Site-directed mutagenesis via TAL effector nucleases and demonstrated that a lack of DNA Ligase4 affects the kinetics of TALEN-induced DSB repair.
Abstract
We have established methods for site-directed mutagenesis via transcription activator-like effector nucleases (TALENs) in the endogenous rice (Oryza sativa) waxy gene and demonstrated stable inheritance of TALEN-induced somatic mutations to the progeny. To analyze the role of classical nonhomologous end joining (cNHEJ) and alternative nonhomologous end joining (altNHEJ) pathways in TALEN-induced mutagenesis in plant cells, we investigated whether a lack of DNA Ligase4 (Lig4) affects the kinetics of TALEN-induced double-strand break repair in rice cells. Deep-sequencing analysis revealed that the frequency of all types of mutations, namely deletion, insertion, combination of insertion with deletion, and substitution, in lig4 null mutant calli was higher than that in a lig4 heterozygous mutant or the wild type. In addition, the ratio of large deletions (greater than 10 bp) and deletions repaired by microhomology-mediated end joining (MMEJ) to total deletion mutations in lig4 null mutant calli was higher than that in the lig4 heterozygous mutant or wild type. Furthermore, almost all insertions (2 bp or greater) were shown to be processed via copy and paste of one or more regions around the TALENs cleavage site and rejoined via MMEJ regardless of genetic background. Taken together, our findings indicate that the dysfunction of cNHEJ leads to a shift in the repair pathway from cNHEJ to altNHEJ or synthesis-dependent strand annealing.
The introduction of targeted DNA double-strand breaks (DSBs) by sequence-specific nucleases (SSNs), such as meganucleases (Chevalier et al., 2002), zinc finger nucleases (ZFNs; Kim et al., 1996), transcription activator-like effector nucleases (TALENs; Christian et al., 2010), and the bacterial clustered regularly interspaced short palindromic repeat (CRISPR)/CRISPR-associated protein 9 (Cas9) system (Jinek et al., 2012), results in deletions, insertions, and substitutions around the nuclease cleavage site. DSBs are repaired via two major pathways: homologous recombination (HR) and nonhomologous end joining (NHEJ; Gaj et al., 2013; Voytas, 2013). The HR pathway is used less frequently but is more precise because it employs homologous DNA sequences for DSB repair. In contrast, the NHEJ pathway rejoins DSB ends directly and is used preferentially for DSB repair in higher eukaryotes, including higher plants. Almost all DSBs generated by SSNs can be rejoined precisely via the NHEJ pathway; however, this pathway sometimes leads to imprecise repair and results in the introduction of mutations at the DSB site, referred to as site-directed mutagenesis. DSB repair via HR using exogenous donor DNA, resulting in the replacement or insertion of new nucleotide sequences, is referred to as gene targeting (GT). Consequently, SSN-mediated DSB induction at the target locus could lead to an increased frequency of GT in several organisms (Puchta and Fauser, 2013; Lin et al., 2014; Pauwels et al., 2014; Shin et al., 2014; Voytas and Gao, 2014).
SSNs have become a useful tool for genome engineering in several organisms, including plants (Carroll, 2011; Gaj et al., 2013; Voytas, 2013). TALENs have some advantages over other SSNs, such as specificity of binding to target DNA and less restrictive definition of target DNA sequences (Christian et al., 2010). Recent reports describe the introduction of preassembled complexes of Cas9 protein and guide RNA (Woo et al., 2015), or TALEN proteins (Luo et al., 2015), directly into plant protoplasts, leading to the successful induction of targeted mutagenesis. TALENs recognize target sequences and digest DNA using only dimerized proteins without requiring a nucleic acid such as the guide RNA of the CRISPR/Cas9 system. Thus, the direct introduction of TALENs into protoplasts may allow the establishment of a more publicly acceptable approach.
TALEN-mediated target mutagenesis of endogenous genes in higher plants has succeeded in several plant species, such as Arabidopsis (Arabidopsis thaliana; Cermak et al., 2011; Christian et al., 2013; Forner et al., 2015), tobacco (Nicotiana tabacum; Zhang et al., 2013), rice (Oryza sativa; Li et al., 2012; Shan et al., 2013, 2015; Wang et al., 2015; Zhang et al., 2015), Nicotiana benthamiana (Li et al., 2015), Brachypodium spp. (Shan et al., 2013), barley (Hordeum vulgare; Wendt et al., 2013), wheat (Triticum aestivum; Wang et al., 2014) Brassica oleracea (Sun et al., 2013), potato (Solanum tuberosum; Sawai et al., 2014; Clasen et al., 2015), soybean (Glycine max; Haun et al., 2014), maize (Zea mays; Char et al., 2015), and tomato (Solanum lycopersicum; Lor et al., 2014). However, there are only a few reports of TALEN-mediated mutations being transmitted to the next generation: in Arabidopsis (Christian et al., 2013; Forner et al., 2015), rice (Li et al., 2012; Shan et al., 2015; Wang et al., 2015; Zhang et al., 2015), wheat (Wang et al., 2014), soybean (Haun et al., 2014), barley (Gurushidze et al., 2014), and maize (Char et al., 2015). Christian et al. (2013) reported that TALEN-mediated mutations were transmitted to the next generation at low frequency. In Arabidopsis, TALEN-mediated mutations are thought to continue to occur in individual cells at various stages of plant development and, consequently, may show a mosaic pattern in mutant plants. As a result, it may be difficult to generate heritable mutations in germ cells (Christian et al., 2013). On the other hand, Li et al. (2012) demonstrated that more than half of T1 plants generated from self-pollination of T0 rice plants transformed with a TALEN expression cassette carried heterozygous or homozygous TALEN-mediated mutations. The chimerism observed in transgenic rice callus is thought to be eliminated during the process of regeneration, while somatic mutations derived from TALEN-mediated DNA cleavage also showed a mosaic pattern in transgenic rice callus. Therefore, TALEN-mediated mutations could be transmitted stably through the germ line to the next generation in rice. However, it remains unclear whether somatic mutations generated by TALEN-mediated DNA cleavage in transgenic rice calli were transmitted stably through the germ line to T1 and T2 plants in rice and whether de novo mutations could be also induced at the nonmutated TALEN target locus during the development of TALEN-transformed rice.
The NHEJ pathway is thought to comprise both the classical nonhomologous end joining (cNHEJ) and alternative nonhomologous end joining (altNHEJ) pathways (Symington and Gautier, 2011; Deriano and Roth, 2013). In mammalian cells, Ku70/Ku80 heterodimer complexes bind to DSB ends to stabilize and protect them from degradation via exonuclease activity. The Ku complex recruits a DNA-dependent protein kinase, which phosphorylates and activates many proteins such as X-ray cross complementing 4 (XRCC4), DNA Ligase4 (Lig4), and XRCC4-like factor (XLF). Ultimately, the Lig4/XRCC4/XLF complex rejoins the two DSB ends (Chapman et al., 2012; Deriano and Roth, 2013). In Arabidopsis and rice, Ku70, Ku80, Lig4, and XRCC4 proteins have been identified, but DNA-dependent protein kinase and XLF have not (West et al., 2000; Tamura et al., 2002; Hong et al., 2010; Singh et al., 2010; Edlinger and Schlögelhofer, 2011). When dysfunction, or a failure to repair, of the cNHEJ pathway occurs, DSBs are repaired by the altNHEJ pathway, which is independent of cNHEJ-related proteins, including Ku70/Ku80 and Lig4 (Chapman et al., 2012; Deriano and Roth, 2013).
DSBs produced by SSNs leave breaks with compatible overhangs or blunt ends that can be repaired precisely by cNHEJ. However, DSBs are often repaired imprecisely via the error-prone altNHEJ pathway (Shrivastav et al., 2008; Mladenov and Iliakis, 2011). Most DSB repairs via the altNHEJ pathway rely on the use of terminal microhomology, resulting in deletion at the repair junction. Thus, this pathway has been designated as microhomology-mediated end joining (MMEJ). Many proteins involved in the altNHEJ pathway, such as poly (ADP-ribose) polymerase1 (PARP1), meiotic recombination 11 (MRE11)-radiation sensitive 50 (Rad50)-Nijmegen Breakage Syndrome 1 (NBS1), histone H, DNA Ligase III, and XRCC1, have been identified in mammalian cells (Audebert et al., 2004; Rosidi et al., 2008; Cheng et al., 2011). Recent studies have shown that Arabidopsis XRCC1, XPF, Lig1, and PARP1/PARP2 also function in the altNHEJ pathway (Waterworth et al., 2009; Charbonnel et al., 2010, 2011; Jia et al., 2013).
In our previous study, we showed that a lack of Arabidopsis Ku80, which plays an important role in the cNHEJ pathway, led to an increase in the length of deletions in zinc finger nuclease (ZFN)-mediated mutagenesis but no change in efficiency (Osakabe et al., 2010). In agreement with our findings, Qi et al. (2013) reported that the absence of cNHEJ-related proteins, such as Ku70 and Lig4, induced predominantly large deletions following ZFN-mediated DNA cleavage in Arabidopsis, but with no enhancement of targeted mutation frequency. These findings can be explained by the use of the altNHEJ pathway in cNHEJ-deficient Arabidopsis. However, the function of the altNHEJ pathway in rice plants remains largely unknown.
This study undertook a detailed examination of the patterns, frequencies, and heritability of TALEN-mediated somatic mutations in transgenic rice calli and plants. Because the waxy gene has been used in several studies as a model gene for GT-induced modification (Terada et al., 2002, 2010; Ozawa et al., 2012), we chose the first intron of this gene as the TALEN target site to investigate the effect of TALEN-induced DSBs on GT frequency in rice. Thus, although TALEN-induced mutations occur at the target site in rice plants, the waxy mutant phenotype will not be observed in the seeds of mutant rice unless large deletions occur. Furthermore, next-generation sequencing revealed that TALEN-mediated DNA cleavage can lead to effective gene modifications and large deletions at TALEN cleavage sites in lig4 knockout rice. In addition, we demonstrated that the lig4 mutation affects the frequency and patterns of TALEN-induced mutagenesis at another locus, the 33-kD globulin (Glb33) gene. These results suggest that Lig4 plays an important role in the cNHEJ pathway in rice plants and that highly efficient TALEN-mediated site-directed mutagenesis could be established using Lig4 knockout rice plants.
RESULTS
TALEN-Mediated Target Mutation in the waxy Gene in Rice
TALEN pairs wx_L1/R2 and wx_L2/R2 targeting the first intron at 270 bp upstream of the translation initiation site of the waxy gene encoding a granule-bound starch synthase were selected using the single-strand annealing assay in yeast (Saccharomyces cerevisiae; Supplemental Text S1; Supplemental Fig. S1). The DNA cleavage activity of TALENs is reported to be enhanced by truncation of the N- and C-terminal regions flanking the DNA-binding domain (Miller et al., 2011; Mussolino et al., 2011; Zhang et al., 2013). Thus, the TAL effector repeat arrays of wx_L1, wx_L2, and wx_R2 TALENs were recloned into a vector with a TALE backbone harboring N- and C-terminal truncations of 152 and 215 amino acids, respectively (Zhang et al., 2013). To investigate whether TALEN-mediated target mutagenesis can occur in plant cells, expression vectors carrying a maize polyubiquitin1 promoter directing expression of the wx_L1/R2 (Pubi:L1/R2) or wx_L2/R2 (Pubi:wx_L2/R2) TALEN pairs separated by a T2A translational skipping sequence were constructed. Four-week-old calli derived from mature seeds of cv Nipponbare were infected with Agrobacterium tumefaciens harboring the Pubi:wx_L1/R2 or Pubi:wx_L2/R2 TALEN expression vector (Supplemental Fig. S2). After a 2-week selection period, G418-tolerant calli were cloned and propagated for 2 weeks. Genomic DNA was extracted from each of 96 lines of Pubi:wx_L1/R2 and Pubi:wx_L2/R2 transformed calli, and a CelI-nuclease mismatch cleavage assay was performed. Positive signals were detected in 21 (21.9%) and two (2.1%) lines of Pubi:wx_L1/R2 and Pubi:wx_L2/R2 transgenic calli, respectively (Supplemental Fig. S3).
To determine the frequency and pattern of TALEN-mediated target mutagenesis at the waxy locus, genomic DNA was isolated from six independent clones of wx_L1/R2 TALEN transgenic calli that showed a positive signal in the CelI-nuclease mismatch cleavage assay, and mutagenized sequences amplified with PCR using primers flanking the TALEN recognition sequences were cloned into the pCRII-Blunt-TOPO vector. Finally, 46 to 48 PCR products were sequenced per independent clone. The mutation frequency ranged from 4.2% to 30.4% depending on the callus clone (Table I). In the case of clones 3 and 14, one type of mutation was detected (Table I), whereas a variety of mutations, such as small deletions, large deletions, and substitutions of nucleotides, were detected in calli from lines 11, 12, 15, and 36 (Table I).
Table I. Mutation types and frequencies in the waxy locus in Pubi:wx_L1/R2 TALEN-expressing cv Nipponbare calli.
The spacer region between the two TALEN binding sites is shown in lowercase letters. Deletions are shown as hyphens, and other types of mutations are shown in bold. d#, Number of bases deleted; s#, number of bases substituted.
| Line No. | Total No. of Clones Analyzed | Sequence | Mutation Type | No. of Mutant Clones | Frequency of Mutations |
|---|---|---|---|---|---|
| % | |||||
| Wild type | TCCTTATAAGCACATATggcattgtaatatatATGTTTGAGTTTTAGCGACA | ||||
| 3 | 48 | TCCTTATAAGCACATATggcat----atatatATGTTTGAGTTTTAGCGACA | d4 | 4 | 8.33 |
| 11 | 48 | TCCTTATAAGCACATATggcatt-taatatatATGTTTGAGTTTTAGCGACA | d1 | 1 | 10.42 |
| TCCTTATAAGCACATAT-------------atATGTTTGAGTTTTAGCGACA | d13 | 1 | |||
| TCCTTATAAGCA---------------------------AGTTTTAGCGACA | d27 | 1 | |||
| TCCTTATAAGCACATATggcat----atatatATGTTTGAGTTTTAGCGACA | d4 | 1 | |||
| TCCTTATAAGCACATATgg---------------------53-bp deletion---- | d53 | 1 | |||
| 12 | 48 | TCCTTATAAGCACATATggcat---aatatatATGTTTGAGTTTTAGCGACA | d3 | 1 | 4.17 |
| TCCTTATAAGCACATAT-----------atatATGTTTGAGTTTTAGCGACA | d11 | 1 | |||
| 14 | 46 | TCCTTATAAGCACATATggcatt---atatatATGTTTGAGTTTTAGCGACA | d3 | 14 | 30.43 |
| 15 | 48 | TCCTTATAAGCACATATggcat------------GTTTGAGTTTTAGCGACA | d12 | 1 | 14.58 |
| TCCTTATAAGCACATATagcattgtaatatatATGTTTGAGTTTTAGCGACA | s1 | 1 | |||
| TCCTTATAAGCACATATggcatt-taatatatATGTTTGAGTTTTAGCGACA | d1 | 2 | |||
| TCCTTATAAGCACATATggcat----atatatATGTTTGAGTTTTAGCGACA | d4 | 1 | |||
| TCCTTATAAGCACATATggcat------atatATGTTTGAGTTTTAGCGACA | d6 | 1 | |||
| TCCTTATAAGCACATATggcat--------atATGTTTGAGTTTTAGCGACA | d8 | 1 | |||
| 36 | 48 | TCCTTATAAGCACATATggcat-ataatatatATGTTTGAGTTTTAGCGACA | d1s1 | 4 | 16.67 |
| TCCTTATAAGCACATATggcatt-----atatATGTTTGAGTTTTAGCGACA | d5 | 4 |
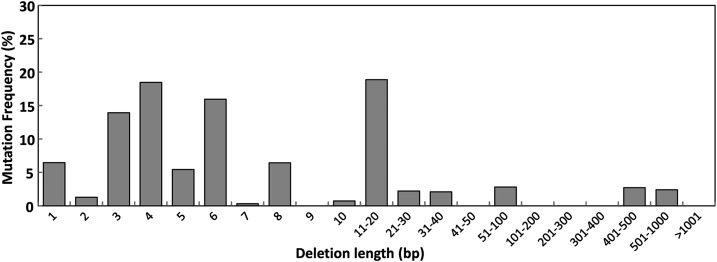
To further evaluate the frequency and pattern of mutations, approximately 2-kb PCR products derived from 11 independent clones of wx_L1/R2 TALEN transgenic calli that showed a positive signal in the CelI-nuclease mismatch cleavage assay were digested and indexed, and short read sequences (less than 300 bp) were then analyzed by Illumina MiSeq, as described in “Materials and Methods.” As shown in Supplemental Table S1, deletion mutations were detected most frequently (0.06%–1.52%), while insertion mutations, including combinations of insertions with deletions, were hardly observed in cv Nipponbare calli. Using deep sequencing, the mutation frequency was calculated as the number of reads containing mutations at the target locus per total number of reads analyzed. The majority of analyzed reads did not contain TALEN target sites; therefore, the mutation frequency determined using deep sequencing (Supplemental Table S1) was underestimated compared with the data obtained from the sequencing analysis of PCR products (Table I). Finally, we observed that small deletions (10 bp or less) account for a large proportion (68.9%) of the deletion mutations, whereas large deletions (greater than 10 bp) were detected infrequently in cv Nipponbare calli (Fig. 1).
Figure 1.
TALEN-mediated mutation frequency of different lengths of deletions in cv Nipponbare calli assessed by next-generation sequencing. The DNA fragment surrounding the TALEN target sites from 11 independent lines of wx_L1/R2 TALEN-expressing cv Nipponbare calli was amplified and sequenced by Illumina MiSeq as described in “Materials and Methods.” Mutation frequency was calculated as follows: mutation frequency (%) = number of reads containing a different length of deletion mutations/total number of reads harboring deletion mutation in the target locus × 100.
Stable Transmission of TALEN-Mediated Mutagenesis to T1 and T2 Progeny
Plants regenerated from six independent calli clones that were confirmed to have TALEN-mediated mutations were analyzed for the transmission of mutations using the CelI-nuclease mismatch cleavage assay. Mutations were detected in zero of 32 (0%), two of 32 (6.3%), 10 of 32 (31.3%), 19 of 19 (100%), six of 43 (14%), and 34 of 38 (89.5%) plants regenerated from lines 3, 11, 12, 14, 15, and 36 of calli clones, respectively. To further investigate the mutation pattern, genomic DNA was extracted from three leaves derived from independent tillers of the reproductive stage of regenerated plants. Sequencing analysis revealed that different types of mutations were detected in independent regenerated plants derived from a single callus clone (Table II). In addition, there were some differences between the mutation patterns detected in the regenerated plants derived from calli clones 12, 15, and 36 (Table I) and those in the calli clones shown in Table II, because of the chimeric nature of mutations in TALEN-transformed callus clones. Although the mutations found in the waxy gene were largely monoallelic (Table II), regenerated plants with biallelic mutations (line no. 12-5) or chimeric mutations including at least three different sequences (lines 36-9, 36-12, and 36-32) in the waxy gene were obtained at low frequency (Table II).
Table II. Mutation patterns in the waxy locus in Pubi:wx_L1/R2 TALEN-expressing T0 regenerated cv Nipponbare plants.
The spacer region between two TALEN binding sites is shown in lowercase letters. Deletions are shown as hyphens, and other types of mutations are shown in bold. d#, Number of bases deleted; i#, number of bases inserted; wt, wild-type sequence.
| Line No. | Zygosity | Sequence | Mutation Type |
|---|---|---|---|
| Wild type | TCCTTATAAGCACATATggcattgtaatatatATGTTTGAGTTTTAGCGACA | ||
| 12-2 | Monoallelic | TCCTTATAAGCACATATggcattgtaaatatatATGTTTGAGTTTTAGCGACA | i1 |
| 12-4 | Monoallelic | TCCTTATAAGCACATATggcat----atatatATGTTTGAGTTTTAGCGACA | d4 |
| 12-5 | Biallelic | TCCTTATAAGCACATATggcat----atatatATGTTTGAGTTTTAGCGACA | d4 |
| TCCTTATAAGCACATATggcattgtaaatatatATGTTTGAGTTTTAGCGACA | i1 | ||
| 12-8 | Monoallelic | TCCTTATAAGCACATATggcattgtaaatatatATGTTTGAGTTTTAGCGACA | i1 |
| 12-11 | Monoallelic | TCCTTATAAGCACATATggcattgtaaatatatATGTTTGAGTTTTAGCGACA | i1 |
| 14-8 | Monoallelic | TCCTTATAAGCACATATggcatt---atatatATGTTTGAGTTTTAGCGACA | d3 |
| 14-10 | Monoallelic | TCCTTATAAGCACATATggcatt---atatatATGTTTGAGTTTTAGCGACA | d3 |
| 14-11 | Monoallelic | TCCTTATAAGCACATATggcatt---atatatATGTTTGAGTTTTAGCGACA | d3 |
| 15-39 | Monoallelic | TCCTTATAAGCACATATggcatt-----atatATGTTTGAGTTTTAGCGACA | d5 |
| 15-40 | Monoallelic | TCCTTATAAGCACATATggcatt-----atatATGTTTGAGTTTTAGCGACA | d5 |
| 15-43 | Monoallelic | TCCTTATAAGCACATATgg------------------TCCTTTTTAGCGACA | d18i4 |
| 36-2 | Monoallelic | TCCTTATAAGCACATATggcat------atatATGTTTGAGTTTTAGCGACA | d6 |
| 36-9 | Chimeric | TCCTTATAAGCACATATggcattgtaatatatATGTTTGAGTTTTAGCGACA | wt |
| TCCTTATAAGCACATATggcatt-----atatATGTTTGAGTTTTAGCGACA | d5 | ||
| TCCTTATAAGCACATATggcat------atatATGTTTGAGTTTTAGCGACA | d6 | ||
| 36-12 | Chimeric | TCCTTATAAGCACATATggcattgtaatatatATGTTTGAGTTTTAGCGACA | wt |
| TCCTTATAAGCACATATggcat----atatatATGTTTGAGTTTTAGCGACA | d4 | ||
| TCCTTATAAGCACATATggcattg---------------AGTTTTAGCGACA | d15 | ||
| TCCTTATAAGCACATATggcat----------ATGTTTGAGTTTTAGCGACA | d10 | ||
| ----------28 bp-------------atatATGTTTGAGTTTTAGCGACA | d28 | ||
| 36-20 | Monoallelic | TCCTTATAAGCACATATggcatt--aatatatATGTTTGAGTTTTAGCGACA | d2 |
| 36-26 | Monoallelic | TCCT-------------------cagagatatATGTTTGAGTTTTAGCGACA | d19i5 |
| 36-32 | Chimeric | TCCTTATAAGCACATATggcattgtaatatatATGTTTGAGTTTTAGCGACA | wt |
| TCCTTATAAGCACATATggcatt-----atatATGTTTGAGTTTTAGCGACA | d5 | ||
| TCCTTATAAGCACATATggcat----atatatATGTTTGAGTTTTAGCGACA | d4 |
In addition, we obtained T1 progeny produced from four independent lines (12, 14, 15, and 36) with monoallelic (12-2, 12-4, 12-8, 14-8, 14-10, 14-11, 15-39, 15-40, 15-43, and 36-2), biallelic (12-5), and chimeric (36-9 and 36-12) mutations. To evaluate the mutation pattern and segregation in T1 progeny, PCR products derived from leaves of T1 plants were sequenced directly. Almost all monoallelic mutations that were identical to those of T0 plants were inherited according to a Mendelian ratio in T1 progeny (Table III). However, line 12-5, which has a biallelic mutation, passed on to T1 progeny not only the two mutation patterns derived from T0 plants but also a wild-type allele. Meanwhile, no heritable mutation was detected in T1 progeny of line 36-9 carrying a chimeric mutation (wt/d5/d6), and only one mutation pattern (d4) was inherited from a 36-12 T0 plant with a chimeric mutation (wt/d4/d10/d15/d28).
Table III. Germinal transmission of TALEN-induced mutations in the waxy locus from T0 plants to T1 progeny.
c, Chimeric mutation; d#, number of bases deleted; i#, number of bases inserted, wt, wild-type sequence.
| Line No. | T0 Plants |
Total No. of T1 Plants Analyzed | Mutation Segregation in T1 Progeny | |
|---|---|---|---|---|
| Zygosity | Target Genotype | |||
| 12-2 | Monoallelic | wt/i1 | 11 | wt/wt, 4; wt/i1, 4; i1/i1, 3 |
| 12-4 | Monoallelic | wt/d4 | 17 | wt/wt, 6; wt/d4, 7; d4/d4, 4 |
| 12-5 | Biallelic | d4/i1 | 16 | wt/d4, 1; d4/d4, 4; d4/i1, 8; wt/i1, 3 |
| 12-8 | Monoallelic | wt/i1 | 18 | wt/wt, 4; wt/i1, 10; i1/i1, 4 |
| 14-8 | Monoallelic | wt/d3 | 15 | wt/wt, 2; wt/d3, 8; d3/d3, 5 |
| 14-10 | Monoallelic | wt/d3 | 16 | wt/wt, 1; wt/d3, 10; d3/d3, 5 |
| 14-11 | Monoallelic | wt/d3 | 12 | wt/wt, 3; wt/d3, 5; d3/d3, 4 |
| 15-39 | Monoallelic | wt/d5 | 26 | wt/wt, 7; wt/d5, 12; d5/d5, 7 |
| 15-40 | Monoallelic | wt/d5 | 12 | wt/wt, 4; wt/d5, 5; d5/d5, 3 |
| 15-43 | Monoallelic | wt/d18i4 | 14 | wt/wt, 6; wt/d18i4, 4; d18i4/d18i4, 4 |
| 36-2 | Monoallelic | wt/d6 | 14 | wt/wt, 5; d3/d6, 4; d3/d3, 4; c, 1 |
| 36-9 | Chimeric | c (wt/d5/d6) | 18 | wt/wt, 18 |
| 36-12 | Chimeric | c (wt/d4/d10/d15/d28) | 14 | wt/wt, 3; wt/d4, 9; d4/d4, 2 |
Furthermore, to examine whether the mutations detected in T1 plants can be inherited in T2 progeny and/or whether new TALEN-mediated mutations appear in T2 progeny, T2 progeny were obtained from three independent lines carrying a heterozygous mutation (12-2-3, 14-11-10, and 15-39-22) and the wild-type allele (12-2-11, 14-11-1, and 15-39-27). Sequencing analysis of PCR products revealed that the mutations detected in T1 plants were inherited in a Mendelian fashion and new mutations were not detected in T2 progeny, although these plants did contain TALEN expression cassettes (Table IV). However, sequencing chromatograms of the TALEN target sites in T2 plants showed the presence of additional mutations at low abundance (Supplemental Fig. S4). Therefore, PCR products derived from T2 plants (two plants each of 12-2-11, 14-11-10, and 15-39-27) were cloned into the pCRII-Blunt-TOPO vector, and DNA from eight randomly picked clones was sequenced. T2 plants of 12-2-11 and 15-39-27, but not 14-11-10, harbored new mutations that were not detected in T1 plants at TALEN target sites in somatic cells (Table V). Thus, these results suggest that, although additive mutations could be induced at a low frequency during vegetative growth, they were not induced during reproductive growth but rather in somatic tissues without being subsequently transferred to reproductive tissues.
Table IV. Germinal transmission of TALEN-induced mutations in the waxy locus from T1 plants to T2 progeny.
d#, Number of bases deleted; i#, number of bases inserted; wt, wild-type sequence; +, number of T2 plants with TALEN expression cassette; −, number of T2 plants without TALEN expression cassette.
| Line No. | Target Genotype of T1 Plants | Total No. of T2 Plants Analyzed | Mutation Segregation in T2 Progeny | TALEN Expression Cassette |
|
|---|---|---|---|---|---|
| + | − | ||||
| 12-2-3 | wt/i1 | 16 | wt/wt, 2; wt/i1, 11; i1/i1, 3 | 16 | 0 |
| 12-2-11 | wt/wt | 15 | wt/wt, 15 | 15 | 0 |
| 14-11-1 | wt/wt | 13 | wt/wt, 13 | 11 | 2 |
| 14-11-10 | wt/d3 | 10 | wt/wt, 2; wt/d3, 6; d3/d3, 2 | 10 | 0 |
| 15-39-22 | wt/d5 | 16 | wt/wt, 5; wt/d5, 8; d5/d5, 3 | 11 | 5 |
| 15-39-27 | wt/wt | 14 | wt/wt, 14 | 10 | 4 |
Table V. Patterns of additive mutations detected at the waxy locus in somatic tissues of Pubi:L1/R2 transgenic T1 plants.
The spacer region between the two TALEN binding sites is shown in lowercase letters. Mutations are shown in bold, and deletions are shown as hyphens. wt, Wild-type sequence.
| Line No. | Target Genotype of T1 Plants | Plant No. | Sequence | No. of Mutant Clones |
|---|---|---|---|---|
| 12-2-11 | wt/wt | 1 | TCCTTATAAGCACATATggc--------atatATGTTTGAGTTTTAGCGACA | 1 |
| 1 | TCCTTATAAGCACATATgg--------------TGTTTGAGTTTTAGCGACA | 1 | ||
| 2 | TCCTTATAAGCACATATggcat----atatatATGTTTGAGTTTTAGCGACA | 2 | ||
| 2 | TCCTTATAAGCACATATggcat-----catatATGTTTGAGTTTTAGCGACA | 1 | ||
| 2 | TCCTTATAAGCACATATggcat------atatATGTTTGAGTTTTAGCGACA | 1 | ||
| 2 | TCCTTATAAGCACATATggcat-----tatatATGTTTGAGTTTTAGCGACA | 1 | ||
| 15-39-27 | wt/wt | 2 | TCCTTATAAGCACATATggcattg--atatatATGTTTGAGTTTTAGCGACA | 1 |
| 9 | TCCTTATAAGCACATATggcat------atatATGTTTGAGTTTTAGCGACA | 1 | ||
| 9 | TCCTTATAAGCACATATggcat----atatatATGTTTGAGTTTTAGCGACA | 1 | ||
| 9 | TCCTTATAAGCACATATggca---------atATGTTTGAGTTTTAGCGACA | 1 | ||
| 9 | TCCTTATAAGCACATATggcatt----tatatATGTTTGAGTTTTAGCGACA | 1 | ||
| 9 | TCCTTATAAGCACATATggcatt---atatatATGTTTGAGTTTTAGCGACA | 1 |
Induction of de Novo Mutations at the Target Gene Depends on the Dedifferentiation of TALEN-Expressing T1 Seeds
The additive mutations occurring in somatic tissues of rice during vegetative growth cannot be transferred to their progeny; hence, plants must be regenerated from the piece of callus carrying the TALEN-mediated mutations. We assumed that the dedifferentiation of TALEN-expressing T1 seeds without TALEN-mediated mutagenesis might be a valuable approach for the regeneration of plants carrying mutations. To test whether TALEN-mediated mutations are induced de novo by dedifferentiation, callus was induced from T1 seeds harboring wx_L1/R2 TALEN expression cassettes. T1 seeds that carry the wild-type waxy gene in both alleles and wx_L1/R2 TALEN expression cassettes (lines 12-8 and 14-8) were selected by PCR and incubated on callus induction medium (Supplemental Fig. S5). After 8 weeks of culture, approximately 80 pieces of calli were chosen arbitrarily from each line and TALEN-mediated mutations were confirmed by the CelI assay. Mutations at the waxy gene were detected in 60 out of 86 calli (69.8%) and in one out of 78 calli (1.3%) derived from lines 12-8 and 14-8, respectively. The difference in the mutation frequency between two independent calli lines might be explained by the difference in the expression levels of TALEN in these callus lines. In addition, CelI assay-positive PCR products were cloned and sequenced. TALEN-mediated de novo mutations that were not observed in T0 regenerated plants and T1 plants, a 1-bp insertion in callus of line 12-8 and a 3-bp deletion in callus of line 14-8, were identified (Table VI).
Table VI. Patterns of de novo mutation at the waxy locus depend on the dedifferentiation of Pubi:wx_L1/R2 transgenic T1 seeds.
The spacer region between the two TALEN binding sites is shown in lowercase letters. Mutations are shown in bold, and deletions are shown as hyphens. d#, Number of bases deleted; i#, number of bases inserted.
| Line No. | Generation | Sequence | Mutation Type |
|---|---|---|---|
| Wild type | TCCTTATAAGCACATATggcattgtaatatatATGTTTGAGTTTTAGCGACA | ||
| 12-8 | T0 plants | TCCTTATAAGCACATATggcattgtaaatatatATGTTTGAGTTTTAGCGACA | i1 |
| T1 plants | TCCTTATAAGCACATATggcattgtaaatatatATGTTTGAGTTTTAGCGACA | i1 | |
| T1 calli | TCCTTATAAGCACATATggcattg----atatATGTTTGAGTTTTAGCGACA | d4 | |
| TCCTTATAAGCACATATggcat------atatATGTTTGAGTTTTAGCGACA | d6 | ||
| TCCTTATAA------------------------------TTTTTTAGCGACA | d30 | ||
| TCCTTATAAGCACATATggcatt---86 bp--------------------- | d86 | ||
| TCCTTATAAGCACATATggcat--------atATGTTTGAGTTTTAGCGACA | d8 | ||
| TCCTTATAAGCACATATggcatttttatgaacgtttggtcctttttaTGAGTTTTAGCGACA | i24 | ||
| TCCTTATAAGCACATATgg---------tcatATGTTTGAGTTTTAGCGACA | d9i2 | ||
| TCCTTATAAGCACATAT---------------aaggagcAGTTTTAGCGACA | d15i7 | ||
| TCCTTATAAGCACATATggcat--------atATGTTTGAGTTTTAGCGACA | d8 | ||
| TCCTTATAAGCACATATggcat---aatatatATGTTTGAGTTTTAGCGACA | d3 | ||
| 14-8 | T0 plants | TCCTTATAAGCACATATggcatt---atatatATGTTTGAGTTTTAGCGACA | d3 |
| T1 plants | TCCTTATAAGCACATATggcatt---atatatATGTTTGAGTTTTAGCGACA | d3 | |
| T1 calli | TCCTTATAAGCACATATggcatt-----atatATGTTTGAGTTTTAGCGACA | d5 |
A Lig4 Defect Affects the Efficiency and Fidelity of the NHEJ Pathway
To analyze whether an enhanced frequency of TALEN-mediated mutations would be observed in OsLig4 mutants, we isolated and characterized transfer DNA (T-DNA) tagging- and γ-ray-induced OsLig4 mutant lines, oslig4-1 and oslig4-2, respectively. Line oslig4-1 is a T-DNA tagging mutant of cv Dongjin kindly provided by POSTECH (no. 3A-01729). oslig4-2 is a γ-irradiation mutant of cv Koshihikari identified by TILLING (Morita et al., 2009; Supplemental Fig. S6A). There were no significant differences in vegetative growth between the wild type (cv Dongjin or cv Koshihikari) and mutants oslig4-1 and oslig4-2, whereas the null mutation in OsLig4 inhibited callus proliferation slightly compared with wild-type rice calli.
Transcript levels of OsLig4 were analyzed by quantitative reverse transcription (RT)-PCR analysis in 4-week-old calli of heterozygous (oslig4-1+/−) and homozygous (oslig4-1−/−) T-DNA insertional mutants, cv Dongjin, homozygous TILLING mutants (oslig4-2−/−), and cv Koshihikari. The OsLig4 transcripts in oslig4-1+/− were decreased to about 50% of the level in cv Dongjin, whereas transcripts of OsLig4 were barely detectable in oslig4-1−/− (Supplemental Fig. S6B). Quantitative RT-PCR analysis revealed that mRNA levels of OsLig4 were not completely lacking in oslig4-2−/− (Supplemental Fig. S6C). However, we confirmed that mRNA carrying a deletion of exon 14 produced a truncated Lig4 protein product (Supplemental Fig. S6D).
Four-week-old calli of cv Dongjin, oslig4-1+/−, oslig4-1−/−, cv Koshihikari, and oslig4-2−/− plants were infected with A. tumefaciens harboring the Pubi:wx_L1/R2 TALEN construct. After a 3-week selection period, a CelI-nuclease mismatch cleavage assay was performed with genomic DNA extracted from each of more than 90 lines of L1/R2 TALEN-transformed oslig4 mutants and wild-type calli. Positive signals were detected in 20 of 92 (21.7%), 19 of 94 (20.2%), 49 of 95 (51.6%), five of 102 (4.9%), and 60 of 137 (43.8%) wx_L1/R2 TALEN-transformed cv Dongjin, oslig4-1+/−, oslig4-1−/−, cv Koshihikari, and oslig4-2−/− calli, respectively (Table VII).
Table VII. Frequency of TALEN-mediated mutations at the waxy locus in cv Dongjin (genetic background of oslig4-1), oslig4-1, cv Koshihikari (genetic background of oslig4-2), and oslig4-2 calli.
Mutation frequencies indicate the rate of calli bearing mutations per total calli analyzed by CelI assay. +/−, Heterozygous mutation; −/−, homozygous mutation.
| Experiment | Genotype | No. of Calli Analyzed | No. of Calli Bearing Mutations | Mutation Frequency |
|---|---|---|---|---|
| % | ||||
| Dongjin | 92 | 20 | 21.7 | |
| oslig4-1+/− | 94 | 19 | 20.2 | |
| oslig4-1−/− | 95 | 49 | 51.6 | |
| 1 | Koshihikari | 16 | 1 | 6.3 |
| oslig4-2−/− | 23 | 10 | 43.5 | |
| 2 | Koshihikari | 26 | 2 | 7.7 |
| oslig4-2−/− | 17 | 5 | 29.4 | |
| 3 | Koshihikari | 60 | 2 | 3.3 |
| oslig4-2−/− | 97 | 45 | 46.4 | |
| Total | Koshihikari | 102 | 5 | 4.9 |
| oslig4-2−/− | 137 | 60 | 43.8 |
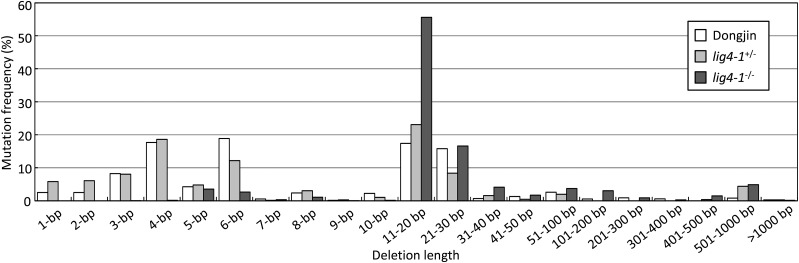
PCR products derived from 10, 11, and 20 independent lines of wx_L1/R2 TALEN-transformed cv Dongjin, oslig4-1+/−, and oslig4-1−/− calli, respectively, were sequenced, and the TALEN target site was analyzed by Illumina MiSeq. Deep sequencing analysis of PCR products demonstrated that the frequency of mutations containing substitutions, insertions, combinations of insertions with deletions, and deletions in oslig4-1−/− was higher than in oslig4-1+/− and cv Dongjin (Fig. 2). In addition, the ratio of large deletions (greater than 10 bp) to total deletion mutations in oslig4-1−/− was 89.5%, considerably higher than that in oslig4-1+/− and cv Dongjin (Fig. 3), while small deletions (10 bp or less) predominated in oslig4-1+/− and cv Dongjin (68.2% and 64.7%, respectively; Fig. 3). Furthermore, PCR products covering the TALEN target site from four and 19 independent lines of wx_L1/R2 TALEN-expressing cv Koshihikari and oslig4-2−/− calli, respectively, were subjected to deep sequencing by Illumina MiSeq. Consistent with the findings from sequencing analysis of oslig4-1, the defect in Lig4 led to increased mutation frequency and enhanced deletion size in the oslig4-2−/− mutant as compared with cv Koshihikari (Supplemental Figs. S7 and S8). Thus, these results suggest that TALEN-induced DSBs that cannot be rejoined by cNHEJ may be repaired via the error-prone altNHEJ pathway in lig4 mutants.
Figure 2.
TALEN-mediated mutation frequency at the waxy locus summarized by mutation type in wild-type (cv Dongjin) and oslig4-1 mutant calli. Box plots show substitution (A); insertion, including the combination of insertion with deletion (B); deletion (C); and total (D) mutation frequency, determined as the number of mutation reads per number of total reads. Boxes indicate the range of the 25th to 75th quartiles of the data, and the horizontal lines in the boxes show the median value for each genetic background. Vertical lines represent the maximum and minimum of all of the data. +/−, Heterozygous oslig4-1 mutants; −/−, homozygous oslig4-1 mutants. The wild type (cv Dongjin), n = 10; heterozygous oslig4-1 mutants, n = 11; homozygous oslig4-1 mutants, n = 20.
Figure 3.
Mutation frequency at the waxy locus summarized by the length of deletion in wild-type (cv Dongjin) and oslig4-1 mutant calli. The graph represents the proportion of sequencing reads containing different deletion lengths (1–1,000 bp or greater) among total reads carrying deletion mutations in the wild type (cv Dongjin), heterozygous oslig4-1 mutant (+/−), and homozygous oslig4-1 mutant (−/−). Mutation frequency was calculated as follows: mutation frequency (%) = number of reads containing a different length of deletion mutations/total number of reads harboring deletion mutation in the target locus × 100.
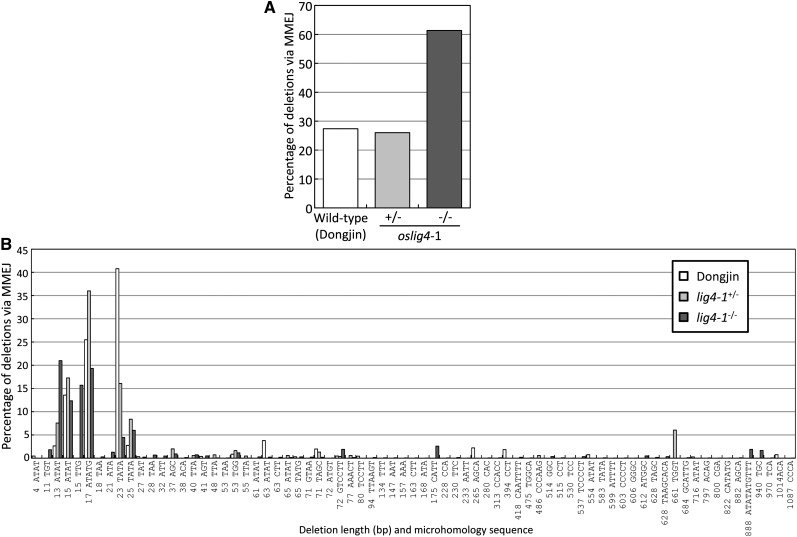
Next, we attempted to identify microhomology-based deletion events among all deletion events, because the altNHEJ pathway repairs TALEN-induced DSBs using microhomology at the DSB ends and thus is also known as MMEJ. About 60% of sequencing reads carrying deletion events at the repair site in oslig4-1−/− and oslig4-2−/− were shown to be repaired via a microhomology of more than 3 bp, while the frequencies of deletions via MMEJ in all deletion events were 25%, 25%, and 5% in cv Dongjin, oslig4-1+/−, and cv Koshihikari, respectively (Fig. 4A; Supplemental Fig. S9A). Furthermore, we examined the deletion length distribution in the sequencing reads containing greater than 3 bp microhomology at the repair site in the wild type and oslig4 mutants. In both the wild type and oslig4 mutants, a 13-bp deletion accompanying a 4-bp (ATAT) microhomology, a 15-bp deletion accompanying a 4-bp (ATAT) microhomology, a 17-bp deletion accompanying a 5-bp (ATATG) microhomology, a 23-bp deletion accompanying a 4-bp (TATA) microhomology, and a 25-bp deletion accompanying a 4-bp (TATA) microhomology were commonly used in MMEJ repair (Fig. 4B; Supplemental Fig. S9B).
Figure 4.
The frequency of deletions at the waxy locus repaired via the MMEJ pathway in wild-type (cv Dongjin) and oslig4-1 mutant calli. A, Proportion of deletions repaired by the MMEJ pathway in total deletions in wild-type (cv Dongjin), heterozygous oslig4-1 mutant (oslig4-1+/−) and homozygous oslig4-1 mutant (oslig4-1−/−) calli. B, Profile of deletions generated by the MMEJ pathway.
Analysis of the insertion sequences showed that almost all insertions greater than 2 bp were repaired via a copy-and-paste mechanism from one or more regions around the TALEN cleavage site that were rejoined via end joining that was dependent on or independent of microhomology regardless of the genetic background (Supplemental Fig. S10). In addition, 1-bp insertions potentially resulting from cNHEJ-induced error were detected only in the wild type and the heterozygous oslig4 mutant but not in oslig4 null mutants (Supplemental Fig. S11).
In addition, we designed TALENs targeting the Glb33 gene encoding glyoxalase I (also known as a major allergen of rice; Usui et al., 2001; Supplemental Fig. S12) and analyzed the frequency and pattern of TALEN-induced target mutagenesis at the Glb33 gene locus in wild-type and lig4 mutant rice (Supplemental Text S1). Consistent with the results at the waxy locus, the oslig4-deficient mutation increased the frequency of TALEN-induced mutations and the length of deletions and insertions at the Glb33 gene locus (Supplemental Tables S2–S4).
DISCUSSION
Using the single-strand annealing assay in yeast, two TALEN pairs, wx_L1/R2 and wx_L2/R2, among 20 TALEN pairs analyzed, displayed relatively high levels of DNA cleavage activity (Supplemental Fig. S1). The frequency of target mutagenesis in wx_L1/R2 TALEN transgenic calli was significantly higher than that of wx_L2/R2 TALEN transgenic calli. The TALEN activity of each TALEN pair in yeast and rice might be affected by protein stability or by the binding affinity between the TALE repeat and its target DNA via interference from an adjacent TALE repeat array.
We describe here the detailed characterization of TALEN-mediated mutations at the waxy locus both in rice calli (i.e. in actively proliferating cells) and in leaves of regenerated plants with fewer dividing cells. At the callus stage, several kinds of mutations occurred in a clonally propagated callus harboring a TALEN expression cassette in the same locus (Table I), suggesting that the induction of TALEN-induced DSBs and the subsequent occurrence of mutations due to DSB repair errors arose stochastically in each cell of the cloned callus and that mutated cells were propagated by cell division. Meanwhile, the chimerism of several kinds of mutated and nonmutated cells was barely detected in vegetative tissues (Table II) of regenerated plants and their progeny. Thus, TALEN-mediated mutations might not occur in rice cells during vegetative and reproductive growth, and any mutations that did occur could not be passed on through the germline (Tables II–V).
The maize polyubiquitin1 promoter, which was used to express high levels of TALEN proteins in rice, has been reported to be regulated by the cell cycle and to be active in rapidly dividing cells, such as callus and meristematic tissues, and the elongation and differentiation zones of root (Cornejo et al., 1993; Takimoto et al., 1994). However, its expression in vascular tissues of mature leaves under normal growth conditions is limited (Cornejo et al., 1993; Takimoto et al., 1994). In addition, loss of DNA methylation was found in rice calli and rice plants regenerated from calli, indicating that epigenetic changes were induced during the tissue culture step in rice (Stroud et al., 2013). Besides, the DNA-binding affinity of the TALE motif was affected by the presence of 5-methylated cytosine in its target sequence (Valton et al., 2012). Taking into consideration the results from the dedifferentiation of T1 seeds harboring TALEN expression cassettes (Table VI), rice calli might be an ideal tissue for TALEN-mediated mutagenesis via stable transformation.
It has been reported that the accurate repair of I-SceI-mediated DSBs via NHEJ was almost totally abolished and the extent of deletion was significantly larger in Ku80-defective mammalian cells, suggesting that the microhomology-dependent altNHEJ pathway can repair DSBs very efficiently in the absence of Ku protein (Guirouilh-Barbat et al., 2004, 2007). In contrast to previous findings in Arabidopsis ku70 or lig4 mutants, here we found not only an increment in deletion length but also an enhancement of the frequency of TALEN-mediated mutation in TALEN-expressing rice lig4 mutants (Figs. 3 and 4; Table VII; Supplemental Figs. S7 and S8; Supplemental Tables S2–S4).
Deep sequencing analysis of PCR products, including TALEN target sites, revealed that microhomology-dependent altNHEJ was involved in TALEN-mediated large deletions in rice lig4 mutant calli (Figure 4 and Supplemental Fig. S9), in good agreement with previous reports. However, not all deletion events were generated by microhomology-dependent altNHEJ, even in the rice lig4 mutants (Figure 5 and Supplemental Fig. S9). In higher plants, two DNA ligase genes, Lig1 and Lig6, have been identified in addition to Lig4 (Waterworth et al., 2009, 2010; Andreuzza et al., 2010), suggesting that these proteins might be able to partially complement the defect of Lig4 in the cNHEJ pathway. Meanwhile, the inconsistent results observed in the frequency of SSN-induced mutations between Arabidopsis and rice cNHEJ mutants might be attributed to differences in the frequency of mutation other than deletion, because we found an increased frequency of all types of mutations, including substitutions, deletions, and insertions (including a combination of insertion with deletion), in rice lig4 mutants (Fig. 3; Supplemental Fig. S7). Interestingly, our findings revealed that cNHEJ-mediated insertions (1 bp) were decreased (Supplemental Fig. S11) and insertion events (greater than 2 bp) involving copying the homologous DNA template around the TALEN cleavage sites were increased at TALEN cleavage sites in lig4 mutants (Fig. 3B; Supplemental Figs. S7B and S10).
Lig4 deficiency was reported to significantly increase insertion frequency but not deletion frequency during the joining of I-SceI-mediated DSBs at a transposable P-element in Drosophila spp. (Yu and McVey, 2010). In addition, many inserted sequences at I-SceI break sites have been shown to be templated from sequences near the DSB sites and to be rejoined via MMEJ; therefore, a model termed synthesis-dependent MMEJ has been proposed (Yu and McVey, 2010). On the other hand, it has been demonstrated that sequences from elsewhere in the genome or around the DSB site may be inserted into I-SceI break sites in exogenous genes via a synthesis-dependent strand annealing (SDSA)-like model in tobacco (Salomon and Puchta, 1998) and barley (Vu et al., 2014). These findings have suggested that the SDSA-like model may result in insertions within DSB sites by homology search via microhomology and copying from sister chromatid DNA. The synthesized strand can then be rejoined with the original strand via a microhomology-dependent or -independent pathway. Therefore, our findings indicate that the increased frequency of TALEN-induced deletion and insertion mutations might be explained by shifting the repair pathway from cNHEJ to altNHEJ and the synthesis-dependent MMEJ or SDSA-like pathway due to dysfunction of the cNHEJ pathway.
The discrepancy in the frequency of SSN-induced mutation between Arabidopsis and rice cNHEJ mutants could be explained by the difference in the mechanism used to escape DNA damage. It has been reported that DNA damage derived from treatment with genotoxic agents induced the onset of endocycle in Arabidopsis (Endo et al., 2006; Adachi et al., 2011). However, endocycle has never been observed in rice cells, even after genotoxic stress treatment (Endo et al., 2012). Accordingly, the frequency of TALEN-induced mutation in Arabidopsis cells might be underestimated compared with that in rice cells. In addition, differences in the assay system, including culture duration following the introduction of TALEN expression cassettes and the kinds of cells used for analysis, may also explain the different results of previous studies compared with this study.
It is widely accepted that DSBs mediated by SSNs at a target gene stimulate GT in several organisms, such as mammals (Pauwels et al., 2014), fish (Shin et al., 2014), insects (Lin et al., 2014), and plants (Puchta and Fauser, 2013; Voytas and Gao, 2014). Here, the induction of DSBs mediated via SSNs in a lig4 mutant background provided evidence that the SDSA-like pathway was enhanced by the disruption of cNHEJ in rice. We previously reported that the suppression of cNHEJ-related genes, including OsKu70, OsKu80, and OsLig4, led to decreased A. tumefaciens-mediated stable transformation and increased frequency of HR in rice (Nishizawa-Yokoi et al., 2012). Most recently, it has been reported that the treatment of mammalian cells with the Lig4 inhibitor Scr7 increased the frequency of break-induced GT (Chu et al., 2015; Maruyama et al., 2015). This approach also might be applicable to GT in plants. Taken together, the combination of the induction of DSBs mediated via SSNs at a target gene and the suppression of cNHEJ-related genes or treatment with Lig4 inhibitors can be expected to synergistically enhance the frequency of GT in rice.
MATERIALS AND METHODS
Vector Construction
TAL effector repeat arrays of the DNA-binding domain were constructed using the Golden Gate Assembly method as described previously (Cermak et al., 2011). Five left TALE repeats (wx_L1–wx_L5) and four right TALE repeats (wx_R1–wx_R4) targeting the waxy gene (Fig. 1) were cloned into yeast expression vectors, pTAL3 (with the HIS3 gene encoding His biosynthetic enzyme; Cermak et al., 2011) and pTAL4 (with the LEU2 gene encoding Leu synthesis enzyme; Cermak et al., 2011), respectively, for the DNA cleavage assay in yeast. The 165-bp wild-type and mutated TALEN target sequences were amplified by PCR and expression PCR (Lanar and Kain, 1994), respectively, using the following primer sets: for the wild-type TALEN target site, waxy TALrs-F (5′-AACGGCCAAGATCTTTATTGTG-3′; BglII site in italics) and waxy TALrs-R (5′-AAGACAGTGAAACTAGTAACGTTCATAA-3′; SpeI site in italics); for first expression PCR of mutated TALEN target site, waxy TALrs-F and waxy TALENm-R (5′-ATATTACAATGCCCTCTCCGCTTATAAG-3′), waxy TALENm-F (5′-GGCATTGTAATATCTCTCCTTGAGTTTTAG-3′) and waxy TALrs-R; for second expression PCR of mutated TALEN target site, waxy TALrs-F and waxy TALrs-R. These PCR fragments were cloned into the BglII/SpeI site between the two partially duplicated fragments of the lacZ gene of pCP5 (with URA3 and TRP1 genes encoding enzymes involved in the synthesis of pyrimidine ribonucleotides and Trp, respectively; Zhang et al., 2013), yielding pCP5/waxyTALENrs-wild and pCP5/waxyTALENrs-mut.
The left TALE repeats wx_L1 and wx_L2 and the right TALE repeat wx_R2, which have higher DNA cleavage activity in yeast, and the left TALE repeats Glb_TALE1-3l and Glb_TALE1-3r, targeting the Glb33 gene, were cloned into pZHY500 (for left TALE repeats) and pZHY501 (for right TALE repeats), respectively, containing truncated TALE backbone (NΔ152/CΔ63; Zhang et al., 2013). The left TALE repeats wx_L1, wx_L2, and Glb_TALE1-3l of pZHY500 and the right TALE repeats wx_R2 and Glb_TALE1-3r were digested with XbaI/BamHI and integrated sequentially into the XbaI/BamHI and compatible NheI/BglII sites of pZHY013 (Zhang et al., 2013) containing two heterodimeric FokI nuclease domains separated by a T2A translational skipping sequence. The wx_L1/R2, wx_L2/R2, Glb_TALEN1, Glb_TALEN2, and Glb_TALEN3 TALEN pairs were recloned into the binary vector pZN_ccdB (a derivative of pPN/hyPBase [Nishizawa-Yokoi et al., 2014] with a kanamycin resistance [nptII gene] cassette) between the maize polyubiquitin1 promoter (Pubi) and the Arabidopsis ribulose bisphosphate carboxylase small subunit gene terminator (TrbcS) using a Gateway LR Clonase II reaction (Life Technologies), yielding Pubi:wx_L1/R2, Pubi:wx_L2/R2, Pubi:Glb_TALEN1, Pubi:Glb_TALEN2, and Pubi:Glb_TALEN3, respectively.
Single-Strand Annealing Assay in Yeast
Twenty TALEN pair constructs (pTAL3 wx_L1/pTAL4 wx_R1-R4, pTAL3 wx_L2/pTAL4 wx_R1-R4, pTAL3 wx_L3/pTAL4 wx_R1-R4, pTAL3 wx_L4/pTAL4 wx_R1-R4, and pTAL3 wx_L5/ pTAL4 wx_R1-R4) were cotransformed into yeast strain EGY40 (Saccharomyces cerevisiae, a-mating type, trp1/his3/ura3/leu2; Wako), and reporter constructs pCP5/waxyTALENrs-wild and pCP5/waxyTALENrs-mut were transformed into yeast strain EGY188 (Saccharomyces cerevisiae, α-mating type, trp1/his3/ura3/leu2; Wako) according to the manufacturer’s instructions. Yeast mating procedures were performed according to the protocol of Cermak et al. (2011). The activity of β-galactosidase in yeast was measured using a Yeast β-Galactosidase Assay Kit (Thermo Scientific) according to the manufacturer’s instructions.
Plant Materials
Rice (Oryza sativa) ‘Nipponbare’, ‘Dongjin’ (genetic background of the oslig4-1 mutant [T-DNA insertional mutant]), and ‘Koshihikari’ (genetic background of the oslig4-2 mutant [γ-ray-induced mutant]) were used in this study. The oslig4-1 mutant line was obtained from the Rice T-DNA Insertion Sequence Database (http://signal.salk.edu/cgi-bin/RiceGE). Plant genotypes were determined by PCR using the T-DNA right border primer pGA2715 RB (5′-TTGGGGTTTCTACAGGACGTAAC-3′) and OsLig4-specific primers (5′-GAACAAATCTTTCCCGAGCA-3′ and 5′-GTCGAGGAGGAGAGGTGTGT-3′). The oslig4-2 mutant line was generated and isolated as follows. We screened 1,968 lines of γ-ray-induced mutants (Morita et al., 2009). To identify nonfunctional alleles of OsLig4 by the TILLING method, we designed primers to target five amplicons covering the open reading frame (ORF) of OsLig4. The target nucleotide sequences were amplified with sequence-specific primer sets (OsLig4-F5 and OsLig4-R5: 5′-TGCAGTTGTGCTGTATCCTTG-3′ and 5′-TGCCATGGCTTATCGTATCTC-3′) from pooled template DNAs that consist of four independent mutant DNAs. PCR was performed with PrimeSTAR GXL (TAKARA) in a GeneAmp PCR System9700 (Applied Biosciences) using 384-well plates. After heteroduplex formation through a heat-denaturing/annealing cycle, each amplicon was treated with a mismatch-specific nuclease, CEL I. Then, DNA fragments were prestained with the Gel Red dye (Wako) and separated with 2% (w/v) agarose gel electrophoresis with Lithium borate (LB) buffer (Funakoshi). Each nucleotide sequence of the PCR amplicon obtained from mutant candidates was determined with a Big Dye Terminator Cycle Sequencing Kit version 3.1 (Applied Biosciences) using the primers described above. Sequencing was performed on a 3130 Genetic Analyzer (Applied Biosciences) and analyzed by Sequence Scanner software version 1.0 (Applied Biosciences).
Agrobacterium tumefaciens-Mediated Transformation
A. tumefaciens-mediated transformation of rice was performed following the procedure of Toki et al. (2006). Mature seeds of cv Nipponbare, cv Dongjin, oslig4-1, cv Koshihikari, and oslig4-2 were inoculated on callus induction medium N6D (Saika and Toki, 2010) for cv Nipponbare, cv Dongjin, and oslig4-1 mutant or A1 (Supplemental Table S5) for cv Koshihikari and oslig4-2 mutant and grown for 4 weeks at 33°C. Four-week-old rice calli were transformed with A. tumefaciens harboring Pubi:L1/R2 or Pubi:L2/R2 and were selected on N6D or A1 medium solidified with 0.8% (w/v) agar (BD) containing 35 mg L−1 geneticin (Nacalai Tesque) and 25 mg L−1 meropenem (Wako Pure Chemical Industries). For regeneration, transgenic calli (cv Nipponbare) were transferred to regeneration medium ReIII (Saika and Toki, 2010) with 25 mg L−1 meropenem, and shoots arising from callus were transferred to Murashige and Skoog medium (Murashige and Skoog, 1962) without phytohormones.
CelI-Nuclease Mismatch Cleavage Assay
Genomic DNA was extracted from small pieces of rice calli or leaves of rice plants using Agencourt Chloropure (Beckman Coulter) according to the manufacturer’s protocol. PCR amplifications were performed with PrimeSTAR GXL (TAKARA) using the primer sets Waxy CelI F1 and Waxy CelI R1 (5′-TCACCATTCCTTCAGTTCTTTGTC-3′ and 5′-CTTCTCCAGGAATGACGGATGGT-3′) for the waxy gene locus and Glb33 F and Glb33 R (5′-GGGGGATGGGATGGTGAGATCATTCTTTCT-3′ and 5′-CAAAAACCTGAGCATATGCGTTGCCCTTGG-3′) for the Glb33 gene locus. PCR products were denatured and reannealed with a PCR program of 98°C for 10 min, 98°C to 85°C (−0.01°C s−1), 85°C for 1 min, and 85°C to 25°C (−0.05°C s−1) and were subjected to CelI nuclease cleavage assay.
CelI nuclease was purified from 20 kg of celery (Apium graveolens) stalks as reported (Oleykowski et al., 1998; Yang et al., 2000) with several modifications. Briefly, CelI nuclease was purified from celery juice with sequential ammonium sulfate precipitation, ConA-Sepharose chromatography (GE Healthcare), and Q-Sepharose FF chromatography (GE Healthcare). The CelI nuclease cleavage assay was performed as follows. Approximately 500 ng of reannealed PCR amplicons was incubated with purified CelI nuclease in buffer (0.4 m HEPES-NaOH [pH 6], 20 mm KCl, 6 mm MgCl2, and 0.004% (v/v) Triton X-100) at 42°C for 20 min. The reaction was terminated by adding stop solution (25 mm EDTA, 10% (v/v) glycerol, 0.1% (v/v) Sodium Orange G, and 0.0025% (v/v) Gel Red [Wako Pure Chemical Industries]) and analyzed on a 2% (w/v) agarose gel with Luria-Bertani buffer (Funakoshi).
Sequencing Analysis of TALEN-Induced Mutations at the waxy Gene
The 1- and 2-kb fragments surrounding the wx_L1/R2 TALEN and Glb_TALEN1 pair recognition sites for the waxy and Glb33 genes were amplified by PCR with PrimeSTAR GXL (TAKARA) using the primer sets Waxy CelI F2 and Waxy CelI R2 (5′-GAGTCTAGATCTTGTGTTCAACTCTCGT-3′ and 5′-GACGAACACGACGTTCATGC-3′) and Glb33 F and Glb33 R, respectively, and was cloned into the pCRII-Blunt-TOPO vector using TOPO cloning methods (Life Technologies). Plasmid DNA was purified using a QIAprep Spin Miniprep Kit (Qiagen) and sequenced using the primer Waxy 843F (5′-GTGCAAGAATTCAGTGTGAAGG-3′) for the waxy gene and Glb33 Seq F1 (5′-CAAGCGGTAGTGAAGCTGAGAA-3′) or Glb33 Seq F2 (5′-CAAAGGTGGGGATCTTTTTG-3′) for the Glb33 gene.
RNA Extraction and Quantitative RT-PCR Analysis
Total RNA was extracted from rice calli using an RNeasy Plant Mini Kit (Qiagen) according to the manufacturer’s protocol. Quantitative RT-PCR was performed with a Power SYBR Green PCR Master Mix (Life Technologies) using the primers OsLig4 RT-F and RT-R (5′-TTGGTGAATGCGGACTACAA-3′ and 5′-AATGCTGCACACTTGACCAC-3′) and an ABI7300 (Life Technologies).
Next-Generation DNA Sequencing
Total genomic DNA was extracted from rice calli using Agencourt Chloropure (Beckman Coulter). The 2-kb fragment surrounding the L1/R2 TALEN pair recognition site for the waxy gene was amplified by PCR with PrimeSTAR GXL (TAKARA) using the primers Waxy CelI F1 and Waxy CelI R and purified by Agencourt AMPure XP (Beckman Coulter) according to the manufacturer’s instructions.
For amplicon sequencing, 1 ng of each amplicon DNA was subsequently used for tagmented sequencing library preparation using the Nextera XT DNA Sample Prep Kit (Illumina; FC-131-1096) and the Nextera XT Index Kit (96 indices; Illumina; FC-131-1002). Sequencing was done on a MiSeq sequencer with 300-bp reads on both sides. Each library was indexed with a barcode, and reads were automatically partitioned after sequencing with MiSeq Reporter (Illumina). Sequenced reads were mapped on reference sequences with bwa (Li and Durbin, 2009), converting the mapped data to a Sequence Alignment/Map (SAM) file and then generating a pileup of the sequence data. Single-nucleotide polymorphisms, deletions, and insertions on alignment were detected.
Mutations detected on less than 10 reads and detected in a location other that the spacer region between the two TALEN binding sites were defined as false positive, such as PCR errors.
Sequence data from this article can be found in the Rice Genome Annotation Project (http://rice.plantbiology.msu.edu/) data libraries under the following accession numbers: rice waxy (LOC_Os06g04200), rice Lig4 (LOC_Os04g51700), and rice Glb33 (LOC_Os08g09250).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. The design and construction of TALENs and their target sequence in the waxy gene and the evaluation of TALENs activity using yeast single-strand annealing (SSA) assay.
Supplemental Figure S2. Experimental scheme for TALEN-induced mutagenesis in rice.
Supplemental Figure S3. Representative results of CelI assay.
Supplemental Figure S4. An example of sequencing chromatogram of the wx_L1/R2 TALENs-target sites in T2 progenies.
Supplemental Figure S5. Experimental scheme of TALEN-mediated de novo mutagenesis in Pubi:wx_L1/R2 T1 rice calli.
Supplemental Figure S6. Molecular analysis of Oslig4 knock-out lines.
Supplemental Figure S7. TALEN-mediated mutation frequency in wild-type (Koshihikari) and oslig4-2 null mutant calli at the waxy locus.
Supplemental Figure S8. Mutation frequency categorized by the deletion length in wild-type (Koshihikari) and oslig4-2 mutant calli.
Supplemental Figure S9. The frequency of deletions repaired via MMEJ pathway in wild-type (Koshihikari) and oslig4-2 mutant calli.
Supplemental Figure S10. Representative sequences of TALEN-mediated insertional-mutations in Dongjin.
Supplemental Figure S11. Enhanced length of insertions was observed in oslig4 mutant calli compared to wild-type calli.
Supplemental Figure S12. DNA sequences of TALEN target sites in the Glb33 gene.
Supplemental Table S1. TALEN-mediated mutations frequency of different mutation types in the waxy locus in Nipponbare calli using Illumina MiSeq.
Supplemental Table S2. Frequency of TALEN-mediated mutations in the Glb33 locus in Dongjin (genetic background of oslig4-1), oslig4-1, Koshihikari (genetic background of oslig4-2), and oslig4-2 calli.
Supplemental Table S3. Mutation types and frequencies in the Glb33 locus in Pubi:Glb_TALEN1-expressing Dongjin and oslig4-1−/− calli.
Supplemental Table S4. Mutation types and frequencies in the Glb33 locus in Pubi:Glb_TALEN1-expressing Koshihikari and oslig4-2−/− calli.
Supplemental Table S5. Composition of culture media used for callus induction (A1).
Supplemental Text S1. The evaluation of TALENs activity using yeast single-strand annealing (SSA) assay and the analysis of TALEN-induced target mutagenesis at Glb33 gene in lig4 mutant rice.
Supplementary Material
Acknowledgments
We thank Dr. Minoru Nishimura (Niigata University) for providing the γ-ray-irradiated library, Dr. Helen Rothnie (Dr. Helen M. Rothnie Manuscript Services) for English editing, Dr. Masaki Endo, Namie Ohtsuki, and Masafumi Mikami (National Institute of Agrobiological Sciences) for insightful discussions, and Kiyoko Amagai, Akemi Nagashii, Fukuko Suzuki, and Rieko Aoto (National Institute of Agrobiological Sciences) for general experimental technical support.
Glossary
- DSB
double-strand break
- SSN
sequence-specific nuclease
- HR
homologous recombination
- NHEJ
nonhomologous end joining
- GT
gene targeting
- cNHEJ
classical nonhomologous end joining
- altNHEJ
alternative nonhomologous end joining
- MMEJ
microhomology-mediated end joining
- T-DNA
transfer DNA
- RT
reverse transcription
- SDSA
synthesis-dependent strand annealing
Footnotes
This work was supported by the Ministry of Agriculture, Forestry, and Fisheries of Japan (Genomics for Agricultural Innovation grant no. PGE1002), KAKENHI (grant nos. 24241028 and 23310142), the Cross-Ministerial Strategic Innovation Promotion Program, and the Program for the Promotion of Basic and Applied Researches for Innovations in Bio-oriented Industry.
Articles can be viewed without a subscription.
References
- Adachi S, Minamisawa K, Okushima Y, Inagaki S, Yoshiyama K, Kondou Y, Kaminuma E, Kawashima M, Toyoda T, Matsui M, et al. (2011) Programmed induction of endoreduplication by DNA double-strand breaks in Arabidopsis. Proc Natl Acad Sci USA 108: 10004–10009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreuzza S, Li J, Guitton AE, Faure JE, Casanova S, Park JS, Choi Y, Chen Z, Berger F (2010) DNA LIGASE I exerts a maternal effect on seed development in Arabidopsis thaliana. Development 137: 73–81 [DOI] [PubMed] [Google Scholar]
- Audebert M, Salles B, Calsou P (2004) Involvement of poly(ADP-ribose) polymerase-1 and XRCC1/DNA ligase III in an alternative route for DNA double-strand breaks rejoining. J Biol Chem 279: 55117–55126 [DOI] [PubMed] [Google Scholar]
- Carroll D. (2011) Genome engineering with zinc-finger nucleases. Genetics 188: 773–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cermak T, Doyle EL, Christian M, Wang L, Zhang Y, Schmidt C, Baller JA, Somia NV, Bogdanove AJ, Voytas DF (2011) Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res 39: e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman JR, Taylor MR, Boulton SJ (2012) Playing the end game: DNA double-strand break repair pathway choice. Mol Cell 47: 497–510 [DOI] [PubMed] [Google Scholar]
- Char SN, Unger-Wallace E, Frame B, Briggs SA, Main M, Spalding MH, Vollbrecht E, Wang K, Yang B (2015) Heritable site-specific mutagenesis using TALENs in maize. Plant Biotechnol J 13: 1002–1010 [DOI] [PubMed] [Google Scholar]
- Charbonnel C, Allain E, Gallego ME, White CI (2011) Kinetic analysis of DNA double-strand break repair pathways in Arabidopsis. DNA Repair (Amst) 10: 611–619 [DOI] [PubMed] [Google Scholar]
- Charbonnel C, Gallego ME, White CI (2010) Xrcc1-dependent and Ku-dependent DNA double-strand break repair kinetics in Arabidopsis plants. Plant J 64: 280–290 [DOI] [PubMed] [Google Scholar]
- Cheng Q, Barboule N, Frit P, Gomez D, Bombarde O, Couderc B, Ren GS, Salles B, Calsou P (2011) Ku counteracts mobilization of PARP1 and MRN in chromatin damaged with DNA double-strand breaks. Nucleic Acids Res 39: 9605–9619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier BS, Kortemme T, Chadsey MS, Baker D, Monnat RJ, Stoddard BL (2002) Design, activity, and structure of a highly specific artificial endonuclease. Mol Cell 10: 895–905 [DOI] [PubMed] [Google Scholar]
- Christian M, Cermak T, Doyle EL, Schmidt C, Zhang F, Hummel A, Bogdanove AJ, Voytas DF (2010) Targeting DNA double-strand breaks with TAL effector nucleases. Genetics 186: 757–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian M, Qi Y, Zhang Y, Voytas DF (2013) Targeted mutagenesis of Arabidopsis thaliana using engineered TAL effector nucleases. G3 (Bethesda) 3: 1697–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu VT, Weber T, Wefers B, Wurst W, Sander S, Rajewsky K, Kühn R (2015) Increasing the efficiency of homology-directed repair for CRISPR-Cas9-induced precise gene editing in mammalian cells. Nat Biotechnol 33: 543–548 [DOI] [PubMed] [Google Scholar]
- Clasen BM, Stoddard TJ, Luo S, Demorest ZL, Li J, Cedrone F, Tibebu R, Davison S, Ray EE, Daulhac A, et al. (2016) Improving cold storage and processing traits in potato through targeted gene knockout. Plant Biotechnol J 14: 169–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornejo MJ, Luth D, Blankenship KM, Anderson OD, Blechl AE (1993) Activity of a maize ubiquitin promoter in transgenic rice. Plant Mol Biol 23: 567–581 [DOI] [PubMed] [Google Scholar]
- Deriano L, Roth DB (2013) Modernizing the nonhomologous end-joining repertoire: alternative and classical NHEJ share the stage. Annu Rev Genet 47: 433–455 [DOI] [PubMed] [Google Scholar]
- Edlinger B, Schlögelhofer P (2011) Have a break: determinants of meiotic DNA double strand break (DSB) formation and processing in plants. J Exp Bot 62: 1545–1563 [DOI] [PubMed] [Google Scholar]
- Endo M, Ishikawa Y, Osakabe K, Nakayama S, Kaya H, Araki T, Shibahara K, Abe K, Ichikawa H, Valentine L, et al. (2006) Increased frequency of homologous recombination and T-DNA integration in Arabidopsis CAF-1 mutants. EMBO J 25: 5579–5590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M, Nakayama S, Umeda-Hara C, Ohtsuki N, Saika H, Umeda M, Toki S (2012) CDKB2 is involved in mitosis and DNA damage response in rice. Plant J 69: 967–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forner J, Pfeiffer A, Langenecker T, Manavella P, Lohmann JU (2015) Germline-transmitted genome editing in Arabidopsis thaliana using TAL-effector-nucleases. PLoS ONE 10: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaj T, Gersbach CA, Barbas CF III (2013) ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol 31: 397–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guirouilh-Barbat J, Huck S, Bertrand P, Pirzio L, Desmaze C, Sabatier L, Lopez BS (2004) Impact of the KU80 pathway on NHEJ-induced genome rearrangements in mammalian cells. Mol Cell 14: 611–623 [DOI] [PubMed] [Google Scholar]
- Guirouilh-Barbat J, Rass E, Plo I, Bertrand P, Lopez BS (2007) Defects in XRCC4 and KU80 differentially affect the joining of distal nonhomologous ends. Proc Natl Acad Sci USA 104: 20902–20907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurushidze M, Hensel G, Hiekel S, Schedel S, Valkov V, Kumlehn J (2014) True-breeding targeted gene knock-out in barley using designer TALE-nuclease in haploid cells. PLoS ONE 9: e92046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haun W, Coffman A, Clasen BM, Demorest ZL, Lowy A, Ray E, Retterath A, Stoddard T, Juillerat A, Cedrone F, et al. (2014) Improved soybean oil quality by targeted mutagenesis of the fatty acid desaturase 2 gene family. Plant Biotechnol J 12: 934–940 [DOI] [PubMed] [Google Scholar]
- Hong JP, Byun MY, An K, Yang SJ, An G, Kim WT (2010) OsKu70 is associated with developmental growth and genome stability in rice. Plant Physiol 152: 374–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Q, den Dulk-Ras A, Shen H, Hooykaas PJ, de Pater S (2013) Poly(ADP-ribose)polymerases are involved in microhomology mediated back-up non-homologous end joining in Arabidopsis thaliana. Plant Mol Biol 82: 339–351 [DOI] [PubMed] [Google Scholar]
- Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E (2012) A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337: 816–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YG, Cha J, Chandrasegaran S (1996) Hybrid restriction enzymes: zinc finger fusions to Fok I cleavage domain. Proc Natl Acad Sci USA 93: 1156–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanar DE, Kain KC (1994) Expression-PCR (E-PCR): overview and applications. PCR Methods Appl 4: S92–S96 [DOI] [PubMed] [Google Scholar]
- Li H, Durbin R (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25: 1754–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Stoddard TJ, Demorest ZL, Lavoie PO, Luo S, Clasen BM, Cedrone F, Ray EE, Coffman AP, Daulhac A, et al. (2015) Multiplexed, targeted gene editing in Nicotiana benthamiana for glyco-engineering and monoclonal antibody production. Plant Biotechnol J (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Liu B, Spalding MH, Weeks DP, Yang B (2012) High-efficiency TALEN-based gene editing produces disease-resistant rice. Nat Biotechnol 30: 390–392 [DOI] [PubMed] [Google Scholar]
- Lin SC, Chang YY, Chan CC (2014) Strategies for gene disruption in Drosophila. Cell Biosci 4: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lor VS, Starker CG, Voytas DF, Weiss D, Olszewski NE (2014) Targeted mutagenesis of the tomato PROCERA gene using transcription activator-like effector nucleases. Plant Physiol 166: 1288–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S, Li J, Stoddard TJ, Baltes NJ, Demorest ZL, Clasen BM, Coffman A, Retterath A, Mathis L, Voytas DF, et al. (2015) Non-transgenic plant genome editing using purified sequence-specific nucleases. Mol Plant 8: 1425–1427 [DOI] [PubMed] [Google Scholar]
- Maruyama T, Dougan SK, Truttmann MC, Bilate AM, Ingram JR, Ploegh HL (2015) Increasing the efficiency of precise genome editing with CRISPR-Cas9 by inhibition of nonhomologous end joining. Nat Biotechnol 33: 538–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JC, Tan S, Qiao G, Barlow KA, Wang J, Xia DF, Meng X, Paschon DE, Leung E, Hinkley SJ, et al. (2011) A TALE nuclease architecture for efficient genome editing. Nat Biotechnol 29: 143–148 [DOI] [PubMed] [Google Scholar]
- Mladenov E, Iliakis G (2011) Induction and repair of DNA double strand breaks: the increasing spectrum of non-homologous end joining pathways. Mutat Res 711: 61–72 [DOI] [PubMed] [Google Scholar]
- Morita R, Kusaba M, Iida S, Yamaguchi H, Nishio T, Nishimura M (2009) Molecular characterization of mutations induced by gamma irradiation in rice. Genes Genet Syst 84: 361–370 [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for the rapid growth and bioassay with tobacco tissue cultures. Physiol Plant 15: 473–497 [Google Scholar]
- Mussolino C, Morbitzer R, Lütge F, Dannemann N, Lahaye T, Cathomen T (2011) A novel TALE nuclease scaffold enables high genome editing activity in combination with low toxicity. Nucleic Acids Res 39: 9283–9293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa-Yokoi A, Endo M, Osakabe K, Saika H, Toki S (2014) Precise marker excision system using an animal-derived piggyBac transposon in plants. Plant J 77: 454–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa-Yokoi A, Nonaka S, Saika H, Kwon YI, Osakabe K, Toki S (2012) Suppression of Ku70/80 or Lig4 leads to decreased stable transformation and enhanced homologous recombination in rice. New Phytol 196: 1048–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleykowski CA, Bronson Mullins CR, Godwin AK, Yeung AT (1998) Mutation detection using a novel plant endonuclease. Nucleic Acids Res 26: 4597–4602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osakabe K, Osakabe Y, Toki S (2010) Site-directed mutagenesis in Arabidopsis using custom-designed zinc finger nucleases. Proc Natl Acad Sci USA 107: 12034–12039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa K, Wakasa Y, Ogo Y, Matsuo K, Kawahigashi H, Takaiwa F (2012) Development of an efficient Agrobacterium-mediated gene targeting system for rice and analysis of rice knockouts lacking granule-bound starch synthase (Waxy) and β1,2-xylosyltransferase. Plant Cell Physiol 53: 755–761 [DOI] [PubMed] [Google Scholar]
- Pauwels K, Podevin N, Breyer D, Carroll D, Herman P (2014) Engineering nucleases for gene targeting: safety and regulatory considerations. New Biotechnol 31: 18–27 [DOI] [PubMed] [Google Scholar]
- Puchta H, Fauser F (2013) Gene targeting in plants: 25 years later. Int J Dev Biol 57: 629–637 [DOI] [PubMed] [Google Scholar]
- Qi Y, Zhang Y, Zhang F, Baller JA, Cleland SC, Ryu Y, Starker CG, Voytas DF (2013) Increasing frequencies of site-specific mutagenesis and gene targeting in Arabidopsis by manipulating DNA repair pathways. Genome Res 23: 547–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosidi B, Wang M, Wu W, Sharma A, Wang H, Iliakis G (2008) Histone H1 functions as a stimulatory factor in backup pathways of NHEJ. Nucleic Acids Res 36: 1610–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saika H, Toki S (2010) Mature seed-derived callus of the model indica rice variety Kasalath is highly competent in Agrobacterium-mediated transformation. Plant Cell Rep 29: 1351–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon S, Puchta H (1998) Capture of genomic and T-DNA sequences during double-strand break repair in somatic plant cells. EMBO J 17: 6086–6095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai S, Ohyama K, Yasumoto S, Seki H, Sakuma T, Yamamoto T, Takebayashi Y, Kojima M, Sakakibara H, Aoki T, et al. (2014) Sterol side chain reductase 2 is a key enzyme in the biosynthesis of cholesterol, the common precursor of toxic steroidal glycoalkaloids in potato. Plant Cell 26: 3763–3774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan Q, Wang Y, Chen K, Liang Z, Li J, Zhang Y, Zhang K, Liu J, Voytas DF, Zheng X, et al. (2013) Rapid and efficient gene modification in rice and Brachypodium using TALENs. Mol Plant 6: 1365–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan Q, Zhang Y, Chen K, Zhang K, Gao C (2015) Creation of fragrant rice by targeted knockout of the OsBADH2 gene using TALEN technology. Plant Biotechnol J 13: 791–800 [DOI] [PubMed] [Google Scholar]
- Shin J, Chen J, Solnica-Krezel L (2014) Efficient homologous recombination-mediated genome engineering in zebrafish using TALE nucleases. Development 141: 3807–3818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivastav M, De Haro LP, Nickoloff JA (2008) Regulation of DNA double-strand break repair pathway choice. Cell Res 18: 134–147 [DOI] [PubMed] [Google Scholar]
- Singh SK, Roy S, Choudhury SR, Sengupta DN (2010) DNA repair and recombination in higher plants: insights from comparative genomics of Arabidopsis and rice. BMC Genomics 11: 443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud H, Ding B, Simon SA, Feng S, Bellizzi M, Pellegrini M, Wang GL, Meyers BC, Jacobsen SE (2013) Plants regenerated from tissue culture contain stable epigenome changes in rice. eLife 2: e00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Li N, Huang G, Xu J, Pan Y, Wang Z, Tang Q, Song M, Wang X (2013) Site-specific gene targeting using transcription activator-like effector (TALE)-based nuclease in Brassica oleracea. J Integr Plant Biol 55: 1092–1103 [DOI] [PubMed] [Google Scholar]
- Symington LS, Gautier J (2011) Double-strand break end resection and repair pathway choice. Annu Rev Genet 45: 247–271 [DOI] [PubMed] [Google Scholar]
- Takimoto I, Christensen AH, Quail PH, Uchimiya H, Toki S (1994) Non-systemic expression of a stress-responsive maize polyubiquitin gene (Ubi-1) in transgenic rice plants. Plant Mol Biol 26: 1007–1012 [DOI] [PubMed] [Google Scholar]
- Tamura K, Adachi Y, Chiba K, Oguchi K, Takahashi H (2002) Identification of Ku70 and Ku80 homologues in Arabidopsis thaliana: evidence for a role in the repair of DNA double-strand breaks. Plant J 29: 771–781 [DOI] [PubMed] [Google Scholar]
- Terada R, Nagahara M, Furukawa K, Shimamoto M, Yamaguchi K, Iida S (2010) Cre-loxP mediated marker elimination and gene reactivation at the waxy locus created in rice genome based on strong positive-negative selection. Plant Biotechnol 27: 29–37 [Google Scholar]
- Terada R, Urawa H, Inagaki Y, Tsugane K, Iida S (2002) Efficient gene targeting by homologous recombination in rice. Nat Biotechnol 20: 1030–1034 [DOI] [PubMed] [Google Scholar]
- Toki S, Hara N, Ono K, Onodera H, Tagiri A, Oka S, Tanaka H (2006) Early infection of scutellum tissue with Agrobacterium allows high-speed transformation of rice. Plant J 47: 969–976 [DOI] [PubMed] [Google Scholar]
- Usui Y, Nakase M, Hotta H, Urisu A, Aoki N, Kitajima K, Matsuda T (2001) A 33-kDa allergen from rice (Oryza sativa L. japonica): cDNA cloning, expression, and identification as a novel glyoxalase I. J Biol Chem 276: 11376–11381 [DOI] [PubMed] [Google Scholar]
- Valton J, Dupuy A, Daboussi F, Thomas S, Maréchal A, Macmaster R, Melliand K, Juillerat A, Duchateau P (2012) Overcoming transcription activator-like effector (TALE) DNA binding domain sensitivity to cytosine methylation. J Biol Chem 287: 38427–38432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytas DF. (2013) Plant genome engineering with sequence-specific nucleases. Annu Rev Plant Biol 64: 327–350 [DOI] [PubMed] [Google Scholar]
- Voytas DF, Gao C (2014) Precision genome engineering and agriculture: opportunities and regulatory challenges. PLoS Biol 12: e1001877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu GT, Cao HX, Watanabe K, Hensel G, Blattner FR, Kumlehn J, Schubert I (2014) Repair of site-specific DNA double-strand breaks in barley occurs via diverse pathways primarily involving the sister chromatid. Plant Cell 26: 2156–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Liu Y, Zhang C, Liu J, Liu X, Wang L, Wang W, Chen H, Wei C, Ye X, et al. (2015) Gene editing by co-transformation of TALEN and chimeric RNA/DNA oligonucleotides on the rice OsEPSPS gene and the inheritance of mutations. PLoS ONE 10: e0122755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Cheng X, Shan Q, Zhang Y, Liu J, Gao C, Qiu JL (2014) Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat Biotechnol 32: 947–951 [DOI] [PubMed] [Google Scholar]
- Waterworth WM, Kozak J, Provost CM, Bray CM, Angelis KJ, West CE (2009) DNA ligase 1 deficient plants display severe growth defects and delayed repair of both DNA single and double strand breaks. BMC Plant Biol 9: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterworth WM, Masnavi G, Bhardwaj RM, Jiang Q, Bray CM, West CE (2010) A plant DNA ligase is an important determinant of seed longevity. Plant J 63: 848–860 [DOI] [PubMed] [Google Scholar]
- Wendt T, Holm PB, Starker CG, Christian M, Voytas DF, Brinch-Pedersen H, Holme IB (2013) TAL effector nucleases induce mutations at a pre-selected location in the genome of primary barley transformants. Plant Mol Biol 83: 279–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West CE, Waterworth WM, Jiang Q, Bray CM (2000) Arabidopsis DNA ligase IV is induced by gamma-irradiation and interacts with an Arabidopsis homologue of the double strand break repair protein XRCC4. Plant J 24: 67–78 [DOI] [PubMed] [Google Scholar]
- Woo JW, Kim J, Kwon SI, Corvalán C, Cho SW, Kim H, Kim SG, Kim ST, Choe S, Kim JS (2015) DNA-free genome editing in plants with preassembled CRISPR-Cas9 ribonucleoproteins. Nat Biotechnol 33: 1162–1164 [DOI] [PubMed] [Google Scholar]
- Yang B, Wen X, Kodali NS, Oleykowski CA, Miller CG, Kulinski J, Besack D, Yeung JA, Kowalski D, Yeung AT (2000) Purification, cloning, and characterization of the CEL I nuclease. Biochemistry 39: 3533–3541 [DOI] [PubMed] [Google Scholar]
- Yu AM, McVey M (2010) Synthesis-dependent microhomology-mediated end joining accounts for multiple types of repair junctions. Nucleic Acids Res 38: 5706–5717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Gou F, Zhang J, Liu W, Li Q, Mao Y, Botella JR, Zhu JK (2016) TALEN-mediated targeted mutagenesis produces a large variety of heritable mutations in rice. Plant Biotechnol J 14: 186–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang F, Li X, Baller JA, Qi Y, Starker CG, Bogdanove AJ, Voytas DF (2013) Transcription activator-like effector nucleases enable efficient plant genome engineering. Plant Physiol 161: 20–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.