicine. 2006;355:1253–1261. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- 76.Challen GA, Little MH. A side order of stem cells: the SP phenotype. Stem cells. 2006;24:3–12. doi: 10.1634/stemcells.2005-0116. [DOI] [PubMed] [Google Scholar]
- 77.Golebiewska A, Brons NH, Bjerkvig R, Niclou SP. Critical appraisal of the side population assay in stem cell and cancer stem cell research. Cell stem cell. 2011;8:136–147. doi: 10.1016/j.stem.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 78*.Yin P, Ono M, Moravek MB, et al. Human Uterine Leiomyoma Stem/Progenitor Cells Expressing CD34 and CD49b Initiate Tumors In Vivo. The Journal of clinical endocrinology and metabolism. 2015;100:E601–606. doi: 10.1210/jc.2014-2134. Reported ovel technique of isolating fibroid stem cells that avoids the pitfalls of side population technique, making further study of fibroid stem cells more attainable. Additionally, the technique revealed a third population of fibroid cells that has yet to be characterized, but likely has a role in interactions between fibroid stem cells and differentiated cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Simon C. Introduction: Are we advancing in our scientific understanding and therapeutic improvement of uterine fibroids… or not? Fertility and sterility. 2014;102:611–612. doi: 10.1016/j.fertnstert.2014.07.1210. [DOI] [PubMed] [Google Scholar]
- 80*.Mehine M, Makinen N, Heinonen HR, et al. Genomics of uterine leiomyomas: insights from high-throughput sequencing. Fertility and sterility. 2014;102:621–629. doi: 10.1016/j.fertnstert.2014.06.050. A comprehensive review of the current state of knowledge on fibroid genomics that would be a good starting point for researchers interested in this field for informing further studies. [DOI] [PubMed] [Google Scholar]
- 81.Bertsch E, Qiang W, Zhang Q, et al. MED12 and HMGA2 mutations: two independent genetic events in uterine leiomyoma and leiomyosarcoma. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2014 doi: 10.1038/modpathol.2013.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Heinonen HR, Sarvilinna NS, Sjoberg J, et al. MED12 mutation frequency in unselected sporadic uterine leiomyomas. Fertility and sterility. 2014;102:1137–1142. doi: 10.1016/j.fertnstert.2014.06.040. [DOI] [PubMed] [Google Scholar]
- 83.Hodge JC, Kim TM, Dreyfuss JM, et al. Expression profiling of uterine leiomyomata cytogenetic subgroups reveals distinct signatures in matched myometrium: transcriptional profilingof the t(12;14) and evidence in support of predisposing genetic heterogeneity. Hum Mol Genet. 2012;21:2312–2329. doi: 10.1093/hmg/dds051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hammond SM, Sharpless NE. HMGA2, microRNAs, and stem cell aging. Cell. 2008;135:1013–1016. doi: 10.1016/j.cell.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Markowski DN, Helmke BM, Belge G, et al. HMGA2 and p14Arf: major roles in cellular senescence of fibroids and therapeutic implications. Anticancer Res. 2011;31:753–761. [PubMed] [Google Scholar]
- 86.Peng Y, Laser J, Shi G, et al. Antiproliferative effects by Let-7 repression of high-mobility group A2 in uterine leiomyoma. Mol Cancer Res. 2008;6:663–673. doi: 10.1158/1541-7786.MCR-07-0370. [DOI] [PubMed] [Google Scholar]
- 87.Makinen N, Mehine M, Tolvanen J, et al. MED12, the mediator complex subunit 12 gene, is mutated at high frequency in uterine leiomyomas. Science. 2011;334:252–255. doi: 10.1126/science.1208930. [DOI] [PubMed] [Google Scholar]
- 88.Kim S, Xu X, Hecht A, Boyer TG. Mediator is a transducer of Wnt/beta-catenin signaling. The Journal of biological chemistry. 2006;281:14066–14075. doi: 10.1074/jbc.M602696200. [DOI] [PubMed] [Google Scholar]
- 89.Huang S, Holzel M, Knijnenburg T, et al. MED12 Controls the Response to Multiple Cancer Drugs through Regulation of TGF-beta Receptor Signaling. Cell. 2012;151:937–950. doi: 10.1016/j.cell.2012.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guo X, Wang XF. A mediator lost in the war on cancer. Cell. 2012;151:927–929. doi: 10.1016/j.cell.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 91.Lee BS, Nowak RA. Human leiomyoma smooth muscle cells show increased expression of transforming growth factor-beta 3 (TGF beta 3) and altered responses to the antiproliferative effects of TGF beta. The Journal of clinical endocrinology and metabolism. 2001;86:913–920. doi: 10.1210/jcem.86.2.7237. [DOI] [PubMed] [Google Scholar]
- 92.Catherino WH, Leppert PC, Stenmark MH, et al. Reduced dermatopontin expression is a molecular link between uterine leiomyomas and keloids. Genes, chromosomes & cancer. 2004;40:204–217. doi: 10.1002/gcc.20035. [DOI] [PMC free article] [PubMed] [Google Scholar]


