Adventitious roots have varied origins and functions, as illustrated by three case studies that highlight their physiology under flooding, nutrient deficiency, and wounding stress.
Abstract
Adventitious roots are plant roots that form from any nonroot tissue and are produced both during normal development (crown roots on cereals and nodal roots on strawberry [Fragaria spp.]) and in response to stress conditions, such as flooding, nutrient deprivation, and wounding. They are important economically (for cuttings and food production), ecologically (environmental stress response), and for human existence (food production). To improve sustainable food production under environmentally extreme conditions, it is important to understand the adventitious root development of crops both in normal and stressed conditions. Therefore, understanding the regulation and physiology of adventitious root formation is critical for breeding programs. Recent work shows that different adventitious root types are regulated differently, and here, we propose clear definitions of these classes. We use three case studies to summarize the physiology of adventitious root development in response to flooding (case study 1), nutrient deficiency (case study 2), and wounding (case study 3).
ECONOMY, ECOLOGY, AND EXISTENCE
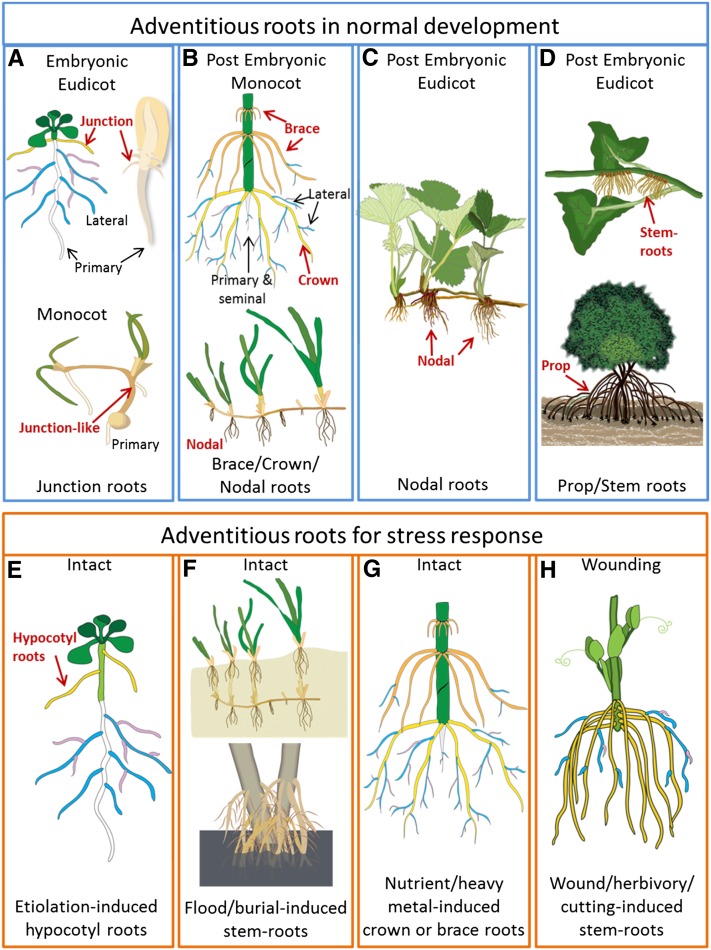
In plants, roots that form from nonroot tissues are known as adventitious roots. This general definition distinguishes adventitious roots from primary and lateral roots. However, there are subgroups of adventitious roots that can be formed as a stress response and during normal development. Figure 1 illustrates some examples of this diversity of adventitious root types, including but not restricted to junction roots; nodal roots (both crown and brace roots in monocots and nodal roots in eudicots such as strawberry [Fragaria spp.]); nonnodal prop or stem roots used for support (as in ivy and mangroves); stress-induced roots (Arabidopsis [Arabidopsis thaliana] etiolated hypocotyl, flooding, burial, and dark induced); and roots formed in response to soil chemicals (nutrient deficiency and heavy metals) or wounding (on cuttings).
Figure 1.
Examples of adventitious root types. This figure highlights a few examples of the diversity of adventitious roots. A to D show types of adventitious roots that form during normal development, including those potentially established in the embryo (A); the dominant root system of monocots, including maize (top image) crown roots (yellow) and brace roots (orange) and nodal roots on other grasses (bottom image; B) and on eudicots such as strawberry (C); and nonnodal roots that provide support for plants such as ivy (top image) and mangroves (bottom image; D). E to H show adventitious root development under stressed conditions: Arabidopsis under low or no light (used as a model for adventitious root regulation; E); burial (top image) or flooding (bottom image) can induce adventitious roots from either nodal or nonnodal stem positions (F); nutrient or heavy metal stress increases adventitious root development (G); and wounding such as cutting induces de novo adventitious root development (H). Primary and seminal roots are depicted in white, first order lateral roots in blue, and second order lateral roots in pink.
Recent work has shown that many of these root types are differentially regulated (Hochholdinger et al., 2004; Atkinson et al., 2014; Bellini et al., 2014; Pacurar et al., 2014), and this likely impacts their function and physiology. As a result, we suggest that descriptions of adventitious roots be precise; to this end, we have composed Table I to provide clear descriptions, which include the conditions triggering each specific type of adventitious root development. For example, roots that form on stems in response to flooding are described as flood-induced stem roots; likewise, crown roots that form as a result of flooding are described as flood-induced crown roots. This scheme can be applied to most plant root systems and will help the plant community clarify differences among root types.
Table I. Adventitious root descriptions based on physical characteristics and induction conditions.
| Parameter | Junction Roots | Hypocotyl Roots | Crown Roots | Brace Roots | Nodal Roots | Stem Roots | Prop Roots |
|---|---|---|---|---|---|---|---|
| Definition | Roots that form specifically at the root-shoot junction (e.g. Arabidopsis and bean) | Roots that form on the hypocotyl (e.g. bean and tomato) | Nodal roots that form below soil level as part of normal postembryonic development (e.g. rice, wheat, and maize) | Nodal roots that form above soil level as part of normal postembryonic development (e.g. maize) | Roots that form from nodes (e.g. strawberry and grass rhizomes) | Roots that form from internode (e.g. ivy) | Roots used for physical support (e.g. mangroves, where they also aid in aeration via lenticels and aerenchyma) |
| Induced by | |||||||
| Flood | Flood-induced hypocotyl roots | Flood-induced crown roots | Flood-induced brace roots | Flood-induced nodal roots | Flood-induced stem roots | ||
| Burial (including layering in propagation) | Burial-induced nodal roots | Burial-induced stem roots | |||||
| Etiolation | Etiolation-induced hypocotyl roots | ||||||
| Wound | Wound-induced stem roots | Wound-induced prop roots | |||||
| Heavy metals | Heavy-metal-induced crown roots | Heavy metal-induced brace roots | Heavy metal-induced nodal roots | Heavy metal-induced stem roots | |||
| Nutrient | Nutrient-induced crown roots | Nutrient-induced brace roots | Nutrient-induced nodal roots |
Economically, adventitious roots are very important. Propagation using cuttings is central to many forestry and horticulture industries, including the production of woody crops like apple (Malus domestica), grapes (Vitis vinifera), and stone fruit. In addition, cereal crops depend on a root system dominated by crown and brace roots.
Ecologically, adventitious roots are important for stabilizing shifting environments such as coastal regions (seagrasses; Ondiviela et al., 2014), estuaries, and river flood plains (Krauss et al., 2003). They are important for plant survival under abiotic and biotic stress conditions and are induced during flooding in a wide range of species (see case study 1). They are also important in response to other stresses, including heavy metals (for review, see Steffens, 2014), burial (Dech and Maun, 2006), drought (Liao et al., 2012), nutrient deficiencies (see case study 2), and biotic or abiotic wounding (Simberloff et al., 1978). This wound-induced adventitious rooting is the basis of cutting propagation (see case study 3).
In addition to the economic and ecological importance of adventitious roots, they play a key role for our existence. The cereal crops wheat (Triticum aestivum), rice (Oryza sativa), and maize (Zea mays) provide 60% of global caloric intake (Food and Agriculture Organisation of the United Nations). Cereal crops, like most monocots, rely on root systems composed almost exclusively of adventitious roots. If we are to achieve global food security, we need to improve food production in the face of increasing extreme weather events such as floods. In addition, we must do this more sustainably through reduced fertilizer applications. Adventitious roots have evolved to help plants tolerate a variety of stressful conditions, and understanding the importance of these adventitious root types in many crops will aid our development of nutrient-efficient and environmentally resilient crops.
To highlight the potential of exploiting adventitious roots for crop improvement, we now present three case studies of adventitious root physiology, focusing on responses to flooding, nutrient deficiency, and wounding.
CASE STUDY 1: FLOOD-INDUCED ADVENTITIOUS ROOTS
Flooding is a severe abiotic stress that is increasing in frequency worldwide (Brakenridge). One key aspect of flooding is the 10,000-fold slower diffusion rate of gases in water compared with that in air (Armstrong et al., 1991; Blom and Voesenek, 1996; Bailey-Serres and Voesenek, 2008), resulting in low oxygen availability and the trapping of gases in submerged tissues. Higher plants have evolved many metabolic and morphological adaptations to flooding (Bailey-Serres et al., 2012; Voesenek and Bailey-Serres, 2013; Abbas et al., 2015). A key response of many species, including rice (Lorbiecke and Sauter, 1999), Rumex spp. (Visser et al., 1996), tamarack (Larix laricina; Calvo-Polanco et al., 2012), Eucalyptus spp. (Argus et al., 2015), and tomato (Solanum lycopersicum), is the emergence of adventitious roots.
In rice, bittersweet (Solanum dulcamara), and Rumex palustris, adventitious root primordia form during normal development and, upon flooding, can emerge as roots. In sunflower (Helianthus annuus), some tomato cultivars (Kramer, 1951; Negi et al., 2010; Dawood et al., 2014), and trees such as Eucalyptus spp. and tamarack, adventitious roots develop de novo upon flooding stress (Table II). Submergence-induced adventitious root growth is a complex process mediated by cell division in the root apical meristem and elongation of basal cells in root primordia (Lorbiecke and Sauter, 1999).
Table II. Comparison of adventitious root growth induced by flooding in some model species.
| Species | Adventitious Root Primordia | Adventitious Root Emergence | Reference |
|---|---|---|---|
| Poaceae | |||
| Deepwater rice | Preformed at nodes | 10 h after onset of partial submergence | Lorbiecke and Sauter (1999) |
| Asteraceae | |||
| Sunflower | De novo upon flooding | 2 d after onset of waterlogging | Wample and Reid (1978) |
| Solanaceae | |||
| Bittersweet | Preformed at the main stem and branches | 2 to 3 d after onset of partial submergence at 20°C or after 7 d at 13°C | Dawood et al. (2014); Zhang et al. (2015) |
| Tomato | Preformed or de novo upon flooding | 1 d after onset of flooding at hypocotyls; 2 to 3 d after onset of flooding in 4-week-old plants | Vidoz et al. (2010) |
| Wetland species | |||
| R. palustris | Preformed | 2 d after onset of waterlogging | Visser et al. (1996) |
| Flood-tolerant trees | |||
| E. camaldulensis ssp. refulgens | De novo upon flooding | 5 d of waterlogging at seedling stage | Argus et al. (2015) |
| Tamarack | De novo upon flooding | Time unknown; analyzed after 6 months of flooding | Calvo-Polanco et al. (2012) |
The timing of flood-induced adventitious root emergence is species specific (Lorbiecke and Sauter, 1999; Dawood et al., 2014; Argus et al., 2015) and depends on the developmental stage of the plant, the water temperature (Zhang et al., 2015) and depth (e.g. soil waterlogging, partial or complete submergence), and the flood duration (summarized in Table II). In rice, root emergence also depends on the age and developmental stage of the respective node (Lorbiecke and Sauter, 1999; Steffens et al., 2012). At the third and fourth nodes, adventitious root emergence occurs earlier than at more apical nodes, because in older nodes, almost all root primordia tips are in direct contact with the epidermis, while in younger nodes, parenchymal cells cover the root primordia (Steffens et al., 2012).
Adventitious roots facilitate gas transport and water and nutrient uptake during flooding. Following flooding, they help take up nutrients and ensure plant survival (Sauter, 2013). In the next sections, we summarize the signals and morphological changes involved in flood-induced adventitious root formation and growth in both monocots (e.g. rice) and eudicots (e.g. tomato).
Adventitious Root Growth Regulation upon Flooding
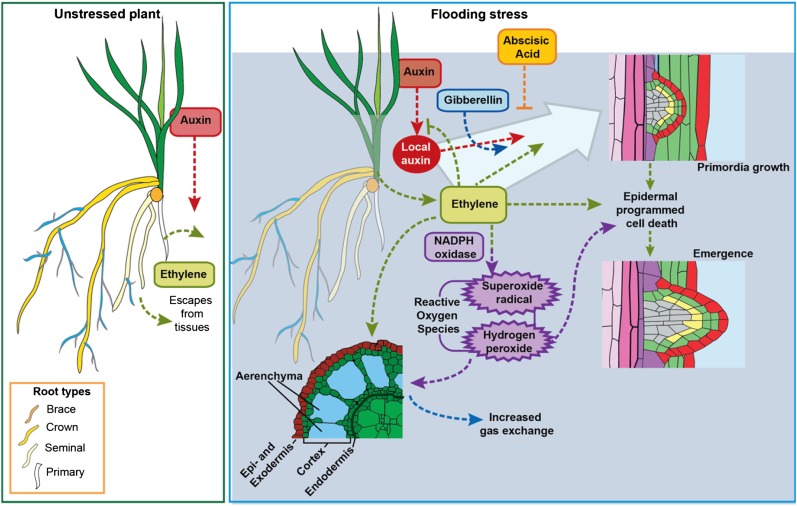
During submergence, ethylene biosynthesis increases in deepwater rice plants (Raskin and Kende, 1984a, 1984b; Kende et al., 1998), and because it is a gas, it also accumulates due to physical entrapment (Fig. 2; Musgrave et al., 1972). Ethylene is the major hormone that induces adventitious root growth in rice (Lorbiecke and Sauter, 1999) and tomato (Kim et al., 2008; Negi et al., 2010; Vidoz et al., 2010). The production of ethylene increases in submerged tomato plants due to enhanced ethylene biosynthesis via the rate-limiting enzyme 1-aminocyclopropane-1-carboxylic acid synthase (Vidoz et al., 2010). This increased ethylene promotes adventitious root formation through NEVER-RIPE (NR; Kim et al., 2008; Negi et al., 2010; Vidoz et al., 2010), which encodes the ethylene receptor LeETR3 of subfamily I of the LeETR1 to LeETR6 gene family (Wilkinson et al., 1995). NR-deficient tomato plants are ethylene insensitive and exhibit a reduced number of adventitious roots either upon submergence or after ethylene treatment (Clark et al., 1999; Vidoz et al., 2010).
Figure 2.
Adventitious root development in response to flooding. Under aerated conditions, gaseous ethylene escapes from plant tissues, but during flooding, water acts as a physical barrier, trapping ethylene in the plant. GA enhances the ethylene-promoted adventitious root growth, while abscisic acid reduces the effect. Ethylene triggers reactive oxygen species production, and together they trigger epidermal programmed cell death for root emergence and cortical programmed cell death lysigenous aerenchyma formation. The main difference in some eudicots (e.g. tomato) is the requirement for de novo adventitious root initiation via auxin and ethylene signaling. In the cross section, epidermis and exodermis are combined, but the exodermis can be several cell layers adjacent to the epidermis. Yellow roots are adventitious roots, blue and pink roots are lateral roots, and white roots are primary roots. Pointed arrows represent positive interactions, and flat-ended arrows represent negative interactions.
In rice, ethylene-mediated adventitious root development also requires signaling via auxin (Fig. 2; Zhou et al., 2003; Pacurar et al., 2014). Auxin is well known to regulate adventitious root development. In flooded rice plants, inhibitor studies using N-1-naphthylphthalamic acid indicate that polar auxin transport through the PIN-FORMED (PIN) family of auxin efflux carriers is required for adventitious root growth both in adult plants (B. Steffens, unpublished data) and in seedlings (Xu et al., 2005). OsPIN1 RNA interference transgenic plants exhibit the same number of adventitious root primordia but show fewer emerged adventitious roots, indicating that PIN1 is involved in root emergence but not initiation (Xu et al., 2005). In addition, auxin transport is a prerequisite for adventitious root development in tomato (Tyburski and Tretyn, 2004; Negi et al., 2010; Vidoz et al., 2010). Together with ethylene, auxin positively regulates adventitious root initiation through DIAGEOTROPICA (DGT; Vidoz et al., 2010; Lombardi-Crestana et al., 2012), which encodes SlCYP1, a cyclophilin A-type protein. SlCYP1 changes the abundance of auxin efflux carriers of the PIN family at the plasma membrane and, hence, modulates polar auxin transport (Oh et al., 2006; Ivanchenko et al., 2015; Retzer and Luschnig, 2015; Spiegelman et al., 2015). dgt mutant plants exhibited low sensitivity to auxin and inhibited submergence-induced ethylene biosynthesis, resulting in a reduced number of adventitious roots relative to the wild type (Vidoz et al., 2010).
In rice, the development of nodal adventitious root primordia requires the transcription factors CROWN ROOTLESS5 (CRL5) and ADVENTITIOUS ROOTLESS1 (ARL1 [also named CRL1]; Suge, 1985; Bleecker et al., 1986; Inukai et al., 2005; Liu et al., 2005). CRL5 belongs to the APETALA2/ETHYLENE RESPONSE FACTOR gene family, and ARL1 is an ethylene- and auxin-responsive gene that belongs to the ASYMMETRIC LEAVES2/LATERAL ORGAN BOUNDARIES domain gene family (Inukai et al., 2005; Liu et al., 2005). Both CRL5 and ARL1 are targets of transcription factors of the AUXIN RESPONSE FACTOR gene family, which link crown root development to auxin signaling (for review, see Sauter and Steffens, 2014).
Another class of hormones suggested to modulate polar auxin transport is the terpenoid lactone strigolactones (see case studies 2 and 3). However, the involvement of strigolactones in submergence-induced adventitious root development has yet to be determined.
Ethylene-induced adventitious root growth in adult rice plants upon flooding is mediated not only by hormonal signals but also by reactive oxygen species (Fig. 2; Steffens et al., 2012). Reactive oxygen species such as superoxide anion and hydrogen peroxide are created as a normal part of development, and antioxidant enzymes maintain normal homeostatic levels (for review, see Steffens, 2014). Superoxide anions are converted to hydrogen peroxide by superoxide dismutase and/or peroxidase enzymes (for review, see Steffens, 2014). Catalase then detoxifies hydrogen peroxide, which is involved in both signaling and programmed cell death. Under stress conditions, the amount of reactive oxygen species changes via either increased production of reactive oxygen species or changes in antioxidant levels (Bouranis et al., 2003; Steffens, 2014). In flooded rice plants, ethylene enhances superoxide anion generation by plasma membrane-located NADPH oxidase (Fig. 2). The accumulation of endogenous reactive oxygen species enhanced root growth through inhibition of the scavenging enzyme catalase (with aminotriazole), whereas hydrogen peroxide scavenging (with potassium iodide) inhibited this growth (Steffens et al., 2012), demonstrating the importance of hydrogen peroxide in crown root growth.
In rice, nodal adventitious root primordia never break through the epidermis without an exogenous trigger and internal ethylene accumulation (Steffens et al., 2006). When stimulated to grow, these nodal adventitious roots exert a mechanical force on the overlying epidermal cells, resulting in epidermal programmed cell death (Steffens et al., 2012). Epidermal programmed cell death above root primordia ensures that the tip of the growing root is not damaged during emergence (Mergemann and Sauter, 2000). Both epidermal programmed cell death and adventitious root growth are regulated through the interaction of ethylene, GA, and abscisic acid (Fig. 2; Steffens and Sauter, 2005; Steffens et al., 2006). GA promotes ethylene-triggered adventitious root growth (Suge, 1985; Lorbiecke and Sauter, 1999; Steffens et al., 2006), and abscisic acid inhibits both ethylene-induced and GA-promoted adventitious root emergence (Steffens et al., 2006). In adult mhz4 mutant rice plants, a decreased abscisic acid level and an increased ethylene concentration resulted in enhanced adventitious root growth (Ma et al., 2014), supporting the idea that alterations in abscisic acid and ethylene concentrations are a prerequisite for adventitious root growth in rice. It is unknown whether adventitious root emergence in tomato is facilitated by cell wall loosening of stem tissue through expansins such as LeEXP1 (Rose et al., 2000) or by programmed cell death of covering epidermal cells, as was described for rice (Mergemann and Sauter, 2000; Steffens et al., 2012).
Flooded tamarack seedlings possess fewer but longer adventitious roots compared with seedlings grown in air (Calvo-Polanco et al., 2012). Interestingly, the endodermis of these longer adventitious roots is poorly developed, cell walls are less suberized, tracheids possess a smaller diameter, and cortex cells are filled with starch (Calvo-Polanco et al., 2012). Despite the reduced chlorophyll content in needles of flooded seedlings, photosynthesis and transpiration were not altered, suggesting that the morphological changes of these adventitious roots contribute to seedling survival. These nonwoody secondary roots may serve as sinks for carbohydrates, as perhaps indicated by the abundance of starch in cortex cells. This adaptive response is considered to be a typical mechanism of flooding-tolerant trees (Gravatt and Kirby, 1998).
Adventitious Root Aerenchyma Facilitate Gas Transport
Another type of morphological response to flooding is the production of air-filled aerenchyma (Fig. 2). Newly formed adventitious roots of many species develop aerenchyma in the root cortex together with an inducible barrier of thickened cell walls to prevent radial oxygen loss upon flooding (Drew et al., 1979; Colmer et al., 2006; Argus et al., 2015). Root aerenchyma connected to the shoot help to maintain the diffusion of gases and, hence, enable the plant to survive under flooded and oxygen-deficient conditions. Radial oxygen loss supports longitudinal gas transport toward the apex, as demonstrated in roots of different deepwater, paddy, or upland rice varieties (Armstrong, 1971; Colmer, 2003).
In rice, aerenchyma formation in adventitious roots in stagnant water is induced within 12 h (Webb and Jackson, 1986) and results in a relatively high porosity of 30% to 40% depending on the genotype (Colmer, 2003). Ethylene mediates aerenchyma formation in adventitious roots but does not induce the barrier to radial oxygen loss (Colmer et al., 2006), indicating differential regulation of both processes.
Flood-tolerant trees also develop adventitious root aerenchyma upon flooding. In adventitious roots of soil-flooded Eucalyptus camaldulensis ssp. refulgens seedlings, root porosity increased about 14% in comparison with unflooded seedlings (Argus et al., 2015), indicating that the presence of aerenchyma upon flooding is an adaptive response of this riparian tree species. Aerenchyma formation was linked to flood tolerance in Rumex spp., with the more flood-tolerant Rumex palustris producing more aerenchyma than the less tolerant Rumex acetosa (Herzog and Pedersen, 2014).
CASE STUDY 2: ADVENTITIOUS ROOTS FOR IMPROVED NUTRIENT USE EFFICIENCY
Plants require a combination of three structural nutrients (carbon, hydrogen, and oxygen), six macronutrients (nitrogen, phosphorus, potassium, calcium, magnesium, and sulfur), and eight micronutrients (boron, chlorine, cobalt, copper, iron, manganese, molybdenum, and zinc; Timilsena et al., 2015). In most intensive farming situations, nutrients are added to the soil in the form of fertilizer. In 2010 to 2011, global application of nitrogen, phosphorus, and potassium totaled 104.1, 40.5 and 27.6 million tons, respectively (Timilsena et al., 2015). However, between 50% and 70% of nitrogen (for example) is lost through volatilization or runoff, polluting waterways through eutrophication (Robinson et al., 2011; Timilsena et al., 2015). Fertilizer production is also both energy and financially expensive, highlighting the importance of developing nutrient-efficient crops. To efficiently improve food production, we need an understanding of nutrient mobility in the rhizosphere and in the plant.
Responses to nutrient deficiencies begin with changes in deficiency-responsive genes, which then lead to physiological changes. There is less known about the deficiency-responsive genes in adventitious roots, and we recommend reviews on these genes in Arabidopsis and other model species (Atkinson et al., 2014; Bellini et al., 2014). Using cereals and the eudicot bean (Phaseolus vulgaris), the following section will focus on nutrient uptake by different adventitious roots and physiological responses to changing nutrient conditions.
Adventitious Root Responses to Nutrient Deficiency
Nutrient uptake occurs via transporters in the root (Fig. 3). Rice has 13 known phosphate transporters and an additional 13 putative transporters (Rose et al., 2013). Upon detection of low nutrient levels, the expression of these transporter genes increases to improve uptake capacity. For example, in rice crown roots, low potassium (Chen et al., 2015) or zinc (Widodo et al., 2010) increases expression of the potassium (OsHAK1 and OsHAK5; Chen et al., 2015) or zinc (ZIP family; Widodo et al., 2010) transporters, respectively, and maize nitrogen transporter gene expression increases in different root types under low-nitrogen conditions (Yu et al., 2014).
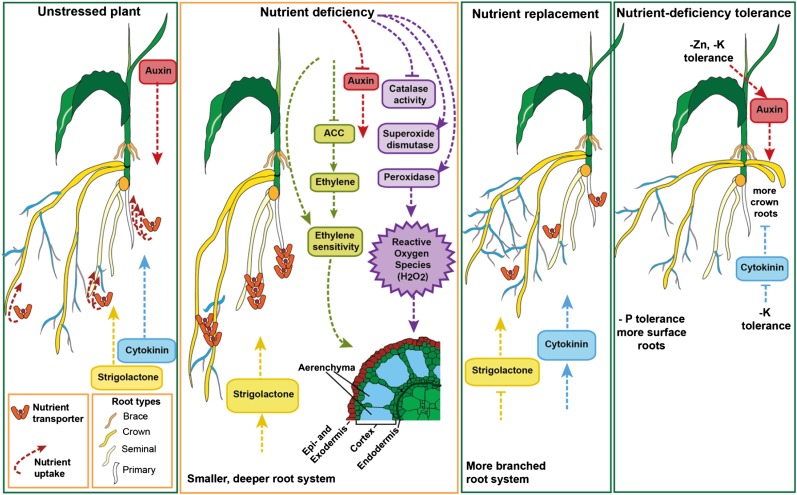
Figure 3.
Nutrient responses in adventitious roots. Under nutrient-replete conditions, crown roots have the lowest nutrient uptake rates, followed by seminal roots, while the primary roots have the highest uptake. When nutrients are deficient, the expression of nutrient transporters increases. In addition, strigolactone increases while auxin decreases, resulting in long roots with minimal lateral roots. Reactive oxygen species increase via changes in antioxidant enzyme activity (catalase, superoxide dismutase, and peroxidase) and, together with enhanced ethylene sensitivity, trigger lysigenous aerenchyma formation. When nutrients are replaced, nutrient transporter expression decreases systemically, cytokinin production increases, strigolactone levels decrease, and lateral root initiation increases on the adventitious roots. In potassium and zinc deficiency-tolerant lines, auxin signaling increases, and in potassium-efficient lines, cytokinin signaling decreases, together resulting in more adventitious roots. In phosphorus-efficient lines, more adventitious roots are found in the surface layers of the soil. In the cross section, epidermis and exodermis are combined, but the exodermis can be several cell layers adjacent to the epidermis. Pointed arrows represent positive interactions, and flat-ended arrows represent negative interactions. –K, Potassium deficiency; –P, phosphorus deficiency; –Zn = zinc deficiency. Yellow roots are crown roots, orange roots (the upper, short ones here) are brace roots (both adventitious root types), cream roots are seminal roots, white roots are primary roots, and blue and pink roots are lateral roots. ACC, 1-Aminocyclopropane-1-carboxylic acid (an ethylene precursor).
Perhaps surprisingly, there are differences in nutrient uptake ability among different types of roots (Table III). For example, using labeled uptake studies, under high-nitrogen (maize) or high-sulfur (rice) conditions, primary roots show greater nitrogen uptake than seminal roots (embryonic roots that emerge adjacent to the radicle; Fig. 3). The crown roots had the lowest nitrogen uptake (although the crown root measurements for the nitrogen study were done in plants different in age from those used for the primary and seminal measurements; Maniou et al., 2014; Yu et al., 2014). For low-nitrogen conditions, crown root uptake is reduced less (Yu et al., 2014), whereas for sulfur, the percentage reduction was similar for each root type (except for mesocotyl roots, which are similar to hypocotyl roots in monocots and had a much bigger reduction in sulfate uptake; Maniou et al., 2014). In wheat plants grown in aerated conditions, phosphorus uptake was initially higher in seminal roots than in crown roots, but this trend reversed in slightly older plants (Wiengweera and Greenway, 2004). However, in stagnant, flooded conditions, crown roots showed consistently higher phosphorus and potassium uptake than did seminal roots (Wiengweera and Greenway, 2004). This trend was also found in the eudicot bean, in which basal roots (i.e. roots at the base of the bean hypocotyl) had higher phosphate uptake under low phosphorus (Rubio et al., 2004).
Table III. Trends in nutrient uptake of different root types of monocot species.
| Growth Condition | Nutrient Measured | Trends for Monocot Root Type Uptake | Species/Reference |
|---|---|---|---|
| Replete nitrogen | Nitrogen | Primary > seminal > > crown | Maize/Yu et al. (2014) |
| Low nitrogen | Nitrogen | Primary > crown > seminal | Maize/Yu et al. (2014) |
| Replete sulfur | Sulfur | Primary > seminal > mesocotyl > crown | Rice/Maniou et al. (2014) |
| Low sulfur | Sulfur | Seminal > primary > mesocotyl > crown | Rice/Maniou et al. (2014) |
| Replete sulfur | Sulfate | Seminal > mesocotyl > primary > crown | Rice/Maniou et al. (2014) |
| Low sulfur | Sulfate | Seminal > primary > crown > mesocotyl | Rice/Maniou et al. (2014) |
| Aerated | Phosphorus | Seminal > crown (reversed over time) | Wheat/Wiengweera and Greenway (2004) |
| Stagnant | Phosphorus | Crown > seminal (all times measured) | Wheat/Wiengweera and Greenway (2004) |
| Aerated | Potassium | Crown > seminal (all times measured) | Wheat/Wiengweera and Greenway (2004) |
| Stagnant | Potassium | Crown > seminal (all times measured) | Wheat/Wiengweera and Greenway (2004) |
| Root type | Trends for nitrogen (N) uptake (Yu et al., 2014) | ||
| Primary | Local high N > homogenous high N > local low N > homogenous low N (60% reduction) | ||
| Seminal | Local low N > homogenous high N > local high N > homogenous low N (71% reduction) | ||
| Crown | Local high N > homogenous low N > homogenous high N > local low N (86% reduction) | ||
When maize roots are exposed to patchy nutrient conditions, nutrient uptake responds differently for each root type. This is summarized in the lower part of Table III (Yu et al., 2014). For example, crown roots take up more 15N under homogenous low-nitrogen conditions than they do under homogenous high nitrogen, a trend that is reversed for primary and seminal roots (Yu et al., 2014). However, both primary and crown roots have the highest uptake of nitrogen when that root type is exposed to local high nitrogen, whereas seminal roots had the highest uptake under locally low levels of nitrogen (Yu et al., 2014). These findings demonstrate significant physiological differences between each root type.
Root Architecture Response to Nutrient Deficiency
Each root type forms in different vertical positions, exposing them to different layers of the soil. Because nutrients are not evenly distributed in the soil, changes in root architecture can change the efficiency of nutrient uptake. For example, phosphorus is more available in the 10- to 15-cm depth (Miller et al., 2003), so increased tolerance to phosphorus-deficient soils may be a result of having more surface roots (Fig. 3). Phosphorus-efficient bean lines grew more adventitious roots (either stem or basal roots) in the surface soil layers, whereas less efficient lines either increased the number of deeper roots or failed to respond to phosphorus deficiency (Bonser et al., 1996; Liao et al., 2001; Miller et al., 2003; Miguel et al., 2013). This was also the case in pea (Pisum sativum) and soybean (Glycine max; Bonser et al., 1996).
Furthermore, the root system response is systemic, such that if any root is exposed to high phosphorus, the root system as a whole responded as if it were exposed to uniform high phosphorus (Bonser et al., 1996). Increases in phosphorus uptake with shallower roots also corresponded with increased yield in bean (Bonser et al., 1996; Liao et al., 2001; Richardson et al., 2011), so improvements in phosphorus efficiency could be achieved by selecting shallower root systems. However, the tradeoff with shallow roots is a reduction in drought tolerance because the deeper soil layers contain more water, so the ideal scenario for tolerating drought and phosphorus deficiency is a combination of deep and shallow roots (Uga et al., 2011, 2012; Rose et al., 2013). Recent identification of quantitative trait loci for both shallow and deep rooting in rice (Uga et al., 2011, 2012) and bean (Liao et al., 2004; Richardson et al., 2011) suggest that this may be a real possibility (Rose et al., 2013).
The growth and response of adventitious roots vary between nutrients and between tolerant and intolerant varieties. For example, zinc deficiency reduces the number of crown roots by up to 75% in a sensitive rice cultivar, whereas crown root number is maintained in a tolerant cultivar, a trait shared by many tolerant cultivars (Widodo et al., 2010; Rose et al., 2013).
Work has begun on improving tolerance to nutrient-deficient conditions by introducing genes linked to changes in root architecture, such as PHOSPHORUS-STARVATION TOLERANCE1 (PSTOL1; Gamuyao et al., 2012) or the WUSCHEL-related homeobox gene OsWOX11 (Chen et al., 2015). PSTOL1 is a phosphorus deficiency-tolerant protein kinase found in the aus-type rice varieties but not in all other types (Gamuyao et al., 2012). The OsWOX11 lines have used a potassium deficiency-induced gene promotor that drives the expression of OsWOX11 (Chen et al., 2015). The OsWOX11 and PSTOL1 rice varieties produced bigger root systems than the wild types under low potassium (Chen et al., 2015) and phosphorus (Gamuyao et al., 2012), respectively. These larger root systems also showed improved uptake of other nutrients (Gamuyao et al., 2012; Chen et al., 2015), resulting in higher yield (Chen et al., 2015). These two examples demonstrate that manipulating the adventitious root system can benefit the uptake of more than just that single nutrient. PSTOL1 expression correlates with the expression of ARL1/CRL1 and RR2 (a cytokinin type A response regulator), suggesting that changes in architecture could be linked to these networks (Gamuyao et al., 2012). Interestingly, PSTOL1 expression is present in primordia of crown roots but not in seminal roots (Gamuyao et al., 2012), highlighting differences in root types.
Not only do root types differ in the number of roots produced and their growth response to different nutrient deficiencies, but they also differ in the number of lateral roots that form on each root type. In rice, phosphate or nitrate deficiency results in longer roots with fewer lateral roots on the seminal roots (Rose et al., 2013; Sun et al., 2014), whereas zinc deficiency reduced the number on crown roots but had little effect on root length (Widodo et al., 2010). Using split-root experiments, Yu et al. (2014, 2015) demonstrated that, although lateral root density increased on maize crown roots that were exposed to locally high concentrations of nitrate, lateral root density was not affected on seminal roots (Yu et al., 2014). This difference in lateral root initiation between seminal and crown roots further highlights the complex differences between the different root classes in maize.
Lateral root density also increased on adventitious roots of phosphorus-efficient bean lines (Miller et al., 2003). Low phosphorus increased the distance from the root tip to the first lateral root in adventitious roots, but in basal roots there was no change except in one inefficient line (Miller et al., 2003), again demonstrating differences between adventitious root types.
Nutrient Changes Alter Hormone Signaling in Adventitious Roots
Root induction is dependent on the interaction of different hormone networks (for a summary comparing adventitious and lateral roots, see Atkinson et al. [2014] and Bellini et al. [2014]). Generally, auxin promotes adventitious (and lateral) root initiation and decreases elongation, whereas cytokinin and strigolactones inhibit root initiation. Strigolactone levels increase systemically under low-phosphorus or low-nitrogen conditions in monocots, including rice and sorghum (Sorghum bicolor; Fig. 3; Yoneyama et al., 2007, 2015; López-Ráez et al., 2008; Umehara, 2011; Sun et al., 2014), and in dicots, such as pea and tomato (López-Ráez et al., 2008; Balzergue et al., 2011; Kohlen et al., 2012). Nitrogen and phosphorus deficiency responses were lost in the rice strigolactone mutants (Sun et al., 2014), demonstrating the importance of the strigolactone signaling pathway for nutrient responses in monocot roots (Umehara, 2011). However, the exact nature of the interaction between low nutrients, increased strigolactones, and changes in root architecture is not well understood (Rasmussen et al., 2013).
Because nutrient efficiency is linked to changes in root architecture, and because root architecture is regulated by signaling molecules, it is perhaps not surprising that tolerance of nutrient deficiency can result from changes in hormone signaling (Fig. 3). For example, in rice, zinc deficiency-tolerant lines and the potassium-induced WOX11 lines have increased expression of genes linked to auxin signaling compared with the intolerant line (Widodo et al., 2010; Chen et al., 2015). Furthermore, WOX11 rice lines had reduced cytokinin signaling (Chen et al., 2015). The combination of down-regulating cytokinin signaling while up-regulating auxin signaling may explain the increase in adventitious root number and growth in these nutrient-efficient rice lines.
Lateral root induction also relies on changes in hormone signaling. Lateral root induction in maize crown roots exposed to local nitrogen patches occurs via a nitrate-induced increase in auxin levels. This includes increased expression of the monocot-specific PIN9 followed by cell cycle induction in the phloem pole pericycle cells (Yu et al., 2015). The presence of monocot-specific PIN transporters involved in crown root developmental patterns supports the idea that they have evolved separately or divergently to control features of monocot-specific morphogenesis such as adventitious root development (Yu et al., 2015).
Nutrient Deficiency-Induced Aerenchyma
In addition to flooding (see case study 1), many nutrient deficiencies, including phosphorus (Drew et al., 1989; He et al., 1992; Siyiannis et al., 2012; Rose et al., 2013; Fu et al., 2014; Hu et al., 2014), nitrogen (Drew et al., 1989; He et al., 1992; Siyiannis et al., 2012), and sulfur (Bouranis et al., 2003; Siyiannis et al., 2012; Maniou et al., 2014), have been shown to induce root aerenchyma formation. This induction varies in speed of onset and severity depending on the specific nutrient deficiency. For example, in maize, nitrogen deficiency caused the fastest production of aerenchyma followed by sulfur; the least production was caused by phosphorus (Drew et al., 1989; Siyiannis et al., 2012), although the final percentage of aerenchyma was similar between both nitrogen and phosphorus deficiency (Drew et al., 1989).
Lysigenous aerenchyma forms through the lysis of cortical cells and helps improve the movement of gasses (Maniou et al., 2014), initiating in the center of the cortex (Bouranis et al., 2003). The spaces increase in size, leaving bridges of intact cells linking the epidermal layers to the endodermis. Similar to aerenchyma formation in flooded conditions, nutrient deficiency-induced aerenchyma formation depends on ethylene signaling (He et al., 1992). However, in contrast to during and following flooding, ethylene production decreases under nitrogen- or phosphorus-deficient conditions (Drew et al., 1989) but sensitivity to ethylene is increased (He et al., 1992). After the inductive ethylene signal (Fig. 3), cellulase activity peaks (Siyiannis et al., 2012), probably leading to the controlled destruction of cortical cells. In nitrogen-deprived roots, this peak in cellulase activity was earlier and higher than in phosphorus-deficient roots (Siyiannis et al., 2012), corresponding to the faster production of aerenchyma under nitrogen-deficient conditions.
In addition to changes in ethylene signaling, nutrient stress increases the production of reactive oxygen species (Bouranis et al., 2003; Fu et al., 2014). A deficiency of phosphorus (Fu et al., 2014) or sulfur (Bouranis et al., 2003) leads to increased levels of superoxide anion and hydrogen peroxide in crown roots of maize (Bouranis et al., 2003) and rice (Fu et al., 2014). In flooded conditions, hydrogen peroxide is known to be involved in programmed cell death (for review, see Quan et al., 2008), and in sulfur-deficient maize, superoxide anions and hydrogen peroxide were found in the degenerating cells of the root cortex (Bouranis et al., 2003) where aerenchyma form (Fig. 3). Changes in reactive oxygen species levels and peroxidase activity also increase lignification (Quan et al., 2008), which occurs in the exodermis, sclerenchyma (Fu et al., 2014), and endodermis (Bouranis et al., 2003) under phosphorus or sulfur deficiency (Fu et al., 2014). This potentially stabilizes the remaining cells, avoiding complete tissue collapse. Concurrent with the formation of aerenchyma, root porosity increased, resulting in higher levels of oxygen and hydrogen peroxide released from the roots (Fu et al., 2014).
CASE STUDY 3: WOUND-INDUCED ADVENTITIOUS ROOTS: CUTTING PROPAGATION
Wound-induced adventitious roots are central to the propagation of forestry and horticultural species, and recent work has begun to unravel the molecular and physiological steps leading to rooting. We focus here on the physiology of adventitious root initiation and emergence, including the wound response and the networks regulating adventitious root induction (summarized in Fig. 4).
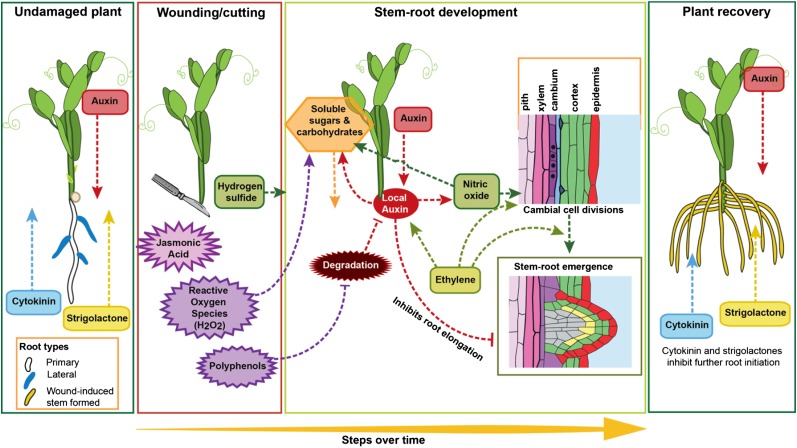
Figure 4.
Adventitious root formation on cuttings. In intact plants, cytokinin and strigolactones are predominantly produced in the root, while auxin is predominantly produced in the shoot. On wounding, jasmonic acid peaks within 30 min and is required for successful root development. Reactive oxygen species, polyphenols, and hydrogen sulfide also increase and promote adventitious rooting. Polyphenols do this via reducing auxin degradation. Auxin builds up in the base of the cutting, acting upstream of nitric oxide to promote adventitious root initiation. Auxin, nitric oxide, and hydrogen peroxide (H2O2) increase soluble sugars, which can be used for root development. Furthermore, levels of root initiation inhibitors (cytokinin and strigolactone) are reduced with the removal of the original root system. At later stages, auxin inhibits primordia elongation while ethylene promotes adventitious root emergence. As the new root system establishes, the production of cytokinin and strigolactones is restored. Pointed arrows represent positive interactions, and flat-ended arrows represent negative interactions. Yellow roots are adventitious roots, the white root is a primary roots, and blue roots are lateral roots.
Wound Response
Immediately after cutting, wound response signaling pathways are initiated at the base of the cutting (Creelman et al., 1992; Schilmiller and Howe, 2005), with a fast increase in jasmonic acid levels peaking 30 min after cutting (Fig. 4; Ahkami et al., 2009; Rasmussen et al., 2015). The presence of this peak correlated with adventitious root formation (Ahkami et al., 2009; Fattorini et al., 2009; Rasmussen et al., 2015), and a short pulse of jasmonic acid increased adventitious root formation (Rasmussen et al., 2015). However, in Bupleurum kaoi cuttings and intact Arabidopsis hypocotyls, prolonged exposure to jasmonic acid inhibited adventitious root formation (Chen et al., 2007; Gutierrez et al., 2012). In natural conditions, this wound response may be triggered by herbivory, physical damage, disease, or parasites (Schilmiller and Howe, 2005; Wasternack et al., 2006; Wasternack and Hause, 2013).
Similar to other stresses, including flooding and nutrient deficiency, physical damage also increases the production of reactive oxygen species (Fig. 4; Wasternack et al., 2006). Hydrogen peroxide is important for the wound response (Quan et al., 2008), as it increases adventitious root formation (Li et al., 2009; Santos Macedo et al., 2009; Li and Xue, 2010; Liao et al., 2010; She et al., 2010) via diamine oxidases (She et al., 2010). By contrast, catalase and ascorbic acid, which reduce hydrogen peroxide levels, both inhibited adventitious rooting (Li et al., 2009). Furthermore, the reduction in adventitious rooting that occurs by reducing auxin signaling (see below) can be partially rescued by treatment with hydrogen peroxide (Li et al., 2009). Hydrogen peroxide production begins to increase from 12 h after cutting and can reach seven times higher after 36 h (Li et al., 2009). Indole-3-butryic acid also increased hydrogen peroxide production (Li et al., 2009), suggesting feedback loops between auxin biosynthesis and signaling and reactive oxygen species signaling.
Because phenolic compounds help protect against reactive oxygen species (Jaleel et al., 2009), it is not surprising that they also increase in response to wounding. De Klerk and others (2011) tested a wide range of polyphenols and found that all of them promoted adventitious rooting, with ferulic acid having the strongest effect.
Adventitious Root Initiation
Adventitious root induction is promoted by high auxin and low cytokinin levels (Fig. 4; Bollmark and Eliasson, 1986; Bollmark et al., 1988; De Klerk et al., 1999; Kuroha et al., 2002, 2005). Auxin levels peak early after cutting in petunia (Petunia hybrida) and pea (Ahkami et al., 2013; Rasmussen et al., 2015) and then decrease, while cytokinin levels rapidly plummet with the removal of roots and then begin to recover at later stages (Bollmark et al., 1988; Rasmussen et al., 2015).
Auxin levels can be regulated by biosynthesis, transport, conjugation, and degradation. Reductions in any of these also alter adventitious rooting (Ahkami et al., 2013; da Costa et al., 2013). Auxin transport inhibitors significantly reduce adventitious rooting (Garrido et al., 1998, 2002; Koukourikou-Petridou, 1998; Ford et al., 2001). More recently, however, it has been suggested that auxin degradation may also be responsible for changes in the rooting ability of pea or Prunus spp. cuttings (Liao et al., 2010; De Klerk et al., 2011; Osterc and Štampar, 2011; Rasmussen et al., 2015). De Klerk and others (2011) found that, at optimal concentrations of many of the phenolics tested, auxin degradation by decarboxylation was almost completely blocked, resulting in higher active auxin levels (Fig. 4). This protective role of auxin may explain the improvement in adventitious rooting with phenolic applications.
In addition to auxin and cytokinin, strigolactones also regulate adventitious root initiation (Kohlen et al., 2012; Rasmussen et al., 2012b). Strigolactones are produced predominantly in the roots (Gomez-Roldan et al., 2008; Umehara et al., 2008), so the main strigolactone source has been removed in cuttings. Shoot removal by decapitation (which is often used in cutting propagation) also reduces strigolactone levels (Gomez-Roldan et al., 2008; Umehara et al., 2008). Inhibition of strigolactones using mutants or chemical inhibitors improved the adventitious rooting of pea and tomato cuttings (Kohlen et al., 2012; Rasmussen et al., 2012a, 2012b), and strigolactones act independently of cytokinins in intact Arabidopsis hypocotyls (Rasmussen et al., 2012b). Strigolactones may act by altering auxin transport (Bennett et al., 2006; Rasmussen et al., 2012b); however, an independent role for strigolactones on adventitious rooting cannot be fully ruled out (Rasmussen et al., 2012b).
The effect of ethylene on adventitious rooting under nonwaterlogged conditions has been shown to be contradictory. For example, in tobacco (Nicotiana tabacum; McDonald and Visser, 2003), sunflower (Liu et al., 1990), and Prunus avium (Biondi et al., 1990), ethylene treatments reduced adventitious rooting. By contrast, the ethylene precursor 1-aminocyclopropane-1-carboxylic acid enhanced adventitious rooting in stem cuttings of grape (Riov and Yang, 1989) and Norway spruce (Picea abies; Bollmark and Eliasson, 1990), whereas ethylene appeared to have no effect on adventitious rooting in apple (Harbage and Stimart, 1996). Adding even more complexity, at high auxin levels, ethylene is inhibitory in mung bean (Vigna radiata) and Eucalyptus spp. (De Klerk and Hanecakova, 2008; Kilkenny et al., 2012), but at low auxin levels, ethylene promoted adventitious rooting in mung bean (De Klerk and Hanecakova, 2008). Ethylene is known to interact with both auxin (Růzicka et al., 2007; Lewis et al., 2011) and cytokinin (Bollmark and Eliasson, 1990; Ramírez-Carvajal et al., 2009), but the precise nature of this interaction in cutting propagation requires further study.
Nitric oxide and hydrogen sulfide increase adventitious rooting in a wide range of species (Fig. 4; Zhang et al., 2009; Li and Xue, 2010; Liao et al., 2010; Li et al., 2011). Zhang and others (2009) showed that hydrogen sulfide is produced 24 h after cutting, followed by auxin, followed by nitric oxide. Nitric oxide mediates the auxin response leading to adventitious root formation (Pagnussat et al., 2003, 2004; Zhang et al., 2009), and using treatments and inhibitors, it has been demonstrated that auxin acts upstream of nitric oxide (Zhang et al., 2009).
Aside from hormonal signals, resource availability is also an important factor in adventitious root formation on cuttings (Fig. 4; da Costa et al., 2013). During adventitious root development in teak (Tectona grandis) cuttings, the soluble sugar and starch levels decreased (Jasik and Klerk, 1997; Husen and Pal, 2007). Higher levels of soluble sugars improve adventitious rooting and survival in many species, including petunia (Druege and Kadner, 2008), Pelargonium spp. (Druege et al., 2004), Chrysanthemum spp. (Druege et al., 2000), and Eucalyptus spp. (Hoad and Leakey, 1996). Auxin (Jasik and Klerk, 1997; Husen and Pal, 2007), nitric oxide, and hydrogen peroxide (Liao et al., 2010) treatments increase total soluble sugar levels. Furthermore, it has been demonstrated that increased rooting of cuttings kept in low light can be linked to an increase in soluble sugar (Druege et al., 2004; Druege and Kadner, 2008; Husen, 2008; Klopotek et al., 2010).
These signaling pathways control the cell division and differentiation that leads to a new root primordium. In many species, adventitious roots form from cambial cell divisions, which either develop directly into a new primordium or first divide into a callus tissue before tracheid differentiation and primordia establishment (Bollmark et al., 1988; Kevers et al., 1997; De Klerk et al., 1999; Naija et al., 2009; Rasmussen et al., 2009; Rasmussen and Hunt, 2010). As the root tissues form, hormone signaling changes with the restoration of higher strigolactone and cytokinin production, possibly preventing uncontrolled cell division and root initiation. At these later stages of root development, the hormone signaling requirements also change, with auxin inhibiting root elongation (Kevers et al., 1997; De Klerk et al., 1999; da Costa et al., 2013) and cytokinin (Bollmark and Eliasson, 1986) and ethylene enhancing adventitious root emergence and elongation.
CONCLUSION
Clear evidence is emerging demonstrating that each type of adventitious root is regulated and responds to environmental cues in unique ways. Because adventitious roots are important for tolerance to stresses such as flooding, nutrient deficiency, and wounding in both monocot and eudicot species, it is important that we understand the commonalities and differences among these important root types.
In each of the case studies, the timing of both hormonal interaction and reactive oxygen species homeostasis is very important. In addition, a core signaling network regulates root initiation and emergence, with auxin and ethylene promoting and cytokinin and strigolactones inhibiting. The formation of aerenchyma in adventitious roots is common to both flooding and nutrient deficiency and reduces the energy requirement for growth and maintenance. In flooded plants, aerenchyma is also crucial for enhancing gas exchange in this low-oxygen environment. Under nutrient deficiency, being able to adapt root architecture enables maximum nutrient capture, improving plant survival and crop yield. By manipulating recently identified nutrient transporters and quantitative trait loci for root angle, we now have the potential to improve breeding programs for nutrient-efficient crop lines. In future studies with combined stresses, this will prove extremely important. When flooding was combined with nutrient uptake studies, it was found that the adventitious roots had higher nutrient uptake ability compared with other root types (see case study 2). This could mean that nutrient-efficient lines, depending on surface adventitious roots, may also have improved flood tolerance. However, much more work is still required.
In summary, we have precisely defined and described the different adventitious root types and their physiological responses in particular to three stress conditions. Understanding the functional similarities and differences shared by these advantageous adventitious roots is crucial for maximizing efficient and resilient crop production.
Acknowledgments
We thank Ute Voss for reading and commenting on the article.
Footnotes
This work was supported by a Nottingham Research Fellowship to A.R.
References
- Abbas M, Berckhan S, Rooney DJ, Gibbs DJ, Vicente Conde J, Sousa Correia C, Bassel GW, Marín-de la Rosa N, León J, Alabadí D, et al. (2015) Oxygen sensing coordinates photomorphogenesis to facilitate seedling survival. Curr Biol 25: 1483–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahkami AH, Lischewski S, Haensch KT, Porfirova S, Hofmann J, Rolletschek H, Melzer M, Franken P, Hause B, Druege U, et al. (2009) Molecular physiology of adventitious root formation in Petunia hybrida cuttings: involvement of wound response and primary metabolism. New Phytol 181: 613–625 [DOI] [PubMed] [Google Scholar]
- Ahkami AH, Melzer M, Ghaffari MR, Pollmann S, Ghorbani Javid M, Shahinnia F, Hajirezaei MR, Druege U (2013) Distribution of indole-3-acetic acid in Petunia hybrida shoot tip cuttings and relationship between auxin transport, carbohydrate metabolism and adventitious root formation. Planta 238: 499–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argus RE, Colmer TD, Grierson PF (2015) Early physiological flood tolerance is followed by slow post-flooding root recovery in the dryland riparian tree Eucalyptus camaldulensis subsp. refulgens. Plant Cell Environ 38: 1189–1199 [DOI] [PubMed] [Google Scholar]
- Armstrong W. (1971) Oxygen diffusion from the roots of rice grown under non-waterlogged conditions. Physiol Plant 24: 242–247 [Google Scholar]
- Armstrong W, Justin SHFW, Beckett PM, Lythe S (1991) Root adaptation to soil waterlogging. Aquat Bot 39: 57–73 [Google Scholar]
- Atkinson JA, Rasmussen A, Traini R, Voß U, Sturrock C, Mooney SJ, Wells DM, Bennett MJ (2014) Branching out in roots: uncovering form, function, and regulation. Plant Physiol 166: 538–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey-Serres J, Fukao T, Gibbs DJ, Holdsworth MJ, Lee SC, Licausi F, Perata P, Voesenek LACJ, van Dongen JT (2012) Making sense of low oxygen sensing. Trends Plant Sci 17: 129–138 [DOI] [PubMed] [Google Scholar]
- Bailey-Serres J, Voesenek LACJ (2008) Flooding stress: acclimations and genetic diversity. Annu Rev Plant Biol 59: 313–339 [DOI] [PubMed] [Google Scholar]
- Balzergue C, Puech-Pagès V, Bécard G, Rochange SF (2011) The regulation of arbuscular mycorrhizal symbiosis by phosphate in pea involves early and systemic signalling events. J Exp Bot 62: 1049–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini C, Pacurar DI, Perrone I (2014) Adventitious roots and lateral roots: similarities and differences. Annu Rev Plant Biol 65: 639–666 [DOI] [PubMed] [Google Scholar]
- Bennett T, Sieberer T, Willett B, Booker J, Luschnig C, Leyser O (2006) The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport. Curr Biol 16: 553–563 [DOI] [PubMed] [Google Scholar]
- Biondi S, Diaz T, Iglesias I, Gamberini G, Bagni N (1990) Polyamines and ethylene in relation to adventitious root formation in Prunus avium shoot cultures. Physiol Plant 78: 474–483 [Google Scholar]
- Bleecker AB, Schuette JL, Kende H (1986) Anatomical analysis of growth and developmental patterns in the internode of deepwater rice. Planta 169: 490–497 [DOI] [PubMed] [Google Scholar]
- Blom CWPM, Voesenek LACJ (1996) Flooding: the survival strategies of plants. Trends Ecol Evol 11: 290–295 [DOI] [PubMed] [Google Scholar]
- Bollmark M, Eliasson L (1986) Effects of exogenous cytokinins on root formation in pea cuttings. Physiol Plant 68: 662–666 [Google Scholar]
- Bollmark M, Eliasson L (1990) Ethylene accelerates the breakdown of cytokinins and thereby stimulates rooting in Norway spruce hypocotyl cuttings. Physiol Plant 80: 534–540 [Google Scholar]
- Bollmark M, Kubat B, Eliasson L (1988) Variation in endogenous cytokinin content during adventitious root formation in pea cuttings. J Plant Physiol 132: 262–265 [Google Scholar]
- Bonser AM, Lynch J, Snapp S (1996) Effect of phosphorus deficiency on growth angle of basal roots in Phaseolus vulgaris. New Phytol 132: 281–288 [DOI] [PubMed] [Google Scholar]
- Bouranis DL, Chorianopoulou SN, Siyiannis VF, Protonotarios VE, Hawkesford MJ (2003) Aerenchyma formation in roots of maize during sulphate starvation. Planta 217: 382–391 [DOI] [PubMed] [Google Scholar]
- Brakenridge GR. Global Active Archive of Large Flood Events. Dartmouth Flood Observatory, University of Colorado.; http://floodobservatory.colorado.edu/Archives/index.html (January 6, 2016) [Google Scholar]
- Calvo-Polanco M, Señorans J, Zwiazek JJ (2012) Role of adventitious roots in water relations of tamarack (Larix laricina) seedlings exposed to flooding. BMC Plant Biol 12: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Feng H, Hu Q, Qu H, Chen A, Yu L, Xu G (2015) Improving rice tolerance to potassium deficiency by enhancing OsHAK16p:WOX11-controlled root development. Plant Biotechnol J 13: 833–848 [DOI] [PubMed] [Google Scholar]
- Chen LR, Chen YJ, Lee CY, Lin TY (2007) MeJA-induced transcriptional changes in adventitious roots of Bupleurum kaoi. Plant Sci 173: 12–24 [Google Scholar]
- Clark DG, Gubrium EK, Barrett JE, Nell TA, Klee HJ (1999) Root formation in ethylene-insensitive plants. Plant Physiol 121: 53–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmer TD. (2003) Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant Cell Environ 26: 17–36 [Google Scholar]
- Colmer TD, Cox MCH, Voesenek LACJ (2006) Root aeration in rice (Oryza sativa): evaluation of oxygen, carbon dioxide, and ethylene as possible regulators of root acclimatizations. New Phytol 170: 767–777 [DOI] [PubMed] [Google Scholar]
- Creelman RA, Tierney ML, Mullet JE (1992) Jasmonic acid/methyl jasmonate accumulate in wounded soybean hypocotyls and modulate wound gene expression. Proc Natl Acad Sci USA 89: 4938–4941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa CT, de Almeida MR, Ruedell CM, Schwambach J, Maraschin FS, Fett-Neto AG (2013) When stress and development go hand in hand: main hormonal controls of adventitious rooting in cuttings. Front Plant Sci 4: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawood T, Rieu I, Wolters-Arts M, Derksen EB, Mariani C, Visser EJW (2014) Rapid flooding-induced adventitious root development from preformed primordia in Solanum dulcamara. AoB Plants 6: plt058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dech JP, Maun MA (2006) Adventitious root production and plastic resource allocation to biomass determine burial tolerance in woody plants from central Canadian coastal dunes. Ann Bot (Lond) 98: 1095–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Klerk GJ, Guan H, Huisman P, Marinova S (2011) Effects of phenolic compounds on adventitious root formation and oxidative decarboxylation of applied indoleacetic acid in Malus ‘Jork 9’. Plant Growth Regul 63: 175–185 [Google Scholar]
- De Klerk GJ, Hanecakova J (2008) Ethylene and rooting of mung bean cuttings: the role of auxin induced ethylene synthesis and phase-dependent effects. Plant Growth Regul 56: 203–209 [Google Scholar]
- De Klerk GJ, Van der Krieken W, De Jong JC (1999) The formation of adventitious roots: new concepts, new possibilities. In Vitro Cell Dev Biol Plant 35: 189–199 [Google Scholar]
- Drew MC, He CJ, Morgan PW (1989) Decreased ethylene biosynthesis, and induction of aerenchyma, by nitrogen- or phosphate-starvation in adventitious roots of Zea mays L. Plant Physiol 91: 266–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew MC, Jackson MB, Giffard S (1979) Ethylene-promoted adventitious rooting and development of cortical air spaces (aerenchyma) in roots may be adaptive responses to flooding in Zea mays L. Planta 147: 83–88 [DOI] [PubMed] [Google Scholar]
- Druege U, Kadner R (2008) Response of post-storage carbohydrate levels in Pelargonium cuttings to reduced air temperature during rooting and the relationship with leaf senescence and adventitious root formation. Postharvest Biol Technol 47: 126–135 [Google Scholar]
- Druege U, Zerche S, Kadner R (2004) Nitrogen- and storage-affected carbohydrate partitioning in high-light-adapted Pelargonium cuttings in relation to survival and adventitious root formation under low light. Ann Bot (Lond) 94: 831–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druege U, Zerche S, Kadner R, Ernst M (2000) Relation between nitrogen status, carbohydrate distribution and subsequent rooting of Chrysanthemum cuttings as affected by pre-harvest nitrogen supply and cold storage. Ann Bot (Lond) 85: 687–701 [Google Scholar]
- Fattorini L, Falasca G, Kevers C, Rocca LM, Zadra C, Altamura MM (2009) Adventitious rooting is enhanced by methyl jasmonate in tobacco thin cell layers. Planta 231: 155–168 [DOI] [PubMed] [Google Scholar]
- Food and Agriculture Organisation of the United Nations http://www.fao.org/docrep/u8480e/u8480e07.htm (January 6, 2016)
- Ford YY, Bonham EC, Cameron RWF, Blake PS, Judd HL, Harrison-Murray RS (2001) Adventitious rooting: examining the role of auxin in an easy- and a difficult-to-root plant. Plant Growth Regul 36: 149–159 [Google Scholar]
- Fu YQ, Yang XJ, Shen H (2014) The physiological mechanism of enhanced oxidizing capacity of rice (Oryza sativa L.) roots induced by phosphorus deficiency. Acta Physiol Plant 36: 179–190 [Google Scholar]
- Gamuyao R, Chin JH, Pariasca-Tanaka J, Pesaresi P, Catausan S, Dalid C, Slamet-Loedin I, Tecson-Mendoza EM, Wissuwa M, Heuer S (2012) The protein kinase Pstol1 from traditional rice confers tolerance of phosphorus deficiency. Nature 488: 535–539 [DOI] [PubMed] [Google Scholar]
- Garrido G, Cano EA, Acosta M, Sanchez-Bravo J (1998) Formation and growth of roots in carnation cuttings: influence of cold storage period and auxin treatment. Sci Hortic (Amsterdam) 74: 219–231 [Google Scholar]
- Garrido G, Ramón Guerrero J, Angel Cano E, Acosta M, Sánchez-Bravo J (2002) Origin and basipetal transport of the IAA responsible for rooting of carnation cuttings. Physiol Plant 114: 303–312 [DOI] [PubMed] [Google Scholar]
- Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pagès V, Dun EA, Pillot JP, Letisse F, Matusova R, Danoun S, Portais JC, et al. (2008) Strigolactone inhibition of shoot branching. Nature 455: 189–194 [DOI] [PubMed] [Google Scholar]
- Gravatt DA, Kirby CJ (1998) Patterns of photosynthesis and starch allocation in seedlings of four bottomland hardwood tree species subjected to flooding. Tree Physiol 18: 411–417 [DOI] [PubMed] [Google Scholar]
- Gutierrez L, Mongelard G, Floková K, Păcurar DI, Novák O, Staswick P, Kowalczyk M, Păcurar M, Demailly H, Geiss G, et al. (2012) Auxin controls Arabidopsis adventitious root initiation by regulating jasmonic acid homeostasis. Plant Cell 24: 2515–2527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbage JF, Stimart DP (1996) Ethylene does not promote adventitious root initiation on apple microcuttings. J Am Soc Hortic Sci 121: 880–885 [Google Scholar]
- He CJ, Morgan PW, Drew MC (1992) Enhanced sensitivity to ethylene in nitrogen- or phosphate-starved roots of Zea mays L. during aerenchyma formation. Plant Physiol 98: 137–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog M, Pedersen O (2014) Partial versus complete submergence: snorkelling aids root aeration in Rumex palustris but not in R. acetosa. Plant Cell Environ 37: 2381–2390 [DOI] [PubMed] [Google Scholar]
- Hoad SP, Leakey RRB (1996) Effects of pre-severance light quality on the vegetative propagation of Eucalyptus grandis W. Hill ex Maiden: cutting morphology, gas exchange and carbohydrate status during rooting. Trees (Berl) 10: 317–324 [Google Scholar]
- Hochholdinger F, Woll K, Sauer M, Dembinsky D (2004) Genetic dissection of root formation in maize (Zea mays) reveals root-type specific developmental programmes. Ann Bot (Lond) 93: 359–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Henry A, Brown KM, Lynch JP (2014) Root cortical aerenchyma inhibits radial nutrient transport in maize (Zea mays). Ann Bot (Lond) 113: 181–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husen A. (2008) Stock-plant etiolation causes drifts in total soluble sugars and anthraquinones, and promotes adventitious root formation in teak (Tectona grandis L. f.) coppice shoots. Plant Growth Regul 54: 13–21 [Google Scholar]
- Husen A, Pal M (2007) Metabolic changes during adventitious root primordium development in Tectona grandis Linn. f. (teak) cuttings as affected by age of donor plants and auxin (IBA and NAA) treatment. New For 33: 309–323 [Google Scholar]
- Inukai Y, Sakamoto T, Ueguchi-Tanaka M, Shibata Y, Gomi K, Umemura I, Hasegawa Y, Ashikari M, Kitano H, Matsuoka M (2005) Crown rootless1, which is essential for crown root formation in rice, is a target of an AUXIN RESPONSE FACTOR in auxin signaling. Plant Cell 17: 1387–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanchenko MG, Zhu J, Wang B, Medvecká E, Du Y, Azzarello E, Mancuso S, Megraw M, Filichkin S, Dubrovsky JG, et al. (2015) The cyclophilin A DIAGEOTROPICA gene affects auxin transport in both root and shoot to control lateral root formation. Development 142: 712–721 [DOI] [PubMed] [Google Scholar]
- Jaleel C, Riadh K, Gopi R, Manivannan P, Inès J, Al-Juburi H, Chang-Xing Z, Hong-Bo S, Panneerselvam R (2009) Antioxidant defense responses: physiological plasticity in higher plants under abiotic constraints. Acta Physiol Plant 31: 427–436 [Google Scholar]
- Jasik J, Klerk GJD (1997) Anatomical and ultrastructural examination of adventitious root formation in stem slices of apple. Biol Plant 39: 79–90 [Google Scholar]
- Kende H, van der Knaap E, Cho HT (1998) Deepwater rice: a model plant to study stem elongation. Plant Physiol 118: 1105–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevers C, Hausman JF, Faivre-Rampant O, Evers D, Gaspar T (1997) Hormonal control of adventitious rooting: progress and questions. J Appl Bot 71: 71–79 [Google Scholar]
- Kilkenny AJ, Wallace HM, Walton DA, Adkins MF, Trueman SJ (2012) Improved root formation in eucalypt cuttings following combined auxin and anti-ethylene treatments. J Plant Sci 7: 138–153 [Google Scholar]
- Kim HJ, Lynch JP, Brown KM (2008) Ethylene insensitivity impedes a subset of responses to phosphorus deficiency in tomato and petunia. Plant Cell Environ 31: 1744–1755 [DOI] [PubMed] [Google Scholar]
- Klopotek Y, Haensch KT, Hause B, Hajirezaei MR, Druege U (2010) Dark exposure of petunia cuttings strongly improves adventitious root formation and enhances carbohydrate availability during rooting in the light. J Plant Physiol 167: 547–554 [DOI] [PubMed] [Google Scholar]
- Kohlen W, Charnikhova T, Lammers M, Pollina T, Tóth P, Haider I, Pozo MJ, de Maagd RA, Ruyter-Spira C, Bouwmeester HJ, et al. (2012) The tomato CAROTENOID CLEAVAGE DIOXYGENASE8 (SlCCD8) regulates rhizosphere signaling, plant architecture and affects reproductive development through strigolactone biosynthesis. New Phytol 196: 535–547 [DOI] [PubMed] [Google Scholar]
- Koukourikou-Petridou MA. (1998) Etiolation of stock plants affects adventitious root formation and hormone content of pea stem cuttings. Plant Growth Regul 25: 17–21 [Google Scholar]
- Kramer PJ. (1951) Causes of injury to plants resulting from flooding of the soil. Plant Physiol 26: 722–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss KW, Allen JA, Cahoon DR (2003) Differential rates of vertical accretion and elevation change among aerial root types in Micronesian mangrove forests. Estuar Coast Shelf Sci 56: 251–259 [Google Scholar]
- Kuroha T, Kato H, Asami T, Yoshida S, Kamada H, Satoh S (2002) A trans-zeatin riboside in root xylem sap negatively regulates adventitious root formation on cucumber hypocotyls. J Exp Bot 53: 2193–2200 [DOI] [PubMed] [Google Scholar]
- Kuroha T, Ueguchi C, Satoh S (2005) The defect of Arabidopsis histidine kinase genes leads retarded vascular system of hypocotyls and the accumulation of auxin resulting in the inhibition of lateral root formation and induction of adventitious root formation. Plant Cell Physiol 46: S48 [Google Scholar]
- Lewis DR, Negi S, Sukumar P, Muday GK (2011) Ethylene inhibits lateral root development, increases IAA transport and expression of PIN3 and PIN7 auxin efflux carriers. Development 138: 3485–3495 [DOI] [PubMed] [Google Scholar]
- Li MY, Cao ZY, Shen WB, Cui J (2011) Molecular cloning and expression of a cucumber (Cucumis sativus L.) heme oxygenase-1 gene, CsHO1, which is involved in adventitious root formation. Gene 486: 47–55 [DOI] [PubMed] [Google Scholar]
- Li SW, Xue LG (2010) The interaction between H2O2 and NO, Ca2+, cGMP, and MAPKs during adventitious rooting in mung bean seedlings. In Vitro Cell Dev Biol Plant 46: 142–148 [Google Scholar]
- Li SW, Xue LG, Xu SJ, Feng HY, An LZ (2009) Hydrogen peroxide acts as a signal molecule in the adventitious root formation of mung bean seedlings. Environ Exp Bot 65: 63–71 [Google Scholar]
- Liao H, Rubio G, Yan X, Cao A, Brown KM, Lynch JP (2001) Effect of phosphorus availability on basal root shallowness in common bean. Plant Soil 232: 69–79 [PubMed] [Google Scholar]
- Liao H, Yan X, Rubio G, Beebe SE, Blair MW, Lynch JP (2004) Genetic mapping of basal root gravitropism and phosphorus acquisition efficiency in common bean. Funct Plant Biol 31: 959–970 [DOI] [PubMed] [Google Scholar]
- Liao WB, Huang GB, Yu JH, Zhang ML (2012) Nitric oxide and hydrogen peroxide alleviate drought stress in marigold explants and promote its adventitious root development. Plant Physiol Biochem 58: 6–15 [DOI] [PubMed] [Google Scholar]
- Liao WB, Xiao HL, Zhang ML (2010) Effect of nitric oxide and hydrogen peroxide on adventitious root development from cuttings of ground-cover Chrysanthemum and associated biochemical changes. J Plant Growth Regul 29: 338–348 [Google Scholar]
- Liu H, Wang S, Yu X, Yu J, He X, Zhang S, Shou H, Wu P (2005) ARL1, a LOB-domain protein required for adventitious root formation in rice. Plant J 43: 47–56 [DOI] [PubMed] [Google Scholar]
- Liu JH, Mukherjee I, Reid DM (1990) Adventitious rooting in hypocotyls of sunflower (Helianthus annuus) seedlings. III. The role of ethylene. Physiol Plant 78: 268–276 [Google Scholar]
- Lombardi-Crestana S, da Silva Azevedo M, e Silva GF, Pino LE, Appezzato-da-Glória B, Figueira A, Nogueira FTS, Peres LEP (2012) The tomato (Solanum lycopersicum cv. Micro-Tom) natural genetic variation Rg1 and the DELLA mutant procera control the competence necessary to form adventitious roots and shoots. J Exp Bot 63: 5689–5703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Ráez JA, Charnikhova T, Gómez-Roldán V, Matusova R, Kohlen W, De Vos R, Verstappen F, Puech-Pages V, Bécard G, Mulder P, et al. (2008) Tomato strigolactones are derived from carotenoids and their biosynthesis is promoted by phosphate starvation. New Phytol 178: 863–874 [DOI] [PubMed] [Google Scholar]
- Lorbiecke R, Sauter M (1999) Adventitious root growth and cell-cycle induction in deepwater rice. Plant Physiol 119: 21–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B, Yin CC, He SJ, Lu X, Zhang WK, Lu TG, Chen SY, Zhang JS (2014) Ethylene-induced inhibition of root growth requires abscisic acid function in rice (Oryza sativa L.) seedlings. PLoS Genet 10: e1004701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniou F, Chorianopoulou SN, Bouranis DL (2014) New insights into trophic aerenchyma formation strategy in maize (Zea mays L.) organs during sulfate deprivation. Front Plant Sci 5: 581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald MP, Visser EJW (2003) A study of the interaction between auxin and ethylene in wild type and transgenic ethylene-insensitive tobacco during adventitious root formation induced by stagnant root zone conditions. Plant Biol 5: 550–556 [Google Scholar]
- Mergemann H, Sauter M (2000) Ethylene induces epidermal cell death at the site of adventitious root emergence in rice. Plant Physiol 124: 609–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel MA, Widrig A, Vieira RF, Brown KM, Lynch JP (2013) Basal root whorl number: a modulator of phosphorus acquisition in common bean (Phaseolus vulgaris). Ann Bot (Lond) 112: 973–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CR, Ochoa I, Nielsen KL, Beck D, Lynch JP (2003) Genetic variation for adventitious rooting in response to low phosphorus availability: potential utility for phosphorus acquisition from stratified soils. Funct Plant Biol 30: 973–985 [DOI] [PubMed] [Google Scholar]
- Musgrave A, Jackson MB, Ling E (1972) Callitriche stem elongation is controlled by ethylene and gibberellin. Nature 238: 93–96 [Google Scholar]
- Naija S, Elloumi N, Ammar S, Kevers C, Dommes J (2009) Involvement of polyamines in the adventitious rooting of micropropagated shoots of the apple rootstock MM106. In Vitro Cell Dev Biol Plant 45: 83–91 [Google Scholar]
- Negi S, Sukumar P, Liu X, Cohen JD, Muday GK (2010) Genetic dissection of the role of ethylene in regulating auxin-dependent lateral and adventitious root formation in tomato. Plant J 61: 3–15 [DOI] [PubMed] [Google Scholar]
- Oh K, Ivanchenko MG, White TJ, Lomax TL (2006) The diageotropica gene of tomato encodes a cyclophilin: a novel player in auxin signaling. Planta 224: 133–144 [DOI] [PubMed] [Google Scholar]
- Ondiviela B, Losada IJ, Lara JL, Maza M, Galván C, Bouma TJ, van Belzen J (2014) The role of seagrasses in coastal protection in a changing climate. Coast Eng 87: 158–168 [Google Scholar]
- Osterc G, Štampar F (2011) Differences in endo/exogenous auxin profile in cuttings of different physiological ages. J Plant Physiol 168: 2088–2092 [DOI] [PubMed] [Google Scholar]
- Pacurar DI, Perrone I, Bellini C (2014) Auxin is a central player in the hormone cross-talks that control adventitious rooting. Physiol Plant 151: 83–96 [DOI] [PubMed] [Google Scholar]
- Pagnussat GC, Lanteri ML, Lamattina L (2003) Nitric oxide and cyclic GMP are messengers in the indole acetic acid-induced adventitious rooting process. Plant Physiol 132: 1241–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagnussat GC, Lanteri ML, Lombardo MC, Lamattina L (2004) Nitric oxide mediates the indole acetic acid induction activation of a mitogen-activated protein kinase cascade involved in adventitious root development. Plant Physiol 135: 279–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan LJ, Zhang B, Shi WW, Li HY (2008) Hydrogen peroxide in plants: a versatile molecule of the reactive oxygen species network. J Integr Plant Biol 50: 2–18 [DOI] [PubMed] [Google Scholar]
- Ramírez-Carvajal GA, Morse AM, Dervinis C, Davis JM (2009) The cytokinin type-B response regulator PtRR13 is a negative regulator of adventitious root development in Populus. Plant Physiol 150: 759–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin I, Kende H (1984a) Regulation of growth in stem sections of deep-water rice. Planta 160: 66–72 [DOI] [PubMed] [Google Scholar]
- Raskin I, Kende H (1984b) Role of gibberellin in the growth response of submerged deep water rice. Plant Physiol 76: 947–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen A, Beveridge CA, Geelen D (2012a) Inhibition of strigolactones promotes adventitious root formation. Plant Signal Behav 7: 694–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen A, Depuydt S, Goormachtig S, Geelen D (2013) Strigolactones fine-tune the root system. Planta 238: 615–626 [DOI] [PubMed] [Google Scholar]
- Rasmussen A, Hosseini SA, Hajirezaei M-R, Druege U, Geelen D (2015) Adventitious rooting declines with the vegetative to reproductive switch and involves a changed auxin homeostasis. J Exp Bot 66: 1437–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen A, Hunt MA (2010) Ageing delays the cellular stages of adventitious root formation in pine. Aust For 73: 41–46 [Google Scholar]
- Rasmussen A, Mason MG, De Cuyper C, Brewer PB, Herold S, Agusti J, Geelen D, Greb T, Goormachtig S, Beeckman T, et al. (2012b) Strigolactones suppress adventitious rooting in Arabidopsis and pea. Plant Physiol 158: 1976–1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen A, Smith TE, Hunt MA (2009) Cellular stages of root formation, root system quality and survival of Pinus elliottii var. elliottii × P. caribaea var. hondurensis cuttings in different temperature environments. New For 38: 285–294 [Google Scholar]
- Retzer K, Luschnig C (2015) DIAGEOTROPICA: news from the auxin swamp. Trends Plant Sci 20: 328–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson A, Lynch J, Ryan P, Delhaize E, Smith FA, Smith S, Harvey P, Ryan M, Veneklaas E, Lambers H, et al. (2011) Plant and microbial strategies to improve the phosphorus efficiency of agriculture. Plant Soil 349: 121–156 [Google Scholar]
- Riov J, Yang SF (1989) Ethylene and auxin-ethylene interaction in adventitious root formation in mung bean (Vigna radiata) cuttings. J Plant Growth Regul 8: 131–141 [Google Scholar]
- Robinson N, Brackin R, Vinall K, Soper F, Holst J, Gamage H, Paungfoo-Lonhienne C, Rennenberg H, Lakshmanan P, Schmidt S (2011) Nitrate paradigm does not hold up for sugarcane. PLoS ONE 6: e19045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JKC, Cosgrove DJ, Albersheim P, Darvill AG, Bennett AB (2000) Detection of expansin proteins and activity during tomato fruit ontogeny. Plant Physiol 123: 1583–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose TJ, Impa SM, Rose MT, Pariasca-Tanaka J, Mori A, Heuer S, Johnson-Beebout SE, Wissuwa M (2013) Enhancing phosphorus and zinc acquisition efficiency in rice: a critical review of root traits and their potential utility in rice breeding. Ann Bot (Lond) 112: 331–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio G, Sorgonà A, Lynch JP (2004) Spatial mapping of phosphorus influx in bean root systems using digital autoradiography. J Exp Bot 55: 2269–2280 [DOI] [PubMed] [Google Scholar]
- Růzicka K, Ljung K, Vanneste S, Podhorská R, Beeckman T, Friml J, Benková E (2007) Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell 19: 2197–2212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos Macedo E, Cardoso HG, Hernández A, Peixe AA, Polidoros A, Ferreira A, Cordeiro A, Arnholdt-Schmitt B (2009) Physiologic responses and gene diversity indicate olive alternative oxidase as a potential source for markers involved in efficient adventitious root induction. Physiol Plant 137: 532–552 [DOI] [PubMed] [Google Scholar]
- Sauter M. (2013) Root responses to flooding. Curr Opin Plant Biol 16: 282–286 [DOI] [PubMed] [Google Scholar]
- Sauter M, Steffens B (2014) Biogenesis of adventitious roots and their involvement in the adaptation to oxygen limitations. In VanDongen JT, Licausi F, eds, Low-Oxygen Stress in Plants: Oxygen Sensing and Adaptive Responses to Hypoxia, Vol 21 pp 299–312 [Google Scholar]
- Schilmiller AL, Howe GA (2005) Systemic signaling in the wound response. Curr Opin Plant Biol 8: 369–377 [DOI] [PubMed] [Google Scholar]
- She XP, Huang AX, Ren Y (2010) Hydrogen peroxide generated by copper amine oxidase involved in adventitious root formation in mung bean hypocotyl cuttings. Aust J Bot 58: 656–662 [Google Scholar]
- Simberloff D, Brown BJ, Lowrie S (1978) Isopod and insect root borers may benefit Florida mangroves. Science 201: 630–632 [DOI] [PubMed] [Google Scholar]
- Siyiannis VF, Protonotarios VE, Zechmann B, Chorianopoulou SN, Müller M, Hawkesford MJ, Bouranis DL (2012) Comparative spatiotemporal analysis of root aerenchyma formation processes in maize due to sulphate, nitrate or phosphate deprivation. Protoplasma 249: 671–686 [DOI] [PubMed] [Google Scholar]
- Spiegelman Z, Ham BK, Zhang Z, Toal TW, Brady SM, Zheng Y, Fei Z, Lucas WJ, Wolf S (2015) A tomato phloem-mobile protein regulates the shoot-to-root ratio by mediating the auxin response in distant organs. Plant J 83: 853–863 [DOI] [PubMed] [Google Scholar]
- Steffens B. (2014) The role of ethylene and ROS in salinity, heavy metal, and flooding responses in rice. Front Plant Sci 5: 685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens B, Kovalev A, Gorb SN, Sauter M (2012) Emerging roots alter epidermal cell fate through mechanical and reactive oxygen species signaling. Plant Cell 24: 3296–3306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens B, Sauter M (2005) Epidermal cell death in rice is regulated by ethylene, gibberellin, and abscisic acid. Plant Physiol 139: 713–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens B, Wang J, Sauter M (2006) Interactions between ethylene, gibberellin and abscisic acid regulate emergence and growth rate of adventitious roots in deepwater rice. Planta 223: 604–612 [DOI] [PubMed] [Google Scholar]
- Suge H. (1985) Ethylene and gibberellin: regulation of internodal elongation and nodal root development in floating rice. Plant Cell Physiol 26: 607–614 [Google Scholar]
- Sun H, Tao J, Liu S, Huang S, Chen S, Xie X, Yoneyama K, Zhang Y, Xu G (2014) Strigolactones are involved in phosphate- and nitrate-deficiency-induced root development and auxin transport in rice. J Exp Bot 65: 6735–6746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timilsena YP, Adhikari R, Casey P, Muster T, Gill H, Adhikari B (2015) Enhanced efficiency fertilisers: a review of formulation and nutrient release patterns. J Sci Food Agric 95: 1131–1142 [DOI] [PubMed] [Google Scholar]
- Tyburski J, Tretyn A (2004) The role of light and polar auxin transport in root regeneration from hypocotyls of tomato seedling cuttings. Plant Growth Regul 42: 39–48 [Google Scholar]
- Uga Y, Hanzawa E, Nagai S, Sasaki K, Yano M, Sato T (2012) Identification of qSOR1, a major rice QTL involved in soil-surface rooting in paddy fields. Theor Appl Genet 124: 75–86 [DOI] [PubMed] [Google Scholar]
- Uga Y, Okuno K, Yano M (2011) Dro1, a major QTL involved in deep rooting of rice under upland field conditions. J Exp Bot 62: 2485–2494 [DOI] [PubMed] [Google Scholar]
- Umehara M. (2011) Strigolactone, a key regulator of nutrient allocation in plants. Plant Biotechnol 28: 429–437 [Google Scholar]
- Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N, Magome H, Kamiya Y, Shirasu K, Yoneyama K, et al. (2008) Inhibition of shoot branching by new terpenoid plant hormones. Nature 455: 195–200 [DOI] [PubMed] [Google Scholar]
- Vidoz ML, Loreti E, Mensuali A, Alpi A, Perata P (2010) Hormonal interplay during adventitious root formation in flooded tomato plants. Plant J 63: 551–562 [DOI] [PubMed] [Google Scholar]
- Visser E, Cohen JD, Barendse G, Blom C, Voesenek L (1996) An ethylene-mediated increase in sensitivity to auxin induces adventitious root formation in flooded Rumex palustris Sm. Plant Physiol 112: 1687–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voesenek LA, Bailey-Serres J (2013) Flooding tolerance: O2 sensing and survival strategies. Curr Opin Plant Biol 16: 647–653 [DOI] [PubMed] [Google Scholar]
- Wample RL, Reid DM (1978) Control of adventitious root production and hypocotyl hypertrophy of sunflower (Helianthus annuus) in response to flooding. Physiologia Plantarum 44: 351–358 [Google Scholar]
- Wasternack C, Hause B (2013) Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann Bot (Lond) 111: 1021–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasternack C, Stenzel I, Hause B, Hause G, Kutter C, Maucher H, Neumerkel J, Feussner I, Miersch O (2006) The wound response in tomato: role of jasmonic acid. J Plant Physiol 163: 297–306 [DOI] [PubMed] [Google Scholar]
- Webb J, Jackson MB (1986) A transmission and cryo-scanning electron microscopy study of the formation of aerenchyma (cortical gas-filled space) in adventitious roots of rice (Oryza sativa). J Exp Bot 37: 832–841 [Google Scholar]
- Widodo, Broadley MR, Rose T, Frei M, Pariasca-Tanaka J, Yoshihashi T, Thomson M, Hammond JP, Aprile A, Close TJ, et al. (2010) Response to zinc deficiency of two rice lines with contrasting tolerance is determined by root growth maintenance and organic acid exudation rates, and not by zinc-transporter activity. New Phytol 186: 400–414 [DOI] [PubMed] [Google Scholar]
- Wiengweera A, Greenway H (2004) Performance of seminal and nodal roots of wheat in stagnant solution: K+ and P uptake and effects of increasing O2 partial pressures around the shoot on nodal root elongation. J Exp Bot 55: 2121–2129 [DOI] [PubMed] [Google Scholar]
- Wilkinson JQ, Lanahan MB, Yen HC, Giovannoni JJ, Klee HJ (1995) An ethylene-inducible component of signal transduction encoded by never-ripe. Science 270: 1807–1809 [DOI] [PubMed] [Google Scholar]
- Xu M, Zhu L, Shou H, Wu P (2005) A PIN1 family gene, OsPIN1, involved in auxin-dependent adventitious root emergence and tillering in rice. Plant Cell Physiol 46: 1674–1681 [DOI] [PubMed] [Google Scholar]
- Yoneyama K, Kisugi T, Xie X, Arakawa R, Ezawa T, Nomura T, Yoneyama K (2015) Shoot-derived signals other than auxin are involved in systemic regulation of strigolactone production in roots. Planta 241: 687–698 [DOI] [PubMed] [Google Scholar]
- Yoneyama K, Yoneyama K, Takeuchi Y, Sekimoto H (2007) Phosphorus deficiency in red clover promotes exudation of orobanchol, the signal for mycorrhizal symbionts and germination stimulant for root parasites. Planta 225: 1031–1038 [DOI] [PubMed] [Google Scholar]
- Yu P, Eggert K, von Wirén N, Li C, Hochholdinger F (2015) Cell-type specific gene expression analyses by RNA-Seq reveal local high nitrate triggered lateral root initiation in shoot-borne roots of maize by modulating auxin-related cell cycle-regulation. Plant Physiol 169: 690–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P, White PJ, Hochholdinger F, Li C (2014) Phenotypic plasticity of the maize root system in response to heterogeneous nitrogen availability. Planta 240: 667–678 [DOI] [PubMed] [Google Scholar]
- Zhang H, Tang J, Liu XP, Wang Y, Yu W, Peng WY, Fang F, Ma DF, Wei ZJ, Hu LY (2009) Hydrogen sulfide promotes root organogenesis in Ipomoea batatas, Salix matsudana and Glycine max. J Integr Plant Biol 51: 1086–1094 [DOI] [PubMed] [Google Scholar]
- Zhang Q, Visser EJW, de Kroon H, Huber H (2015) Life cycle stage and water depth affect flooding-induced adventitious root formation in the terrestrial species Solanum dulcamara. Ann Bot (Lond) 116: 279–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou DX, Yin K, Xue HW (2003) Effect of polar auxin transport on rice root development. Acta Bot Sin 45: 1421–1427 [Google Scholar]