Abstract
Since the 2013 description of Blastomyces gilchristii, research describing the virulence or clinical outcome of B. gilchristii infection has been lacking. We report molecular evidence of B. gilchristii as an etiologic agent of fatal acute respiratory distress syndrome. B. gilchristii infection was confirmed by PCR and sequence analysis.
Keywords: acute respiratory distress syndrome, Blastomyces dermatitidis, Blastomyces gilchristii, fungi, blastomycosis, respiratory infections, virulence
Differences in virulence have long been observed between different strains of Blastomyces dermatitidis (1,2). Following the 2013 description of B. gilchristii as a cryptic species of Blastomyces spp., B. dermatitidis and B. gilchristii have been recognized as etiologic agents of blastomycosis (3). However, research evaluating the differences in clinical outcome between B. dermatitidis and B. gilchristii infection has been lacking. Here we demonstrate that B. gilchristii infection can cause fatal acute respiratory distress syndrome (ARDS) in humans.
The Study
A 27-year-old woman sought care at an emergency department in a rural community in northwestern Ontario, Canada, for a nonproductive cough, right-sided chest heaviness, nausea, and abdominal pain that had developed over the previous week. She had a previous diagnosis of type 1 diabetes mellitus and a history of intravenous drug use.
On examination, deep and labored breathing was observed, and decreased air entry in the left upper lung was heard on auscultation. A chest radiograph revealed left upper lobe consolidation (Figure 1, panel A). Blood chemical analysis revealed diabetic ketoacidosis (25.4 mmol/L glucose, 3.2 mmol/L K+, 6.0 mmol/L HCO3−, anion gap 27 mg/dL, and pH 7.0 venous blood gas). Intravenous fluoroquinolone was administered for suspected left upper lobe bacterial pneumonia, and fluid resuscitation and intravenous insulin were administered for diabetic ketoacidosis.
Figure 1.
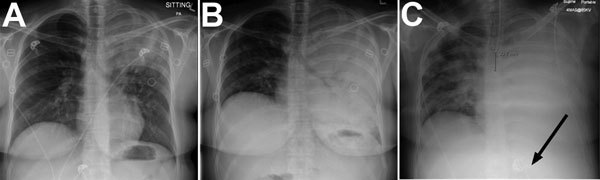
Chest radiograph at various stages of Blastomyces gilchristii infection in a 27-year-old woman, Ontario, Canada. A) Day 0: posterior–anterior (PA) chest radiograph at initial emergency department examination. Discrete confluent left upper lobe consolidation with air bronchograms are visible. B) Day 5, 15:10: PA chest radiograph demonstrating complete confluent opacification of the left hemithorax with extensive air bronchograms. C) Day 6, 23:30: PA chest radiograph postintubation with confluent left lung consolidation and new right patchy airspace opacification. Arrow indicates the correct placement of a nasogastric tube.
During the first 2 days in hospital, the patient was stable and afebrile, despite remaining tachycardic and tachypneic. From day 3 to day 4, the patient became febrile (38.3°C) and tachypnea worsened to the point that >4 L of O2 was required to maintain O2 saturation >90%. The patient’s leukocyte count was elevated at 13.5 × 109 cells/L.
On day 5, the patient was observed to be using accessory muscles of respiration. A chest radiograph was performed and showed a complete whiteout of the left lung (Figure 1, panel B). Air transfer to a tertiary hospital was requested but unavailable. The patient’s mean arterial blood pressure decreased from 80 to 65 mm Hg, leukocyte count increased to 18.8 × 109 cells/L, and hemoglobin decreased from 140 to 102 g/L. A chest radiograph revealed patchy opacification of the right lung that was not observed several hours earlier (Figure 1, panel C). After intubation and sedation, the patient was successfully transferred to the intensive care unit of a tertiary hospital.
On arrival at the intensive care unit, the patient was put on a mechanical ventilator requiring positive-end expiratory pressure to oxygenate. Antimicrobial drugs (vancomycin, piperacillin/tazobactam, and azithromycin), an antifungal drug (amphotericin B), and a vasopressor (norepinephrine) were administered, and sputum cultures were sent for bacterial and fungal culture. One day after entering the intensive care unit, the patient became bradycardic and went into cardiac arrest. Cause of death was determined to be ARDS (Figure 2).
Figure 2.
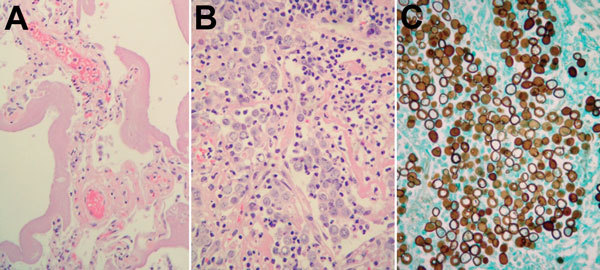
Histologic manifestations of Blastomyces gilchristii infection in a 27-year-old woman, Ontario, Canada. A) Nonconsolidated lung. Thick hyaline membranes, characteristic of diffuse alveolar damage and acute respiratory distress syndrome, line the alveoli. Hematoxylin and eosin (H&E) stain, original magnification ×400. B) Consolidated lung. Alveoli are completely filled with B. gilchristii yeast cells and neutrophils. B. gilchristii cells are pale bluish-gray with a distinct cell border. H&E stain, original magnification ×100. C) Consolidated lung containing abundant B. gilchristii yeast cells with characteristic broad-based buds. Grocott methenamine silver stain, original magnification ×400.
A sputum specimen was prepared for direct microscopic examination for fungal elements by staining with Calcofluor White (Sigma-Aldrich, St. Louis, MO, USA) and for fungal culture by plating onto BHICCGE (brain heart infusion + 5% sheep blood + 50 μg/mL chloramphenicol, 0.01% cycloheximide, 20 μg/mL gentamicin, and egg white) agar and inhibitory mold agar plates and incubated at 28°C. Direct microscopic examination of the sputum specimen revealed broad-based budding yeast with refractile cell walls characteristic of Blastomyces. After appropriate culture and incubation, small colonies of mold were observed after 1 week. The identification of a Blastomyces sp. was confirmed with a molecular probe (AccuProbe Blastomyces dermatitidis Culture Identification Test; Gen-Probe, San Diego, CA, USA) and by conversion of the mold-form to the yeast-phase using Blasto-D medium and incubation at 37°C (4). The culture was preserved at −80°C as a glycerol stock with the designation 13BL347.
The isolate was later resurrected and incubated as previously described (4). Using a similar method described in Brown et al. (3), fungal DNA was extracted, the internal transcribed spacer 2 (ITS2) region was amplified by PCR using the primers described in White et al. (5), and the resulting product was sequenced. Sequences were analyzed, and the single nucleotide polymorphism in ITS2 at position 19 (used for differentiation of B. dermatitidis from B. gilchristii) was noted (3). The sequence of 13BL347 ITS2 possessed a cytosine at position 19, which is diagnostic for B. gilchristii, whereas thymine at that position is conserved in B. dermatitidis. By using a multiple sequence alignment tool, we aligned the 13BL347 ITS2 sequence to sequences of several well-characterized representative sequences of both species (1,3); it clustered with other B. gilchristii strains (Technical Appendix) (6).
Variation in clinical presentation among different genetic groups of B. dermatitidis has long been observed (1–3). However, since the 2013 identification of B. gilchristii, it is unclear as to whether the previous reports were actually reporting differences in virulence between B. dermatitidis and B. gilchristii. Therefore, we refer to the fungi that were the subject of previous blastomycosis studies as Blastomyces spp. rather than B. dermatitidis.
Known virulence factors of Blastomyces spp. include the cell surface carbohydrate polymer alpha-1,3-glucan and the Blastomyces adhesion 1 protein (formerly WI-1) surface adhesion molecule (2). To our knowledge, differences in expression of these virulence factors in B. dermatitidis and B. gilchristii have not been compared. It has been demonstrated previously that African strains of B. dermatitidis differ from North American strains in their growth and morphology and are thought to cause less severe disease (2). Genetic analysis has identified 2 distinct genetic groups of African B. dermatitidis that differ in expression of the Blastomyces adhesion 1 protein surface adhesion molecule (2). Whether the differences in growth, morphology, and apparent virulence in the African Blastomyces sp. is attributable to B. gilchristii and B. dermatitidis, intraspecies variation, or a separate undescribed genetic group remains unexplored.
There are several challenges in evaluating the virulence of B. dermatitidis and B. gilchristii. First, the clinical course of blastomycosis has been found to be correlated with the amount of inoculum (conidia) initially acquired (typically through inhalation) (7). In the context of human infection, it is difficult to determine if differences in disease process are attributable to differences in fungal virulence or the magnitude of inoculum. Furthermore, host factors such as immunodeficiency and variation in human leukocyte antigen profile have been shown to cause variation in immune response to Blastomyces spp. (8).
Blastomyces spp. have also been observed to lose virulence during laboratory processing, making the results of previous laboratory-based virulence studies questionable (7). Another challenge is that the commercially available molecular probe that is often used to confirm the identification of Blastomyces spp. cannot differentiate between B. dermatitidis and B. gilchristii. Currently, B. dermatitidis and B. gilchristii cannot be distinguished based on phenotype; PCR followed by sequence analysis is the only method of differentiating these species.
Conclusions
Most cases of blastomycosis-induced ARDS are preceded by a prodrome of pneumonia weeks to months before development of ARDS (9,10). However, in a minority of cases, such as the one we describe, the clinical course rapidly progresses to fatal ARDS (9,10). This patient died within 7 days of hospital admission and <24 hours after intubation. In this case, it is difficult to comment on the virulence of B. gilchristii because the patient’s immune status is uncertain. The patient had uncontrolled hyperglycemia secondary to type 1 diabetes mellitus, history of intravenous drug use, and an unknown HIV status; these factors are known to cause immune dysfunction and might have contributed to the acuity and severity of disease. Nevertheless, our findings demonstrate that B. gilchristii infection can cause fatal ARDS in humans and form a basis for further virulence and epidemiologic studies of B. dermatitidis and B. gilchristii.
Clustal alignment of sequenced region of internal transcribed spacer 2 of Blastomyces dermatitidis and B. gilchristii.
Acknowledgments
The authors are grateful to Marina Ulanova for providing a critical review of the manuscript and to Michael Wilson for helpful advice.
D.D. was funded by a Professional Student Research Award from the Canadian Institute of Health Research.
Biography
Mr. Dalcin is a medical student at the Northern Ontario School of Medicine in Thunder Bay, Ontario, Canada, with an interest in infectious diseases.
Footnotes
Suggested citation for this article: Dalcin D, Rothstein A, Spinato J, Escott N, Kus JV. Blastomyces gilchristii as cause of fatal acute respiratory distress syndrome. Emerg Infect Dis. 2016 Feb [date cited]. http://dx.doi.org/10.3201/eid2202.151183
References
- 1.Meece JK, Anderson JL, Gruszka S, Sloss BL, Sullivan B, Reed KD. Variation in clinical phenotype of human infection among genetic groups of Blastomyces dermatitidis. J Infect Dis. 2013;207:814–22. 10.1093/infdis/jis756 [DOI] [PubMed] [Google Scholar]
- 2.Klein BS, Aizenstein BD, Hogan LH. African strains of Blastomyces dermatitidis that do not express surface adhesin WI-1. Infect Immun. 1997;65:1505–9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown EM, McTaggart LR, Zhang SX, Low DE, Stevens DA, Richardson SE. Phylogenetic analysis reveals a cryptic species Blastomyces gilchristii, sp. nov. within the human pathogenic fungus Blastomyces dermatitidis. PLoS ONE. 2013;8:e59237. 10.1371/journal.pone.0059237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kane J. Conversion of Blastomyces dermatitidis to the yeast form at 37 degrees C and 26 degrees C. J Clin Microbiol. 1984;20:594–6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White TJ, Bruns T, Lee S, Taylor JW. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. New York: Academic Press; 1990. p. 315. [Google Scholar]
- 6.Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 2011;7:539 . 10.1038/msb.2011.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevens DA, Brummer E, DiSalvo AF, Ganer A. Virulent isolates and mutants of Blastomyces in mice: a legacy for studies of pathogenesis. Semin Respir Infect. 1997;12:189–95 . [PubMed] [Google Scholar]
- 8.Chang WL, Audet RG, Aizenstein BD, Hogan LH, DeMars RI, Klein BS. T-Cell epitopes and human leukocyte antigen restriction elements of an immunodominant antigen of Blastomyces dermatitidis. Infect Immun. 2000;68:502–10. 10.1128/IAI.68.2.502-510.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyer KC, McManus EJ, Maki DG. Overwhelming pulmonary blastomycosis associated with the adult respiratory distress syndrome. N Engl J Med. 1993;329:1231–6. 10.1056/NEJM199310213291704 [DOI] [PubMed] [Google Scholar]
- 10.Lemos LB, Baliga M, Guo M. Acute respiratory distress syndrome and blastomycosis: presentation of nine cases and review of the literature. Ann Diagn Pathol. 2001;5:1–9. 10.1053/adpa.2001.21473 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clustal alignment of sequenced region of internal transcribed spacer 2 of Blastomyces dermatitidis and B. gilchristii.


