The calcium sensor protein CML38 is a core hypoxia-induced calcium sensor protein that associates with stress granule mRNA complexes during the response of Arabidopsis to low-oxygen stress.
Abstract
During waterlogging and the associated oxygen deprivation stress, plants respond by the induction of adaptive programs, including the redirected expression of gene networks toward the synthesis of core hypoxia-response proteins. Among these core response proteins in Arabidopsis (Arabidopsis thaliana) is the calcium sensor CML38, a protein related to regulator of gene silencing calmodulin-like proteins (rgsCaMs). CML38 transcripts are up-regulated more than 300-fold in roots within 6 h of hypoxia treatment. Transfer DNA insertional mutants of CML38 show an enhanced sensitivity to hypoxia stress, with lowered survival and more severe inhibition of root and shoot growth. By using yellow fluorescent protein (YFP) translational fusions, CML38 protein was found to be localized to cytosolic granule structures similar in morphology to hypoxia-induced stress granules. Immunoprecipitation of CML38 from the roots of hypoxia-challenged transgenic plants harboring CML38pro::CML38:YFP followed by liquid chromatography-tandem mass spectrometry analysis revealed the presence of protein targets associated with messenger RNA ribonucleoprotein (mRNP) complexes including stress granules, which are known to accumulate as messenger RNA storage and triage centers during hypoxia. This finding is further supported by the colocalization of CML38 with the mRNP stress granule marker RNA Binding Protein 47 (RBP47) upon cotransfection of Nicotiana benthamiana leaves. Ruthenium Red treatment results in the loss of CML38 signal in cytosolic granules, suggesting that calcium is necessary for stress granule association. These results confirm that CML38 is a core hypoxia response calcium sensor protein and suggest that it serves as a potential calcium signaling target within stress granules and other mRNPs that accumulate during flooding stress responses.
Exposure of plants to oxygen deprivation stress resulting from flooding, waterlogging, and poor soil aeration leads to depression of respiration, reduced adenylate energy charge, accumulation of toxic metabolites, and cytosolic acidification (Voesenek and Bailey-Serres, 2013, 2015). In response to these conditions, plants employ a variety of short-term and long-term adaptation strategies, including (1) increases in glycolytic flux to provide ATP; (2) elevation of fermentation metabolism to regenerate NAD+ for glycolysis; (3) induction of morphological and developmental changes (e.g. aerenchyma, adventitious root formation, and root and stem elongation) to elevate oxygen levels in waterlogged roots; and (4) increased capacity to avoid/repair the oxidative damage that occurs during posthypoxia reoxygenation (Blokhina et al., 2003; Ella et al., 2003; Tamang et al., 2014).
In the model plant Arabidopsis (Arabidopsis thaliana), the response to hypoxia is orchestrated by a coordinated change in transcript profiles and the translatome to favor the expression of a small set of core hypoxia-induced genes. These include glycolytic and fermentation enzymes and other metabolic proteins as well as various signal transduction proteins, transcription factors, and other genes involved in the adaptation response to anaerobiosis (Klok et al., 2002; Branco-Price et al., 2008; Mustroph et al., 2009, 2010). These regulatory controls rely on cis-acting hypoxia regulatory elements in the promoters of hypoxia-responsive genes (Dolferus et al., 2001; Mohanty et al., 2005), posttranscriptional control by silencing RNAs (Moldovan et al., 2010; Licausi et al., 2011), mRNA sequestration in ribonucleoprotein complexes (Weber et al., 2008; Sorenson and Bailey-Serres, 2014), and the selective targeting of core hypoxia-response transcripts to polysomes (Branco-Price et al., 2008; Mustroph et al., 2009). Overall, these developmental programs prepare the plant at various fronts for a coordinated survival response to low oxygen stress.
A number of metabolic cues and signaling pathways are involved in triggering the hypoxia response, including (1) low energy charge and pH changes associated with a shift from respiration to fermentation; (2) the release of reactive oxygen species; (3) ethylene signaling pathways; and (4) cytosolic calcium signals (Licausi and Perata, 2009; Bailey-Serres and Voesenek, 2010; Bailey-Serres et al., 2012; Voesenek and Bailey-Serres, 2013, 2015). Ca2+ is a ubiquitous secondary messenger and is involved in responses to myriad developmental, hormonal, and environmental cues in plant cells, including the hypoxia response (White and Broadley, 2003; Gao et al., 2011; Oh et al., 2014). Initial evidence for a role of calcium in hypoxia responses came from work with maize (Zea mays) seedlings, where pretreatment with Ruthenium Red (RR; an inhibitor of organellar Ca2+ release) followed by exposure to low-oxygen stress inhibited increases in ALCOHOL DEHYDROGENASE1 (ADH1) transcript and impaired poststress survival of the seedlings (Subbaiah et al., 1994a, 1994b). Similarly, analyses of RR pretreatment of Arabidopsis seedlings followed by anaerobic stress treatment supported the observation that ADH1 expression is under the control of cytosolic Ca2+ (Sedbrook et al., 1996; Chung and Ferl, 1999). Moreover, RR treatment causes inhibition of root and leaf growth during the posthypoxia survival period (Sedbrook et al., 1996). Taken together, these studies support a role for calcium signaling as a component of the hypoxia response.
Calcium signals are decoded by a collection of calcium sensor proteins that have specialized EF hand calcium-binding domains that detect micromolar changes in cytosolic calcium and mediate downstream responses. The most well characterized of these is the ubiquitous calcium sensor calmodulin (Chin and Means, 2000); however, higher plants have a large collection of calmodulin-like proteins (CMLs) that contain EF hands but have diverged from calmodulin in structure and function (McCormack and Braam, 2003; Bender and Snedden, 2013). To gain insight into the calcium sensors involved in the hypoxia-response program in Arabidopsis, the interaction targets and subcellular localization of CML38 in Arabidopsis were investigated.
RESULTS
Expression of CML38 during Hypoxia Stress in Arabidopsis Seedlings
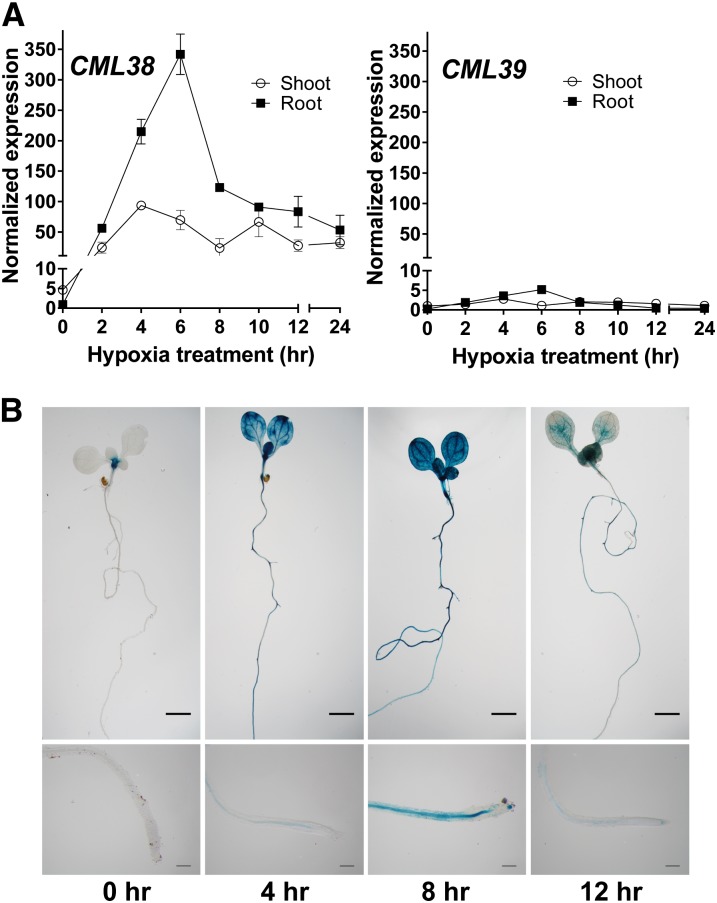
Analysis of the flooding- and hypoxia-induced transcript levels from a variety of microarray studies (Liu et al., 2005a; Loreti et al., 2005; Branco-Price et al., 2008; Christianson et al., 2009; Mustroph et al., 2009; Licausi et al., 2010; Gibbs et al., 2011; Lee et al., 2011; Yang et al., 2011; Chang et al., 2012) shows that, among the 50 CML and seven CALMODULIN (CaM) calcium sensor genes, CML38 is uniquely and acutely up-regulated in response to hypoxia (Supplemental Fig. S1). Phylogenetic analysis of CML38 (Supplemental Fig. S2) shows that it is part of a small subfamily of genes in Arabidopsis with structural and sequence homology to the regulator of gene silencing rgsCaM protein from tobacco (Nicotiana tabacum; Anandalakshmi et al., 2000). The most closely related protein to CML38 (At1g76650) is a tandem gene, CML39 (At1g76640), with 76% amino acid sequence identity to CML38 (Supplemental Fig. S3). Since microarray results might not resolve the expression of these two closely related gene products, CML38 and CML39 transcript levels in Arabidopsis seedlings challenged with hypoxia by complete submergence were assayed by quantitative reverse transcription (Q)-PCR (Fig. 1). CML38 showed acute elevation of transcript levels (greater than 300-fold increase in expression in the root tissue and approximately 100-fold in the shoot at 6 h of hypoxia), while CML39 showed only a minor (5-fold) increase (Fig. 1). The tissue-specific expression profile of CML38 during submergence stress was analyzed using CML38 promoter::GUS transgenic plants. Under normoxic conditions, CML38::GUS seedlings showed a basal level of expression at the hypocotyl-root junction in untreated seedlings (0 h). However, submergence of seedlings resulted in a rapid elevation of GUS activity in both root and shoot tissue (Fig. 1B), in support of the Q-PCR results. These findings confirm previous work that has placed CML38 into the small group of core hypoxia-response genes (Mustroph et al., 2009) and suggest that CML38 may play a specialized role as a calcium sensor during this response.
Figure 1.
Expression of CML38 and CML39 in hypoxia-stressed Arabidopsis seedlings. A, Q-PCR analysis of CML38 and CML39 expression in roots (squares) and shoots (circles) of hypoxia-challenged 2-week-old Arabidopsis seedlings. Normalized expression represents 2−ΔΔCt values, obtained by subtracting the threshold cycle value (Ct) of CML38 or CML39 from that of the internal reference gene, UBIQUITIN10 (UBQ10), and using the 0-h shoot sample as a calibrator. Error bars indicate se of three biological replicates. B, CML38::GUS expression in 10-d-old hypoxia-treated seedlings. Untreated negative control and hypoxia-treated (4, 8, and 12 h) seedlings are shown. The bottom images show magnifications of the root tip area. Bars = 500 µm (top) and 25 µm (bottom).
cml38 Transfer DNA Knockout Seedling Survival under Anaerobic Stress
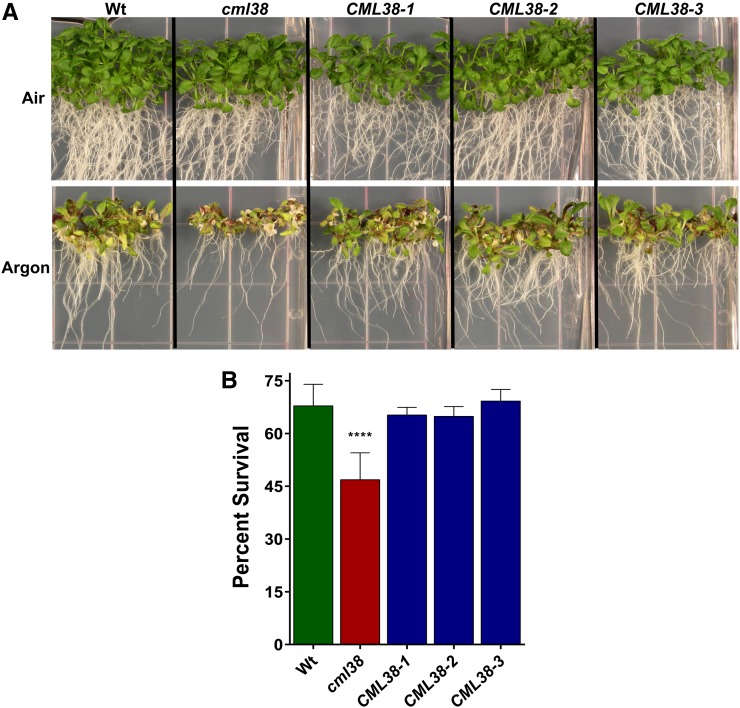
Mutations that target core hypoxia-response genes in general result in a lower survival and/or increased sensitivity to anaerobic stress (Ismond et al., 2003; Kürsteiner et al., 2003; Licausi et al., 2010; Giuntoli et al., 2014; Sorenson and Bailey-Serres, 2014). To determine the effects of the elimination of CML38 expression on Arabidopsis survival to anaerobic stress, a transfer DNA (T-DNA) mutant (Salk_066538C; referred to here as cml38) line with an insertion site within the coding region of CML38 (Supplemental Fig. S4) was characterized. Q-PCR analysis confirmed that cml38 plants do not express the CML38 transcript in response to hypoxia induction (Supplemental Fig. S4). Argon-induced anaerobiosis resulted in chlorosis of the apical shoot, reduction in the length of the primary root, and reduction in seedling growth of most of the cml38 mutant seedlings, with a survival rate significantly lower than wild-type plants (Fig. 2).
Figure 2.
Effects of oxygen deprivation on the survival of cml38 T-DNA insertional mutant seedlings. A, Eight-day-old, vertically grown seedlings corresponding to the wild type (Wt), the cml38 T-DNA insertional mutant (cml38), and three independent 35S::CML38:YFP complemented lines (CML38) were subjected to a 15-h treatment of air or argon gas and allowed to recover as described in “Materials and Methods.” B, Histogram showing the percentage survival of wild-type, cml38, and CML38 seedlings in response to argon treatment. Error bars show the se of nine biological replicates for the wild type and the cml38 mutant and three biological replicates for CML38 complementation lines, with each replicate containing 25 to 40 seedlings. Asterisks indicate a significant paired Student’s t test value (P < 0.0001) between cml38 and wild-type or CML38 plants. A similar Student’s t test showed no significant difference between wild-type and CML38 plants.
To test whether this effect was the result of reduced CML38 expression, cml38 plants were transformed with an expression construct consisting of CML38 fused to C-terminal yellow fluorescent protein (YFP) under the control of the cauliflower mosaic virus 35S promoter. These plants showed recovery of CML38 expression (Supplemental Fig. S5) as well as restoration of survival frequencies to argon-mediated stress that were indistinguishable from wild-type plants (Fig. 2). This suggests that the loss of CML38 expression was responsible for the greater sensitivity of cml38 to anaerobic stress.
Association of CML38 Protein with Cytosolic Granules in Hypoxia-Challenged Arabidopsis
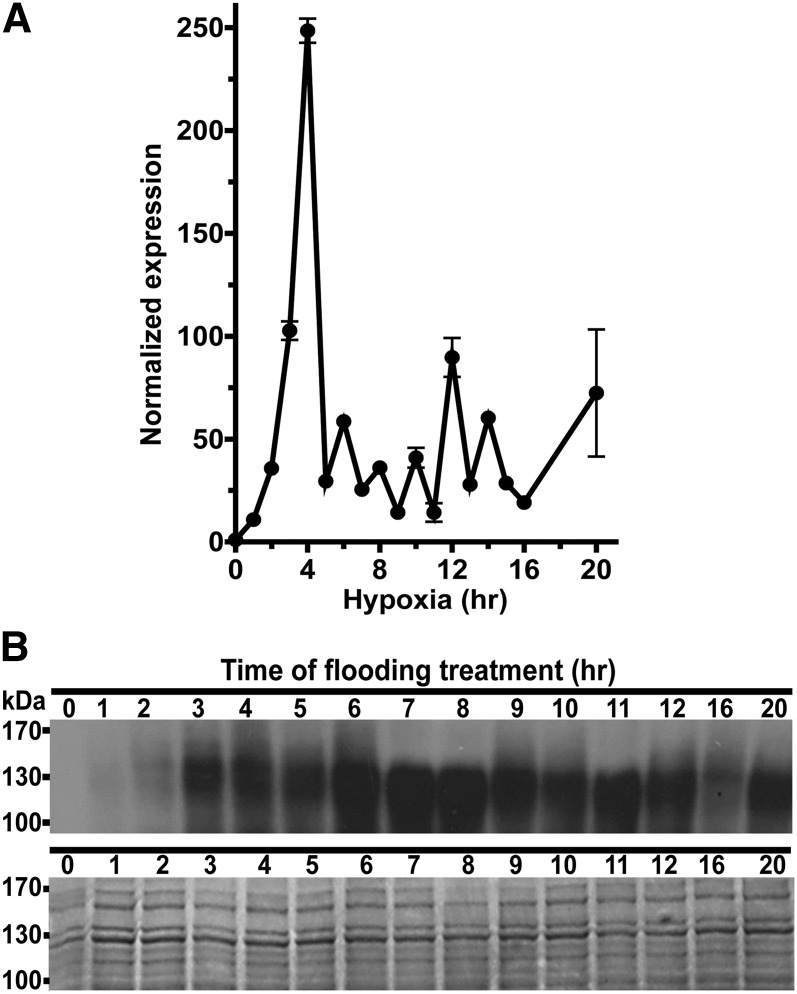
To investigate the dynamics of CML38 protein expression as well as its subcellular localization, transgenic Arabidopsis plants expressing CML38 with an in-frame C-terminal YFP fusion (CML38-YFP) were generated using a recombineering method (Zhou et al., 2011). The advantage of this approach is that it results in in situ production of a gene encoding the protein of interest tagged with a fluorescent reporter within the larger genomic context, which avoids the exclusion of cis-regulatory elements (Zhou et al., 2011). To verify that the CML38-YFP recombineering plants respond to hypoxia treatment, plants grown hydroponically were perfused with nitrogen gas and the levels of CML38-YFP transcripts and CML38-YFP protein were analyzed (Fig. 3). Q-PCR confirmed that CML38-YFP was induced by low-oxygen conditions, peaking at 4 h and remaining elevated throughout the assay period (Fig. 3). Analysis of CML38 protein levels by western blot with an anti-GFP antibody showed rapid accumulation of CML38-YFP protein, with a peak reached between 6 and 8 h after hypoxia induction (Fig. 3).
Figure 3.
Transcript and protein levels in hypoxia-challenged CML38-YFP recombineering plants. A, Time course of CML38-YFP transcript expression assayed by Q-PCR in the root tissue of 31-d-old hydroponically grown plants in response to hypoxia. Normalized expression was determined as described in Figure 1. Error bars indicate se of three technical replicates. B, Time course of CML38-YFP fusion protein (102.1 kD) expression assayed by western blot in the root tissue of 31-d-old hydroponically grown plants in response to hypoxia. Top gel, Western-blot chemiluminescent signal; bottom gel, Coomassie Blue-stained gel from the same extracts. Each lane contained 30 μg of protein. The electrophoretic mobility positions of the molecular mass markers are shown at left.
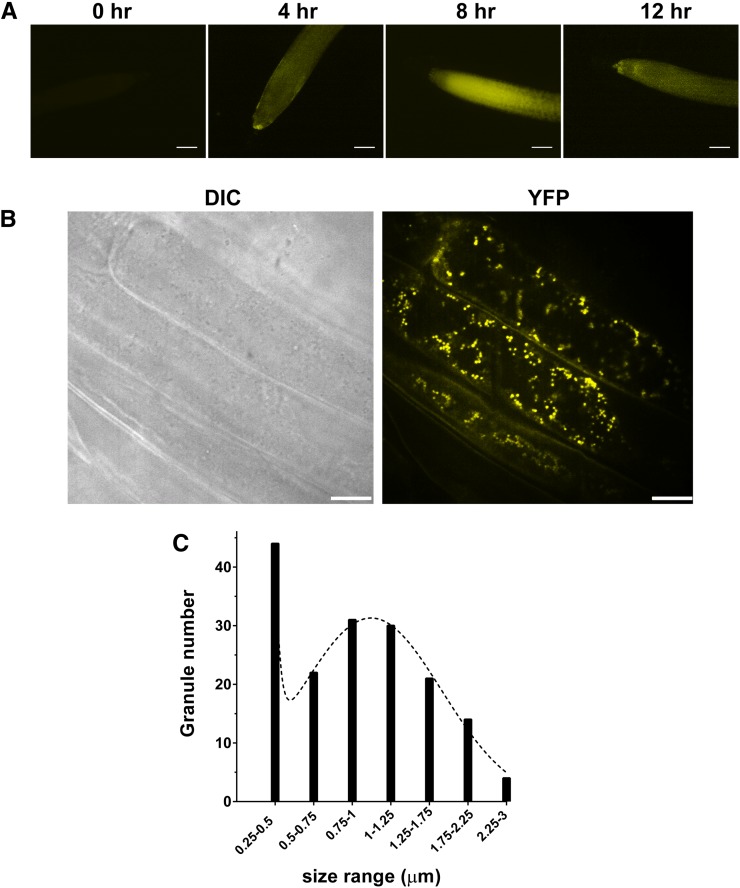
The subcellular localization of CML38 was investigated by fluorescence microscopy of the CML38-YFP recombineering plants (Fig. 4). Consistent with western-blot analysis, normoxic plants showed the absence of fluorescent signal while hypoxia triggered the appearance of the CML38-YFP fluorescent signal detected by 4 h after the onset of hypoxia and peaking by 8 h (Fig. 4A). Higher resolution images obtained by confocal fluorescence microscopy showed that the fluorescent signal consists of distinct cytosolic granule structures with sizes ranging to 3 μm (Fig. 4A). Statistical analysis of the size distribution of particles indicated two size classes, one approximately 0.2 to 0.5 μm and a second with an average of 1 μm.
Figure 4.
Subcellular localization of CML38-YFP in the roots of hypoxia-challenged 10-d-old CML38-YFP recombineering seedlings. A, Ten-day-old vertically grown CML38-YFP seedlings were subjected to anaerobic stress by complete submergence in water, and the appearance CML38-YFP protein was monitored by fluorescence microscopy. B, Higher resolution confocal micrographs of root cells from seedlings after 8 h of submergence. DIC represents the image obtained with differential interference contrast optics, while YFP represents the image obtained with the YFP filter set. C, Histogram of the size distribution of CML38-YFP granules (between 0.4 and 3 μm) from two representative cells as determined by ImageJ analysis. The dotted line shows the fit to a sum of two Gaussian distributions. The median particle size was 0.9 μm, and the average was 1 μm. Bars = 100 µm (A) and 25 µm (B).
Identification of Proteins Associated with CML38 from Hypoxic Arabidopsis Roots
To identify the potential targets of CML38, as well as the identity of the granulated structures in hypoxic roots, immunoprecipitation of CML38-YFP from extracts of root tissue from hypoxia-treated Arabidopsis CML38-YFP plants was performed, followed by tryptic digestion and liquid chromatography-tandem mass spectrometry peptide fingerprinting (Supplemental Table S1). Analyses of mock negative control immunoprecipitation samples from the hypoxia-treated wild type and a GFP transgenic background were used to identify and exclude false positives. By this approach, a total of 106 proteins were identified as CML38-YFP-interacting proteins (Supplemental Table S2), which could be divided into nine functional categories based on PANTHER analysis (Mi et al., 2013; Supplemental Table S2; Supplemental Fig. S6). Potential insight into the nature of the CML38 granulated structures observed in Figure 4 came from the observation that a subset (48 proteins) of the CML38-interacting proteins have been characterized previously as proteins associated with messenger RNA nucleoprotein (mRNP) particles and/or RNA-processing functions (Supplemental Table S2, sheet 2). A summary of this protein subset (Table I) shows that they can be classified into several functional mRNP categories, including ribosomal proteins, translation initiation factors, heat shock proteins and chaperones, proteins associated with ubiquitination and protein degradation, mRNA-processing and degradation proteins, other RNA-binding proteins, and proteins associated with posttranscriptional gene silencing.
Table I. Summary of mRNP and RNA-processing protein classes within CML38 immunoprecipitates based on tandem mass spectrometry peptide fingerprinting.
| Functional Group | Proteina | mRNP Typeb | Referencesc |
|---|---|---|---|
| Ribosomes | RPL, 60S subunit (12 proteins) | U3 snoRNPs | 1 |
| RPS, 40S subunit (9 proteins) | SG, U3 snoRNP | 1–3 | |
| Translation factors | eEF1A (α-1,2,3,4) | SG, PB | 4 |
| eiF4A-1, eiF4A-2 | SG | 5 and 6 | |
| PABP [poly(A)-binding protein] | SG, PB | 2–4 and 7 | |
| Heat shock proteins and chaperones | HSP70 | SG, HSG | 8 and 9 |
| HSP81,89 and HSP90,91 | HSG, U3 snoRNP | 1, 8, and 9 | |
| TCP-1 (CPN60) | HSG, U3 snoRNP | 1 and 9 | |
| Ubiquitination and protein degradation | RPN10, RPT1 and RPT2A, RPT3, AAA-type ATPases | SG, PB | 1, 10 and 11 |
| mRNA processing and degradation | Varicose (VCS) | PB | 12 |
| DEAD/DEAH box RNA helicases | SG | 13 and 14 | |
| Tudor-streptococcal nuclease protein (TSN1) | SG and PB | 15 and 16 | |
| Other RNA-binding proteins | GRP8 | SG | 17 |
Listed candidates are present in at least two biological replicates. Proteins may have overlapping/predicted functions and can belong to more than one mRNP type.
mRNP type is as follows: stress granule (SG), processing body (PB), heat shock granule (HSG), and small nucleolar RNA U3 (U3 snoRNP).
References are as follows: 1, Samaha et al. (2010); 2, Kedersha et al. (2002); 3, Sorenson and Bailey-Serres (2014); 4, Hafrén et al. (2013); 5, Buchan and Parker (2009); 6, Bailey-Serres et al. (2009); 7, Kimball et al. (2003); 8, Weber et al. (2008); 9, Nover et al. (1989); 10, Mazroui et al. (2007); 11, Sako et al. (2012); 12, Xu et al. (2011); 13, Kobayashi et al. (2007); 14, Linder and Owttrim (2009); 15, Yan et al. (2014); 16, Gutierrez-Beltran et al. (2015); and 17, Schmidt et al. (2010).
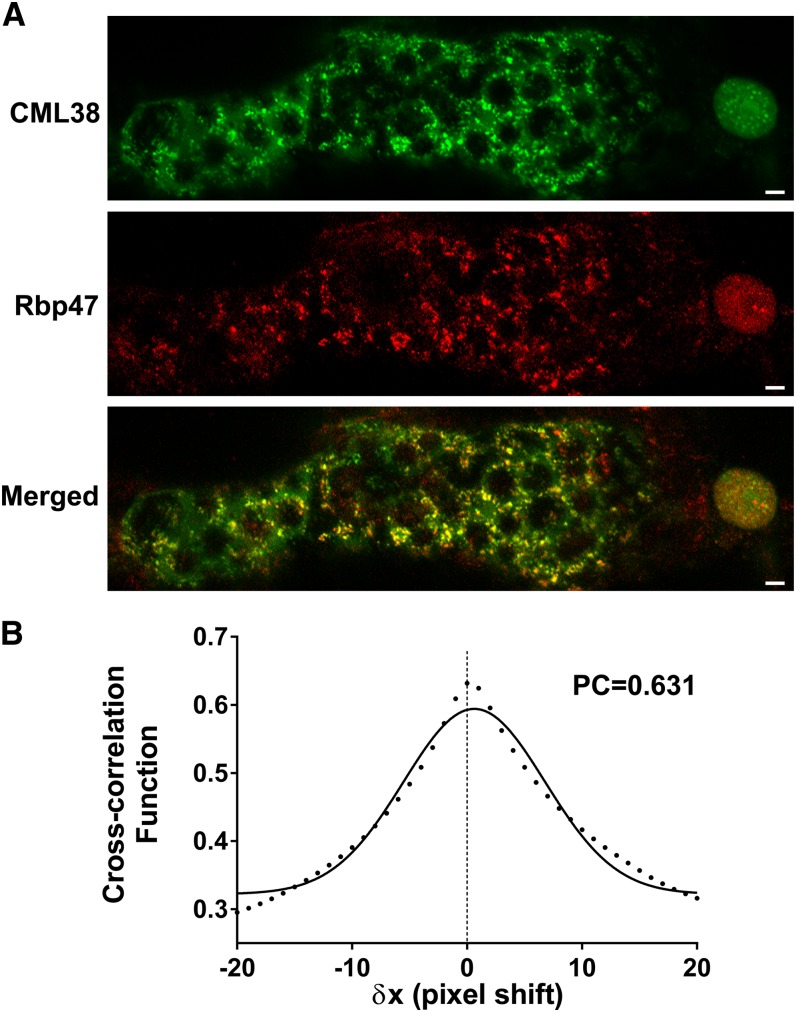
Calcium-Dependent Colocalization of CML38 with Stress Granule mRNPs
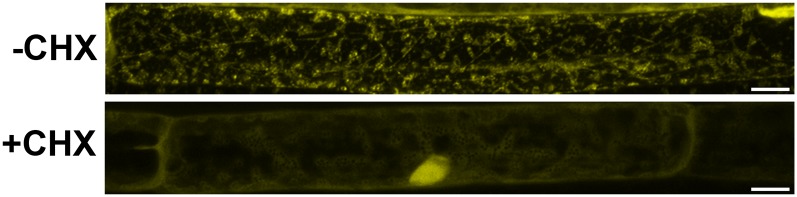
The appearance of CML38-YFP granulated bodies in the hypoxia-challenged seedlings, and the finding of mRNP granule markers in CML38 immunoprecipitates, suggest that the appearance of CML38 granules may be the result of localization to hypoxia-induced stress granules (SG). It is well established that part of the hypoxia response in plants is the rapid formation of SG mRNP bodies that serve as translationally-repressed mRNA triage centers that facilitate the storage and/or transfer of mRNA to other RNA nucleoproteins during adaptation to hypoxia stress (Weber et al., 2008; Sorenson and Bailey-Serres, 2014). To test this hypothesis, the colocalization of CML38 with the SG-specific marker protein RBP47 (Weber et al., 2008) under hypoxia stress was investigated by transient cotransfection assays of Nicotiana benthamiana leaves with CML38:YFP and RBP47:cyan fluorescent protein (CFP) constructs (Fig. 5). As described previously (Sorenson and Bailey-Serres, 2014), N. benthamiana cells expressing fluorescently tagged RBP47-CFP show the appearance of fluorescent signal within cytosolic granule structures within minutes of hypoxia initiation (Fig. 5). Similarly, transfection of N. benthamiana with CML38-YFP shows the accumulation of cytosolic granules (Fig. 5A; Supplemental Fig. S7). In cells cotransfected with CML38-YFP and Rbp47-CFP, both fluorescent proteins show similar appearance of cytosolic granules, as well as appearance in the cell nucleus, within 30 min of hypoxia induction. Comparison of the merged images of cotransfected cells shows substantial colocalization of the two proteins (Fig. 5A) within cytosolic granules as well as the nucleus. The degree of colocalization between CML38 and Rbp47 was quantified by cross-correlation analysis (Bolte and Cordelières, 2006) using the method of van Steensel et al. (1996; Fig. 5B). This method relies on cross-correlation analysis by computationally shifting one image (e.g. CML38) relative to the other image (e.g. Rbp47) and calculating the Pearson coefficient (PC). The PC is a quantitative measure of the pixel overlap in superimposed, merged dual-channel images (Bolte and Cordelières, 2006). PC values range from +1 to −1, with +1 representing theoretical complete (100%) correlation between the two fluorophores, −1 representing complete exclusion of the images, and 0 representing a random distribution of each image. Analysis of merged RBP47-CFP and CML38-YFP images shows a calculated PC value of 0.631, supporting strong colocalization of the two fluorescent signals (Fig. 5B). This result supports the prediction from the mass spectrometry (MS) experiments, as well as the known properties of SG dynamics in hypoxia-stressed Arabidopsis, and argues that the fluorescent granules found in hypoxic Arabidopsis roots in CML-YFP plants (Fig. 4) correspond, at least in part, to hypoxia-induced SGs. In further support of this, treatment of complementation plants expressing CML38-YFP with cycloheximide, which prevents the assembly of SGs (Kedersha et al., 2002; Sorenson and Bailey-Serres, 2014), results in the loss of YFP signal in particulate structures and accumulation in the cell nucleus (Fig. 6).
Figure 5.
CML38 and RBP47 colocalization in transfected N. benthamiana leaf epidermal cells. N. benthamiana leaves were cotransfected with D35S::CML38:YFP and D35S::RBP47:CFP and subjected to 30 min of hypoxia. A, CML38, confocal micrograph with the YFP filter set (false-colored green) showing CML38-YFP localization; RBP47, confocal micrograph with the CFP filter set (false-colored red) showing RBP47-CFP localization; Merged, merged image showing the colocalization of RBP47-CFP and CML38-YFP. Bars = 10 µm. B, Quantitative analysis of the colocalization of RBP47-CFP and CML38-YFP was performed using the Just Another Colocalization Plugin in ImageJ (Bolte and Cordelières, 2006) and is expressed as a van Steensel plot (van Steensel et al., 1996). The cross-correlation function between CML38-YFP and RBP47-CFP granule signals is plotted against the pixel shift (δx). The fit to a Gaussian function (r2 = 0.9771) with the calculated PC is shown.
Figure 6.
Effects of cycloheximide on hypoxia-induced CML38 granule formation. Fourteen-day-old Arabidopsis seedlings expressing CML38:YFP were treated with cycloheximide (+CHX) or a dimethyl sulfoxide (DMSO) mock treatment (−CHX) and subjected to hypoxic conditions as described in “Materials and Methods.” Shown are confocal micrographs of representative root cells. Bars = 10 µm.
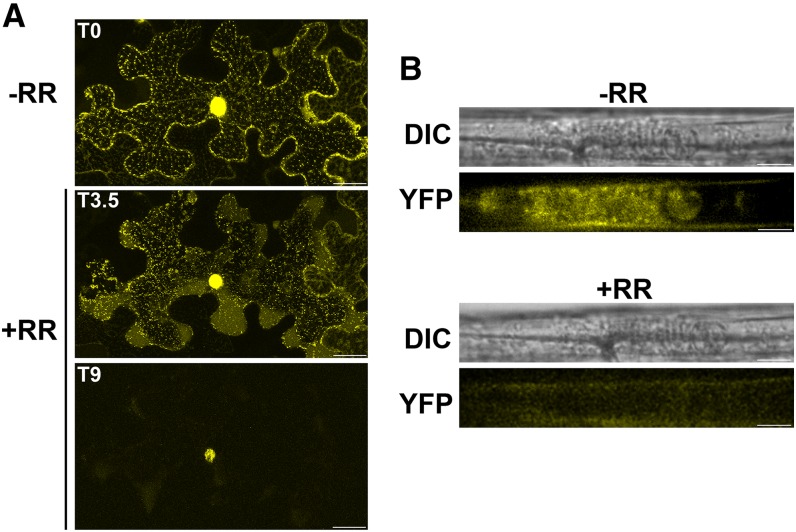
Calcium sensor EF hand proteins, including CML38 (Vanderbeld and Snedden, 2007), exhibit calcium-dependent conformational changes that are associated with the regulation of function through interaction with cellular target proteins (Bender and Snedden, 2013). To investigate the role of calcium binding in CML38’s association with cytosolic granules, the effects of RR were investigated (Figs. 7 and 8). RR lowers cytosolic calcium levels by the inhibition of intracellular Ca2+ channels, and it has been shown previously to suppress the hypoxia-induced response in plant roots (Sedbrook et al., 1996; Dolferus et al., 1997; Subbaiah et al., 1998). Application of RR to N. benthamiana leaves led to the loss of cytosolic CML38-YFP granules within 10 min of application, with CML38 fluorescence detected solely within foci in the nucleus (Fig. 7A). Similarly, RR treatment of hypoxia-challenged CML38-YFP recombineering plants results in a rapid (within 5 min) loss of fluorescent signal in granules (Fig. 7B).
Figure 7.
Effects of RR on CML38-YFP granule structures. A, Confocal images of an N. benthamiana leaf epidermal section transfected with D35S::CML38:YFP. Granule formation was induced by 30 min of hypoxia treatment, and then RR was applied as described in “Materials and Methods.” T0 represents the starting time before RR was applied (−RR), while T3.5 and T9 mark the treatment in minutes after RR application (+RR). Bars = 25 µm. B, Confocal images of a 10-d-old recombineering CML38-YFP seedling root subjected to 3 h of hypoxia stress followed by the application of 0.1 mm RR. −RR represents the hypoxic root sample before RR treatment, while +RR represents the same sample 2.5 min after RR treatment. DIC represents the image obtained with differential interference contrast optics, while YFP represents the image obtained with the YFP filter set. Bars = 10 µm.
Figure 8.
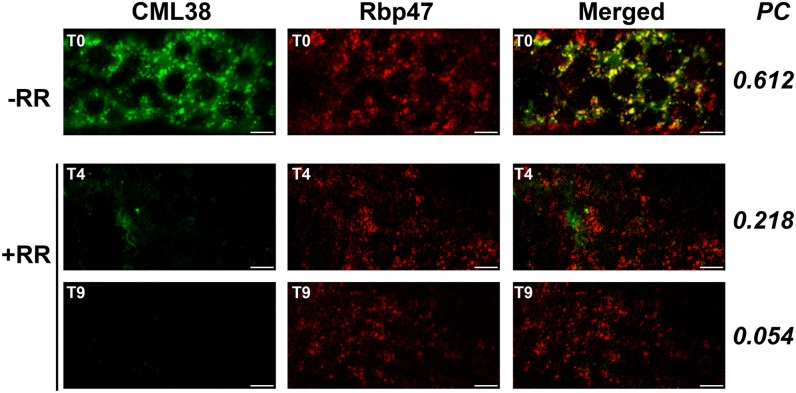
Effects of RR on CML38-YFP and RBP47-CFP colocalization. Confocal images from z-stacks of three 0.2-µm N. benthamiana leaf epidermal sections cotransfected with CML38:YFP (CML38) and RBP47:CFP (Rbp47), and merged images show the colocalization of RBP47-CFP and CML38-YFP. Granule formation was induced by 30 min of hypoxia treatment, and then RR was applied as described in “Materials and Methods.” T0 represents the starting time before RR was applied (−RR), while T4 and T9 mark the treatment in minutes after the RR application (+RR). The PC value for each time point is shown on the right. Bars = 10 µm.
To determine whether the effect of RR was on CML38’s association with SG or on the general integrity of these structures, its effect on N. benthamiana leaf cells cotransfected with CML38-YFP and RBP47-CFP was assessed (Fig. 8). The results show that RR treatment exhibited little effect on the association of RBP47 with SGs while the CML38 signal associated with these structures was lost in a time-dependent manner, suggesting that RR has no effect on SG integrity. Thus, it appears as if calcium is necessary for CML38’s association with SG structures, presumably through the calcium-dependent sensor function of CML38.
CML38 Expression and Granule Localization during Reaeration
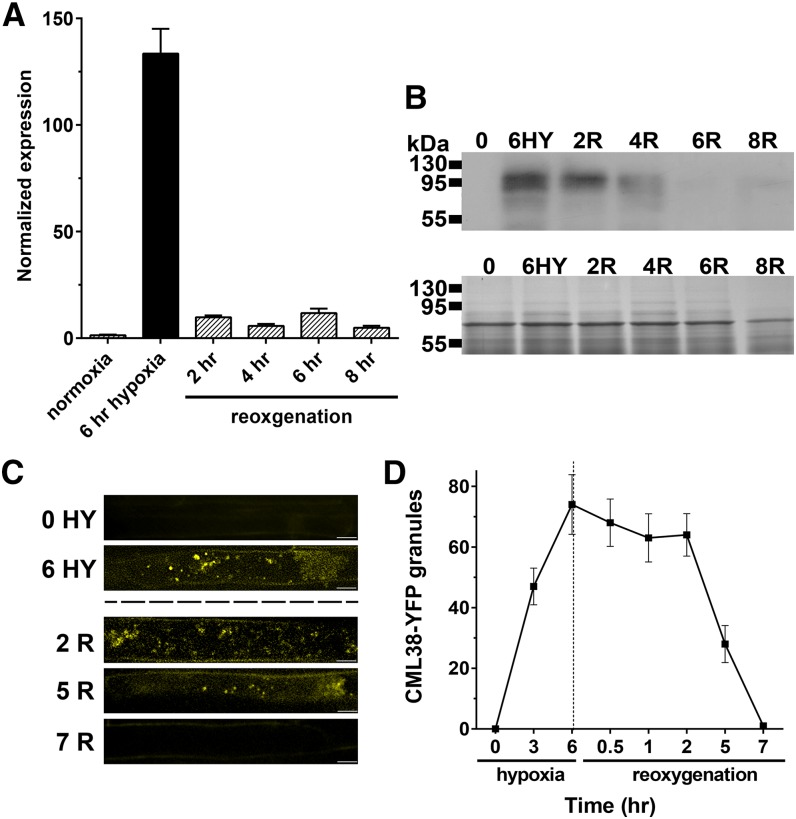
Previous studies in both animals and plants show that recovery from stress conditions is accompanied by the disassembly of SG structures and the degradation of SG-associated proteins (Gilks et al., 2004; Buchan and Parker, 2009; Sorenson and Bailey-Serres, 2014). Consistent with this observation, reoxygenation of hypoxia-treated Arabidopsis CML-YFP recombineering seedlings results in a decrease in transcript levels within 2 h of reoxygenation (Fig. 9A). This is followed by a decline in CML38-YFP protein levels (Fig. 9B) and the disappearance of CML38-YFP granule structures (Fig. 9, C and D), with the western-blot protein signal and fluorescent granules no longer apparent at 6 to 7 h after reoxygenation (Fig. 9).
Figure 9.
Effects of reaeration on CML38 expression and granule structures. A, Q-PCR of CML38-YFP from root tissue of 10-d-old CML38-YFP seedlings untreated (normoxia), challenged with 6 h of hypoxia stress, and then reoxygenated for 2 to 8 h. Normalized expression was determined as described in Figure 1. Error bars indicate se of five biological replicates. B, Western-blot analysis of CML38-YFP (102.1 kD) for the indicated treatments (top gel) and a Coomassie Blue-stained gel with the same extracts as a loading control (bottom gel). Hypoxia (HY) and reoxygenation (R) time points are indicated. The electrophoretic mobility positions of molecular mass marker proteins are shown at left. C, Confocal images from z-stacks of three 0.1-µm slices of 10-d-old CML38-YFP seedling roots that were untreated (0 HY), subjected to 6 h of hypoxia (6 HY), or treated with 6 h of hypoxia followed by 2 h (2 R), 5 h (5 R), or 7 h (7 R) of reoxygenation as described in “Materials and Methods.” Bars = 10 µm. D, Quantitative analysis of CML38-YFP granule number from three root cells in 10-d-old CML38-YFP seedlings after the indicated hypoxia and reoxygenation treatments. Error bars represent se of three CML38-YFP seedling roots.
DISCUSSION
In response to flooding and the associated hypoxic stress, plants have developed various morphological and metabolic strategies to conserve energy and sustain life. Fundamental to these responses is the reorientation of gene expression networks toward the targeted synthesis of a core set of anaerobic response proteins that mediate the adaptive response (Mustroph et al., 2010, 2014). An additional key strategy to the anaerobic responses in plants is the regulation of mRNA translation and homeostasis, mediated by the formation of mRNP aggregates, including SGs (Weber et al., 2008; Sorenson and Bailey-Serres, 2014). This study shows that the Arabidopsis calmodulin-like protein CML38, which is highly up-regulated during hypoxia stress and is a member of the collection of core hypoxia-induced gene products, enhances the survival of the plant to low-oxygen challenge. Under hypoxia stress, CML38 colocalizes with mRNP particles, including hypoxia-induced SGs, in a calcium-dependent manner. Given the previous demonstration of calcium signals in regulating hypoxia responses in plants (Subbaiah et al., 1994a, 1994b; Dolferus et al., 1997; Peng et al., 2001), it is proposed that CML38 may serve as a target for calcium signals in the potential regulation of mRNP function during hypoxia.
SG aggregates have been reported in most eukaryotes and form in response to conditions under which mRNA translation is impaired or restricted (Anderson and Kedersha, 2009; Bailey-Serres et al., 2009; Buchan and Parker, 2009). SG particles consist of mRNA bound in an arrested state as a preinitiation (48S) complex composed of small 40S ribosomal subunits, eIFs, and mRNA-associated proteins like the poly(A)-binding protein (Kedersha et al., 2002; Kimball et al., 2003). Besides these core components, proteomic analyses of SGs reveal that these structures are complex, with a large array of protein components, including RNA-processing proteins, metabolic enzymes, chaperone proteins, and cell signaling components, associated with the granule complex (Samaha et al., 2010; Isabelle et al., 2012; Sorenson and Bailey-Serres, 2014). Functionally, SGs are proposed to be in dynamic equilibrium with other RNA nucleoprotein particles, such as processing bodies (PB) involved in mRNA degradation, and polysomes involved in mRNA translation. SGs serve as RNA triage centers that sort transcripts for various cellular fates (Decker and Parker, 2012; Kedersha et al., 2013).
The formation of SG complexes is one of the hallmarks of the plant response to oxygen deprivation stress (Sorenson and Bailey-Serres, 2014). Translation-competent transcripts associated with polysomes decrease by up to 90% during hypoxia stress in Arabidopsis (Branco-Price et al., 2008), an event that is accompanied by the rapid generation of SGs and PBs, which serve as sequestration or degradation sites for these mRNAs (Weber et al., 2008; Sorenson and Bailey-Serres, 2014), presumably to reduce the energy cost of translating nonessential gene products. Upon reoxygenation, these transcripts are then released from SGs as part of the recovery process (Sorenson and Bailey-Serres, 2014). Several findings in this study support the association of CML38 with hypoxia-induced SGs. First, CML38 is expressed in a temporally controlled fashion in roots during hypoxia stress and becomes associated with cytosolic foci that correspond to the known size of SG particles (Anderson and Kedersha, 2009; Sorenson and Bailey-Serres, 2014). Similar to previous studies of time-resolved SG disassembly in plant tissues recovering from hypoxia (Sorenson and Bailey-Serres, 2014) and related heat stress (Gutierrez-Beltran et al., 2015), the CML38 foci disassemble upon reoxygenation. Second, MS analysis of immunoprecipitates of CML38 from hypoxic root samples reveals the presence of established SG-associated proteins, including poly(A)-binding protein (Kedersha et al., 2002; Kimball et al., 2003; Sorenson and Bailey-Serres, 2014), eukaryotic initiation factors (Li et al., 2010), eukaryotic elongation factors (Hafrén et al., 2013), RNA helicase (Koroleva et al., 2009), small ribosomal subunit proteins (Kedersha et al., 2002; Sorenson and Bailey-Serres, 2014), and others (Mazroui et al., 2007; Buchan et al., 2013; Yan et al., 2014, Gutierrez-Beltran et al., 2015). Third, coexpression of the core SG-nucleating RNA-binding protein RBP47 (Weber et al., 2008) with CML38 shows that, under hypoxia conditions, the two proteins colocalize to cytosolic granules.
RBP47 is related to the TIA-1 protein of animals (Lorković et al., 2000) and contains both RNA-binding motifs as well as prion-like sequences that are involved in SG assembly induced by hypoxia or heat stress (Weber et al., 2008). The oligouridylate-binding protein UBP1C has been shown to play a similar role during hypoxia in Arabidopsis (Sorenson and Bailey-Serres, 2014). These proteins show both nuclear and cytoplasmic localization and are proposed to play a multifunctional role in SG assembly and mRNA recruitment in the cytosol as well as nuclear functions, including pre-mRNA processing, exon splicing, and nucleocytoplasmic transport (Lambermon et al., 2000; Weber et al., 2008; Sorenson and Bailey-Serres, 2014). The localization of CML38 to both the nucleus and cytosol suggests that this protein may also be associated with RBP47 mRNPs in both locations. Consistent with this, the Arabidopsis ortholog of the mammalian DEAD box helicase (Table I), eIF4A-III, and the putative anchor protein of the exon junction complex (Koroleva et al., 2009; Supplemental Table S1, sheet 3), are among the CML38-interacting proteins detected by MS analysis. In addition, CML38 immunoprecipitates also contained proteins associated with the nuclear U3 snoRNP complex (small and large ribosomal subunit proteins, TCH-1 chaperones, and the 26S proteasome regulatory subunit S2; Table I; Samaha et al., 2010), which is involved in ribosome biogenesis, suggesting another potential function in RNA metabolism.
As noted above, SGs are in dynamic equilibrium with mRNP PBs, and some proteins associate with both SGs and PBs (Pomeranz et al., 2010; Bogamuwa and Jang, 2013; Gutierrez-Beltran et al., 2015). Consistent with these findings, CML38 interactome analysis showed the presence of TSN1 and TSN2 proteins (Table I; Supplemental Table S1, sheet 3), which were recently found to associate with both SGs and PBs under heat stress (Gutierrez-Beltran et al., 2015), an abiotic stress with overlapping responses with low-oxygen stress (Banti et al., 2010). These observations strengthen the argument that CML38 is a core component of both SG and PB mRNPs and may play a role in mRNA homeostasis during the stress challenge.
Treatment of hypoxic tobacco mesophyll cells with RR, which perturbs the release of intracellular calcium stores, results in the rapid loss of CML38 signal associated with the cytosolic granules. Similar treatments on hypoxic roots of CML38-YFP recombineering Arabidopsis plants also resulted in complete loss of its association with granules. RR treatments showed no effect on the RBP47 localization to cytosolic granules, suggesting that this treatment did not disrupt SGs but only CML38’s association with these structures. This observation suggests that the calcium sensor function of CML38 may be necessary for its assembly into SGs. Furthermore, the kinetics of CML38’s appearance in cytosolic granules in Arabidopsis roots (3–6 h after the induction of hypoxia) is slower than the establishment of SGs, which occurs within minutes after the establishment of hypoxia (Weber et al., 2008; Sorenson and Bailey-Serres, 2014). Overall, the data suggest that CML38 is not necessary for the formation of SG structures during the initial phases of hypoxia stress but may function as a calcium sensor in SG function at a later stage of the low-oxygen stress response.
To date, there is no evidence of calcium- or calmodulin-related proteins in regulating mRNP particle dynamics in either plants or animals, and the functional role of CML38 during the hypoxia response remains unknown. However, a CML38 function in the regulation of mRNA expression and posttranscriptional silencing is suggested from the observation that CML38 is phylogenetically and structurally related to the rgsCaM protein from tobacco (Supplemental Figs. S2 and S3). rgsCaM was originally identified in tobacco as an interacting protein of the potyviral helper-component proteinase (HC-Pro; Anandalakshmi et al., 2000). HC-Pro suppresses the virus-induced posttranscriptional gene silencing pathway of the host by interfering with small interfering RNA (siRNA)-based silencing pathways (Mallory et al., 2001). Overexpression of rgsCaM itself leads to the suppression of posttranscriptional gene silencing, and it is has been proposed to be an endogenous regulator of this process (Anandalakshmi et al., 2000) in addition to serving as a potential target for viral suppressors of virus-induced posttranscriptional gene silencing (Anandalakshmi et al., 2000; Nakahara et al., 2012). Besides its structural and sequence similarity to rgsCaM, Arabidopsis CML38 also has been demonstrated to be induced by HC-Pro and is proposed to be an orthologous endogenous suppressor of gene silencing in Arabidopsis (Endres et al., 2010).
Previous studies have shown that the RNA-induced silencing complex and siRNAs also accumulate in cytosolic mRNP particles (Jakymiw et al., 2005; Liu et al., 2005b; Sen and Blau, 2005; Jouannet et al., 2012). Besides their function in posttranscriptional gene silencing and targeting of mRNA for degradation, an alternative function in the regulation of the translation of selected mRNAs has also been ascribed to siRNA and microRNA in plants (Brodersen et al., 2008). Whether CML38, similar to rgsCaM, possesses suppressor of posttranscriptional gene silencing function during the hypoxia response remains a topic for future study. One observation of interest from this study is the finding that some of the components of potyvirus RNA nucleoprotein complexes, such as eukaryotic elongation factor 1A and poly(A)-binding protein (Thivierge et al., 2008; Hafrén et al., 2013), are among the CML38-interacting proteins detected by MS analysis (Table I).
A number of critical questions regarding a mechanistic function of CML38 and its role in hypoxia responses and mRNP function remain. First, while CML38 appears to interact with SGs in a calcium-dependent fashion, the specific protein targets within these mRNP structures that CML38 binds and modulates remain to be determined. Based on the calmodulin paradigm (Chin and Means, 2000), the interaction of calcium with CML38 would likely expose a binding surface for its association and activation of downstream regulatory targets. The protein complement of SGs is complex (Kedersha et al., 2013), and the identification of calcium-dependent specific binding targets of CML38 among the collection of proteins identified by immunoprecipitation is an essential step regarding its regulatory role in mRNP function and dynamics.
A second challenge for future work is to determine the spatiotemporal changes in the calcium signaling program during hypoxia perception, adaptation/response, and recovery, as well as the corresponding calcium sensor targets. Previous work using aequorin reporter constructs in Arabidopsis (Sedbrook et al., 1996) illustrated the complexity of the calcium signaling responses during anaerobiosis, with acute influx of cytosolic calcium observed early in the onset of anoxia followed by a lower sustained level of calcium that rises and persists for an extended period during sustained anoxia. Reoxygenation results in an initial decline in cytosolic calcium followed by another acute burst of calcium (Sedbrook et al., 1996). Given the time course for CML38 appearance during the hypoxia response, its function as an early sensor of hypoxia (i.e. within minutes of the response) is not likely. Rather, it may play a role as a sensor of calcium signals later in the adaptive response or during oxygen recovery. Besides the identification of molecular targets for CML38, additional analyses of mRNP dynamics in CML38 mutant backgrounds will hopefully shed additional light on its calcium sensor roles during the hypoxia/reoxygenation response program.
As a final note, phylogenetic analysis reveals that CML38 and rgsCaM are part of a larger family of structurally related calcium sensor proteins (Supplemental Fig. S2). Structural features characteristic of this family are (1) the presence of four EF hand-like calcium-binding domain structures; (2) the common finding that EF hand III in these structures possesses substitutions predicted to abrogate calcium binding; and (3) the presence of an N-terminal basic, amphipathic extension (Supplemental Fig. S3). In Arabidopsis there are three additional genes associated with the rgsCaM-like clade (CML37, CML39, and CML41). Previous work shows that these Arabidopsis proteins, as well as orthologs in rice (Oryza sativa), are also regulated by abiotic stresses (Vanderbeld and Snedden, 2007; Xu et al., 2011).
It remains to be determined whether Arabidopsis rgsCaM-like proteins in general share some degree of similarity with respect to participation in mRNA silencing and association with mRNP particles. One observation of interest in this respect is the finding that CML39 interacts with another viral suppressor of silencing, the AL2 suppressor of Tomato golden mosaic virus, and becomes localized within particles in the nucleus (Yong Chung et al., 2014). These structures are reminiscent of mRNP bodies found in the nucleus during stress responses (Weber et al., 2008; Sorenson and Bailey-Serres, 2014). It remains to be verified if these nuclear structures are SGs. More broadly, the investigation of other members of the rgsCaM-like family to determine whether these proteins share a common property of association with mRNP particles during abiotic or biotic stress responses is merited.
MATERIALS AND METHODS
Plant Growth Conditions and Stress Treatment Protocols
Arabidopsis (Arabidopsis thaliana) ecotype Columbia-0 seeds were germinated and grown under a long-day cycle of 16 h of light (approximately 100 μmol m−2 s−1)/8 h of dark at 22°C as described previously (Choi and Roberts, 2007). For hydroponic growth, 10-d-old seedlings were transferred onto a hydroponics growth system (Gibeaut et al., 1997) and were grown under long-day conditions.
Hypoxia was administered by different protocols depending on the age and culture conditions of the plants. For 12-d-old Arabidopsis seedlings grown vertically on Murashige and Skoog agar medium, stress was administered by complete submergence of seedlings with water purged with a continuous supply of nitrogen gas with a dissolved oxygen content of 2% v/v or less. For hydroponically grown Arabidopsis plants, hypoxia was administered by replacing the air supply with nitrogen gas, and time of hypoxia was initiated at 2% v/v or less oxygen. The dissolved oxygen concentration was monitored with an oxygen monitor (YSI model 55).
Nicotiana benthamiana plants were grown and transformed by infiltration of leaves with Agrobacterium tumefaciens by the protocol of Wang et al. (2013). Hypoxia treatment of N. benthamiana leaf sections was performed essentially as described previously (Weber et al., 2008; Sorenson and Bailey-Serres, 2014). Briefly, a small section (1 cm × 0.5 cm) of the infiltrated leaf was cut and submerged in 200 μL of 30 mm Tris-HCl, pH 7, on a glass slide and was covered with a coverslip (Weber et al., 2008). For RR treatment of hypoxic leaf sections, 100 μL of 0.1 mm RR (Sigma-Aldrich) in 30 mm Tris-HCl, pH 7, was perfused onto leaf sections under the coverslip. A similar approach was used for administering RR treatment to hypoxic CML38-YFP recombineering seedlings.
Cycloheximide treatment was done as described previously (Sorenson and Bailey-Serres, 2014). Seedlings were either submerged in 1× Murashige and Skoog medium containing 0.4% (v/v) DMSO and 200 ng µL−1 cycloheximide or mock treated with 0.4% (v/v) DMSO for 35 min at room temperature in darkness. After treatment, seedlings were transferred to slides and were treated with 8 units mL−1 Oxyrase (Oxyrase) to induce hypoxia. Images were acquired within 10 min of slide preparation.
Molecular Cloning and Plant Transformation Techniques
For the generation of CML38 promoter::GUS reporter constructs, a fragment corresponding to 1,570 bp of DNA upstream of the CML38 transcriptional start site was amplified from Arabidopsis genomic DNA by PCR using gene-specific primers (PstI_CML38pro F and BamHI_CML38pro R; Supplemental Table S3). The PCR-amplified fragment was digested with PstI and BamHI and was cloned into the PstI and BamHI sites of pCAMBIA1391Z (Hajdukiewicz et al., 1994) upstream of a promoterless GUS reporter gene. A. tumefaciens strain GV3101 (Koncz and Schell, 1986) was transformed by electroporation as described previously (Jones, 1995). Transformants were identified by selection on Luria-Bertani agar containing 50 μg mL−1 kanamycin. Plant transformation was carried out using the floral dip method (Clough and Bent, 1998).
Recombineering lines containing CML38 fused to three copies of YFP at the C terminus (CML38-YFP) were generated as described (Zhou et al., 2011). The JAtY clone information and primers for CML38 (JAtY53G16) were obtained from resources available at http://www4.ncsu.edu/∼jmalonso/Alonso-Stepanova_Arabidopsis_localizome.html. TJAtY68K23 was purchased from the Genome Analysis Center (Norwich). The Escherichia coli recombineering strain SW105 was purchased from the Frederick National Laboratory for Cancer Research, and the recombineering cassette with 3xYpet was generously provided by Dr. José Alonso (North Carolina State University). The cassette was introduced to the C terminus of CML38 by PCR using Rec_F and R primers (Supplemental Table S3). The 3xYpet-tagged JAtY53G16 clone was transformed into A. tumefaciens GV3101, and transgenic recombineering strains were selected by the procedure of Zhou et al. (2011) using the 3xYFP_SEQ primers listed in Supplemental Table S3. Growth of the recombineering transgenic plants was done by selection on Murashige and Skoog medium supplemented with 25 µg mL−1 Basta (Chem Service; N12111).
To generate the RBP47-CFP and CML38-YFP reporter constructs for expression in N. benthamiana, the open reading frame of each complementary DNA (cDNA) was PCR amplified from Arabidopsis Columbia-0 cDNA using gene-specific primers that incorporate Gateway-compatible attB1 and attB2 recombination sites in a two-step PCR process (for primers, see Supplemental Table S3) developed by Invitrogen (http://www.embl.de/pepcore/pepcore_services/cloning/cloning_methods/gateway/2step/) to generate PCR products for recombination into Gateway-compatible entry vectors (Life Technology). Donor constructs containing CML38 and RBP47 were recombined into the pEarleyGate 101 and pEarleyGate 102 destination vectors (Earley et al., 2006), which incorporate a C-terminal YFP tag (CML38) or CFP tag (RBP47), respectively. These plasmids were transformed into A. tumefaciens GV3101 prior to transformation of N. benthamiana leaves by infiltration. Sequences were verified by automated DNA sequencing using a model 373 DNA sequencer (Applied Biosystems) at the University of Tennessee Molecular Biology Research Facility.
Characterization of a T-DNA Insertion Mutant of CML38
The cml38 T-DNA insertion mutant (Salk_066538C) was obtained from the Arabidopsis Biological Resource Center and was characterized by antibiotic selection followed by PCR genotyping. Selection of cml38 seeds was done by germination and growth on one-half-strength Murashige and Skoog agar with 50 μg mL−1 kanamycin. Selection was done over several subsequent generations until homozygous plants (100% resistance) were obtained. PCR genotyping was performed with gene-specific CML38 primers and a left border T-DNA primer (Supplemental Table S3; Supplemental Fig. S5). The predicted T-DNA insertion site was verified by cloning of the T-DNA/CML38 PCR product into the pCR2.1-TOPO vector (Invitrogen) and DNA sequence analysis.
For complementation, late-generation cml38 homozygous plants were transfected with the CML38-YFP construct under the control of the cauliflower mosaic virus 35S promoter by the approach described above, and selection of transgenic plants was done on Murashige and Skoog agar medium with 30 μg mL−1 Basta. T3 lines of these transformants were used for all experiments.
Sensitivity to oxygen deprivation stress was done by argon gas treatment and assay of survival as described previously (Sorenson and Bailey-Serres, 2014). Eight-day-old seedlings grown vertically on Murashige and Skoog agar with 3% (w/v) Suc were subjected to anaerobic conditions by perfusion with 99.99% (v/v) argon gas for 15 h at room temperature in darkness. Negative control seedlings were treated identically except that perfusion was performed with air. After treatment, the seedlings were returned to standard long-day conditions, and survival was assessed (seedlings with chlorotic shoots were scored as dead) after an additional 10 d of growth.
RNA Purification and PCR
Total RNA was isolated and Q-PCR analysis was performed as described previously (Choi and Roberts, 2007) with the gene-specific and internal control primers are described in Supplemental Table S3. The Arabidopsis UBQ10 gene was used as an internal reference for standardization, and quantitation of gene expression was calculated using the comparative threshold cycle method as described previously (Choi and Roberts, 2007). For end-point reverse transcription-PCR, 50 ng of 2-h hypoxic root cDNA from 12-d-old wild-type, CML38-1 complementation, and cml38 mutant seedlings was combined with 500 nm CML38 or ACTIN2 (internal reference) gene primer sets and 12.5 µL of the GoTaq Green Master Mix (Promega) in a final volume of 25 µL. PCR was performed using the following parameters: one cycle of 5 min at 95°C and 30 cycles of 30 s at 95°C, 30 s at 50°C, 1 min (for CML38 amplification)/2 min (ACTIN2 amplification) at 72°C, and one cycle of 7 min at 72°C for final elongation.
Histochemical and Microscopic Techniques
GUS staining was performed on Arabidopsis seedlings as described by Choi and Roberts (2007). Epifluorescence images were captured with an Axiovert 200M microscope (Zeiss) equipped with filters for YFP fluorescence (Chroma; filter set 52017) and a digital camera (Hamamatsu Orca-ER) controlled by the Openlab software (Improvision). Confocal imaging of CML38-YFP plants was performed with a Hamamatsu camera mounted on an Olympus IX83 microscope with a Visitech confocal system. YFP excitation was kept at 514 nm, and emission scans were taken using a long-pass filter (525LP). Confocal imaging and colocalization experiments in N. benthamiana leaves were performed with either a Leica SP2 or SP8 laser scanning confocal microscope using the 488-nm argon ion laser (for CFP) and 543-nm helium-neon laser (for YFP) at the Advanced Microscopy and Imaging Facility at the University of Tennessee. The emission filter was set to 470 to 500 nm for CFP, 560 to 600 nm for YFP, and 660 to 690 nm for chlorophyll autofluorescence.
For image analyses and statistical evaluation, ImageJ version 1.41 software was used (http://rsb.info.nih.gov/ij/index.html). The size distribution of CML38-YFP granules was measured by the particle-analysis tool for ImageJ. For colocalization analyses, 0.2-µm sections from the z-stack of merged RBP47-CFP and CML38-YFP images were adjusted to the same background threshold and quantified by the Just Another Colocalization Plugin (Bolte and Cordelières, 2006) in ImageJ. Statistical analysis was performed using the method of van Steensel et al. (1996) for cross-correlation analysis and PC determination.
Immunochemical and MS Techniques
For western-blot analysis, plant tissue (1 g) was quick frozen in liquid nitrogen and ground in a mortar with a pestle, and proteins were extracted with 250 µL of 2× Laemmli SDS sample buffer (Laemmli, 1970). Proteins were quantified by the Bradford assay, separated by SDS-PAGE on 12.5% (w/v) polyacrylamide gels, and electroblotted onto polyvinylidene fluoride membranes. Western-blot detection of fusion proteins was done as described in (Wallace and Roberts, 2005) using chemiluminescent detection. A rabbit anti-GFP polyclonal antibody (a kind gift from Dr. Rose Goodchild, The University of Tennessee, Knoxville) was used as the primary antibody. Immunoprecipitation of CML38-YFP-associated proteins from plant tissue extracts was performed by using a magnetic anti-GFP resin (Chromotek). Wild-type (control) and CML38-YFP roots were dissected from 31-d-old hydroponic plant samples exposed to hypoxic conditions for 4 to 8 h and were quickly frozen in liquid nitrogen. For the GFP control, root tissues from seedlings expressing GFP under the control of the 35S promoter (Peng et al., 2014) were used. For immunoprecipitation, root tissue (approximately 1 g) was ground in a mortar with a pestle, and the tissue powder was suspended in 250 μL of resuspension buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 0.1 mm dithiothreitol, 0.5% [v/v] glycerol, 1 mm CaCl2, a cOmplete Roche EDTA-free protease inhibitor tablet [catalog no. 05892791001], and 40 µm MG132 [Sigma-Aldrich C2211]) and then vortexed for 30 s followed by centrifugation at 13,000 rpm at 4°C. The supernatant fraction was removed and combined with 20 μL of magnetic anti-GFP resin preequilibrated in resuspension buffer, and the mixture was incubated by mixing at 4°C for 6 h. The resin was collected and was washed with 5 mL of resuspension buffer. The adsorbed protein was collected by the addition of 100 μL of elution buffer (6 m guanidine HCl and 5 mm Tris-HCl, pH 8) followed by incubation in a boiling water bath for 5 min. The eluents were stored at −80°C until MS analysis.
Prior to chromatographic separation with online MS analysis, immunoprecipitated protein samples were incubated at 60°C for 1 h in elution buffer supplemented with 5 mm dithiothreitol. Samples were diluted 6-fold with 50 mm Tris-HCl and 10 mm CaCl2, pH 7.6, and were digested at 37°C with Trypsin Gold (Promega; catalog no. PRV5111) at a final protease:protein sample ratio of 1:20 (w/w). Peptides were collected by centrifugation at 15,000 rpm through a 10-kD molecular mass cutoff spin filter (Millipore), and the filtrate was loaded directly onto a split strong cation-exchange/reverse-phase back column (Luna 5-µm SCX 100 Å and Kinetex 2.6-µm 100 Å C18 resins; Phenomenex) and then desalted prior to injection into the analytical column for tandem liquid chromatography-tandem mass spectrometry analysis. A six-step multidimensional protein identification technology (Washburn et al., 2001) approach was utilized for enhanced sensitivity beyond a standard reverse-phase separation. Briefly, an Ultimate U3000 HPLC workstation (Dionex) delivered increasing pulses of 500 mm ammonium acetate (three steps: 10%, 50%, and 100%; or six steps: 5%, 10%, 15%, 20%, 50%, and 100%) to liberate fractions of peptides bound to the cation-exchange resin for reverse-phase separation prior to entry into an LTQ-Velos mass spectrometer (Thermo Scientific).
Raw files were transformed into mzXL format using MSConvert (Chambers et al., 2012) and searched by MyriMatch software (Tabb et al., 2007) against a compiled The Arabidopsis Information Resource (TAIR) 10 database containing proteins of interest along with contaminants including trypsin. Results were visualized with IDPicker version 3.0 software (Vanderbilt University Medical Center), and to minimize false-positive identifications, results were subjected to highly stringent filtering criteria for all three biological replicates of CML38-YFP and wild-type peptide-spectrum-match filters (maximum Q value of 0.01% or 1% false discovery rate; minimum spectra per peptide, 3; minimum spectra per match, 3; maximum protein groups, 10) and protein-level filters (minimum distinct peptides, 3; minimum additional peptides, 3; minimum spectra per protein, 3). For the GFP control, the stringency levels were set as follows for peptide-spectrum-match filters (maximum Q value of 5% false discovery rate; minimum spectra per peptide, 1; minimum spectra per match, 1; maximum protein groups, 10) and protein-level filters (minimum distinct peptides, 1; minimum additional peptides, 1; minimum spectra per protein, 1). The results were exported into an Excel format, and accession numbers from the wild type and the GFP control (Supplemental Table S1) were used to remove false-positive hits from the CML38-YFP data set. The final list of CML38-YFP-interacting proteins (Supplemental Table S2) represented proteins present in at least two of the three biological replicates based on comparative analysis using VENNY version 2.0 (Oliveros, 2007; Supplemental Table S2, sheet 1). The functional distribution was determined by the PANTHER classification tool version 9.0 (Mi et al., 2013; Supplemental Table S2, sheet 1), which uses the TAIR 10 database (www.pantherdb.org). Identification of proteins involved in RNA processing, or which are associated with RNA nucleoprotein particles, was performed by searching individual accession numbers in the TAIR 10 database and arranging them according to their biological functions, as shown in Supplemental Table S2, sheet 2.
Other Computational Methods
Protein sequences for CaM1 to CaM7 and CML1 to CML50 were downloaded from TAIR (www.arabidopsis.org) using the accession numbers reported in (McCormack and Braam, 2003). The sequence for tobacco (Nicotiana tabacum) rgsCaM comes from GI: 12963415 (Anandalakshmi et al., 2000). Multiple sequence alignments and tree construction were performed using MEGA 6.06 (Tamura et al., 2013). The full-length sequences for the 58 proteins were aligned using MUSCLE (Edgar, 2004) with multiple iterations performed until convergence was achieved. A maximum likelihood tree was constructed using the Jones-Taylor-Thornton model with 200 bootstrap replications. The figure of the tree was prepared using FigTree version 1.4.2 (http://tree.bio.ed.ac.uk/software/figtree/). Amino acids preceding the start site of CaM7 were identified as N-terminal extensions (Supplemental Fig. S3A), and EF hands were identified based on consensus sequences (Gifford et al., 2007; Supplemental Fig. S3B). Homology modeling of the calcium sensor region of CML38 was done using MOE version 2008.10 (Molecular Operating Environment) as described previously (Li et al., 2011), with the calcium-calmodulin structure (Protein Data Bank identifier 1EXR; Wilson and Brunger, 2000) used as a structural template. An ensemble of 10 possible structures was generated by applying MMFF94x force field, and these models were subsequently ranked using MOE’s packing algorithm. Of the 10 possible models, the CML38 structure with the lowest root-mean-square deviation value (0.71 Å) compared with the carbon backbone of the calmodulin structural template was selected for evaluation.
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: CML38, NM_001198484; UBQ10, HQ693235.1; ACTIN2, AK317305; RBP47, BT026489.1.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Transcript expression changes of CMLs in hypoxic Arabidopsis roots.
Supplemental Figure S2. CML38 is phylogenetically similar to rgsCaM.
Supplemental Figure S3. Structural comparisons of the rgsCML protein subfamily.
Supplemental Figure S4. Characterization of a T-DNA insertion mutant of CML38.
Supplemental Figure S5. Reverse transcription-PCR analysis of the expression of CML38 transcripts in wild-type, cml38 mutant, and CML38 complementation plants.
Supplemental Figure S6. Functional classification of the CML38 immunoprecipitation proteins identified from MS analysis.
Supplemental Figure S7. Subcellular localization of CML38 in transfected N. benthamiana leaf epidermal cells.
Supplemental Table S1. MS analysis of tryptic digests of immunoprecipitates from roots of hypoxic wild-type and CML38-YFP recombineering plants.
Supplemental Table S2. Functional classification of CML38 immunoprecipitation proteins based on the PANTHER classification tool.
Supplemental Table S3. Oligonucleotides used for PCR and plasmid construction.
Supplementary Material
Acknowledgments
We thank Dr. Tessa Burch-Smith (University of Tennessee, Knoxville) for guidance in setting up the A. tumefaciens-mediated transformation of the N. benthamiana leaves and for thoughtful and constructive input during the course of this work; Dr. José Alonso (North Carolina State University) for providing assistance in the generation of the CML38-YFP recombineering plants; Dr. Rose Goodchild (University of Tennessee, Knoxville) for providing a sample of polyclonal rabbit anti-GFP antibody; Dr. Neal Stewart (University of Tennessee Institute of Agriculture) for providing Arabidopsis transgenic lines transfected with GFP under the control of the cauliflower mosaic virus 35S promoter; Dr. Bob Hettich (Oak Ridge National Laboratory) for assistance in conducting the MS analyses in this study; and Dr. Won Gyu Choi (present address: University of Wisconsin, Madison), who provided assistance in early experiments in this study.
Glossary
- RR
Ruthenium Red
- Q
quantitative reverse transcription
- T-DNA
transfer DNA
- mRNP
messenger RNA nucleoprotein
- SG
stress granule
- PC
Pearson coefficient
- MS
mass spectrometry
- PB
processing body
- siRNA
small interfering RNA
- DMSO
dimethyl sulfoxide
- cDNA
complementary DNA
- TAIR
The Arabidopsis Information Resource
Footnotes
This work was supported by a subcontract from the University of South Carolina in support of National Science Foundation grant no. IOS–1029803) and by the National Science Foundation (grant no. IOS–1121465).
Articles can be viewed without a subscription.
References
- Anandalakshmi R, Marathe R, Ge X, Herr JM Jr, Mau C, Mallory A, Pruss G, Bowman L, Vance VB (2000) A calmodulin-related protein that suppresses posttranscriptional gene silencing in plants. Science 290: 142–144 [DOI] [PubMed] [Google Scholar]
- Anderson P, Kedersha N (2009) Stress granules. Curr Biol 19: R397–R398 [DOI] [PubMed] [Google Scholar]
- Bailey-Serres J, Fukao T, Gibbs DJ, Holdsworth MJ, Lee SC, Licausi F, Perata P, Voesenek LACJ, van Dongen JT (2012) Making sense of low oxygen sensing. Trends Plant Sci 17: 129–138 [DOI] [PubMed] [Google Scholar]
- Bailey-Serres J, Sorenson R, Juntawong P (2009) Getting the message across: cytoplasmic ribonucleoprotein complexes. Trends Plant Sci 14: 443–453 [DOI] [PubMed] [Google Scholar]
- Bailey-Serres J, Voesenek LACJ (2010) Life in the balance: a signaling network controlling survival of flooding. Curr Opin Plant Biol 13: 489–494 [DOI] [PubMed] [Google Scholar]
- Banti V, Mafessoni F, Loreti E, Alpi A, Perata P (2010) The heat-inducible transcription factor HsfA2 enhances anoxia tolerance in Arabidopsis. Plant Physiol 152: 1471–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender KW, Snedden WA (2013) Calmodulin-related proteins step out from the shadow of their namesake. Plant Physiol 163: 486–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blokhina O, Virolainen E, Fagerstedt KV (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot (Lond) 91: 179–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogamuwa S, Jang JC (2013) The Arabidopsis tandem CCCH zinc finger proteins AtTZF4, 5 and 6 are involved in light-, abscisic acid- and gibberellic acid-mediated regulation of seed germination. Plant Cell Environ 36: 1507–1519 [DOI] [PubMed] [Google Scholar]
- Bolte S, Cordelières FP (2006) A guided tour into subcellular colocalization analysis in light microscopy. J Microsc 224: 213–232 [DOI] [PubMed] [Google Scholar]
- Branco-Price C, Kaiser KA, Jang CJH, Larive CK, Bailey-Serres J (2008) Selective mRNA translation coordinates energetic and metabolic adjustments to cellular oxygen deprivation and reoxygenation in Arabidopsis thaliana. Plant J 56: 743–755 [DOI] [PubMed] [Google Scholar]
- Brodersen P, Sakvarelidze-Achard L, Bruun-Rasmussen M, Dunoyer P, Yamamoto YY, Sieburth L, Voinnet O (2008) Widespread translational inhibition by plant miRNAs and siRNAs. Science 320: 1185–1190 [DOI] [PubMed] [Google Scholar]
- Buchan JR, Kolaitis RM, Taylor JP, Parker R (2013) Eukaryotic stress granules are cleared by autophagy and Cdc48/VCP function. Cell 153: 1461–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchan JR, Parker R (2009) Eukaryotic stress granules: the ins and outs of translation. Mol Cell 36: 932–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers MC, Maclean B, Burke R, Amodei D, Ruderman DL, Neumann S, Gatto L, Fischer B, Pratt B, Egertson J, et al. (2012) A cross-platform toolkit for mass spectrometry and proteomics. Nat Biotechnol 30: 918–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang R, Jang CJH, Branco-Price C, Nghiem P, Bailey-Serres J (2012) Transient MPK6 activation in response to oxygen deprivation and reoxygenation is mediated by mitochondria and aids seedling survival in Arabidopsis. Plant Mol Biol 78: 109–122 [DOI] [PubMed] [Google Scholar]
- Chin D, Means AR (2000) Calmodulin: a prototypical calcium sensor. Trends Cell Biol 10: 322–328 [DOI] [PubMed] [Google Scholar]
- Choi WG, Roberts DM (2007) Arabidopsis NIP2;1, a major intrinsic protein transporter of lactic acid induced by anoxic stress. J Biol Chem 282: 24209–24218 [DOI] [PubMed] [Google Scholar]
- Christianson JA, Wilson IW, Llewellyn DJ, Dennis ES (2009) The low-oxygen-induced NAC domain transcription factor ANAC102 affects viability of Arabidopsis seeds following low-oxygen treatment. Plant Physiol 149: 1724–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HJ, Ferl RJ (1999) Arabidopsis alcohol dehydrogenase expression in both shoots and roots is conditioned by root growth environment. Plant Physiol 121: 429–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Decker CJ, Parker R (2012) P-bodies and stress granules: possible roles in the control of translation and mRNA degradation. Cold Spring Harb Perspect Biol 4: a012286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolferus R, Ellis M, DeBruxelles G, Trevaskis B, Hoeren F, Dennis ES, Peacock WJ (1997) Strategies of gene action in Arabidopsis during hypoxia. Ann Bot (Lond) 79: 21–31 [Google Scholar]
- Dolferus R, Klok EJ, Ismond K, Delessert C, Wilson S, Good A, Peacock J, Dennis L (2001) Molecular basis of the anaerobic response in plants. IUBMB Life 51: 79–82 [DOI] [PubMed] [Google Scholar]
- Earley KW, Haag JR, Pontes O, Opper K, Juehne T, Song K, Pikaard CS (2006) Gateway-compatible vectors for plant functional genomics and proteomics. Plant J 45: 616–629 [DOI] [PubMed] [Google Scholar]
- Edgar RC. (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32: 1792–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ella ES, Kawano N, Ito O (2003) Importance of active oxygen-scavenging system in the recovery of rice seedlings after submergence. Plant Sci 165: 85–93 [Google Scholar]
- Endres MW, Gregory BD, Gao Z, Foreman AW, Mlotshwa S, Ge X, Pruss GJ, Ecker JR, Bowman LH, Vance V (2010) Two plant viral suppressors of silencing require the ethylene-inducible host transcription factor RAV2 to block RNA silencing. PLoS Pathog 6: e1000729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Jia Y, Guo S, Lv G, Wang T, Juan L (2011) Exogenous calcium affects nitrogen metabolism in root-zone hypoxia-stressed muskmelon roots and enhances short-term hypoxia tolerance. J Plant Physiol 168: 1217–1225 [DOI] [PubMed] [Google Scholar]
- Gibbs DJ, Lee SC, Isa NM, Gramuglia S, Fukao T, Bassel GW, Correia CS, Corbineau F, Theodoulou FL, Bailey-Serres J, et al. (2011) Homeostatic response to hypoxia is regulated by the N-end rule pathway in plants. Nature 479: 415–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibeaut DM, Hulett J, Cramer GR, Seemann JR (1997) Maximal biomass of Arabidopsis thaliana using a simple, low-maintenance hydroponic method and favorable environmental conditions. Plant Physiol 115: 317–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford JL, Walsh MP, Vogel HJ (2007) Structures and metal-ion-binding properties of the Ca2+-binding helix-loop-helix EF-hand motifs. Biochem J 405: 199–221 [DOI] [PubMed] [Google Scholar]
- Gilks N, Kedersha N, Ayodele M, Shen L, Stoecklin G, Dember LM, Anderson P (2004) Stress granule assembly is mediated by prion-like aggregation of TIA-1. Mol Biol Cell 15: 5383–5398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuntoli B, Lee SC, Licausi F, Kosmacz M, Oosumi T, van Dongen JT, Bailey-Serres J, Perata P (2014) A trihelix DNA binding protein counterbalances hypoxia-responsive transcriptional activation in Arabidopsis. PLoS Biol 12: e1001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Beltran E, Moschou PN, Smertenko AP, Bozhkov PV (2015) Tudor staphylococcal nuclease links formation of stress granules and processing bodies with mRNA catabolism in Arabidopsis. Plant Cell 27: 926–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafrén A, Eskelin K, Mäkinen K (2013) Ribosomal protein P0 promotes Potato virus A infection and functions in viral translation together with VPg and eIF(iso)4E. J Virol 87: 4302–4312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajdukiewicz P, Svab Z, Maliga P (1994) The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol Biol 25: 989–994 [DOI] [PubMed] [Google Scholar]
- Isabelle M, Gagné JP, Gallouzi IE, Poirier GG (2012) Quantitative proteomics and dynamic imaging reveal that G3BP-mediated stress granule assembly is poly(ADP-ribose)-dependent following exposure to MNNG-induced DNA alkylation. J Cell Sci 125: 4555–4566 [DOI] [PubMed] [Google Scholar]
- Ismond KP, Dolferus R, de Pauw M, Dennis ES, Good AG (2003) Enhanced low oxygen survival in Arabidopsis through increased metabolic flux in the fermentative pathway. Plant Physiol 132: 1292–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakymiw A, Lian S, Eystathioy T, Li S, Satoh M, Hamel JC, Fritzler MJ, Chan EKL (2005) Disruption of GW bodies impairs mammalian RNA interference. Nat Cell Biol 7: 1267–1274 [DOI] [PubMed] [Google Scholar]
- Jones H, editor (1995) Plant Gene Transfer and Expression Protocols. Humana Press, Totowa, NJ [Google Scholar]
- Jouannet V, Moreno AB, Elmayan T, Vaucheret H, Crespi MD, Maizel A (2012) Cytoplasmic Arabidopsis AGO7 accumulates in membrane-associated siRNA bodies and is required for ta-siRNA biogenesis. EMBO J 31: 1704–1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha N, Chen S, Gilks N, Li W, Miller IJ, Stahl J, Anderson P (2002) Evidence that ternary complex (eIF2-GTP-tRNA(i)(Met))-deficient preinitiation complexes are core constituents of mammalian stress granules. Mol Biol Cell 13: 195–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha N, Ivanov P, Anderson P (2013) Stress granules and cell signaling: more than just a passing phase? Trends Biochem Sci 38: 494–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball SR, Horetsky RL, Ron D, Jefferson LS, Harding HP (2003) Mammalian stress granules represent sites of accumulation of stalled translation initiation complexes. Am J Physiol Cell Physiol 284: C273–C284 [DOI] [PubMed] [Google Scholar]
- Klok EJ, Wilson IW, Wilson D, Chapman SC, Ewing RM, Somerville SC, Peacock WJ, Dolferus R, Dennis ES (2002) Expression profile analysis of the low-oxygen response in Arabidopsis root cultures. Plant Cell 14: 2481–2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Otegui MS, Krishnakumar S, Mindrinos M, Zambryski P (2007) INCREASED SIZE EXCLUSION LIMIT 2 encodes a putative DEVH box RNA helicase involved in plasmodesmata function during Arabidopsis embryogenesis. Plant Cell 19: 1885–1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koncz C, Schell J (1986) The promoter of Tl-DNA gene 5 controls the tissue-specific expression of chimeric genes carried by a novel type of Agrobacterium binary vector. Mol Gen Genet 204: 383–396 [Google Scholar]
- Koroleva OA, Calder G, Pendle AF, Kim SH, Lewandowska D, Simpson CG, Jones IM, Brown JWS, Shaw PJ (2009) Dynamic behavior of Arabidopsis eIF4A-III, putative core protein of exon junction complex: fast relocation to nucleolus and splicing speckles under hypoxia. Plant Cell 21: 1592–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kürsteiner O, Dupuis I, Kuhlemeier C (2003) The Pyruvate decarboxylase1 gene of Arabidopsis is required during anoxia but not other environmental stresses. Plant Physiol 132: 968–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 [DOI] [PubMed] [Google Scholar]
- Lambermon MHL, Simpson GG, Wieczorek Kirk DA, Hemmings-Mieszczak M, Klahre U, Filipowicz W (2000) UBP1, a novel hnRNP-like protein that functions at multiple steps of higher plant nuclear pre-mRNA maturation. EMBO J 19: 1638–1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Mustroph A, Sasidharan R, Vashisht D, Pedersen O, Oosumi T, Voesenek LACJ, Bailey-Serres J (2011) Molecular characterization of the submergence response of the Arabidopsis thaliana ecotype Columbia. New Phytol 190: 457–471 [DOI] [PubMed] [Google Scholar]
- Li CH, Ohn T, Ivanov P, Tisdale S, Anderson P (2010) eIF5A promotes translation elongation, polysome disassembly and stress granule assembly. PLoS ONE 5: e9942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Choi WG, Wallace IS, Baudry J, Roberts DM (2011) Arabidopsis thaliana NIP7;1: an anther-specific boric acid transporter of the aquaporin superfamily regulated by an unusual tyrosine in helix 2 of the transport pore. Biochemistry 50: 6633–6641 [DOI] [PubMed] [Google Scholar]
- Licausi F, Perata P (2009) Low oxygen signaling and tolerance in plants. Adv Bot Res 50: 139–198 [Google Scholar]
- Licausi F, van Dongen JT, Giuntoli B, Novi G, Santaniello A, Geigenberger P, Perata P (2010) HRE1 and HRE2, two hypoxia-inducible ethylene response factors, affect anaerobic responses in Arabidopsis thaliana. Plant J 62: 302–315 [DOI] [PubMed] [Google Scholar]
- Licausi F, Weits DA, Pant BD, Scheible WR, Geigenberger P, van Dongen JT (2011) Hypoxia responsive gene expression is mediated by various subsets of transcription factors and miRNAs that are determined by the actual oxygen availability. New Phytol 190: 442–456 [DOI] [PubMed] [Google Scholar]
- Linder P, Owttrim GW (2009) Plant RNA helicases: linking aberrant and silencing RNA. Trends Plant Sci 14: 344–352 [DOI] [PubMed] [Google Scholar]
- Liu F, Vantoai T, Moy LP, Bock G, Linford LD, Quackenbush J (2005a) Global transcription profiling reveals comprehensive insights into hypoxic response in Arabidopsis. Plant Physiol 137: 1115–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Valencia-Sanchez MA, Hannon GJ, Parker R (2005b) MicroRNA-dependent localization of targeted mRNAs to mammalian P-bodies. Nat Cell Biol 7: 719–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreti E, Poggi A, Novi G, Alpi A, Perata P (2005) A genome-wide analysis of the effects of sucrose on gene expression in Arabidopsis seedlings under anoxia. Plant Physiol 137: 1130–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorković ZJ, Wieczorek Kirk DA, Klahre U, Hemmings-Mieszczak M, Filipowicz W (2000) RBP45 and RBP47, two oligouridylate-specific hnRNP-like proteins interacting with poly(A)+ RNA in nuclei of plant cells. RNA 6: 1610–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallory AC, Ely L, Smith TH, Marathe R, Anandalakshmi R, Fagard M, Vaucheret H, Pruss G, Bowman L, Vance VB (2001) HC-Pro suppression of transgene silencing eliminates the small RNAs but not transgene methylation or the mobile signal. Plant Cell 13: 571–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazroui R, Di Marco S, Kaufman RJ, Gallouzi IE (2007) Inhibition of the ubiquitin-proteasome system induces stress granule formation. Mol Biol Cell 18: 2603–2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack E, Braam J (2003) Calmodulins and related potential calcium sensors of Arabidopsis. New Phytol 159: 585–598 [DOI] [PubMed] [Google Scholar]
- Mi H, Muruganujan A, Thomas PD (2013) PANTHER in 2013: modeling the evolution of gene function, and other gene attributes, in the context of phylogenetic trees. Nucleic Acids Res 41: D377–D386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty B, Krishnan SPT, Swarup S, Bajic VB (2005) Detection and preliminary analysis of motifs in promoters of anaerobically induced genes of different plant species. Ann Bot (Lond) 96: 669–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldovan D, Spriggs A, Yang J, Pogson BJ, Dennis ES, Wilson IW (2010) Hypoxia-responsive microRNAs and trans-acting small interfering RNAs in Arabidopsis. J Exp Bot 61: 165–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustroph A, Barding GA Jr, Kaiser KA, Larive CK, Bailey-Serres J (2014) Characterization of distinct root and shoot responses to low-oxygen stress in Arabidopsis with a focus on primary C- and N-metabolism. Plant Cell Environ 37: 2366–2380 [DOI] [PubMed] [Google Scholar]
- Mustroph A, Lee SC, Oosumi T, Zanetti ME, Yang H, Ma K, Yaghoubi-Masihi A, Fukao T, Bailey-Serres J (2010) Cross-kingdom comparison of transcriptomic adjustments to low-oxygen stress highlights conserved and plant-specific responses. Plant Physiol 152: 1484–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustroph A, Zanetti ME, Jang CJ, Holtan HE, Repetti PP, Galbraith DW, Girke T, Bailey-Serres J (2009) Profiling translatomes of discrete cell populations resolves altered cellular priorities during hypoxia in Arabidopsis. Proc Natl Acad Sci USA 106: 18843–18848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahara KS, Masuta C, Yamada S, Shimura H, Kashihara Y, Wada TS, Meguro A, Goto K, Tadamura K, Sueda K, et al. (2012) Tobacco calmodulin-like protein provides secondary defense by binding to and directing degradation of virus RNA silencing suppressors. Proc Natl Acad Sci USA 109: 10113–10118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nover L, Scharf KD, Neumann D (1989) Cytoplasmic heat shock granules are formed from precursor particles and are associated with a specific set of mRNAs. Mol Cell Biol 9: 1298–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh M, Nanjo Y, Komatsu S (2014) Gel-free proteomic analysis of soybean root proteins affected by calcium under flooding stress. Front Plant Sci 5: 559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveros J (2007) VENNY: an interactive tool for comparing lists with Venn diagrams. http://bioinfogp.cnb.csic.es/tools/venny/index.html Date accessed: September 5, 2015
- Peng HP, Chan CS, Shih MC, Yang SF (2001) Signaling events in the hypoxic induction of alcohol dehydrogenase gene in Arabidopsis. Plant Physiol 126: 742–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Allen S, Millwood RJ, Stewart CN Jr (2014) ‘Fukusensor’: a genetically engineered plant for reporting DNA damage in response to gamma radiation. Plant Biotechnol J 12: 1329–1332 [DOI] [PubMed] [Google Scholar]
- Pomeranz MC, Hah C, Lin PC, Kang SG, Finer JJ, Blackshear PJ, Jang JC (2010) The Arabidopsis tandem zinc finger protein AtTZF1 traffics between the nucleus and cytoplasmic foci and binds both DNA and RNA. Plant Physiol 152: 151–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sako K, Maki Y, Kanai T, Kato E, Maekawa S, Yasuda S, Sato T, Watahiki MK, Yamaguchi J (2012) Arabidopsis RPT2a, 19S proteasome subunit, regulates gene silencing via DNA methylation. PLoS ONE 7: e37086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaha H, Delorme V, Pontvianne F, Cooke R, Delalande F, Van Dorsselaer A, Echeverria M, Sáez-Vásquez J (2010) Identification of protein factors and U3 snoRNAs from a Brassica oleracea RNP complex involved in the processing of pre-rRNA. Plant J 61: 383–398 [DOI] [PubMed] [Google Scholar]
- Schmidt F, Marnef A, Cheung MK, Wilson I, Hancock J, Staiger D, Ladomery M (2010) A proteomic analysis of oligo(dT)-bound mRNP containing oxidative stress-induced Arabidopsis thaliana RNA-binding proteins ATGRP7 and ATGRP8. Mol Biol Rep 37: 839–845 [DOI] [PubMed] [Google Scholar]
- Sedbrook JC, Kronebusch PJ, Borisy GG, Trewavas AJ, Masson PH (1996) Transgenic AEQUORIN reveals organ-specific cytosolic Ca2+ responses to anoxia and Arabidopsis thaliana seedlings. Plant Physiol 111: 243–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen GL, Blau HM (2005) Argonaute 2/RISC resides in sites of mammalian mRNA decay known as cytoplasmic bodies. Nat Cell Biol 7: 633–636 [DOI] [PubMed] [Google Scholar]
- Sorenson R, Bailey-Serres J (2014) Selective mRNA sequestration by OLIGOURIDYLATE-BINDING PROTEIN 1 contributes to translational control during hypoxia in Arabidopsis. Proc Natl Acad Sci USA 111: 2373–2378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaiah CC, Bush DS, Sachs MM (1994a) Elevation of cytosolic calcium precedes anoxic gene expression in maize suspension-cultured cells. Plant Cell 6: 1747–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaiah CC, Bush DS, Sachs MM (1998) Mitochondrial contribution to the anoxic Ca2+ signal in maize suspension-cultured cells. Plant Physiol 118: 759–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaiah CC, Zhang J, Sachs MM (1994b) Involvement of intracellular calcium in anaerobic gene expression and survival of maize seedlings. Plant Physiol 105: 369–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabb DL, Fernando CG, Chambers MC (2007) MyriMatch: highly accurate tandem mass spectral peptide identification by multivariate hypergeometric analysis. J Proteome Res 6: 654–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamang BG, Magliozzi JO, Maroof MAS, Fukao T (2014) Physiological and transcriptomic characterization of submergence and reoxygenation responses in soybean seedlings. Plant Cell Environ 37: 2350–2365 [DOI] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30: 2725–2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thivierge K, Cotton S, Dufresne PJ, Mathieu I, Beauchemin C, Ide C, Fortin MG, Laliberté JF (2008) Eukaryotic elongation factor 1A interacts with turnip mosaic virus RNA-dependent RNA polymerase and VPg-Pro in virus-induced vesicles. Virology 377: 216–225 [DOI] [PubMed] [Google Scholar]
- Vanderbeld B, Snedden WA (2007) Developmental and stimulus-induced expression patterns of Arabidopsis calmodulin-like genes CML37, CML38 and CML39. Plant Mol Biol 64: 683–697 [DOI] [PubMed] [Google Scholar]
- van Steensel B, van Binnendijk EP, Hornsby CD, van der Voort HT, Krozowski ZS, de Kloet ER, van Driel R (1996) Partial colocalization of glucocorticoid and mineralocorticoid receptors in discrete compartments in nuclei of rat hippocampus neurons. J Cell Sci 109: 787–792 [DOI] [PubMed] [Google Scholar]
- Voesenek LA, Bailey-Serres J (2013) Flooding tolerance: O2 sensing and survival strategies. Curr Opin Plant Biol 16: 647–653 [DOI] [PubMed] [Google Scholar]
- Voesenek LACJ, Bailey-Serres J (2015) Flood adaptive traits and processes: an overview. New Phytol 206: 57–73 [DOI] [PubMed] [Google Scholar]
- Wallace IS, Roberts DM (2005) Distinct transport selectivity of two structural subclasses of the nodulin-like intrinsic protein family of plant aquaglyceroporin channels. Biochemistry 44: 16826–16834 [DOI] [PubMed] [Google Scholar]
- Wang Y, Yu B, Zhao J, Guo J, Li Y, Han S, Huang L, Du Y, Hong Y, Tang D, et al. (2013) Autophagy contributes to leaf starch degradation. Plant Cell 25: 1383–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn MP, Wolters D, Yates JR III (2001) Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nat Biotechnol 19: 242–247 [DOI] [PubMed] [Google Scholar]
- Weber C, Nover L, Fauth M (2008) Plant stress granules and mRNA processing bodies are distinct from heat stress granules. Plant J 56: 517–530 [DOI] [PubMed] [Google Scholar]
- White PJ, Broadley MR (2003) Calcium in plants. Ann Bot (Lond) 92: 487–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, Brunger AT (2000) The 1.0 A crystal structure of Ca2+-bound calmodulin: an analysis of disorder and implications for functionally relevant plasticity. J Mol Biol 301: 1237–1256 [DOI] [PubMed] [Google Scholar]
- Xu GY, Rocha PS, Wang ML, Xu ML, Cui YC, Li LY, Zhu YX, Xia X (2011) A novel rice calmodulin-like gene, OsMSR2, enhances drought and salt tolerance and increases ABA sensitivity in Arabidopsis. Planta 234: 47–59 [DOI] [PubMed] [Google Scholar]
- Yan C, Yan Z, Wang Y, Yan X, Han Y (2014) Tudor-SN, a component of stress granules, regulates growth under salt stress by modulating GA20ox3 mRNA levels in Arabidopsis. J Exp Bot 65: 5933–5944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CY, Hsu FC, Li JP, Wang NN, Shih MC (2011) The AP2/ERF transcription factor AtERF73/HRE1 modulates ethylene responses during hypoxia in Arabidopsis. Plant Physiol 156: 202–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong Chung H, Lacatus G, Sunter G (2014) Geminivirus AL2 protein induces expression of, and interacts with, a calmodulin-like gene, an endogenous regulator of gene silencing. Virology 460-461: 108–118 [DOI] [PubMed] [Google Scholar]
- Zhou R, Benavente LM, Stepanova AN, Alonso JM (2011) A recombineering-based gene tagging system for Arabidopsis. Plant J 66: 712–723 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.